Abstract
Objective:
To investigate whether the effects of HD-tDCS and conventional tDCS of the right IFG are superior to the effects of sham stimulation for the improvement of working memory performance in ADHD.
Methods:
15 ADHD patients between 10 and 16 years underwent three tDCS sessions in which conventional, HD and sham tDCS of the right IFG were applied. In all sessions a 2-back working memory task was solved and EEG was recorded. Baseline data were assessed from 15 age matched healthy controls.
Results:
In ADHD patients, increased positive values of P300 and N200 mean amplitudes were found after conventional and HD-tDCS. Thus, both components were more in resemblance to ERPs in healthy controls. Behavioral performance was not generally influenced by tDCS but effects of HD-tDCS depended on individual hyperactive/impulsive symptom load. The rate of responders for HD-tDCS was equivalent to the responder rate for conventional tDCS.
Conclusions:
ERP data indicate that HD-tDCS is equally suitable as conventional tDCS for the recruitment of the right IFG in the context of working memory processing.
Significance:
HD-tDCS of the right IFG is a promising approach for neuromodulation in ADHD but further research is necessary to develop adaptations that produce reliable behavioral benefits.
Keywords: Attention deficit hyperactivity disorder (ADHD), Transcranial direct current stimulation (tDCS), High definition transcranial direct current stimulation (HD-tDCS), Working memory, Right inferior frontal gyrus (IFG), n-back task
1. Introduction
Attention deficit hyperactivity disorder (ADHD) has a childhood prevalence of 7.2% and is therefore one of the most common psychiatric disorders in school age children (Thomas et al., 2015). It is characterized by age inappropriate levels of inattention, impulsivity and hyperactivity (DSM-V) leading to functional and psychosocial impairments that affect school performance as well as family life (Able et al., 2007). Working memory deficits belong to the most prominent cognitive ADHD symptoms and are found in up to 98% of patients (Kasper et al., 2012). They are a better predictor than inattention or hyperactive/impulsive symptoms for academic dysfunction, grade retention, placement in special classes and poor reading and math performance (Fried et al., 2016, Simone et al., 2018).
Working memory is defined as the temporary storage for maintaining and manipulating information. It is often investigated with the n-back paradigm, in which subjects have to indicate if each stimulus of a sequence matches the stimulus presented a specified number (n) of trials previously. ADHD patients show diminished n-back performance associated with reduced amplitudes of the N200 and P300 (Barry et al., 2003, Keage et al., 2008, Johnstone et al., 2013). The N200 peaks over centro-parietal sites (Stroux et al., 2016) and represents a match/mismatch process (Daffner et al., 2011). P300 peaks over parietal sites and has been associated with stimulus classification, updating of mental representations in working memory and the decision how to respond (Helenius et al., 2011). Working memory is modulated by a fronto-parietal network (Darki et al., 2015) including the inferior frontal gyrus (IFG). This region receives information from posterior association areas and organizes information held in working memory (D’Esposito et al., 2000). IFG activity during the n-back task is mainly bilateral (Miró-Padilla et al., 2019). But in ADHD patients, particularly the right IFG shows structural and functional changes such as reduced grey matter volume (Depue et al., 2010) and decreased activity during working memory tasks (Schweitzer et al., 2000, Valera et al., 2010).
Response inhibition is a cognitive function important to consider when understanding working memory deficits in ADHD. On a behavioral level this functions shows moderate to high correlations with working memory (Alderson et al., 2017). This is reflected on the functional level, where the right IFG was identified as a common area being active during working memory and response inhibition (McNab et al., 2008). A study from Clark et al. (2007) indicates that ADHD related deficits in both cognitive functions may stem from a common pathologic process that is driven by the underactivation of the right IFG. They showed that working memory and response inhibition performance are associated with each other in ADHD patients and in patients with right frontal lesions but not in patients with left frontal lesions. Because both functions seem to be closely intertwined, Johnstone et al. (2010) showed effectiveness for a cognitive ADHD training that combined working memory and response inhibition aspects. Accordingly, we applied a combination of both task demands for the improvement of working memory in ADHD.
Transcranial direct current stimulation (tDCS) is a method to modulate cortical excitability, which has been suggested to be of therapeutic use in ADHD (Castellanos et al., 2012, Muszkat et al., 2016). As a non-pharmaceutical alternative it produces less sideeffects (Lee et al., 2011) and can have long-lasting effects when applied repeatedly (Cohen Kadosh et al., 2010). An important factor for the success of tDCS is the degree of activation in the target area. For prefrontal tDCS during the n-back task it was found that online stimulation and a difficult task (3-back task) lead to greatest performance improvements (Martin et al., 2014, Gill et al., 2015). It seems that best effects are achieved when the target brain network is in an activated or pre-activated state so that activation within the network is reinforced by the stimulation (Gill et al., 2015). In the present study design these effects were considered as stimulation was performed online. Moreover, the working memory task was enriched by inhibitory task demands to maximize involvement of the right IFG. This approach was considered most suitable for ADHD patients since increasing the difficulty of the n-back task could have resulted in reduced motivation or cognitive fatigue.
Studies that applied tDCS in ADHD patients have already shown beneficial effects on interference control (Breitling et al., 2016), functional connectivity (Cosmo et al., 2015, Sotnikova et al., 2017), different aspects of executive functions (Soltaninejad et al., 2015, Bandeira et al., 2016, Nejati et al., 2017) and general ADHD symptoms (Cachoeira et al., 2017, Soff et al., 2017). Further, oscillatory tDCS during sleep increased behavioral inhibition (Munz et al., 2015) and declarative memory (Prehn-Kristensen et al., 2014). However, none of these studies directly analyzed the underlying electrophysiology and only one focused on the stimulation of the right IFG.
