Abstract
Objective
The associations between maternal use of antidepressant during pregnancy and preterm birth (PTB) has been the subject of much discussion and controversy. The aim of the present study was to systematically review the association between antidepressant use during pregnancy and the risk of PTB, especially in depressed women.
Methods
A computerized search was conducted in PubMed, PsycINFO, and Embase before June 30, 2019, supplemented with a manual search of the reference lists, to identify original research regarding PTB rates in women taking antidepressants during pregnancy. A random-effects model was used to calculate the summarized relative risks (RRs) and 95% confidence intervals (CIs). The potential for publication bias was examined through Begg' s and Egger' s tests.
Results
A total of 2,279 articles were reviewed, 23 of which were selected. The risk of PTB was increased in women with depression [1.58 (1.23−2.04)] and in the general pregnant female population [1.35 (1.11−1.63)] who used antidepressants during pregnancy. Similar results were observed in depressed women treated with selective serotonin reuptake inhibitors (SSRIs) during pregnancy [1.46 (1.32−1.61)]. There was no significantly increased risk of PTB observed with SSRI use in the general pregnant female population [1.25 (1.00−1.57)], and the heterogeneity of these studies was high.
Conclusions
The results of this meta-analysis indicate maternal antidepressant use is associated with a significantly increased risk of PTB in infants. Health care providers and pregnant women must weigh the risk-benefit potential of these drugs when making decisions about whether to treat with antidepressant during pregnancy.
Keywords: antidepressants, depression, drug safety, meta-analysis, preterm birth
Introduction
Preterm birth (PTB), defined as delivery prior to 37 weeks of gestation, is regarded as a global public health concern, since it is the leading cause of infant mortality worldwide (Blencowe et al., 2012). In comparison with infants born full-term, PTB infants are at a greater risk of multiple health problems, including neurological and long-term developmental disorders (Marlow et al., 2000). Rates of PTB have fortunately declined over the last decade, occurring in approximately 12% of pregnancies in the U.S. (Purisch and Gyamfi-Bannerman, 2017). This decline is due, in part, to declines in the number of births to teens and young mothers. The annual economic cost related to PTB has been approximated at $26 billion dollars in the U.S. and £939 million in the U.K. (Mangham et al., 2009).
Many factors are thought to contribute to the increased PTB rate, including a higher mean maternal age, more frequent use of assisted reproductive technologies and a resulting increase in multiple gestations, and higher rates of preterm inductions and cesarean deliveries (Chang et al., 2013). Interestingly, since the prevalence of depression during pregnancy has increased from 8.3% to 12.7%, many studies have shown that antidepressant use during pregnancy increases the risk of PTB (Lee et al., 2007; El Marroun et al., 2014; Staneva et al., 2015). Additionally, there exists emerging evidence that maternal depression during pregnancy is a risk factor for PTB (El Marroun et al., 2014; Staneva et al., 2015). However, based on the existing literature, it is difficult to draw a unified conclusion regarding the association of antidepressant use during pregnancy and PTB, since the research differs in terms of the timing of antidepressant use during pregnancy, adjustment for potential confounding variables, inclusion of lifestyle factors, presence of comorbidities, and severity of the underlying psychiatric illness. The existing four published meta-analyses (Huybrechts et al., 2014; Ross and Grigoriadis, 2014; Hsiang and Bridge, 2014; Eke et al., 2016) had several limitations. Firstly, these meta-analyses consisted of both retrospective and prospective cohort studies, the former of which may have caused recall bias. Moreover, in these meta-analyses, there were no reports of depressed pregnant women in the study population (Huybrechts et al., 2014; Ross and Grigoriadis, 2014; Hsiang and Bridge, 2014), with the exception of one study (Eke et al., 2016) including eight original studies that examined the effect of selective serotonin reuptake inhibitor (SSRI) use on PTB in pregnant women with depression. Additionally, information regarding subgroup analyses stratified by geographic locations and whether adjustments were made for confounders [e.g., maternal age, ethnicity, education, smoking, alcohol consumption, body mass index (BMI), and parity] were not provided.
Therefore, in the present study, a meta-analysis of all available studies was conducted to quantitate the strength of the relationship between antidepressant use during pregnancy and PTB especially in depressed women.
Materials and Methods
Search Strategy
The PubMed, PsycINFO, and Embase databases were searched for related articles published before June 30, 2019. The search queries used are listed in Supplementary File 1. The articles were imported into the NoteExpress library for removal of the duplicates.
Study Selection
Eligibility criteria were defined as: (1) articles available in English with the full text provided; (2) studies conducted in humans; (3) prospective cohort studies; (4) antidepressant use as the study exposure and PTB as the study outcome; (5) antidepressant use during pregnancy; (6) no psychotropic drug use in the control group; and (7) reported risk ratio or adequate data for the calculation of an effect size as a relative risk (RR) between antidepressant use and PTB.
Data Extraction
The study quality of eligible articles was assessed by two reviewers (X-YM and X-RX), who subsequently extracted data from each selected article including the study design, sample size, data source, criteria for inclusion/exclusion, definition of exposure, definition of outcome, outcomes with risk estimates and 95% confidence intervals (CIs), and adjusted confounders. The two reviewers extracted data independently and any disagreements were resolved by discussion with a third reviewer (QC), when necessary.
For one study (Källén, 2004) that characterized neonates in the Swedish birth registry following maternal antidepressant use in late pregnancy, discussion among the three reviewers resulted in recalculation of the crude RR value according to the control group as the non-exposed population.
Risk of Bias Assessment
The two reviewers independently performed a quality assessment using the Newcastle−Ottawa scale (NOS) (Wells et al., 2011) for cohort studies. The scale of this quality tool consists of three components for which a maximum of 9 points can be given: selection of study participant groups (max. 4 points); comparability of study groups (max. 2 points); and ascertainment of outcome (max. 3 points). Studies were considered to have a low risk of bias if they achieved a full rating in at least two of these categories (Odutayo et al., 2016).
Statistical Analysis
STATA, version 11.0 (StataCorp, College Station, TX) was used for all statistical analyses. The mean effect size approach was used to pool estimates, which has been applied in other studies (McDonald et al., 2010; Gao et al., 2018). The effect size was weighted as per the study sample size. Estimates were pooled using the DerSimonian and Laird random-effects model to calculate the summarized RRs and 95% CI, as large inter-study heterogeneity was expected (DerSimonian and Laird, 1986). For studies reporting the necessary data instead of providing the risk estimates directly, these data were used to calculate the crude RRs. The I2 value represents the percentage of total variation across studies due to heterogeneity rather than chance (Higgins and Green, 2011). Values of 25%, 50%, and 75% are regarded as representing low, moderate, and high heterogeneity (Higgins et al., 2003). A two-tailed P-value <0.05 was considered statistically significant. Potential publication bias was assessed using Begg's test (Begg and Mazumdar, 1994).
