Abstract
Purpose
While extreme physical exertion is known to induce changes in the status of inflammation comparisons of the responses for various mediators of inflammation after acute bouts of high-intensity exercise have been limited.
Subjects and Methods
We examined the responses in serum levels of novel inflammatory proteins, calprotectin, suPAR, CD163, and pro- and anti-inflammatory cytokines in 12 physically active volunteers (10 men, 2 women, mean age 37±14 years) before and after completing various types of extreme physical exertion (marathon run, half-marathon run or 24-h cross-country skiing). For comparisons, the levels of the biomarkers were also measured at rest in 30 healthy controls (25 men, 5 women, mean age 42 ± 12 years) with low or sedentary activity.
Results
Extreme physical exertion induced significant increases in serum calprotectin (p < 0.0005), suPAR (p < 0.01), CD163 (p < 0.05), IL-6 (p < 0.0005), IL-8 (p < 0.01) and IL-10 (p < 0.0005) (pre- vs 3h-post-exercise). These responses were found to normalize within 48 hours. While the increases in blood leukocytes were of similar magnitude following the different types of exercise, markedly more pronounced responses occurred in serum TNF-α (p < 0.01), IL-8 (p < 0.01) and CD163 (p < 0.05) in those with more intense activity. In 3-h post-exercise samples significant correlations were observed between serum calprotectin and IL-6 (rs = 0.720, p < 0.01), IL-10 (rs = 0.615, p < 0.05), TNF-α (rs = 0.594, p < 0.05), suPAR (rs = 0.587, p < 0.05) and blood leukocytes (rs = 0.762, p < 0.01).
Conclusion
The present results suggest distinct exercise-intensity dependent changes in mediators of inflammation (including calprotectin, suPAR and CD163) following extreme physical exertion. Our findings indicate that there is a major reversible impact of high-intensity physical exertion on the status of inflammation.
Keywords: acute exercise, cytokine, immune status, leukocyte, oxidative stress
Introduction
Although the health benefits brought about by regular physical activity are proved and wide ranging,1,2 the intensity and types of physical activity associated with either beneficial or adverse effects on the status of inflammation have, however, remained a matter of controversy.3–7 While regular physical activity with appropriate resting periods is expected to enhance immunity, acute bouts of vigorous activity may also lead to adverse consequences.3–6,8 Several lines of evidence indicate that strenuous exercise is associated with robust inflammatory responses, mobilization of leukocytes and increases in the levels of inflammatory mediators in circulation.4,6,9-15 However, the intensity and duration of exercise needed to elicit clinically notable changes in the mediators of inflammation remain unclear.3–6
Previous studies have indicated that high-intensity physical activity leads to a release of both pro-inflammatory and anti-inflammatory cytokines.4,6,12 While recent advances in inflammation research have provided a wide range of new and emerging serum biomarkers of inflammation, only limited information is available on the levels of such markers in response to exercise. Serum calprotectin is secreted from neutrophil granulocytes and may serve as a marker of neutrophil-driven inflammation.16–19 Its release has been linked to both anti-infective and anti-inflammatory properties including control of myelopoiesis, chelation of divalent cations, scavenging of reactive oxygen species, chemotaxis and direct antimicrobial action.16–19 Soluble urokinase plasminogen activator receptor (suPAR) is expressed on many immunologically active cells, including monocytes, neutrophils and activated T cells.20–22 CD163, an endocytic receptor protein for haptoglobin-hemoglobin complexes, is found on macrophages and monocytes: its serum levels increase in conjunction with the involvement of macrophages and free-radical induced tissue damage.23,24 Both suPAR and CD163 proteins have emerged as prospective risk factors for adverse clinical outcomes in various inflammatory conditions.20–24
In this study, we assessed the magnitude and patterns of acute changes in serum calprotectin with those of suPAR, CD163 and pro- (IL-6, IL-8, TNF-α) and anti-inflammatory (IL-10, TGF-β) cytokines in a group of 12 healthy volunteers participating in various types of high-intensity exercise. For additional comparisons, these biomarkers were also measured in a group of 30 apparently healthy age- and sex-matched individuals with low or sedentary activity. Our findings support a major reversible impact of high-intensity exercise on the status of inflammation.
