Abstract
Background
Marijuana is a commonly used recreational substance with purported analgesic and mood enhancing properties. Many people living with HIV identify marijuana as a palliative substance. However, through its main psychoactive component, tetrahydrocannabinol (THC), is known to influence the immune system. The effects of marijuana use in people with HIV are still controversial, with very scant literature in Black adults.
Methods
The current study determined the differences in the lymphocyte count, specifically the number cluster differentiation 4 and 8 (CD4+ and CD8+), among patients who urine drug tested negative for THC (n = 70) and those who tested positive for THC (n = 25). The sample included 95 Black people living with HIV, 51% female, with a mean age of 46 ± 11 years. Participants provided a urine sample for substance use testing and a trained researcher extracted clinical data from clinical charts on the day of appointment.
Results
After adjusting for demographic and HIV-related covariates, THC-positive patients had significantly higher CD4+ and CD8+ counts than their THC-negative counterparts.
Conclusion
These results extend previous HIV-related immunity findings in an underrepresented group, and suggest that THC use does not reduce immune function as measured by CD count. Further research is warranted on the overall effects of THC on immune function in HIV positive patients.
Keywords: HIV, Marijuana, African American, CD8+, CD4+, THC
1. Introduction
Over one million people are currently living with HIV in the United States (CDC, 2016). Through advances in HIV medications, the use of antiretroviral therapy has led to people living longer with HIV (Palella et al., 2006). In attempts to mitigate the effects of the psychological and physiological symptoms posed by HIV, some people living with HIV (PLWH) are using marijuana for symptom palliation (Prentiss et al., 2004). Although marijuana may offer some relief to PLWH for a variety of symptoms, marijuana usage also has potentially deleterious immunomodulatory effects, leaving individuals more susceptible to infections (Cabral and Pettit, 1998; Klein et al., 1998; Keen et al., 2014; Keen and Turner, 2015). Therefore, PLWH who use marijuana may be at increased risk of infection as their immune system is already compromised.
Cross-sectional studies in PLWH examining the relationship between marijuana use and cluster of differentiation 4 (CD4+) or cluster of differentiation 8 (CD8+) T lymphocyte counts have shown inconsistent results (Whitfield et al., 1997; Furler et al., 2004; Abrams et al., 2003; Kuo et al., 2004; Chang et al., 2001; Chao et al., 2008; Bonn-Miller et al., 2014; Kelly et al., 2016; Okafor et al., 2016). However, longitudinal research suggest that marijuana use frequency is not deleterious or associated with CD4+ T cells in a sample of HIV and Hepatitis C co-infected patients (Marcellin et al., 2017). In PLWH, the CD4+ and CD8+ T lymphocytes counts are reduced due to the HIV virus utilizing the these T lymphocytes to replicate the virus and ultimately destroy infected cells. As a result, the ability to fight infection is reduced because of a reduced number of CD4+ and CD8+ T lymphocytes. The addition of a substance like tetrahydrocannabinol (THC) that further suppresses the activity of immune cells, raises the concern that further inhibition of function in a patient with a diminished cell population would significantly increase the risk for infections. On the other hand, THC inhibiting the same immune cells (i.e. natural killer cells and cytokines) that aid the HIV virus in infecting other cells that are recruited may save such T lymphocytes from further infection (Molina et al., 2011).
The use of marijuana for palliation in PLWH for neurologic symptoms is on the rise (Purohit et al;, 2014), however, there is a dearth of literature that examines the difference in HIV-related immune function among recent users and non-users. Given the immune system dysregulation, paired with the deleterious influence of HIV on immune function may leave PLWH more susceptible to disease, it is important to determine the potential risk/benefit of marijuana in this group. This is especially imperative to examine in African Americans/Blacks (Blacks), who are disproportionately affected by HIV (CDC, 2016). The purpose of the current study was to examine the differences in CD4+ and CD8+ counts between Black PLWH who urine drug test positive for THC compared to Black PLWH who test negative for THC. Based on previous research, we hypothesized that PLWH who urine test positive for THC will have significantly lower CD4+ and CD8+ counts when compared to their negative counterparts.