If more than eight studies were available, potential sources of heterogeneity were explored by conducting subgroup analyses according to the following parameters: geographic location (Europe vs. North America or other regions) and adjustment for potential confounders (adjusted vs. unadjusted) including maternal age, ethnicity, maternal education, tobacco use, alcohol consumption, BMI during pregnancy, and parity. Heterogeneity between subgroups was evaluated by meta-regression analysis. To determine the influence of individual studies on each analysis of the estimated RR, sensitivity analysis was conducted that recalculated the pooled effect by omitting one study at a time. The population was divided into four groups: antidepressant use in the general pregnant female population, antidepressant use in pregnant women with depression, SSRI use in the general pregnant female population, and SSRI use in pregnant women with depression.
Results
Search Results
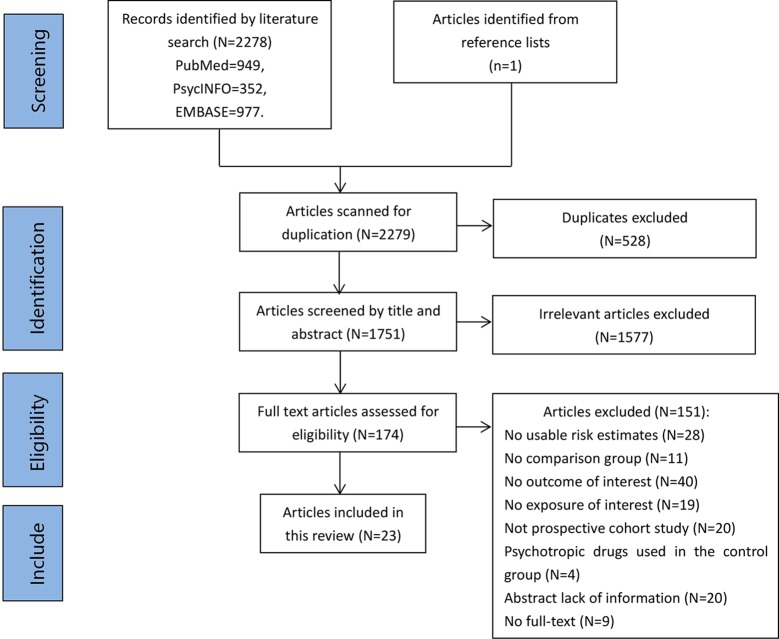
A total of 2,278 potentially eligible articles were identified in PubMed, PsycINFO, and Embase. One additional study was added following a manual search of the reference lists. Following removal of 528 duplicate articles, the titles and abstracts were screened, and 1,577 irrelevant articles were removed. A total of 174 full-text articles were assessed for eligibility, and 23 (published between 2004 and 2019) were ultimately included in the present analysis. A flow diagram of study identification is shown in Figure 1.
Figure 1.
Flowchart of the article selection process.
All prospective cohort studies assessing antidepressant exposure during pregnancy were included. Eight studies (Calderon-Margalit et al., 2009; Lund et al., 2009; El Marroun et al., 2012; Nordeng et al., 2012; Viktorin et al., 2016; Laine et al., 2019; Richardson et al., 2019) were conducted in Europe, 13 (Källén, 2004; Sivojelezova et al., 2005; Djulus et al., 2006; Suri et al., 2007; Wisner et al., 2009; Einarson et al., 2010; Einarson et al., 2011; Latendresse and Ruiz, 2011; Yonkers et al., 2012; Klieger-Grossmann et al., 2012; Sadowski et al., 2013; Winterfeld et al., 2015; Yonkers et al., 2017) in North America, 1 in Asia and southeast Europe (Şahingöz et al., 2014), and 1 in Australia (Lewis et al., 2010). The included studies all defined PTB as gestational weeks <37, with the exception of one study, Malm H, 2015 (Malm et al., 2015), which classified PTB into two types [late preterm (32−36 gestational weeks) and very preterm (< 32 weeks) births]. We did the analysis after combining them. Most studies evaluated the association between SSRI use and the risk of PTB (n = 12) (Källén, 2004; Sivojelezova et al., 2005; Suri et al., 2007; Wisner et al., 2009; McDonald et al., 2010; El Marroun et al., 2012; Yonkers et al., 2012; Huybrechts et al., 2014; Şahingöz et al., 2014; Malm et al., 2015; Viktorin et al., 2016; Yonkers et al., 2017); four studies evaluated the association between the use of all antidepressants and PTB (Suri et al., 2007; Einarson et al., 2010; Nordeng et al., 2012; Laine et al., 2019); four studies evaluated the association between tricyclic antidepressant (TCA) (Källén, 2004), mirtazapine (Djulus et al., 2006; Winterfeld et al., 2015), and venlafaxine (Richardson et al., 2019) use and PTB; three studies evaluated the association between the use of one or more antidepressants (Calderon-Margalit et al., 2009; Lewis et al., 2010; Einarson et al., 2011) and PTB; and one study evaluated the association between second-generation antipsychotics (SGAs) (Sadowski et al., 2013) (e.g., SSRIs, benzodiazepines, anticonvulsants, SNRIs, atypical antidepressants) and PTB. Eight studies recruited patients through the Teratology Information Service (Sivojelezova et al., 2005; Djulus et al., 2006; Einarson et al., 2010; Einarson et al., 2011; Klieger-Grossmann et al., 2012; Sadowski et al., 2013; Winterfeld et al., 2015; Richardson et al., 2019) and four studies recruited participants from the national birth registry (Källén, 2004; Viktorin et al., 2016; Laine et al., 2019). The characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author | Country | Year | Study type | Data period | Definition of preterm | Exposure | Sample size (preterm) | Adjusted confounders |
|---|---|---|---|---|---|---|---|---|
| Richardson et al., 2019 | UK | 2019 | UKTIS | 1995−2018 | — | Venlafaxine | 1,274 (158) | Venlafaxine-exposed pregnancies by both calendar year and maternal age (each ±2 years) at UKTIS referral |
| Laine et al., 2019 | Finnish | 2019 | National birth registry | 2009−2015 | <37 | All ADs | 6263 | Age, cohabitation, smoking, education, body mass index, fertility treatments, previous pregnancies, and gestational diabetes mellitus |
| Yonkers et al., 2017 | Connecticut and southern Massachusetts | 2017 | U.S. prospective cohort | 2005−2009 | <37 | SRIs | 2,654 (225) | Age, race/ethnicity, educational level, and smoking, heavy drinking, and illicit drug use during pregnancy |
| Viktorin et al., 2016 | Swedish | 2016 | National birth registry | 2006−2009 | <259 days |