All prior studies used a bipolar electrode configuration mostly with rectangular pad electrodes that had a size of 7 × 5 cm. This conventional tDCS montage is discussed critically because it induces diffuse distributions of current flow in widespread brain areas, where the largest current density might not occur directly under the electrodes (Datta et al., 2009, Faria et al., 2011). An alternative is high definition tDCS (HD-tDCS). For HD-tDCS small disc electrodes are placed in a 4 × 1 configuration with the stimulation electrode being surrounded by four reference electrodes in a ringlike pattern (Datta et al., 2009). In this montage current flow is restricted to the area under the electrodes, which increases precision. This ensures high current densities mainly in the target area and the risk of side effects is reduced as stimulation of non-target brain areas is kept to a minimum.
The aim of this study was to investigate whether effects of HD-tDCS and of conventional tDCS are superior to the effects of sham stimulation on working memory performance in ADHD patients. The present study is the first to apply HD-tDCS to ADHD patients and one of the first to use this method in children and adolescents. We used a within subjects design, where, in a first step, every patient underwent a training session of the cognitive task to reduce learning effects in later sessions. In the following sessions, anodal stimulation was applied to the right IFG using conventional, HD and sham tDCS. Patients performed a 2-back task enriched with response inhibition requirements during stimulation. Current flow simulations of HD-tDCS were used to place electrodes. For conventional tDCS a bipolar setting of pad electrodes was used with the anode placed over the target area, and this montage was computer-simulated to assess the current density. EEG was recorded subsequent to tDCS, while patients still performed the cognitive task. Additionally, baseline data were assessed in a healthy control group in order to evaluate performance and neurophysiological parameters in the ADHD group. We expected that in verum tDCS conditions working memory performance would improve and that amplitudes of N200 and P300 would increase. In tDCS, as in most treatments, interindividual variability in response is high, with less than 50% responders being not unusual (Lopez Alonso 2014). Therefore, we investigated whether individual characteristics as inattentive and hyperactive/impulsive symptom load predicted responsiveness to stimulation (Fins et al., 2017).
2. Methods
2.1. Participants
30 children and adolescents aged 10–16 years participated in the study. Patients were recruited via the Department of Child and Adolescent Psychiatry and control participants through advertisements in the local newspaper. All participants and their parents were interviewed using the German Adaption (Delmo et al., 2000) of the K-SADS-PL based on DSM-IV criteria (Kaufmann et al., 1997). Fifteen participants met the diagnostic criteria of ADHD. Standardized measures of intelligence (CFT 20-R; Weiss, 2008), concentration performance (d2; Brickenkamp, 2002) and handedness (Edinburgh Handedness Inventory; Oldfield, 1971) were used. In the patient group, participants with an IQ below 80 and over 130 or with psychiatric disorders others than oppositional defiant disorder or conduct disorder were excluded. Patients that were currently taking ADHD medication refrained at least 24 h before each session. Participants of the healthy control group reported no neurological or psychiatric disorders. As one ADHD patient was excluded from all data analyses, sample characteristics in Table 1 are displayed for the remaining participants. Four more patients were excluded from EEG analysis only.
Table 1.
Sample characteristics, M and SD.
| ADHD | Controls | t (p) | |
|---|---|---|---|
| N | 14 | 15 | - |
| Age (years) | 13.3(1.9) | 13.3(1.8) | 0.13(0.896) |
| Number of females | 2 | 2 | - |
| Combined subtype ADHD | 10 | - | - |
| Primarily inattentive subtype ADHD | 4 | - | - |
| Oppositional defiant disorder | 3 | - | - |
| Current medication | 5 | - | - |
| Methylphenidate | 4 | - | - |
| Lisdexamfetamine | 1 | - | - |
| IQ | 100.2(11.2) | 104.3(12.0) | −0.94(0.356) |
| Number of ADHD symptoms (K-SADS-Pl, parent rating present) | 12.6(3.7) | 1.0(2.1) | 10.38(<0.001) |
The study was approved by the local ethics committee of the University of Magdeburg and followed the ethical standards of the Helsinki declaration. All participants and their parents gave written informed assent/consent before participating and none of them reported contraindications to receiving tDCS. Participants obtained a voucher in each session (15 €) for a local shopping center.
2.2. Task and procedure
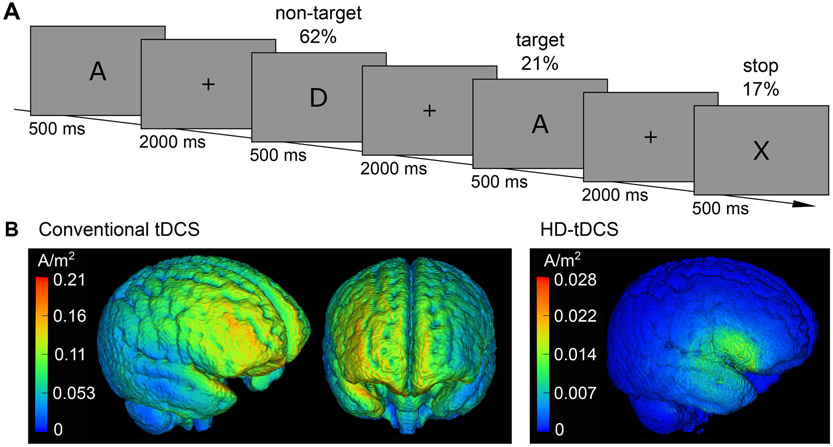
An n-back paradigm (n = 2) was used where a series of capital letters (A, D, E, H, I, N, R, S, T, U) was presented (Fig. 1A). Target trials (21%) had to be identified by button press (right target, left non-target). This task was enriched by stop trials (17%) where the stimulus was an X and participants should not press any button. Afterwards, a new series of letters started. Before the beginning of the task a practice session was conducted for 1.25 min. Participants were instructed to react as fast and as accurately as possible. Stimuli were presented with the software Presentation (version 18.0, www.neurobs.com) and had a visual angle of 0.86° (height). In a pilot experiment, it was validated that the inhibitory task demands did not compromise the ERP component structure (see Supplementary Table S1).