2. Material and methods
2.1. Subjects
One hundred heterosexual Black adults were recruited by flyers and referrals within the University of Florida Center for HIV/AIDS Research and Education Service Rainbow Center in Jacksonville, Florida as a part of the parent study entitled, “The Effects of Racial Discrimination, Social Support, and Executive Function on HIV Health Outcomes in HIV Positive Heterosexual Black Men.” Inclusion criteria for the parent study included individuals who were aged 18 years or older with no history of traumatic brain injury and self-identify as heterosexual. Both the Virginia State University and University of Florida Institutional Review Boards approved this study. A total of 100 individuals participated in the parent study, but only those with complete CD4+ and CD8+ count data were included in the current study (n = 95).
2.2. Procedures
Study procedures entailed one study visit lasting approximately one hour. Participants were screened for inclusion criteria and scheduled to participate on the date of their next doctor’s appointment. After completing their appointment, participants were escorted to the research space in the adjacent building from the clinic. Upon entering the University of Florida Center for AIDS/HIV Research and Education Services research space, researchers obtained informed consent from the participants. After informed consent was received, a registered nurse obtained a urine sample. Following this collection, participants completed demographic, brief medical history, and behavioral health questionnaires. When the participants’ data from their doctor’s appointment were entered into the hospital’s medical database, a member of the research staff extracted primary vitals and immune data from patient charts for the date they participated in the research study. Participation was voluntary and participants were remunerated $25 for their time.
2.3. Assessment of recent marijuana use
Recent marijuana use was assessed using urine drug screening kits. Urine specimens obtained by a trained research assistant, who then utilized a rapid one step screening method with a multi-drug panel dipping stick. The multi-drug panel dipstick applies lateral flow chromatographic immunoassays to detect positive results based on standard cutoffs for THC urinary metabolites. A positive result reflects marijuana use in the past 15–30 days. Positive results were coded as “1″ and negative results were coded as “0″.
2.4. Chart Extraction: CD4+ and CD8+
Cluster Differentiations 4 and 8 were obtained via patient chart extraction. The participants were scheduled to participate on days when they also had an appointment with their doctor in order to obtain HIV-relevant immune function from their charts. A trained researcher abstracted patient’s’ current medical and lab data from their EPIC electronic medical records. A second research coordinator verified accuracy. The clinical laboratory used fluorescence activated cell sorter to enumerate the cell type.
2.5. Assessment of covariates
Age (in years), sex, and annual income were collected via a demographic questionnaire administered by a trained researcher. Height and weight were obtained from their recent doctor’s appointment via chart extraction. HIV-related covariates included 1) “How many years each participant has lived with HIV”, which was confirmed by the clinic (by original diagnosis or from self-report) and 2) detectable viral load, coded as 1 for “yes” and 0 for “no”.
2.6. Data analysis
Data were analyzed using the Statistical Package for Social Sciences, Version 24.0 (SPSS Incorporated). Continuous variables are presented as “mean (standard deviation)”. Frequencies and percentages for categorical variables are reported as the “n” of the subsample and the within group percentage. CD4+ counts and BMI levels were negatively skewed, so values were log transformed prior to analyses. Independent T-tests and Chi-square tests were used to compare respectively continuous and categorical variables between groups. Analyses of covariance were performed in order to examine the differences in CD4+ and CD8+ lymphocyte T cell counts between those who urine tested positive and those who urine tested negative for THC, adjusting for covariates. Demographic and physiological covariates that may confound a significant relationship between marijuana use and immune function were selected based on published literature. Specifically, age (Ader et al., 1990), sex (Keen and Turner, 2015), body mass index (Molina et al., 2015), and income (Galea et al., 2007) were included in the analysis of covariance. Additionally, analyses of covariance included HIV-related covariates.