SSRIs | 390,404 | Mother's education, mother's BMI, parity, mother's age at pregnancy, mother's previous psychiatric history, mother's smoking status at the first visit to maternal care |
| Winterfeld et al., 2015 | Canada | 2015 | TIS | 1995−2011 | <37 | Mirtazapine | 581 (57) | Selected randomly, and cases and control subjects were matched by TIS center, year of TIS contact (± 2 years), maternal age (± 2 years), and gestational age at the time of call (± 4 weeks) |
| Malm et al., 2015 | Finland | 2015 | National birth registry | 1996−2010 | Late preterm (32−36 weeks) |
SSRIs | 56,775 (2,449) | Gender and birth period (1996–2000, 2001–2005, and 2006–2010), maternal age at delivery, place of residence, marital status, parity, smoking, socioeconomic status, purchase of anxiolytics, sedative-hypnotics, or antiepileptic drugs, pre-pregnancy diabetes, and other chronic diseases |
| Very preterm (< 32 weeks) | 56,775 (359) | |||||||
| Şahingöz et al., 2014 | Konya and Istanbul | 2014 | Turkish prospective cohort | — | 20 < weeks < 37 | SSRIs | 89 | — |
| Sadowski et al., 2013 | Canada | 2013 | TIS | 2005−2009 | <37 | Second-generation antipsychotics | 266 (17) | Age at conception (± 3 years) and pregnancy duration at the initial time of contact (± 2 weeks) |
| Nordeng et al., 2012 | Norwegian | 2012 | The MoBa study | 2000−2006 | <37 | All ADs | 62,347 | Level of depression, maternal age at delivery, education, parity, pre-pregnancy BMI, maternal asthma or cardiovascular disease, NSAID use, folic acid use, and smoking during pregnancy |
| Yonkers et al., 2012 | Connecticut and southern Massachusetts | 2012 | U.S. prospective cohort | 2005−2009 | <37 | SRIs | 2,654 (225) | Age, education, race, smoking, illicit drug use and pregnancy history, number of lifetime hospitalizations, age of depressive onset, number of prior depressive episodes, post-traumatic stress disorder, generalized anxiety disorder, panic disorder in pregnancy, and suicidal thoughts in pregnancy |
| El Marroun et al., 2012 | Netherlands | 2012 | The Generation R study | 2002−2006 | <37 | SSRIs | 7,126 (365) | Maternal age at intake, gender of the child, maternal education, ethnicity, maternal smoking and drinking habits, body mass index, parity, and maternal benzodiazepine use |
| Klieger-Grossmann et al., 2012 | Canada | 2012 | TIS | — | <37 | Escitalopram | 425 (28) | Maternal age ±2 years, alcohol consumption and smoking, and gestational age at the time of call ±2 weeks |
| Einarson et al., 2011 | Canada | 2011 | TIS | 1992−2007 | <37 | All ADs | 178 (15) | Maternal age (± 2 years), smoking and alcohol use, time of call to Motherisk |
| Latendresse and Ruiz, 2011 | Utah | 2011 | U.S. prospective cohort | 2007.3−11 | <37 | SSRIs | 100 | — |
| Einarson et al., 2010 | Canada | 2010 | TIS | — | < 37 | All ADs | 1,856 (132) | Maternal age (± 2 years), alcohol, tobacco, concurrent drug use |
| Lewis et al., 2010 | Australia | 2010 | Clinic-based prospective cohort | 2004−2005 | <37 | SSRIs/SNRIs/NaSSAs | 54 (5) | — |
| Lund et al., 2009 | Denmark | 2009 | Aarhus birth prospective cohort | 1989−2006 | <37 | SSRIs | 57,001 (2796) | Maternal age, body mass index, smoking, a previous pregnancy with prematurity, and parity |
| Wisner et al., 2009 | Cleveland and Pitts burgh | 2009 | U.S. prospective cohort | 2000−2001, 2003−2007 | Late preterm (≥34 to <37 weeks) | SSRIs | 279 (24) | Maternal age and race |
| Early preterm (< 34 weeks) | ||||||||
| Calderon-Margalit et al., 2009 | Swedish | 2009 | Omega study | since 1996 | 20 < weeks < 37 | SSRIs/SSRI+SNRI | 2,631 (253) | Maternal age, race, years of education, marital status, smoking during pregnancy, preeclampsia, parity, and singleton/multiple pregnancy |
| Suri et al., 2007 | Los Angeles | 2007 | the University of California | 2000−2005 | — | All ADs | 90 (14) | — |
| Djulus et al., 2006 | Canada | 2006 | TIS | 2002−2005 | <37 | Mirtazapine | 208 (12) | Maternal age at the time of conception (± 2 years), gestational age at the first contact (± 2 weeks), tobacco use, alcohol consumption, and chronic conditions |
| Sivojelezova et al., 2005 | Canada | 2005 | TIS | 1999-2002 | <37 | Citalopram | 264 (16) | Maternal age (± 2 years), gestational stage of pregnancy (± 2 weeks) at the time of recruitment |
| Källén, 2004 | Swedish | 2004 | National birth registry | 1995−2001 | <37 | Tricyclic | 563,656 (28,743) |
Year of birth, maternal age, parity, and maternal smoking in early pregnancy |
| SSRIs |
TIS, Teratogen Information Service; AD, antidepressant; SSRI, selective serotonin reuptake inhibitor; SRI, serotonin reuptake inhibitor; NaSSA, noradrenergic and specific serotonergic antidepressant; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin–norepinephrine reuptake inhibitor; UKTIS, UK Teratology Information Service.
Bias Assessment
Analysis of the included studies using Newcastle−Ottawa criteria indicated that 21 studies were at a low risk and two studies (Källén, 2004; Latendresse and Ruiz, 2011) were at a high risk of bias. All studies achieved a total score of 6−9 (median = 7) (Supplementary File 2).
