Abstract
The activating BRAF mutation p.V600E has been identified in many human cancers, including colon and lung adenocarcinoma, papillary thyroid cancer, malignant melanoma, and hairy cell leukemia. The latter two are of particular interest both because of the high proportion of cases harboring the mutation as well as the dramatic responses to BRAF inhibitor therapy which have been reported in the literature. Here we report for the first time the case of a patient with hairy cell leukemia and malignant melanoma both harboring the BRAF V600E mutation, and the successful treatment of both cancers with the BRAF inhibitor dabrafenib.
Keywords: Hairy Cell Leukemia, Melanoma, BRAF, Targeted therapy, Dabrafenib
Background
The recognition that some cancers can be driven by the presence of a single genetic lesion has helped to usher in a new era of molecular diagnosis and treatment in oncology. Although this phenomenon was first recognized and therapeutically exploited in chronic myelogenous leukemia, more recently there has been a tremendous number of discoveries identifying the genetic underpinnings of many human cancers, along with development of selective therapies to target these lesions. The relationships between genetic events and the resultant neoplasms are varied: some genes are found to be commonly mutated in a wide variety of cancers (for example, TP53 and KRAS) while others seem to be more specifically restricted to a particular malignant histology (NOTCH2 in marginal zone lymphoma).1
The BRAF p.V600E mutation has been recently identified in a number of cancers, both solid and hematologic, including adenocarcinoma of the colon, lung, and ovary (9%, 3–5%, and up to 35% respectively); papillary thyroid cancer (up to 69%), malignant melanoma (40–60%), and hairy cell leukemia (up to 100% in a landmark paper).2–9 Melanoma and hairy cell leukemia are of particular interest because both diseases have demonstrated dramatic responses to therapy with the small-molecule BRAF inhibitor vemurafenib.7,10 The newer BRAF inhibitor dabrafenib has additionally been approved for use in melanoma.
Herein we report for the first time the co-occurrence of malignant melanoma and hairy-cell leukemia both harboring the BRAF p.V600E mutation, and the successful treatment with the BRAF inhibitor dabrafenib.
Hairy Cell Leukemia
A 67-year old man with past medical history of diverticulosis, dyslipidemia, folliculitis, and mitral valve prolapse complained of excessive fatigue and cough; leukocytosis was then detected on screening blood work. Peripheral blood immunophenotyping revealed the presence of lambda restricted B-lymphocytes simultaneously expressing CD11c, CD19, CD20 (bright), CD25, CD103, and FMC-7. A bone marrow biopsy at this time was hypercellular (80%) and approximately 70% of the marrow cellularity consisted of lymphoid aggregates with lymphocytes displaying a characteristic fried-egg appearance and reticulated cytoplasmic borders. On this basis a diagnosis of classical hairy-cell leukemia (HCL) was made.
Because of hemoglobin of 10.7 g/dL and platelets of 43×103/μL, treatment was initiated with cladribine, 0.12 mg/kg/day as a 2-hour IV infusion daily for 5 days and he experienced a hematologic complete remission (CR). Two years later he became progressively more neutropenic and a bone marrow biopsy revealed recurrent disease. The patient was again successfully treated with cladribine, this time 0.09 mg/kg/day as a seven-day continuous infusion, and he again achieved a hematologic CR. This remission was more durable, but he again relapsed almost five years later with neutropenia and thrombocytopenia. He next received 12 doses of pentostatin at 4 mg/m2 and experienced a hematologic CR; however his bone marrow biopsy demonstrated small persistent disease (0.3% of lymphocytes consistent with HCL). He was also found to have new dyserythropoiesis in his marrow as well as clinical neurologic toxicity, and therefore cytotoxic chemotherapy was discontinued. The partial remission was sustained for the next two years with suboptimal, but tolerable, hematologic parameters.