Subjects and Methods
Study Subjects
The sample of individuals attending the different types of strenuous exercise events consisted of 12 healthy physically active volunteers (10 men, two women, mean age 37 ± 14 years), who had no history of diseases or medication at the time of the study. These subjects attended either a marathon run (ie 42.2 km, n = 4) or half-marathon run (ie 21.1 km, n = 7) or in one case, a 24-h skiing event (Table 1). The participants in the skiing event and the full marathon run were healthy endurance trained fitness enthusiasts whereas the half-marathon runners were mainly recreational runners. The details of the exercise protocols and associated medical surveillance have been described previously.12,25 The typical weekly physical activity of the participants prior to the experiment had ranged from three to 15 hours of moderate to high intensity aerobic activity per week. Medical examinations were carried out before undertaking the intensive exercise, within two hours after finishing and one week after the trial. All participants were allowed to follow their individual food strategies prior to the experiment and in the restitution period. A two-day rest before the trial was recommended. During the experiment, liberal amounts of various fluids and food (mostly exogenous carbohydrates) were available ad libitum. After the trial, two of the subjects received intravenous hydration (approximately 300 mL/h) for the next 20 hours while being monitored in a hospital setting. Post-exercise symptoms ranged from muscle pain and stiffness to signs of generalized fatigue and nausea. Both the self-concepts expressed by the participants and the medical and psychological post-exercise assessments suggested a rapid recovery in all subjects, which was completed within one week with no apparent signs of residual emotional or physiological exhaustion.
Table 1.
Main Characteristics of Study Subjects Including Physically Active Individuals Participating in Various Types of Extreme Physical Exertion and Individuals with Low/Sedentary Activity
| Subjects with Extreme Physical Exertion | Subjects with Low/Sedentary Activity | |||
|---|---|---|---|---|
| n = 12 | Exercise Timing Characteristics | |||
| Speed (km/h) Mean ± SD | Pace (Min/km) Mean ± SD | n = 30 | ||
| Age, years, mean ± SD | 36.5 ± 13.7 | 42.2 ± 12.3 | ||
| Males, n (%) | 10 (83.3%) | 25 (83.3%) | ||
| Exercise type, n (%) | ||||
| Marathon run | 4 (33.3%) | 12.7 ± 0.6 | 4:43 ± 0:13 | |
| Half-marathon run | 7 (58.3%) | 10.9 ± 1.7 | 5:37 ± 0:50 | |
| 24-h Cross-country skiing | 1 (8.3%) | 17.0 | 3:32 | |
Blood specimens were collected on the day before and at 3 and 48 hours post-exercise by a trained laboratory nurse using previously described standard operating procedures.12,25 For additional comparisons of the biomarker baseline levels in less physically active individuals, blood samples at rest were also collected from a reference population including 30 healthy but sedentary individuals (25 men, 5 women, mean age 42 ± 12 years), whose physical activity was typically less than 150 minutes of light intensity activity per week over a period of one year prior to sampling. Serum was separated by centrifugation (1500×g for 10 minutes) and stored frozen at −70 °C prior to the analyses.
All subjects were informed about the study and gave their written informed consent for participation. The protocol was approved by the local ethical committee (Northern Ostrobothnia Hospital District Institutional Review Board, Oulu, Finland). The study was conducted according to the provisions of the Declaration of Helsinki.
Laboratory Analyses
Serum was separated by centrifugation (1500 × g for 10 minutes) and stored at –70 °C prior to the measurement of the various biomarkers. Serum calprotectin concentrations were measured using BÜHLMANN MRP8/14 ELISA kit according to the instructions of the manufacturer (BÜHLMANN Laboratories AG, Schönenbuch, Switzerland). Serum suPAR levels were measured using the suPARnostic enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the manufacturer (Virogates, Birkerød, Denmark). The measurements of CD163 were carried out using Quantikine human CD163 ELISA assay (R&D Systems, Abingdon Science Park, UK). The concentrations of interleukins (IL-6, IL-8, IL-10, TNF-α and TGF-β) in serum were determined using Quantikine high sensitivity ELISA kits (R&D Systems, Abingdon, Science Park, UK). Routine blood chemistry analyses were carried out using standard clinical chemical methods on an Abbott Architect c8000 automated clinical chemistry analyser (Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL, USA) and blood cell counts were performed using a Sysmex XE-5000 automated Hematology Analyser. All measurements were carried out in SFS-EN ISO 15189:2013 accredited laboratory.
Statistical Methods
The values are reported as mean ± standard deviation (SD) or medians and interquartile ranges, as indicated. The levels of different mediators of inflammation were compared between the study groups using the Mann–Whitney U-test. The comparisons between the different time points related to physical exercise were made using paired samples t-test. Both absolute changes and relative changes from baseline levels were calculated for each parameter. Relative changes were based on the pre-exercise values of the subjects and are expressed as a percentage (% change=100*absolute change/pre-exercise value). Comparisons of the changes were done using the Mann–Whitney U-test. Spearman’s rank correlation coefficient (rs) was used for evaluating the correlations between the study variables. The correlations between postrace values were calculated after an adjustment for baseline variability using percent change (pre–post) for each outcome variable. A p-value of < 0.05 was considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics 24.0 (Armonk, NY: IBM Corp.).