Antidepressant Use in the General Pregnant Female Population
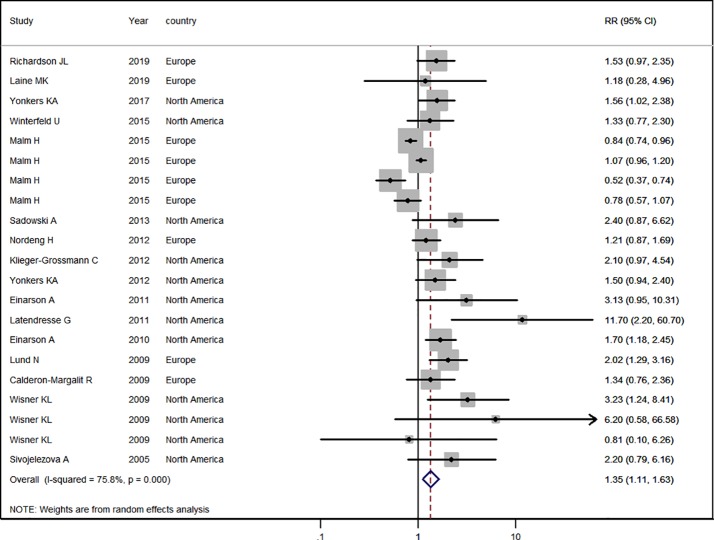
Sixteen studies (Källén, 2004; Sivojelezova et al., 2005; Lund et al., 2009; Calderon-Margalit et al., 2009; Wisner et al., 2009; Einarson et al., 2010; Einarson et al., 2011; Latendresse and Ruiz, 2011; Nordeng et al., 2012; Klieger-Grossmann et al., 2012; Sadowski et al., 2013; Winterfeld et al., 2015; Malm et al., 2015; Yonkers et al., 2017; Richardson et al., 2019; Laine et al., 2019) in the general pregnant female population were included for this analysis. The adjusted RR was 1.35 (95% CI 1.11−1.63, I2 = 75.8%, P < 0.001, Figure 2). A significantly increased risk of PTB was observed with antidepressant use in the general pregnant female population. Subgroup analysis of antidepressant use and the risk of PTB is shown in Table 2. In 10 North American studies, the adjusted RR was 1.78 (1.45−2.18). Following adjustment for maternal age, the RR was statistically significant [1.28 (1.06−1.54)]. If tobacco use and parity were not adjusted for, the RRs were statistically significant [2.01 (1.35−2.98), 1.75 (1.45−2.11)]. There was no evidence of publication bias in any of the studies.
Figure 2.
Meta-analysis of antidepressant use in pregnant women and the risk of preterm birth.
Table 2.
Subgroup analysis of antidepressant use in the general pregnant women and the risk of preterm birth: results of the meta-analysis.
| Antidepressant | |||||
|---|---|---|---|---|---|
| No. of studies | Summary RR (95% CI) | I2, % | P* | P** | |
| Geographic location | 0.01 | ||||
| Europe | 6 | 1.04 (0.84–1.23) | 79.7 | < 0.001 | |
| Northern America | 10 | 1.78 (1.45–2.18) | 5.3 | 0.39 | |
| Adjustment for confounders | |||||
| Maternal age | 0.05 | ||||
| Yes | 14 | 1.28 (1.06–1.54) | 74.9 | < 0.001 | |
| No | 2 | 4.60 (0.99–21.19) | 60.8 | 0.11 | |
| Ethnicity | 0.39 | ||||
| Yes | 4 | 1.59 (1.22–2.06) | 0.0 | 0.52 | |
| No | 12 | 1.27 (1.02–1.57) | 79.5 | < 0.001 | |
| Maternal education | 0.92 | ||||
| Yes | 5 | 1.36 (1.11–1.68) | 0.0 | 0.90 | |
| No | 11 | 1.36 (1.07–1.72) | 80.0 | < 0.001 | |
| Tobacco use | 0.05 | ||||
| Yes | 6 | 1.19 (0.97–1.45) | 79.4 | < 0.001 | |
| No | 10 | 2.01 (1.35–2.98) | 30.7 | 0.18 | |
| Alcohol consumption | 0.22 | ||||
| Yes | 4 | 1.73 (1.34–2.24) | 0.0 | 0.70 | |
| No | 12 | 1.24 (1.01–1.52) | 75.5 | < 0.001 | |
| Pregnancy BMI | 0.55 | ||||
| Yes | 2 | 1.93 (1.26–2.95) | 0.0 | 0.48 | |
| No | 14 | 1.31 (1.07–1.59) | 75.7 | < 0.001 | |
| Parity | 0.01 | ||||
| Yes | 5 | 1.03 (0.83–1.28) | 81.8 | < 0.001 | |
| No | 11 | 1.75 (1.45–2.11) | 0.0 | 0.46 | |
BMI, body mass index; CI, confidence interval; RR, relative risk.
*P for heterogeneity within each subgroup.
**P for heterogeneity between subgroups with meta-regression analysis.
Antidepressant Use in Pregnant Women With Depression
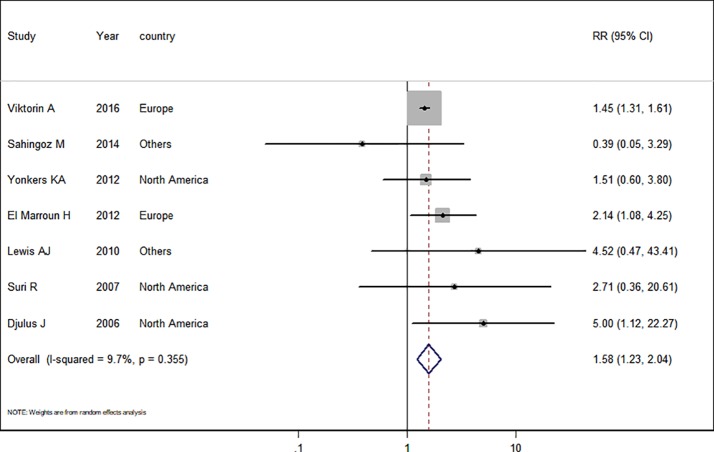
Seven studies (Djulus et al., 2006; Suri et al., 2007; Lewis et al., 2010; Yonkers et al., 2012; El Marroun et al., 2012; Şahingöz et al., 2014; Viktorin et al., 2016) in the pregnant women with depression were included for this analysis. The adjusted RR was 1.58 (95% CI 1.23−2.04, I2 = 9.7%, P = 0.365, Figure 3). A significantly increased risk of PTB was observed when the analysis was restricted to pregnant women with depression. Subgroup analysis of antidepressant use in pregnant women with depression and the risk of PTB was shown in Table 3. In two European studies, the adjusted RR was 1.51 (95% CI 1.20−1.91). Following adjustment for maternal age, ethnicity, maternal education, tobacco use, BMI during pregnancy, and parity, the adjusted RRs were statistically significant: 1.62 (1.20−2.18), 1.89 (1.09−3.28), 1.46 (1.32−1.62), 1.62 (1.20−2.18), 1.51 (1.20−1.91), and 1.46 (1.32−1.62), respectively. There was no evidence of publication bias in any of the studies.