Fig. 1.
Methods. (A) Schematic illustration of the modified n-back task, (B) Simulations of current flow for conventional and HD-tDCS.
ADHD patients underwent four sessions. In the first session they trained the task for six runs (each 4.5 minutes, 110 trials) to reduce learning effects in the following stimulation sessions. Three tDCS conditions, conventional, HD and sham, were conducted in the following sessions in a pseudo-randomized, double blind order, separated by at least six days. After EEG and tDCS electrode placement the experiment started with 5 min of tDCS followed by another 15 min of stimulation while the task was applied (3 runs). Afterwards, the EEG recording started and the task was conducted for another 15 min. At the end of each session participants reported tDCS related skin sensations on a 5-point Likert-scale and at the end of the last session a questionnaire about tDCS side effects was filled in. Each session had a duration of about 1.5 h.
The healthy control group participated in only one session, in which the task was conducted for 15 min while recording EEG. Because of deviating procedures between patients and controls, data of both groups are not entirely comparable. Still, behavioral and neurophysiological data of healthy controls serve as reference and should constitute the margin of improvement that can potentially be achieved in ADHD patients.
2.3. Transcranial direct current stimulation (tDCS)
TDCS was conducted with a battery driven DC stimulator (neuroConn, Munich, Germany). For conventional tDCS 7 × 5 cm rubber electrodes were covered with saline soaked sponges (NaCl 0.9%). The anode was placed centrally over EEG position F8, which corresponds to the right IFG (Koessler et al., 2009). The cathode was placed over the contralateral supra-orbital area. For HD-tDCS a 4 × 1 montage (Kessler et al., 2013) of small circular electrodes (diameter 1 cm) was used with the anode placed centrally. Fig. 1B shows the estimated current magnitude for conventional and HD-tDCS. For details of current flow simulations of both montages and of electrode placement for HD-tDCS see Supplementary Material S2. Sham tDCS was randomly applied with conventional or HD electrode setting. Current intensities were set to 1 mA for conventional and 0.5 mA for HD-tDCS to adjust for higher concentrations of current densitiy during focal stimulation. Three patients were very sensitive to the stimulation so that current intensities were reduced by 50%. In two patients current intensities were reduced during all tDCS sessions, in one patient only current intensity of conventional tDCS was reduced. Stimulation was applied for 20 min with a 30 s ramp up and down.
2.4. EEG recording and analysis
EEG was recorded using a SynAmps amplifier (Neuroscan, Sterling, VA, USA). Data from 21 channels were measured according to the International 10–20 EEG system with Ag/AgCl-electrodes placed in a cap (Easycap GmbH, Herrsching, Germany) at positions Fp1, Fp2, F7, F3, Fz, F4, F8, FT9, FC5, FC6, FT10, C3, Cz, C4, P7, P3, Pz, P4, P8, O1 and O2. Bipolar channels with electrodes placed at the outer canthi of both eyes and at sub- and supra-orbital positions were used to record electro-oculograms. Signals were referenced to linked mastoid electrodes and ground electrode was at AFz. The sampling rate was 500 Hz. Data were filtered with an analog filter between 0.05 and 70 Hz and with a notch filter at 50 Hz. Impedances were kept below 15 kΩ.
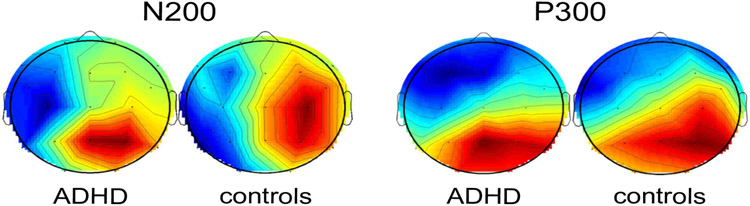
EEG data were analyzed with EEGLAB (Delorme et al., 2004) and ERPLAB (Lopez-Calderon et al., 2014) in the MATLAB environment (version R2013a, The MathWorks, Inc., Nattick, MA, USA). Data were filtered digitally with a 30 Hz low pass and split into epochs of 2000 ms. Epochs were baseline corrected relative to a time window of −200 to 0 ms. Due to extensive eye and facial movement only parietal electrodes were used for further processing. Artifact detection was applied in the time window between 0 and 700 ms. Trials with artifacts that exceeded 100 μV were removed automatically and further artifactual trials were removed by a trained person. Five ADHD patients were excluded from the EEG analysis because of low remaining trial count (less than 15 trials). Thus, a mean number of 34 n-back target trials was analyzed in ADHD patients and of 50 trials in healthy controls (t(23) = −4.23, p < .001). A right parietal region of interest (ROI: P4, P8) was chosen on the basis of topographic distribution of components (see Fig. 2). Mean amplitudes and latencies were defined with the ERPLAB measurement tool for N200 (150–250 ms) and P300 (300–450 ms).
Fig. 2.
Topographic distribution of ERP components. Topographic plots show a right lateralization of N200 (at 220 ms) and P300 (at 320 ms) components in ADHD patients (sham session) and controls during n-back target trials.