3. Results
3.1. Demographic information
Demographic and physiological variable information is displayed in Table 1. The mean age for the overall sample was 46 ± 11 years with 49 (51%) women. Approximately 26% of the sample urine drug tested positive for THC and approximately 73% tested negative.
Table 1.
Demographic Information and Immune Function for Urine Drug Screened THC Positive and Negative Groups.
| Total N = 95 | Negative n = 70 | Positive n = 25 | |||
|---|---|---|---|---|---|
| X /n (SD/%) | X /n (SD/%) | X /n (SD/%) | F/X2 | p | |
| Age (yrs) | 45.76 (11.22) | 45.75 (11.49) | 45.80 (10.63) | .00 | .08 |
| Sex | 2.83 | .09 | |||
| Male | 48 (49.5%) | 32 (44.4%) | 16 (64%) | ||
| Female | 49 (50.5) | 40 (55.6%) | 9 (36%) | ||
| Income | .78 | .67 | |||
| < $20K | 85 (87.6%) | 63 (87.5%) | 22 (88%) | ||
| $21K–$40K | 10 (10.3%) | 7 (9.7%) | 3 (12%) | ||
| $41K–$80K | 2 (2.1%) | 2 (2.8%) | 0 (0%) | ||
| BMI (kg/m2) | 29.63 (8.91) | 30.53 (9.39) | 27.04 (6.90) | 3.05 | .06 |
| Detectable Viral Load | 0.36 | .63 | |||
| No | 58 (61%) | 44 (63%) | 14 (56%) | ||
| Yes | 37 (39%) | 26 (37%) | 11 (44%) | ||
| Years w/HIV | 12.17 (8.62) | 12.32 (8.87) | 11.76 (8.02) | 0.07 | .77 |
| CD8+ (103/mm3) | 805.46 (359.70) | 771.30 (338.34) | 903.84 (406.60) | 2.56 | .11 |
| CD4+ (103/mm3) | 542.23 (356.57) | 514.94 (361.20) | 620.84 (337.53) | 3.05 | .09 |
< .05
> .01.
Note: BMI = Body Mass Index; CD8+ = Cluster of Differentiation 8; CD4+ = Cluster of Differentiation 4.
3.2. THC adjusted mean differences
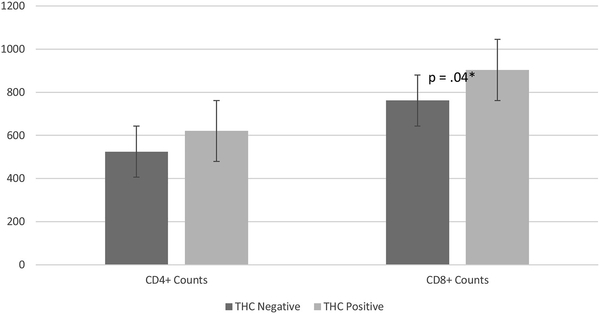
After adjusting for demographic, physiological, and HIV-related covariates, analysis of covariance analyses revealed significant difference in CD4+ and CD8+ counts between THC positive and negative participants (F = 7.06, df = 1, p = .01, partial = .07; F = 4.11, df = 1, p = .04, partial = .04). Specifically, THC positive participants had significantly higher CD4+ levels (621 ± 338) and CD8+ counts (904 ± 461) than their THC negative counterparts (524 ± 360; 762 ± 338, respectively). Additionally, years living with HIV (F = 4.59, df = 1, p = .03, partial = .05) and detectable viral load (F = 9.087, df = 1, p = .01, partial = 0.09) were significant predictors of CD4+ counts in the presences of other variables These results can be seen in Fig. 1.
Fig. 1.
Differences Between THC in CD4+ and CD8+ Counts.