Figure 3.
Meta-analysis of antidepressant use in pregnant women with depression and the risk of preterm birth.
Table 3.
Subgroup analysis of antidepressant use in pregnant women with depression and risk of preterm birth: results of meta-analyses.
| Antidepressant use | |||||
|---|---|---|---|---|---|
| No. of studies | Summary RR (95% CI) | I2,% | P* | P** | |
| Geographic location | 0.75 | ||||
| Europe | 2 | 1.51 (1.20–1.91) | 17.6 | 0.27 | |
| Northern America | 3 | 1.28 (0.12–14.07) | 0.0 | 0.40 | |
| Others | 2 | 2.17 (1.05–4.52) | 58.8 | 0.12 | |
| Adjustment for confounders | |||||
| Maternal age | 0.99 | ||||
| Yes | 4 | 1.62 (1.20–2.18) | 21.1 | 0.28 | |
| No | 3 | 1.64 (0.38–7.01) | 29.0 | 0.25 | |
| Ethnicity | 0.48 | ||||
| Yes | 2 | 1.89 (1.09–3.28) | 0.0 | 0.55 | |
| No | 5 | 1.78 (0.94–3.39) | 26.8 | 0.24 | |
| Maternal education | 0.32 | ||||
| Yes | 3 | 1.46 (1.32–1.62) | 0.0 | 0.54 | |
| No | 4 | 2.42 (0.78–7.49) | 27.2 | 0.25 | |
| Tobacco use | 0.99 | ||||
| Yes | 4 | 1.62 (1.20–2.18) | 21.1 | 0.28 | |
| No | 3 | 1.64 (0.38–7.01) | 29.0 | 0.25 | |
| Alcohol drinking | 0.16 | ||||
| Yes | 2 | 2.50 (1.32–4.73) | 2.3 | 0.31 | |
| No | 5 | 1.45 (1.31–1.61) | 0.0 | 0.58 | |
| Pregnancy BMI | 0.54 | ||||
| Yes | 2 | 1.51 (1.20–1.91) | 17.6 | 0.27 | |
| No | 5 | 2.00 (0.94–4.28) | 15.3 | 0.32 | |
| Parity | 0.32 | ||||
| Yes | 3 | 1.46 (1.32–1.62) | 0.0 | 0.54 | |
| No | 4 | 2.42 (0.78–7.49) | 27.2 | 0.25 | |
BMI, body mass index; CI, confidence interval; RR, relative risk.
*P for heterogeneity within each subgroup.
**P for heterogeneity between subgroups with meta-regression analysis.
SSRI Use in the General Pregnant Female Population
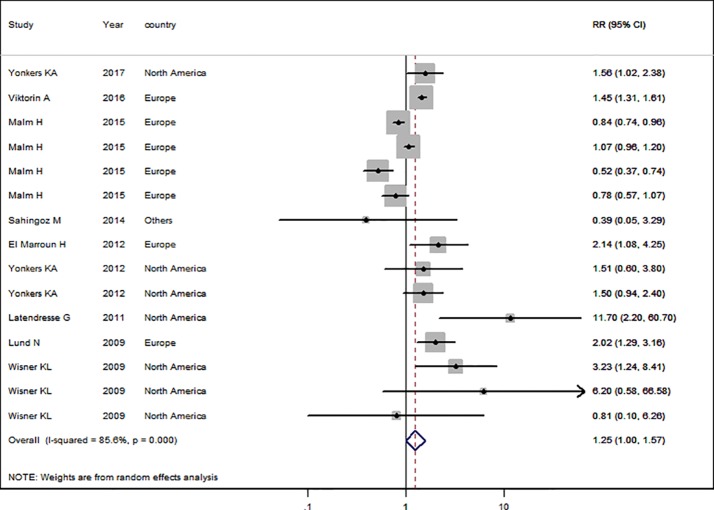
Nine studies (Lund et al., 2009; Wisner et al., 2009; Latendresse and Ruiz, 2011; El Marroun et al., 2012; Yonkers et al., 2012; Şahingöz et al., 2014; Malm et al., 2015; Viktorin et al., 2016; Yonkers et al., 2017) in the general pregnant female population were included for this analysis. The adjusted RR was 1.25 (95% CI 1.01−1.57, I2 = 85.6%, P < 0.001, Figure 4). The heterogeneity of the studies was high and no significantly increased risk of PTB was observed for SSRI use in the general pregnant female population. Subgroup analysis of SSRI use and risk of PTB is shown in Table 4. In four North American studies, the adjusted RR was 1.91 (1.27−2.86). Following adjustment for ethnicity, maternal education, alcohol consumption, and BMI during pregnancy, the adjusted RRs were statistically significant: 1.85 (1.33−2.57), 1.47 (1.33−1.62), 1.70 (1.19−2.44), and 1.63 (1.27−2.09), respectively. If parity was not adjusted, the RR was statistically significant [2.24 (1.03−4.84)]. There was no evidence of publication bias in any of the studies.
Figure 4.
Meta-analysis of selective serotonin reuptake inhibitor use in pregnant women and the risk of preterm birth.
Table 4.
Subgroup analysis of selective serotonin reuptake inhibitor use and risk of preterm birth in infants: results of meta-analyses.
| SSRI use | |||||
|---|---|---|---|---|---|
| No. of studies | Summary RR (95% CI) | I2,% | P* | P** | |
| Geographic location | 0.22 | ||||
| Europe | 4 | 1.07 (0.82–1.39) | 92.30 | < 0.001 | |
| Northern America | 4 | 1.91 (1.27–2.86) | 33.6 | 0.17 | |
| Others | 1 | 0.39 (0.05–3.16) | – | – | |
| Adjustment for confounders | |||||
| Maternal age | 0.34 | ||||
| Yes | 7 | 1.22 (0.97–1.52) | 86.4 | < 0.001 | |
| No | 2 | 2.27 (0.08–63.58) | 83.9 | 0.01 | |
| Ethnicity | 0.13 | ||||
| Yes | 3 | 1.85 (1.33–2.57) | 0.0 | 0.45 | |
| No | 6 | 1.11 (0.86–1.42) | 89.4 | < 0.001 | |
| Maternal education | 0.45 | ||||
| Yes | 3 | 1.47 (1.33–1.62) | 0.0 | 0.52 | |
| No | 6 | 1.14 (0.88–1.47) | 79.7 | < 0.001 | |
| Tobacco use | 0.10 | ||||
| Yes | 6 | 1.16 (0.92–1.45) | 89.0 | < 0.001 | |
| No | 3 | 2.60 (0.86–7.84) | 50.9 | 0.09 | |
| Alcohol drinking | 0.45 | ||||
| Yes | 2 | 1.70 (1.19–2.44) | 0.0 | 0.44 | |
| No | 7 | 1.18 (0.92–1.51) | 86.9 | < 0.001 | |
| Pregnancy BMI | 0.26 | ||||
| Yes | 3 | 1.63 (1.27–2.09) | 77.5 | 0.21 | |
| No | 6 | 1.09 (0.85–1.40) | 0.4 | < 0.001 | |
| Parity | 0.12 | ||||
| Yes | 5 | 1.12 (0.88–1.43) | 89.9 | < 0.001 | |
| No | 4 | 2.24 (1.03–4.84) | 52.0 | 0.06 | |
BMI, body mass index; CI, confidence interval; RR, relative risk; SSRI, selective serotonin reuptake inhibitor.