2.5. Statistics
Working memory performance was calculated as the corrected hit rate (target hits - false alarms). One patient was excluded from behavioral data analysis because working memory performance (mean over all experimental sessions) was below two standard deviations of the group mean. Reaction times were calculated from correct trials with reaction times of 100 ms or more. Performance measures from online and offline tDCS were pooled as no interaction with tDCS condition was found (see Supplementary Table S3). Statistical evaluation was carried out in SPSS (version 24.0, IBM Corp., Armonk, NY, USA). To compare ADHD patients and healthy controls independent t-tests were performed. If Levene’s test for equality of variances was significant, t-tests with a Satterthwaite approximation for the degrees of freedom were reported. For performance measures (working memory performance, misses, reaction times, standard deviation of reaction times) patients’ first sessions and for ERP measures (amplitudes, latencies) patients’ sham sessions were used. We point out that acquisition and trial number of ERP data in controls are not entirely comparable to ADHD patients. However, these data were used as a reference for the interpretation of ERPs in ADHD patients. ANOVAs were conducted with the factor tDCS condition (conventional vs. HD vs. sham) for performance and ERP measures to investigate effects of tDCS. When necessary, results were Greenhouse Geisser corrected. Subsequently, patients were categorized into responders and nonresponders. The difference between working memory performance in verum and sham session was defined as the tDCS effect and served as an indicator for this classification. Patients with a positive difference were defined as responders, all others as nonresponders. Responding rates during conventional and HD-tDCS were compared using McNemar’s test for repeated measures. Finally, a regression analysis of the tDCS effect on working memory performance was calculated from the factors “number of inattentive symptoms” and “number of hyperactive/impulsive symptoms” assessed with the K-SADS-PL as well as from the factors “IQ” and “age” using the method forward.
3. Results
3.1. Behavioral data
ADHD patients showed impaired working memory performance compared to healthy controls (t(27) = −2.67, p = .013) and responded less frequently (misses: t(23) = 2.26, p = .034). Table 2 displays the task performance.
Table 2.
Behavioral results. M and SD of task performance measures in ADHD patients and controls, results of comparisons between ADHD patients (first training session) vs. controls, and within tDCS conditions (conventional vs. HD vs. sham).
| ADHD | controls | d | t | p | ADHD patients |
F | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st session | conv. | HD | sham | ||||||||
| WM1 | 58.7%(19.0%) | 74.7%(12.9%) | −0.985 | −2.67 | 0.013 | 54.3%(16.4%) | 56.5%(18.2%) | 57.9%(13.5%) | 0.042 | 0.57 | 0.570 |
| Misses | 2.7%(1.9%) | 1.3%(1.4%) | 0.387 | 2.26 | 0.034 | 3.4%(3.9%) | 4.8%(4.4%) | 5.8%(8.4%) | 0.051 | 0.69 | 0.510 |
| RT | 829 ms(209 ms) | 710 ms(195 ms) | 0.282 | 1.60 | 0.122 | 787 ms(214 ms) | 817 ms(180 ms) | 785 ms(192 ms) | 0.100 | 1.44 | 0.256 |
| SD of RT | 280 ms(79 ms) | 252 ms(81 ms) | 0.172 | 0.96 | 0.347 | 266 ms(78 ms) | 281 ms(82 ms) | 275 ms(68 ms) | 0.093 | 1.33 | 0.282 |
WM - working memory.
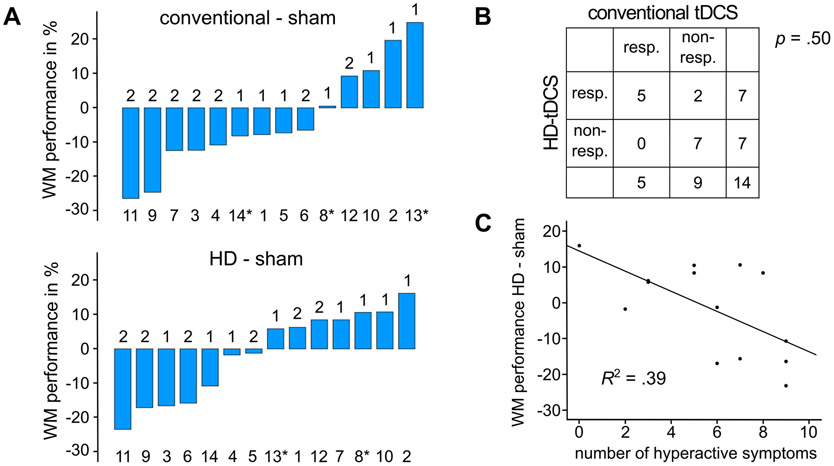
There was no general effect of conventional or HD-tDCS on working memory performance (F(2,26) = 0.57, p = .570) or on other task performance measures. This might be due to high variability in responsivity to tDCS between patients. On a descriptive level, variability (Fig. 3) showed differences between both tDCS montages with the responder rate being higher for HD-tDCS (50% responders) than for conventional tDCS (36% responders) (p = .50). Thereby, all patients who responded to conventional tDCS also responded to HD-tDCS but not vice versa.
Fig. 3.
Interindividual variability. (A) Individual changes of working memory performance (WM) in response to conventional and HD-tDCS, positive values represent performance increase in tDCS conditions, numbers over the bars indicate if the verum tDCS condition was first or second to sham condition, numbers under the bars indicate individual patients with * specifying patients stimulated with reduced current intensities, (B) Number of patients that responded to stimulation for different montages, (C) Association between number of hyperactive ADHD symptoms and HD-tDCS induced working memory improvement.
Regression analyses were used to investigate if the number of inattentive or hyperactive/impulsive ADHD symptoms as well as IQ and age were predictors of tDCS effects. We found that for HD-tDCS the number of hyperactive/impulsive symptoms predicted the effect on working memory performance (b = −0.62, t(12) = −2.74, p = .018) and therefore explained a significant proportion of variance (R2 = 0.39, F(1,12) = 7.50, p = .018). Thus, in individuals with fewer hyperactive/impulsive symptoms HD-tDCS had larger positive effects on working memory performance. As there was no correlation between number of hyperactive/impulsive symptoms and working memory performance in the first training session (r = −0.16, p = .578), the effect was not explained by poor baseline performance of tDCS responders. Interestingly, the effect of conventional tDCS on working memory performance was not predicted by any of the investigated factors.