Malignant Melanoma
Two years after treatment with pentostatin, the patient developed an 8 millimeter red nodule on the extensor surface of his right forearm; a shave biopsy demonstrated nodular melanoma. The patient subsequently underwent a wide excision and sentinel lymph node evaluation. Pathology confirmed the diagnosis of melanoma with a depth of 4.05 mm, mitotic rate 5/mm2, no ulceration, no satellite lesions, and negative sentinel lymph nodes. Adjuvant sargramostim (GM-CSF) was given for twelve months postoperatively. Unfortunately, upon cessation of the sargramostim he developed a new satellite nodule proximal to the site of the prior excision. PET-CT and MRI documented an additional deeper lesion medially in the midportion of the right arm (Figure 1). Notably, the PET-CT also showed intense focal enhancements in the spleen. Excisional biopsy of the new superficial and deep lesions revealed locally recurrent melanoma, with the superficial lesion having a depth of 4.8 mm and mitosis of 12/mm2, while the excised deeper lesion was approximately 1.8 cm in maximum diameter, present in the subcutis having a distance to nearest perpendicular resection margin of 0.8 mm and mitoses of 18/mm2. The excised recurrence was sent for molecular testing, and the patient then began a course of radiotherapy (30 Gy delivered over 14 days) to the affected limb.
Figure 1.
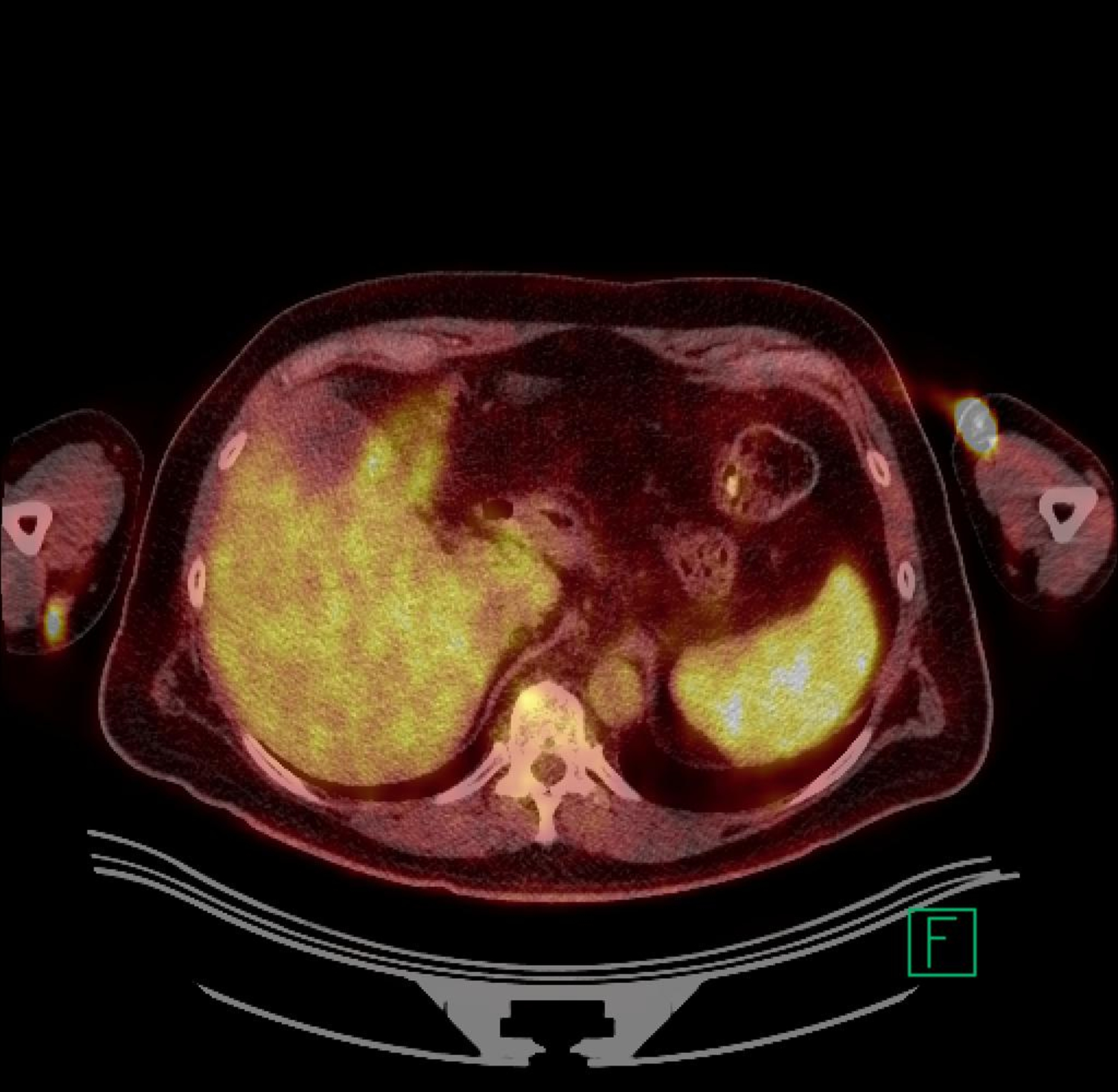
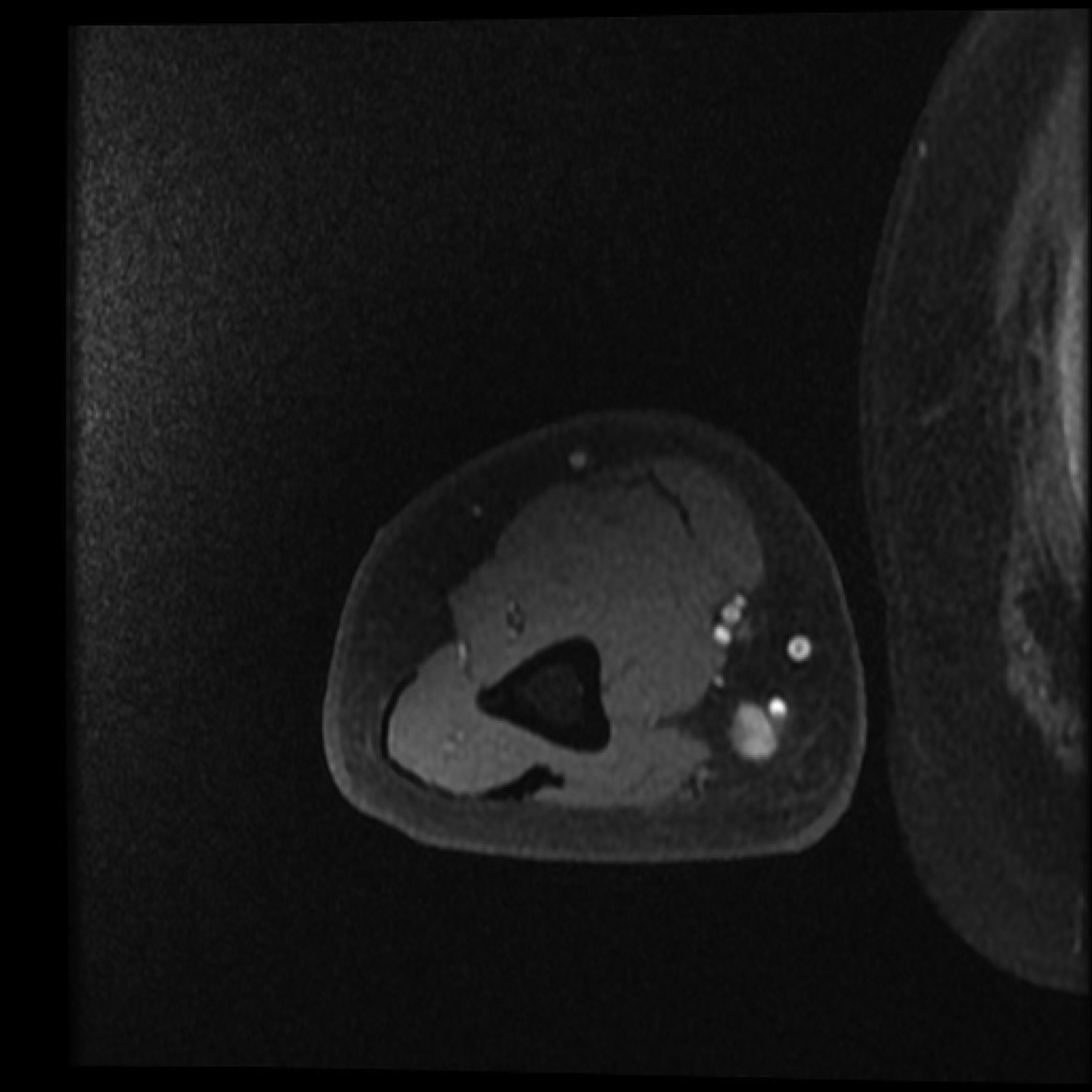
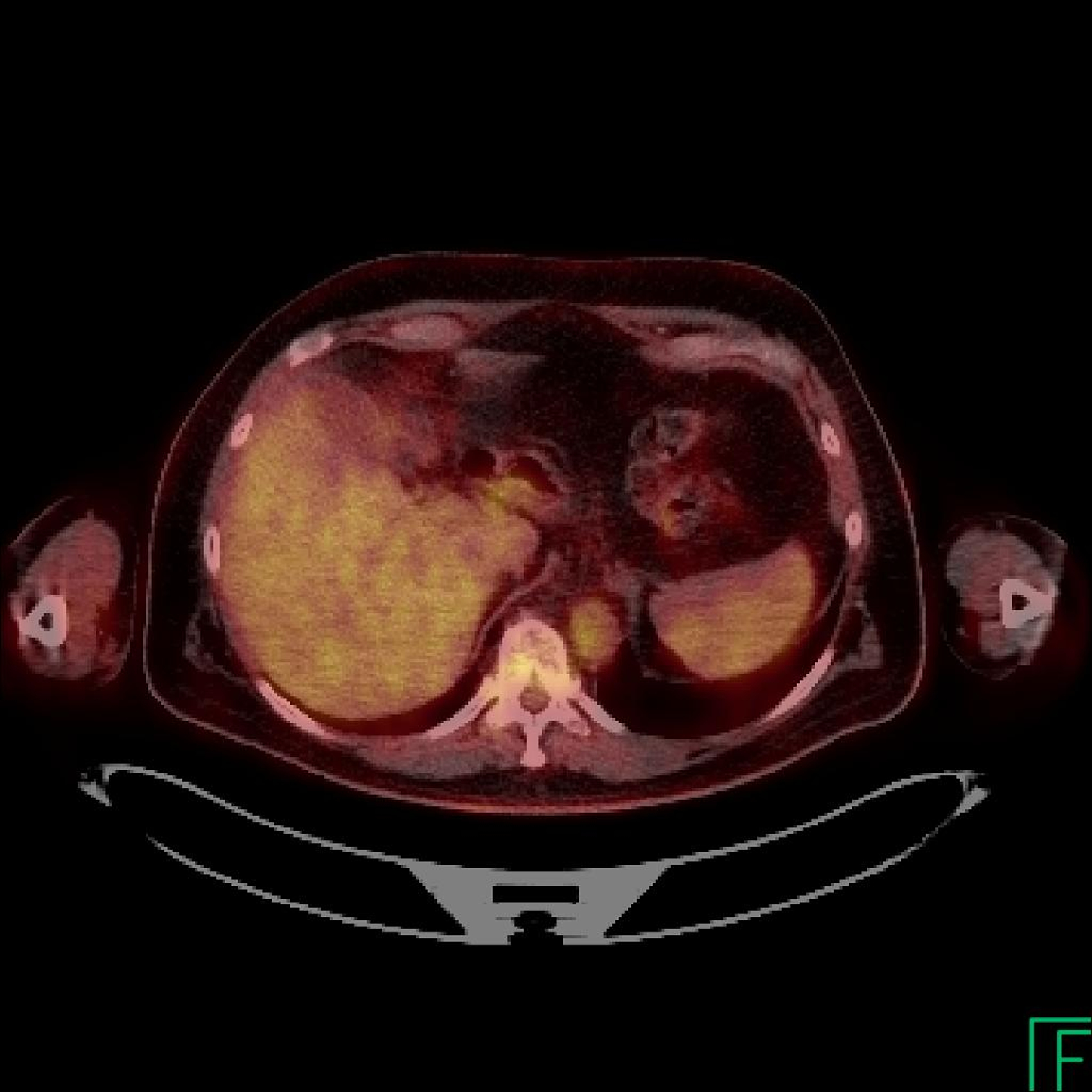
Panel A. PET CT scan and Panel B. MRI of melanoma local recurrence. Panel C. Resolution of FDG avidity in arm and spleen.