*P for heterogeneity within each subgroup.
**P for heterogeneity between subgroups with meta-regression analysis.
SSRI Use in Pregnant Women With Depression
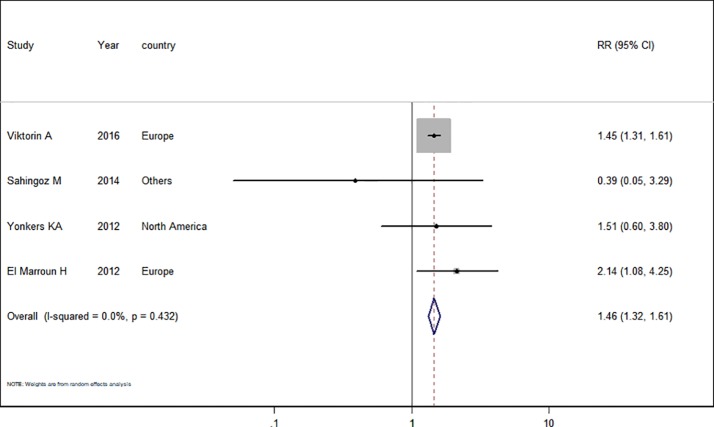
Only four studies (El Marroun et al., 2012; Yonkers et al., 2012; Şahingöz et al., 2014; Viktorin et al., 2016) in the pregnant women with depression were included for this analysis. The adjusted RR was 1.46 (95% CI 1.32−1.61, I2 = 0.0%, P = 0.432, Figure 5). A significantly increased risk of PTB was observed with SSRI use in pregnant women with depression. There was no evidence of publication bias in any of the studies.
Figure 5.
Meta-analysis of selective serotonin reuptake inhibitor use in pregnant women with depression and the risk of preterm birth.
Discussion
Main Findings
To the best of our knowledge, this is the first meta-analysis of the impact of antidepressant use during pregnancy on PTB in depressed women. Our study focused not only on depressed pregnant women but also on the general pregnant female population, with 23 prospective cohort studies finally being selected. It was found that the risk of PTB was increased in depressed women treated with SSRIs during pregnancy. Similar results were observed in women with depression and in the general pregnant female population who used antidepressants during pregnancy. There was no significantly increased risk of PTB observed with SSRI use in the general pregnant female population.
The focus of the present study was to explore the association between maternal use of antidepressants in depressed women during pregnancy and risk of PTB. Subgroup analysis shows that even though the use of antidepressants in the general pregnant female population significantly increased the risk of PTB, the heterogeneity was high, which may be due to the differences in population sources. There was a statistically significant difference in the European cohort study but not in the North American cohort study, which may be one of the sources of heterogeneity. Another reason for the high heterogeneity may be that there were confounding factors that influenced the conclusion. For this reason, a meta-regression analysis was conducted; when maternal age, tobacco use, and parity were defined, there was a statistically significant difference in the meta-regression, indicating that these may be confounding factors.
To further explore whether the heterogeneity originated from different drug types, subgroup analysis of SSRI use in the general pregnant female population was conducted, and it was found that the heterogeneity was elevated. A possible reason could be differences in the sources of the population. Firstly, according to geographic location, studies were divided into European, North American, and others; the P values were 0.27, 0.40, and 0.12, respectively, and the meta-regression was not statistically significant. Subsequently, the data were adjusted for certain confounding factors such as maternal age, ethnicity, maternal education, tobacco use, and alcohol consumption; however, the results were not ideal. Finally, a subgroup analysis of SSRI use in pregnant women with depression was performed, and it was found that the heterogeneity was 0%; therefore, a possible source of the high heterogeneity was not stipulating depression in the study population. However, there have only been four studies to date, and the present study suggests that future studies may be inclined to stipulate depression in the study population and to consider the effects of SSRIs or other antidepressants on preterm PTB, which is of great clinical significance.
Comparison With the Existing Literature
Currently, there exist four meta-analyses of the effects of antidepressant use on PTB (nearly 6 years) (Hsiang and Bridge, 2014; Huybrechts et al., 2014; Ross and Grigoriadis, 2014; Eke et al., 2016). The results of the present study among studies in general pregnant women were consistent with these published meta-analyses. However, these original cohort studies were both retrospective and prospective, which could have caused recall bias. Moreover, there was no stipulation of depression in the study population, with the exception of one study (Eke et al., 2016) including eight original studies, which focused on the effect of SSRIs on PTB in pregnant women with depression. Following adjustment for confounders, the incidence of PTB was significantly higher in pregnant women treated with SSRIs as compared with that in the controls [adjusted odds ratios (aOR) 1.24, 95% CI 1.09−1.41]. Our included studies were all prospective cohort studies with high quality evidence and high credibility. Huybrechts et al. (2014) included 41 original studies, and the pooled adjusted odds ratio was 1.53 (1.40−1.66) for antidepressant use at any time during pregnancy and 1.96 (1.62−2.38) for use during the third trimester; however, confounding factors were not adjusted for. In another study by Rose et al. (2014), 13 original studies providing 14 estimates of the association between antidepressant use and PTB resulted in a pooled OR of 1.55 (95% CI, 1.38−1.74; P < 0.001); after limiting the population to depressed pregnant women, the OR was 1.79 (0.77−4.14). Moreover, a study by Hsiang et al. (2014) included 28 original studies and the meta-analysis showed that antidepressant use in pregnancy was significantly associated with PTB (RR: 1.69, 95% CI: 1.52−1.88).