3.2. Event related potentials (ERPs)
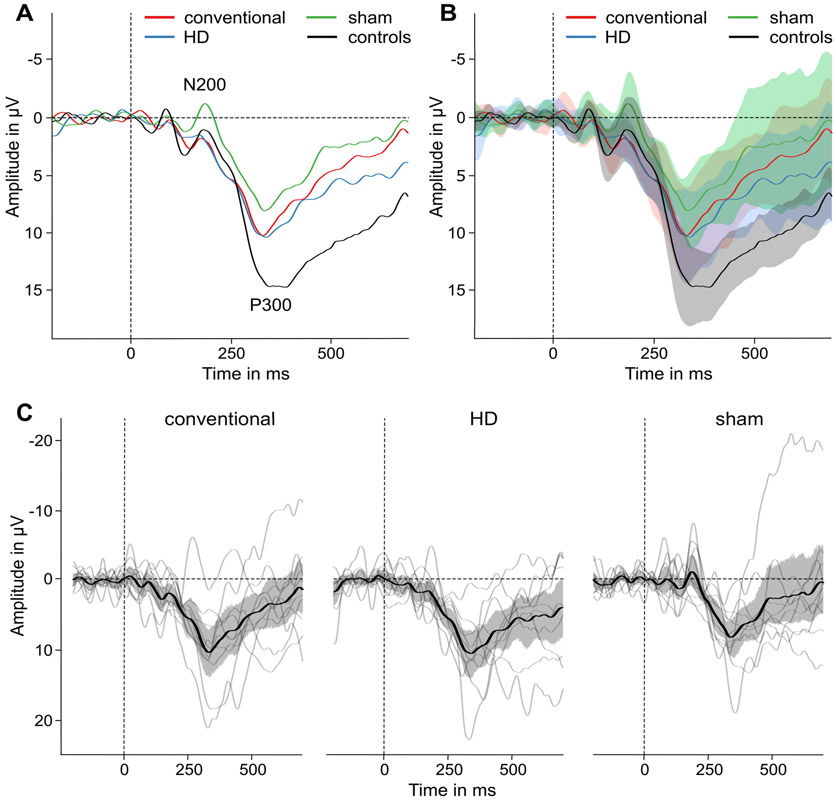
Fig. 4 illustrates ERP waveforms that were analyzed at a right parietal ROI (P4, P8). Over all groups and conditions, components N200 and P300 were evoked at 185 ms and at 358 ms, respectively.
Fig. 4.
ERP results. (A) Grand average ERPs at a right parietal ROI (P4, P8) for conventional, HD and sham tDCS in ADHD patients and healthy controls during n-back target trials (B) with their 95% confidence intervals, (C) ERPs of individual ADHD patients for different experimental conditions.
Between controls and sham condition of ADHD patients there was no difference in amplitudes of N200 (t(23) = −1.13, p = .271) but amplitudes of P300 were reduced in patients (t(23) = −3.46, p = .002). An ANOVA (conventional vs. HD vs. sham) showed a significant difference between tDCS conditions for the N200 (F(2,18) = 7.51, p = .004). Mean amplitudes were more positive after conventional tDCS (t(9) = 2.98, p = .016) and after HD-tDCS (t(9) = 3.20, p = .011) compared to sham stimulation. For the P300, a significant difference between tDCS conditions was also found (F(2,18) = 8.91, p = .002). After conventional tDCS (t(9) = 2.58, p = .030) and after HD-tDCS (t(9) = 5.04, p = .001) amplitudes of P300 were larger compared to sham. Thus, after stimulation, working memory related ERP components in ADHD patients were more in resemblance to ERPs in healthy controls. Latencies of both components were not affected by tDCS. Mean ERP values and results of statistical comparisons are given in Table 3.
Table 3.
ERP results. M and SD of ERP characteristics in ADHD patients and controls, results of ANOVAs and post-hoc t-tests between tDCS conditions (conventional vs. HD vs. sham) within ADHD patients.
| controls | ADHD patients |
F | p | conv. vs. sham |
HD vs. sham |
conv. vs. HD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| conv. | HD | sham | t | p | t | p | t | p | |||||
| N200 amplitude | 2.35 μV (4.80 μV) | 3.16 μV (2.07 μV) | 3.02 μV (1.97 μV) | 0.51 μV (2.21 μV) | 0.455 | 7.51 | 0.004 | 2.98 | 0.016 | 3.20 | 0.011 | 0.23 | 0.821 |
| N200 latency | 191 ms (23 ms) | 187 ms (27 ms) | 181 ms (30 ms) | 179 ms (22 ms) | 0.024 | 0.22 | 0.804 | 0.67 | 0.518 | 0.15 | 0.882 | 0.45 | 0.666 |
| P300 amplitude | 13.87 μV (5.36 μV) | 9.14 μV (5.74 μV) | 9.75 μV (4.93 μV) | 7.11 μV (3.74 μV) | 0.497 | 8.91 | 0.002 | 2.58 | 0.030 | 5.04 | 0.001 | −0.98 | 0.355 |
| P300 latency | 362 ms (39 ms) | 350 ms (36 ms) | 361 ms (47 ms) | 356 ms (39 ms) | 0.033 | 0.31 | 0.739 | −0.49 | 0.636 | 0.38 | 0.713 | −0.65 | 0.531 |
3.3. Side effects
TDCS related sensations were rated with medium intensity on a 5-point Likert-scale (conventional 3.0, HD 2.8, sham 2.3; F(2,28) = 3.55, p = .043; conventional vs. shamp p = .022; HD vs. sham p = .056). Patients reported the following side effects: itching 36%, pain 36%, fatigue 21%, headache 7%, phosphenes 7%.
4. Discussion
We investigated effects of conventional and HD-tDCS over the right IFG on working memory performance in children and adolescents with ADHD. We found increased positive values of mean amplitudes for P300 and N200 components during HD and conventional tDCS, suggesting that the underlying neurophysiological processes were more in resemblance to typically developing peers. Behavioral performance was not generally influenced by tDCS but HD-tDCS effect on working memory depended on the individual hyperactive/impulsive symptom load. Moreover, the rate of responders for HD-tDCS was at least equivalent to the responder rate for conventional tDCS.