At this time the patient was also found to be thrombocytopenic (platelet count approximately 100×103/μL) and he had developed new splenomegaly on physical examination. In addition, the soluble IL-2R (a marker of disease activity in HCL) was rising (peak 3952 U/mL; normal < 970), and a bone marrow biopsy at this time showed a 20% cellular marrow with 40% involvement by classical HCL (Table 1).
Table 1.
Hematologic response to treatment with dabrafenib.
| Pretreatment | Post Cycle 3 | Post Cycle 6 | |
|---|---|---|---|
| WBC, × 103/μL | 2.7 | 4.7 | 5.9 |
| Hemoglobin, g/dL | 14.3 | 14.7 | 15.6 |
| Platelets, × 103/μL | 161 | 149 | 186 |
| Bone marrow | |||
| Cellularity (%) | 20 | 30 | 50 |
| HCL (%) | 40 | 10–15 | Zero |
Treatment with Dabrafenib
Both bone marrow aspirate as well as formalin fixed, paraffin embedded (FFPE) tissue from the second melanoma resection were then sent to the molecular diagnostics lab at The Ohio State University where BRAF mutation testing was performed. Briefly, in a CLIA certified procedure, genomic DNA was amplified with custom in-house PCR primers, after which DNA was sequenced by fluorescent capillary electrophoresis on an ABI DNA Genetic Analyzer (Applied Biosystems, Carlsbad, CA). The presence of the p.V600E mutation was documented in both the bone marrow aspirate as well as the melanoma. Because the mutation was present in his HCL cells, the patient was eligible for and enrolled on a phase I clinical trial of dabrafenib for BRAF p.V600E/K mutant malignancies (NCT01340846). Dabrafenib was initiated orally at a dose of 150 mg twice daily.
After three 28-day cycles, the bone marrow cellularity had improved to 30% with a decrease in the leukemic content to 10–15% of marrow cellularity (Table 1). After 6 cycles, the bone marrow cellularity was normal for age with no residual HCL detectable by immunohistochemical stains or flow cytometric immunophenotyping. PET/CT scan at this time demonstrated no fluorodeoxyglucose (FDG) avid lesions, with resolution of the prior upper extremity mass, resolution of focal splenic FDG avidity, and no splenomegaly. The patient is now status post 18 cycles of therapy with dabrafenib without evidence of either HCL or melanoma. To date he has tolerated therapy well, with only development of characteristic RAF-inhibitor associated skin changes11 and one instance of squamous cell carcinoma that was excised. He will remain on therapy as long as he is deriving clinical benefit per protocol.
Discussion
Activating mutations of BRAF are found overwhelmingly at codon 600 in the activation loop and are primarily a change from valine to glutamic acid, with a lysine substitution being the second-most common and mutations in other codons being found variously in visceral organ cancers.12 The canonical p.V600E mutation permits signaling through downstream MAPK effectors independent of upstream activation by RAS, and is believed to be responsible for driving cancer cell proliferation, although whether the mutation itself is sufficient for oncogenic transformation is not clear. While BRAF p.V600 mutations are present at variable levels in a number of cancers, HCL, melanoma, and papillary thyroid cancer have shown high rates of mutation, with HCL and melanoma being of particular note for having demonstrated dramatic responses to BRAF inhibition. The question then arises as to how to treat individuals with more than one malignancy containing the BRAF mutation, as in our patient with BRAF mutated HCL and melanoma. Our patient experienced a CR in the HCL after six cycles of dabrafenib, although his time to response was slower than that reported by Dietrich et al.10 Anticipated survival for resected locally recurrent melanoma is 12 months.13 The patient is now 23 months from his first melanoma recurrence, with no evidence of melanoma. The impact of BRAF inhibitors as adjuvant therapy for melanoma is unknown and is currently being studied in phase 3 trials.