Strengths and Limitations
The present study describes a meta-analysis of 23 prospective cohort studies investigating the risk of PTB in women taking antidepressants during pregnancy, with additional analyses associated with a diagnosis of depression. The main strength is that it is a large meta-analysis including a large number of prospective cohort studies. Furthermore, to-date, evidence regarding associations between the use of antidepressant in depressed women and risk of PTB is limited. To the best of our knowledge, the present study is the first meta-analysis to investigate the associations between maternal antidepressant use in depressed women and the risk of PTB.
Nevertheless, this study has several limitations, which should be considered when interpreting its findings. First, due to the limited number of included studies, the present study did not account for trimester exposure and either for duration of exposure in the analysis. Both factors may significantly modify the association between maternal use of antidepressant during pregnancy and PTB. The number of studies related to specific antidepressants are small for specific drugs (i.e., SSRIs) and did not allow for investigations into the dose-response. Second, heterogeneity was high among studies focusing on the associations between maternal antidepressant use in the general pregnant women and the risk of PTB. Our subgroup analysis suggested that the high heterogeneity might be attributed to differences in study locations and diagnosing of depression.
Conclusions
The results of this meta-analysis highlights the association between maternal use of antidepressant during pregnancy and the risk of PTB. Health care providers and pregnant women must weigh the risk-benefit potential of these drugs when making decisions about whether to treat with antidepressant during pregnancy.
Author Contributions
Y-HZ and QC designed and conducted the study. X-YM and X-XR collected, managed, and analyzed the data. QC, Q-JW, HS, and Y-HZ prepared, reviewed, and approved the manuscript. Y-HZ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by National Key R&D Program of China (No. 2017YFC0907403 to Y-HZ); Liaoning Revitalization Talents Program (No. XLYC1802095 to Y-HZ); Key R&D Program of Liaoning Province (No. 2019JH8/10300005 to Y-HZ); the Science and Technology Project of Liaoning Province (No. 2019JH6/10400002 to Y-HZ); Young Talents of Education Ministry of Liaoning Province (No. QN2019011 to HS). The other authors declare no conflicts of interest in relation to this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank BioMed Proofreading for English proofreading.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00659/full#supplementary-material
References
- Şahingöz M., Yuksel G., Karsidag C., Uguz F., Sonmez E. O., Annagur B. B., et al. (2014). Birth Weight and Preterm Birth in Babies of Pregnant Women With Major Depression in Relation to Treatment With Antidepressants. J. Clin. Psychopharmacol. 34 (2), 226–229. 10.1097/JCP.0000000000000077 [DOI] [PubMed] [Google Scholar]
- Begg C. B., Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4), 1088–1101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M. Z., Chou D., Moller A. B., Narwal R., et al. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379 (9832), 2162–2172. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- Calderon-Margalit R., Qiu C., Ornoy A., Siscovick D. S., Williams M. A. (2009). Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am. J. Psychiatry 201 (6), 557–566. 10.1016/j.ajog.2009.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. H., Larson J., Blencowe H., Spong C. Y., Howson C. P., Cairns-Smith S., et al. (2013). Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 381 (9862), 223–234. 10.1016/S0140-6736(12)61856-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Control Clin. Trials. 7 (3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Djulus J., Koren G., Einarson T. R., Wilton L., Shakir S., Diav-Citrin O., et al. (2006). Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. J. Clin. Psychiatry 67 (8), 1280–1284. 10.4088/JCP.v67n0817 [DOI] [PubMed] [Google Scholar]
- Einarson A., Choi J., Einarson T. R., Koren G. (2010). Adverse effects of antidepressant use in pregnancy: an evaluation of fetal growth and preterm birth. Depress Anxiety 27 (1), 35–38. 10.1002/da.20598 [DOI] [PubMed] [Google Scholar]
- Einarson A., Choi J., Koren G., Einarson T. (2011). Outcomes of infants exposed to multiple antidepressants during pregnancy: Results of a cohort study. J. Popul. Ther. Clin. Pharmacol. 18 (2), e390–e396. [PubMed] [Google Scholar]
- Eke A. C., Saccone G., Berghella V. (2016). Selective Serotonin Reuptake Inhibitor (SSRI) Use During Pregnancy and Risk of Preterm Birth: A Systematic Review and Meta-Analysis. BJOG 123 (12), 1900–1907. 10.1111/1471-0528.14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H., Jaddoe V. W., Hudziak J. J., Roza S. J., Steegers E. A., Hofman A., et al. (2012). Maternal Use of Selective Serotonin Reuptake Inhibitors, Fetal Growth, and Risk of Adverse Birth Outcomes. Arch. Gen. Psychiatry 69 (7), 706–714. 10.1001/archgenpsychiatry.2011.2333 [DOI] [PubMed] [Google Scholar]
- El Marroun H., White T., Verhulst F. C., Tiemeier H. (2014). Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur. Child Adolesc. Psychiatry 23 (10), 973–992. 10.1007/s00787-014-0558-3 [DOI] [PubMed] [Google Scholar]
- Gao S. Y., Wu Q. J., Sun C., Zhang T. N., Shen Z. Q., Liu C. X., et al. (2018). Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 12;16 (1), 205. 10.1186/s12916-018-1193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration; Available at: https://training.cochrane.org/handbook#how-to-access (Accessed May 13, 2020). [Google Scholar]
- Hsiang H., Bridge J. A. (2014). A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight: letter response. Gen. Hosp. Psychiatry 36 (3), 358–359. 10.1016/j.genhosppsych.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Huybrechts K. F., Sanghani R. S., Avorn J., Urato A. C. (2014). Preterm Birth and Antidepressant Medication Use during Pregnancy: A Systematic Review and Meta-Analysis. PloS One 9 (3), e92778. 10.1371/journal.pone.0092778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B. (2004). Neonate Characteristics After Maternal Use of Antidepressants in Late Pregnancy. Arch. Pediatr. Adolesc. Med. 158 (4), 312–316. 10.1001/archpedi.158.4.312 [DOI] [PubMed] [Google Scholar]