We could show that both tDCS montages increased the amplitude of the P300. Most ERP research on P300 was done in adult ADHD patients and is therefore not entirely comparable to the present study. Still, our results are in line with a meta-analysis of Szuromi et al. (2011) finding decreased P300 amplitudes in ADHD. They stated that over a pathway which includes the lateral prefrontal cortex and the temporoparietal junction, P300 is associated with the ventral attention network. Therefore, they interpreted decreased P300 amplitudes in ADHD as a dysfunction of that network. Accordingly, increased P300 amplitudes in the verum tDCS conditions of this study suggest an enhanced function of the ventral attention network and therefore improved working memory processing in the patients. It is interesting to note that the administration of methylphenidate also increased P300 (Hermens et al., 2005). For the N200 component we found more positive values of mean amplitudes after verum tDCS, which was not in line with our hypothesis. However, this means that after stimulation N200 amplitudes were more similar to control participants, which suggests underlying neurophysiological processes became more comparable with healthy controls.
Although tDCS modulated ERP amplitudes, the primarily targeted behavioral parameter was not generally improved, which is not unusual. Often, tDCS causes changes in related parameters as reaction time (Munz et al., 2015) or network activity (Sotnikova et al., 2017). One possible explanation is that tDCS induced neurophysiological modulation can be too weak to induce behavioral effects in all individuals. Still, our ERP data indicate a positive modification of working memory processing. However, in future studies this promising approach needs to be modified in a way that induces stable improvements on a behavioral level.
Interindividual variability in response to tDCS was high, which prevented a general group effect on behavior. In fact, high variability is a frequent phenomenon in tDCS studies (Lopez-Alonso et al., 2014, Wiethoff et al., 2014). We assume that it was mainly caused by functional differences of pre-activity and excitability in relevant brain areas (Li et al., 2015) and by anatomical differences leading to varied current density distributions (Kim et al., 2014). However, when comparing both stimulation montages there was a trend towards a higher rate of responders to HD-tDCS. Furthermore, all patients that responded to conventional tDCS also responded to HD-tDCS, but not vice versa. Therefore, we consider HD-tDCS to be at least as effective as conventional tDCS.
This result is particularly remarkable as our computer simulations showed reduced average current density magnitudes of approximately 0.014 A/m2 on the right IFG’ brain surface for HD montage compared to approximately 0.14 A/m2 for conventional tDCS (where 1 mA was injected). The current density averages differed by a factor of 10 between both montages. This discrepancy can mostly be attributed to the fact that only half (factor: 2) of the total current intensity was injected for HD-tDCS (compared to conventional tDCS), so while the anodal current intensity was 0.5 mA, the cathodal current intensity of 0.5 mA was splitted equally across 4 electrodes (equals to a total factor of 8). But also volume conduction properties (e.g., tissue conductivity distribution) as well as electrode placement highly influence the current density profile in the right IFG as well as the rest of the brain, whereas the latter may not have been optimal for simulating HD-tDCS to reach a similar level of current density as with conventional tDCS electrode setup. Systematically varying current intensities in future studies may provide clarity regarding this issue. To our knowledge of the literature, no computational algorithm has been proposed to search for optimal electrode scalp locations that maximizes or matches a desired current density profile in the ROI using few HD-tDCS electrodes (e.g., 4 × 1). However, for a large number of HD electrodes with fixed scalp locations, although unknown electrode current intensities, this problem can be solved (Guler et al., 2016a, Guler et al., 2016b).
Regarding ERP modulations both tDCS montages showed similar effects but from a safety point of view HD-tDCS has some advantages. The current flow simulations shown in Fig. 1B illustrate that HD-tDCS stimulated the target area with a much higher precision than conventional tDCS. HD-tDCS induced electrical current peaks in brain areas near the electrodes whereas in conventional tDCS those peaks can also be found in non-target areas inbetween electrodes. Furthermore, during HD-tDCS current flow was restricted to the area circumscribed by the electrodes, while during conventional tDCS widespread brain areas were stimulated, including the whole right frontal lobe and adjacent areas. This unnecessary stimulation of non-target brain areas enhances the risk of unintended changes in brain functions, which is of special importance in the vulnerable ADHD patient group of children and adolescents (Hameed et al., 2017). On the other hand increased precision bears the risk of missing the target area in individuals with varying neuroanatomy. But future approaches could avoid this issue by using individualized tDCS montages. A further downside of HD-tDCS is higher current density on the skin. However, current flow simulations show considerably reduced current density on the cortex during HD compared to conventional tDCS (as discussed above) while comparable effects were induced. In an approach of using the lowest dose necessary, we would expect a reduced risk of side effects by inducing less current flow in the brain. We state that it is preferable to use HD-tDCS over conventional tDCS when possible, for the reasons of higher precision and a potentially reduced dose of current in the brain while inducing similar effects.
We found that effects of HD-tDCS on working memory performance depended on the hyperactive/impulsive symptom load. Patients with fewer symptoms were more likely to respond to HD-tDCS. We assume that this association was modulated by functional (Solanto et al., 2009, Orinstein et al., 2014) or connectivity characteristics (Fair et al., 2012, Park et al., 2016) of ADHD patients with low hyperactivity. However, further studies that compare tDCS effects specifically between high and low hyperactive ADHD patients are necessary to draw reliable conclusions about this factor. If this association will be confirmed, it would allow for the selective use of HD-tDCS in specific individuals making tDCS more efficient by sparing patients unsuccessful stimulations. Interestingly, this relationship was not found for conventional stimulation. But causation of this montage specificity cannot to be explained with the present study as ERP results provide no indication for differential mechanisms of action.