4. Discussion
The current study sought to examine the differences in CD4+ and CD8+ counts between those who tested positive for THC and patients who tested negative for THC in a sample of heterosexual Black PLWH. Both THC negative and THC positive PLWH had CD4+ lymphocyte cell counts above 500 cells/mm3, which suggests the majority of the current sample were in relative good health regarding their HIV progression. Though unadjusted comparisons did not yield statistically significant results, those who tested positive for THC had significantly higher CD4+ and CD8+ cell counts in the presence of demographic, physiological, and HIV-related covariates. The current findings are in line with previous research, reporting daily marijuana users have higher CD4+ cell counts and lower viral load than their non-using and infrequent using counterparts (D’Souza et al., 2012; Milloy et al., 2015). This finding also supports results reported in community based samples of HIV positive marijuana users with higher CD4+ cell counts than their non-using counterparts in Los Angeles (Thames et al., 2016), Houston (Mayben et al., 2007), and Australia (Fogarty et al., 2007).
The progression of HIV varies from patient to patient, with some who progress slowly and others who progress much faster. However, CD4+ and CD8+ cell counts are intimately linked in those that are able to suppress the HIV infection (Walker et al., 1986; Saez-Aninon et al., 2007). Depletion of these markers is associated with increased HIV progression, inflammation, and potentially other end organ complications (Deeks, 2011). The increases in inflammation are critical, as the inflammatory markers are potentially recruiting other immune cells to their death (Doitsh et al., 2014). The decrease in the inflammatory response in marijuana users may account for the increase in HIV-related immune markers, as marijuana usage may attenuate this cycle of HIV-related cell pyroptosis. This notion is in line with previous animal models, which suggest that marijuana may mitigate the transference of the virus from one cell to the next (Molina et al., 2011a). The findings from the current study support this literature, as those who tested positive for marijuana use have higher CD4+ and CD8+ counts than their negative counterparts.
The current study has some limitations. First, this is a cross-sectional design, so results require replication in different sample types. Given the limited sample size of 95, we could not statistically adjust for many other covariates that may be associated with HIV progression. We also could not quantify the marijuana amount used or if any participant has marijuana use disorder. Lastly, the current study did not have any metrics that presented the potential differences in functionality between CD4+ and CD8+ T lymphocytes.
The current findings suggest a potentially beneficial role to marijuana, additional to symptom palliation. This preliminary study shows THC positive patients having better HIV-related immune levels than their negative counterparts, despite not being statistically different on various demographic or HIV-related covariates. Moreover, this provides some insight on heterosexual Black PLWH, as this literature is currently in its infancy. Future research should examine marijuana use history as well as biologically confirmed use to determine the potential points of intervention or even further develop a model based on marijuana’s influence on HIV progression. Additionally, future research should utilize a more comprehensive assessment of immune function in a single sample in order more accurately inform generalization efforts.
Footnotes
Conflict of interest
No conflicts of interest declared by any of the contributors to this manuscript.
References
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M, 2003. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann. Int. Med 139, 258–266. [DOI] [PubMed] [Google Scholar]
- Ader R, Felten D, Cohen N, 1990. Interactions between the brain and the immune system. Ann. Rev. Pharm. Toxicol 30, 561–602. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA, 2014. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J. Behav. Med 37, 1–10. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Pettit DAD, 1998. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmun 83, 116–123. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. HIV Surveillance Report, 2015. pp. 27 Published November 2016. http://www.cdc.gov/hiv/library/reports/hivsurveillance.html.