- Klieger-Grossmann C., Weitzner B., Panchaud A., Pistelli A., Einarson T., Koren G., et al. (2012). Pregnancy Outcomes Following Use of Escitalopram: A Prospective Comparative Cohort Study. J. Clin. Pharmacol. 52 (5), 766–770. 10.1177/0091270011405524 [DOI] [PubMed] [Google Scholar]
- Laine M. K., Masalin S., Rönö K., Kautiainen H., Gissler M., Pennanen P., et al. (2019). Risk of preterm birth in primiparous women with exposure to antidepressant medication before pregnancy and/or during pregnancy-impact of body mass index. Ann. Med. 51 (1), 51–57. 10.1080/07853890.2018.1534265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse G., Ruiz R. J. (2011). Maternal corticotropin-releasing hormone and the use of selective serotonin reuptake inhibitors independently predict the occurrence of preterm birth. J. Midwifery Womens Health 56 (2), 118–126. 10.1111/j.1542-2011.2010.00023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. M., Lam S. K., Sze Mun Lau S. M., Chong C. S., Chui H. W., Fong D. Y. (2007). Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet. Gynecol. 110 (5), 1102–1112. 10.1097/01.AOG.0000287065.59491.70 [DOI] [PubMed] [Google Scholar]
- Lewis A. J., Galbally M., Opie G., Buist A. (2010). Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust. N. Z. J. Psychiatry 44 (5), 482–487. 10.3109/00048670903559593 [DOI] [PubMed] [Google Scholar]
- Lund N., Pedersen L. H., Henriksen T. B. (2009). Selective Serotonin Reuptake Inhibitor Exposure In Utero and Pregnancy Outcomes. Arch. Pediatr. Adolesc. Med. 163 (10), 949–954. 10.1001/archpediatrics.2009.164 [DOI] [PubMed] [Google Scholar]
- Malm H., Sourander A., Gissler M., Gyllenberg D., Hinkka-Yli-Salomäki S., McKeague I. W., et al. (2015). Pregnancy Complications Following Prenatal Exposure to SSRIs or Maternal Psychiatric Disorders: Results From Population-Based National Register Data. Am. J. Psychiatry 172 (12), 1224–1232. 10.1176/appi.ajp.2015.14121575 [DOI] [PubMed] [Google Scholar]
- Mangham L. J., Petrou S., Doyle L. W., Draper E. S., Marlow N. (2009). The Cost of Preterm Birth Throughout Childhood in England and Wales. Pediatrics 123 (2), e312–e327. 10.1542/peds.2008-1827 [DOI] [PubMed] [Google Scholar]
- Marlow N., Wolke D., Bracewell M. A., Samara M., EPICure Study Group (2000). Neurologic and Developmental Disability at Six Years of Age after Extremely Preterm Birth. N. Engl. J. Med. 6 (1), 4–5. [DOI] [PubMed] [Google Scholar]
- McDonald S. D., Han Z., Mulla S., Beyene J. (2010). Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 341, c3428. 10.1136/bmj.c3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeng H., van Gelder M. M., Spigset O., Koren G., Einarson A., Eberhard-Gran M. (2012). Pregnancy Outcome After Exposure to Antidepressants and the Role of Maternal Depression. J. Clin. Psychopharmacol. 32 (2), 186–194. 10.1097/JCP.0b013e3182490eaf [DOI] [PubMed] [Google Scholar]
- Odutayo A., Wong C. X., Hsiao A. J., Hopewell S., Altman D. G., Emdin C. A. (2016). Atrial fibrillation and risks of cardiovascular disease, renal disease, and death:systematic review and meta-analysis. BMJ 354, i4482. 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- Purisch S. E., Gyamfi-Bannerman C. (2017). Epidemiology of preterm birth. Semin. Perinatol. 41 (7), 387–391. 10.1053/j.semperi.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Richardson J. L., Martin F., Dunstan H., Greenall A., Stephens S., Yates L. M., et al. (2019). Pregnancy outcomes following maternal venlafaxine use: A prospective observational comparative cohort study. Reprod. Toxicol. 84, 108–113. 10.1016/j.reprotox.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Ross L. E., Grigoriadis S. (2014). Selected Pregnancy and Delivery Outcomes After Exposure to Antidepressant Medication. JAMA Psychiatry 71 (6), 716. 10.1001/jamapsychiatry.2014.59 [DOI] [PubMed] [Google Scholar]
- Sadowski A., Todorow M., Yazdani Brojeni P., Koren G., Nulman I. (2013). Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open 3 (7), e003062. 10.1136/bmjopen-2013-003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivojelezova A., Shuhaiber S., Sarkissian L., Einarson A., Koren G. (2005). Citalopram use in pregnancy: Prospective comparative evaluation of pregnancy and fetal outcome. Am. J. Obstet. Gynecol. 193 (6), 2004–2009. 10.1016/j.ajog.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Staneva A., Bogossian F., Pritchard M., Wittkowski A. (2015). The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth. 28 (3), 179–193. 10.1016/j.wombi.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Suri R., Altshuler L., Hellemann G., Burt V. K., Aquino A., Mintz J. (2007). Effects of Antenatal Depression and Antidepressant Treatment on Gestational Age at Birth and Risk of Preterm Birth. Am. J. Psychiatry 164 (8), 1206–1213. 10.1176/appi.ajp.2007.06071172 [DOI] [PubMed] [Google Scholar]
- Viktorin A., Lichtenstein P., Lundholm C., Almqvist C., D'Onofrio B. M., Larsson H., et al. (2016). Selective serotonin re-uptake inhibitor use during pregnancy: association with offspring birth size and gestational age. Int. J. Epidemiol. 45 (1), 170–177. 10.1093/ije/dyv351 [DOI] [PubMed] [Google Scholar]
- Wells G. A., Shea B. J., O'Connell D., Peterson J., Welch V., Losos M., et al. (2011). Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 13, 2020).
- Winterfeld U., Klinger G., Panchaud A., Stephens S., Arnon J., Malm H., et al. (2015). Pregnancy Outcome Following Maternal Exposure to Mirtazapine: A Multicenter, Prospective Study. J. Clin. Psychopharmacol. 35 (3), 250–259. 10.1097/JCP.0000000000000309 [DOI] [PubMed] [Google Scholar]
- Wisner K. L., Sit D. K., Hanusa B. H., Bogen D. L., Hunker D. F., Perel J. M., et al. (2009). Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am. J. Psychiatry 166 (5), 557–566. 10.1176/appi.ajp.2008.08081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers K. A., Norwitz E. R., Smith M. V., Lockwood C. J., Gotman N., Luchansky E., et al. (2012). Depression and Serotonin Reuptake Inhibitor Treatment as Risk Factors for Preterm Birth. Epidemiology 23 (5), 677–685. 10.1097/EDE.0b013e31825838e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers K. A., Gilstad-Hayden K., Forray A., Lipkind H. S. (2017). Association of Panic Disorder, Generalized Anxiety Disorder, and Benzodiazepine Treatment During Pregnancy With Risk of Adverse Birth Outcomes. JAMA Psychiatry 74 (11), 1145–1152. 10.1001/jamapsychiatry.2017.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.