A limitation of the study is the small sample size, especially for ERP analysis. Due to ADHD symptoms, the collection of high quality EEG data was extremely difficult resulting in a small number of analyzed trials. To account for the small sample size, confounding variables were avoided. So, participants underwent one training session in the beginning to minimize learning effects between experimental sessions. Strength of the study is the assessment of EEG data from healthy controls. Still, measurements of controls were obtained from a single session, in contrast to repeated sessions in patients. Although data acquisition differed between groups, we assume results to be mainly comparable as it has been found earlier that the target P300 for visual stimuli does not habituate (Geisler et al., 1994), especially in parietal areas (Wintink et al., 2001).
5. Conclusions
We showed that HD-tDCS is at least equally suitable as conventional tDCS for the successful recruitment of the right IFG. Therefore, HD-tDCS is a safe and promising approach for modulating working memory processing in ADHD patients. Further investigations may address the question how the neurophysiological effects found here, can be extended to a stable behavioral effect. Approaches to enlarge effects could be to do repeated tDCS sessions (Ditye et al., 2012) or to apply multifocal stimulation where not only one region but a whole network can be stimulated at the same time (Fischer et al., 2017).
Supplementary Material
HIGHLIGHTS.
Working memory related P300 and N200 mean amplitudes showed more positive values after tDCS.
Behavioral performance was not generally influenced by tDCS.
Behavioral effects of HD-tDCS depended on the hyperactive/impulsive symptom load.
Acknowledgements
The authors thank all families who participated in this study. Furthermore, we thank Kerstin Scheunemann, Greta Leistikow, Asa Maiwald, Anna Skalitz and Fabian Senner for their contribution.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 731827. The work concerning the estimation of current flow related to different tDCS montages was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number P41 GM103545-18.
Abbreviations:
- ANOVA
analysis of variance
- ADHD
attention deficit hyperactivity disorder
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EEG
electroencephalography
- ERP
event related potential
- HD-tDCS
high definition transcranial direct current stimulation
- IFG
inferior frontal gyrus
- tDCS
transcranial direct current stimulation
- WM
working memory
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2019.12.412.
References
- Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med 2007;37:97–107. 10.1017/s0033291706008713. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Patros CH, Tarle SJ, Hudec KL, Kasper LJ, Lea SE. Working memory and behavioral inhibition in boys with ADHD: An experimental examination of competing models. Child Neuropsychol 2017;23:255–72. 10.1080/09297049.2015.1105207. [DOI] [PubMed] [Google Scholar]
- Bandeira ID, Guimaraes RS,Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, et al. Transcranial direct current stimulation in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a pilot study. J Child Neurol 2016;31:918–24. 10.1177/0883073816630083. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 2003;114:184–98. [DOI] [PubMed] [Google Scholar]
- Breitling C, Zaehle T, Dannhauer M, Bonath B, Tegelbeckers J, Flechtner H-H, et al. Improving interference control in ADHD patients with transcranial direct current stimulation (tDCS). Front Cell Neurosci 2016; 10 10.3389/fncel.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickenkamp R Test d2 - Aufmerksamkeits-Belastungs-Test. Göttingen: Hogrefe; 2002. [Google Scholar]
- Cachoeira CT, Leffa DT, Mittelstadt SD, Mendes LST, Brunoni AR, Pinto JV, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder - A pilot randomized controlled study. Psychiatry Res 2017;247:28–32. 10.1016/j.psychres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontalstriatal model. Trends Cogn Sci 2012;16:17–26. 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry 2007;61:1395–401. 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr Biol 2010;20:2016–20. 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmo C, Ferreira C, Miranda JG, do Rosario RS, Baptista AF, Montoya P, et al. Spreading Effect of tDCS in individuals with attention-deficit/hyperactivity disorder as shown by functional cortical networks: a randomized, double-blind, sham-controlled trial. Front Psychiatry 2015; 6: 111 10.3389/fpsyt.2015.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 2000;133:3–11. 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Sun X, Tarbi EC, Riis JL, McGinnis SM, et al. Mechanisms underlying age- and performance-related differences in working memory. J Cogn Neurosci 2011;23:1298–314. 10.1162/iocn.2010.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F, Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb Cortex 2015;25:1587–95. 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2009;2(201–7):7. e1 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmo C, Weiffenbach O, Gabriel M, Bölte S, Marchio E, Poustka F. Fragebogen für Affektive Störungen und Schizophrenie für Kinder im Schulalter (6–18 Jahre). Frankfurt: Klinik für Psychiatrie und Psychotherapie des Kindes- und Jugendalters; 2000. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134:9–21. 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res 2010;182:231–7. 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res 2012;219:363–8. 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci 2012;6:80 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng 2011;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fins JJ, Kubu CS, Mayberg HS, Merkel R, Nuttin B, Schlaepfer TE. Being open minded about neuromodulation trials: Finding success in our “failures”. Brain Stimul 2017;10:181–6. 10.1016/j.brs.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage 2017;157:34–44. 10.1016/j.neuroimage.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R, Chan J, Feinberg L, Pope A, Woodworth KY, Faraone SV, et al. Clinical correlates of working memory deficits in youth with and without ADHD: A controlled study. J Clin Exp Neuropsychol 2016;38:487–96. 10.1080/13803395.2015.1127896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler MW, Polich J. P300 habituation from visual stimuli?. Physiol Behav 1994;56:511–6. [DOI] [PubMed] [Google Scholar]