Of note, HCL and melanoma have also been subdivided into groups in which the BRAF mutation is either enriched or uncommon. In particular, although HCL was initially reported to have a BRAF mutation rate of up to 100%, additional studies have shown that HCL with particular IGH@ V-gene usage has a near zero rate of BRAF mutation.14 Similarly, while nodular (the histologic subtype present in this case) and superficial spreading melanomas are enriched for BRAF mutations, the mutation is virtually absent in uveal melanoma.8
Recently, investigators have demonstrated that the BRAF mutation is present in hematopoietic stem cells (HSC) of HCL patients.15 Further, they showed that induction of mutant BRAF in mature B cells in mice does not give rise to the HCL phenotype, suggesting that the pathologic mutation arises early in hematopoietic or lymphoid development. In addition, a germline BRAF p.V600G mutation has been described in a patient with cardio-facial-cutaneous syndrome, suggesting germline BRAF mutation is compatible with human growth and development.16
In an effort to determine if our patient had germline, common lymphoid progenitor, or HSC BRAF mutation, we collected peripheral blood with patient consent under an IRB approved protocol. We then isolated T-cells and confirmed the absence of HCL by flow cytometry (96% of events were CD3+, while 1.1% were CD3+, CD16+, and CD56+, 1.1% were CD3-, CD16+, CD56+, and no events were CD19+). Genomic DNA was extracted from the T cells and DNA surrounding the BRAF 600 codon was amplified and sequenced by Sanger methodology. There was no evidence of BRAF V600 mutation in the T cell DNA. These results suggest that the BRAF mutation arose independently in the two cancers, but do not exclude the possibility of either chimerism or pluripotent stem cell giving rise to both HSC and neural crest cells. More investigation in this and other patients is required to better understand multiple neoplasms within a patient that exhibit with common genetic lesions.
In conclusion, we have documented the co-occurrence of two BRAF mutant cancers and the successful treatment of both with one molecularly targeted therapy. Given the increased risk of second primary malignancy in patients with HCL, patients with BRAF mutated HCL who develop a second primary malignancy should be considered for BRAF mutation testing of solid tumors in which this mutation has been described, even at very low frequency, as co-treatment may be possible. As we learn more about the molecular underpinnings of cancer, it remains to be seen how often we will discover common mechanisms at work in synchronous or metachronous cancers within individual patients.
References
- 1.Kiel MJ, Velusamy T, Betz BL, et al. Whole-genome sequencing identifies recurrent somatic notch2 mutations in splenic marginal zone lymphoma. J. Exp. Med 2012;209(9):1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the braf v600e mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65(14):6063–6069. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non–small-cell lung cancer harboring braf mutations. J. Clin. Oncol 2011;29(26):3574–3579. [DOI] [PubMed] [Google Scholar]
- 4.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring braf mutations. J. Clin. Oncol 2011;29(15):2046–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grisham RN, Iyer G, Garg K, et al. Braf mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119(3):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen Y, Xing M, Mambo E, et al. Braf mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst 2003;95(8):625–627. [DOI] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with braf v600e mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves WO, Verma S, Patel KP, et al. Frequency and spectrum of braf mutations in a retrospective, single-institution study of 1112 cases of melanoma. J. Mol. Diagn. JMD 2013;15(2):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiacci E, Trifonov V, Schiavoni G, et al. Braf mutations in hairy-cell leukemia. N. Engl. J. Med 2011;364(24):2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich S, Glimm H, Andrulis M, et al. Braf inhibition in refractory hairy-cell leukemia. N. Engl. J. Med 2012;366(21):2038–2040. [DOI] [PubMed] [Google Scholar]
- 11.Su F, Viros A, Milagre C, et al. Ras mutations in cutaneous squamous-cell carcinomas in patients treated with braf inhibitors. N. Engl. J. Med 2012;366(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pakneshan S, Salajegheh A, Smith R, Lam A. Clinicopathological relevance of braf mutations in human cancer. Pathol. June 2013. 2013;45(4):346–356. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann. Surg. Oncol 2001;8(2):101–108. [DOI] [PubMed] [Google Scholar]
- 14.Xi L, Arons E, Navarro W, et al. Both variant and ighv4–34–expressing hairy cell leukemia lack the braf v600e mutation. Blood. 2012;119(14):3330–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of brafv600e mutations in hairy cell leukemia. Sci. Transl. Med 2014;6(238):238ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champion KJ, Bunag C, Estep AL, et al. Germline mutation in braf codon 600 is compatible with human development: de novo p.v600g mutation identified in a patient with cfc syndrome. Clin. Genet 2011;79(5):468–474. [DOI] [PubMed] [Google Scholar]