- Chang YH, Lee ST, Lin WW, 2001. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. Biochem 81, 715–723. [DOI] [PubMed] [Google Scholar]
- Chao C, Jacobson LP, Tashkin D, Martínez-Maza O, Roth MD, Margolick JB, Rinaldo C, Zhang ZF, Detels R, 2008. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend. 94, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G, Matson P, Grady CD, Nahvi S, Merenstein D, Weber K, Greenblatt R, Wilson TE, 2012. Medicinal and recreational marijuana use among HIV-infected women in the women’s interagency HIV cohort (WIHS). J. Acquir. Immune. Defic. Syn 61, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, 2011. HIV infection, inflammation, immunosenescence, and aging. Ann. Readapt. Med. Phys 62, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-AriasGreene IWC, 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R, 2004. Medicinal and recreational marijuana use by patients infected with HIV. AIDS. Pat. Care. STD 18 (215-), 228. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Tracy M, Vlahov D, 2007. Neighborhood income and income distribution and the use of cigarettes, alcohol, and marijuana. Am. J. Prev. Med. Public Health 32, S195–S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen L II, Turner AD, 2015. Differential effects of self-reported lifetime marijuana use on interleukin-1 alpha and tumor necrosis factor in African American adults. J. Behav. Med 38, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen L, Pereira D, Latimer W, 2014. Self-reported lifetime marijuana use and interleukin-6 levels in middle-aged African Americans. Drug Alcohol Depend. 140, 156–160. [DOI] [PubMed] [Google Scholar]
- Kelly EM, Dodge JL, Sarkar M, French AL, Tien PC, Glesby MJ, Golub ET, Augenbraun M, Plankey M, Peters MG, 2016. Marijuana use is not associated with progression to advanced liver fibrosis in HIV/HCV coinfected women. Clin. Infect. Dis, ciw350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Friedman H, Specter S, 1998. Marijuana, immunity and infection. J. Neuroimmun. 83, 102–115. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Wilson TE, Weber KM, Madhava V, Richardson J, Delapenha R, Jarlais DD, 2004. Initiation of regular marijuana use among a cohort of women infected with or at risk for HIV in the women’s interagency HIV study (WIHS). AIDS. Pat. Care STD 18, 702–713. [DOI] [PubMed] [Google Scholar]
- Marcellin F, Lions C, Rosenthal E, Roux P, Sogni P, Wittkop L, Protopopescu C, Spire B, Salmon-Ceron D, Dabis F, Carrieri MP, 2017. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort). Drug Alcohol Rev. 36, 227–238. [DOI] [PubMed] [Google Scholar]
- Milloy MJ, Marshall B, Kerr T, Richardson L, Hogg R, Guillemi S, Montaner JS, Wood E, 2015. High-intensity cannabis use associated with lower plasma HIV-1 RNA viral load among recently-infected people who use injection drugs. Drug Alcohol Rev. 34, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, Stouwe CV, Troxclair D, McGoey R, Varner K, Byerley L, LaMotte L, 2011. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS. Res. Hum. Retrovirus 27, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Amedee AM, Winsauer P, Nelson S, Bagby G, Simon L, 2015. Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: studies in the non-human primate SIV model. J. Neuroimmune Pharmacol 10, 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Zhou Z, Burrell LE, Kelso NE, Whitehead NE, Harman JS, Cook CL, Cook RL, 2016. Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am. J. Drug Alcohol Abs 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holdberg SD, HIV Outpatient Study Investigators, outpatient study, H.I.V., 2006. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the. J. Acquir. Immune Defic. Syndr. 43, 27–34. [DOI] [PubMed] [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM, 2004. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J. Acquir. Immune Defic. Syndr 35, 38–45. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka RS, Rutter J, 2014. Cannabinoid receptor-2 and HIV-associated neurocognitive disorders. J. Neuroimmune Pharmacol 9, 447–453. [DOI] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP, 2016. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care 28, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CM, Moody DJ, Stites DP, Levy JA, 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234, 1563–1566. [DOI] [PubMed] [Google Scholar]
- Whitfield RM, Bechtel LM, Starich GH, 1997. The impact of ethanol and marinol/marijuana usage on HIV+/AIDS patients undergoing azidothymidine, azidothymidine/dideoxycytidine, or dideoxyinosine therapy. Alcohol. Clin. Exp. Res 21, 122–127. [PubMed] [Google Scholar]