- Gill J, Shah-Basak PP, Hamilton R. It’s the thought that counts: examining the taskdependent effects of transcranial direct current stimulation on executive function. Brain Stimul 2015;8:253–9. 10.1016/j.brs.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Guler S, Dannhauer M, Erem B, Macleod R, Tucker D, Turovets S, et al. Optimization of focality and direction in dense electrode array transcranial direct current stimulation (tDCS). J Neural Eng 2016a;13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler S, Dannhauer M, Erem B, Macleod R, Tucker D, Turovets S, et al. Optimizing Stimulus Patterns for Dense Array tDCS With Fewer Sources Than Electrodes Using A branch and Bound Algorithm. Proc IEEE Int Symp Biomed Imaging 2016b;2016:229–32. 10.1109/isbi.2016.7493251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed MQ, Dhamne SC, Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, et al. Transcranial Magnetic and Direct Current Stimulation in Children. Curr Neurol Neurosci Rep 2017;17:11 10.1007/s11910-017-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 2011;49:1889–96. 10.1016/j.neuropsychologia.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Williams LM, Clarke S, Kohn M, Cooper N, Gordon E. Responses to methylphenidate in adolescent AD/HD: Evidence from concurrently recorded autonomic (EDA) and central (EEG and ERP) measures. Int J Psychophysiol 2005;58:21–33. 10.1016/j.ijpsycho.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Clarke AR. Ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin Neurophysiol 2013;124:644–57. 10.1016/j.clinph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Roodenrys S, Phillips E, Watt AJ, Mantz S. A pilot study of combined working memory and inhibition training for children with AD/HD. Atten Defic Hyperact Disord 2010;2:31–42. 10.1007/s12402-009-0017-z. [DOI] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev 2012;32:605–17. 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kaufmann J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children - Present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997;36:980–8. [DOI] [PubMed] [Google Scholar]
- Keage HA, Clark CR, Hermens DF, Williams LM, Kohn MR, Clarke S, et al. ERP indices of working memory updating in AD/HD: differential aspects of development, subtype, and medication. J Clin Neurophysiol 2008;25:32–41. 10.1097/WNP.0b013e318163ccc0. [DOI] [PubMed] [Google Scholar]
- Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One 2013;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim DW, Chang WH, Kim YH, Kim K, Im CH. Inconsistent outcomes of transcranial direct current stimulation may originate from anatomical differences among individuals: electric field simulation using individual MRI data. Neurosci Lett 2014;564:6–10. 10.1016/j.neulet.2014.01.054. [DOI] [PubMed] [Google Scholar]
- Koessler L, Maillard L, Benhadid A, Vignal JP, Felblinger J, Vespignani H, et al. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10–10 system. NeuroImage 2009;46:64–72. 10.1016/j.neuroimage.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Lee J, Grizenko N, Bhat V, Sengupta S, Polotskaia A, Joober R. Relation between therapeutic response and side effects induced by methylphenidate as observed by parents and teachers of children with ADHD. BMC Psychiatry 2011;11:70 10.1186/1471-244x-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci 2015;9:181 10.3389/fncel.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M. Interindividual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 2014;7:372–80. 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci 2014;8:213 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Liu R, Alonzo A, Green M, Loo CK. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp Brain Res 2014;232:3345–51. 10.1007/s00221-014-4022-x. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: an fMRI, within-subiects investigation. Neuropsychologia 2008;46:2668–82. https://doi.Org/10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Miró-Padilla A, Bueichekú E, Ventura-Campos N, Flores-Compañ M-J, Parcet MA, Ávila C. Long-term brain effects of N-back training: an fMRI study. Brain Imaging Behav 2019;13(4):1115–27. 10.1007/s11682-018-9925-x. [DOI] [PubMed] [Google Scholar]
- Munz MT, Prehn-Kristensen A, Thielking F, Molle M, Goder R, Baving L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci 2015;9:307 10.3389/fncel.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkat D, Polanczyk GV, Dias TG, Brunoni AR. Transcranial Direct Current Stimulation in Child and Adolescent Psychiatry. J Child Adolesc Psychopharmacol 2016;26:590–7. 10.1089/cap.2015.0172. [DOI] [PubMed] [Google Scholar]
- Nejati V, Salehinejad MA, Nitsche MA, Najian A, Javadi AH. Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility 1087054717730611. J Atten Disord 2017. 10.1177/1087054717730611. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- Orinstein AJ, Stevens MC. Brain activity in predominantly-inattentive subtype attention-deficit/hyperactivity disorder during an auditory oddball attention task. Psychiatry Res 2014;223:121–8. 10.1016/j.pscychresns.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-y, Kim M, Seo J, Lee J-m, Park H. Connectivity Analysis and Feature Classification in Attention Deficit Hyperactivity Disorder Sub-Types: A Task Functional Magnetic Resonance Imaging Study. Brain Topogr 2016;29:429–39. 10.1007/s10548-015-0463-1. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul 2014;7:793–9. 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. Am J Psychiatry 2000;157:278–80. 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- Simone AN, Marks DJ, Bedard AC, Halperin JM. Low Working Memory rather than ADHD Symptoms Predicts Poor Academic Achievement in School-Aged Children. J Abnorm Child Psychol 2018;46:277–90. 10.1007/s10802-017-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm (Vienna) 2017;124:133–44. 10.1007/s00702-016-1646-y. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J Neuroimaging 2009;19:205–12. 10.1111/j.1552-6569.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltaninejad Z, Nejati V, Ekhtiari H. Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. J Atten Disord 2015. 10.1177/1087054715618792. [DOI] [PubMed] [Google Scholar]
- Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topogr 2017. 10.1007/s10548-017-0552-4. [DOI] [PubMed] [Google Scholar]
- Stroux D, Shushakova A, Geburek-Hofer AJ, Ohrmann P, Rist F, Pedersen A. Deficient interference control during working memory updating in adults with ADHD: An event-related potential study. Clin Neurophysiol 2016;127:452–63. 10.1016/j.clinph.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Szuromi B, Czobor P, Komlosi S, Bitter I. P300 deficits in adults with attention deficit hyperactivity disorder: a meta-analysis. Psychol Med 2011;41:1529–38. 10.1017/s0033291710001996. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015;135:e994–e1001. 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, et al. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry 2010;167:86–94. 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RH. Grundintelligenztest Skala 2 - Revision (CFT 20-R). Göttingen: Hogrefe; 2008. [Google Scholar]
- Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 2014;7:468–75. 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Wintink AJ, Segalowitz SJ, Cudmore LJ. Task complexity and habituation effects on frontal P300 topography. Brain Cogn 2001;46:307–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.