Results
The details of these exercise protocols and associated medical surveillance have been described previously.12,25 Table 1 summarizes the main characteristics of the study subjects and timing details for each type of long-term endurance exercise events. The efforts by all participants represented exercise stress of over 90 minutes at >70% of VO2 max and the self-reported ratings of perceived exertion (RPE) ranged from hard to maximal exertion (Borg scale ≥15 on a 6 to 20 rating scale).
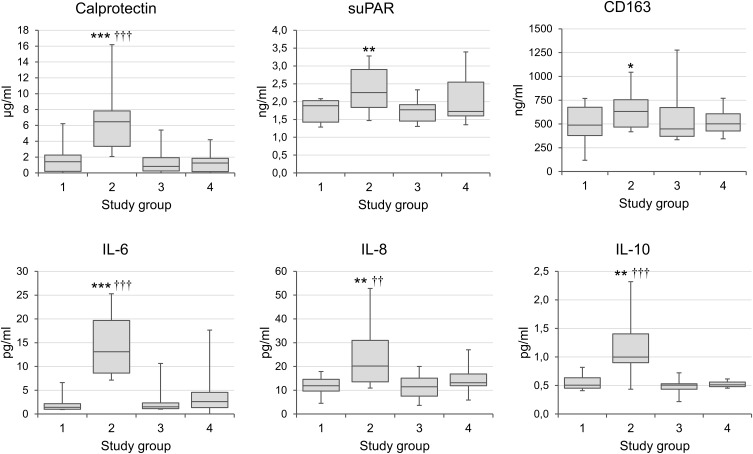
The medians and interquartile ranges of the main biomarkers of inflammation before and after the acute bouts of exercise are summarized in Figure 1. When compared to pre-exercise baseline levels, significant 3-h post-exercise increases occurred in serum calprotectin (p < 0.0005), suPAR (p < 0.01), CD163 (p < 0.05), IL-6 (p < 0.0005), IL-8 (p < 0.01) and IL-10 (p < 0.01). All parameters returned to baseline levels within 48 hours (Figure 1, Table 2). Although the values of IL-6, suPAR and TGF-β tended to be lower in the physically active group at baseline than those in the blood samples taken at rest from a reference group of the individuals with low or sedentary activity (Figure 1, Table 2), the differences did not reach statistical significance.
Figure 1.
Mediators of inflammation in physically active individuals before and after extreme physical exertion.
Notes: The values are shown as medians and interquartile ranges. *p < 0.05; **p < 0.01; ***p < 0.001 for comparisons between pre- and post-exercise values; ††p < 0.01; †††p < 0.001 for comparisons with the group with low or sedentary activity. Study groups: 1= Physically active individuals, pre-exercise baseline; 2= 3-h Post-exercise; 3 = 48-h Post-exercise; 4 = Subjects with low or sedentary activity.
Abbreviations: suPAR, soluble urokinase plasminogen activator receptor, a biomarker of immune activation; CD163, a biomarker of monocyte-macrophage activation; IL, interleukin; TNF-α, tumor necrosis factor-alpha; TGF-β, transforming growth factor-beta.
Table 2.
Mean Absolute and Relative Changes (%) in Mediators of Inflammation as Compared to Baseline (Pre-Exercise) Levels of the Physically Active Individuals
| Physically Active Subjects | Subjects with Low/Sedentary Activity | |||
|---|---|---|---|---|
| Pre-Exercise | 3-h Post | 48-h Post | ||
| Mean ± SD | Exercise-Induced Change from Baseline, Units (%) | Difference from Baseline a, Units (%) | ||
| Calprotectin | 1.8 ± 2.0 μg/mL | +5.8 (+320%) *** | –0.3 (–19%) | –0.4 (–23%) |
| suPAR | 1.8 ± 0.3 ng/mL | +0.6 (+33%) ** | –0.04 (–2%) | +0.8 (+43%) |
| CD163 | 528 ± 208 ng/mL | +142 (+27%) | +51 (+10%) | +30 (+6%) |
| IL-6 | 2.2 ± 1.8 pg/mL | +14.3 (+648%) *** | +0.5 (+22%) | +0.7 (+32%) |
| IL-8 | 12.0 ± 4.2 pg/mL | +12.1 (+101%) ** | –0.7 (–5%) | +2.3 (+19%) |
| IL-10 | 0.6 ± 0.1 pg/mL | +0.8 (+139%) *** | –0.06 (–11%) | –0.02 (–4%) |
| TNF-α | 0.8 ± 0.2 pg/mL | +0.3 (+45%) | +0.2 (+23%) | +0.04 (+5%) |
| TGF-β | 516 ± 284 pg/mL | –68 (–13%) | +85 (+16%) | +135 (+26%) |
| Leukocytes | 5.9 ± 2.2109/l | +8.2 (+139%) | +0.2 (+3%) | −0.1 (−2%) |
Notes: Mean relative change significantly different from baseline (pre-exercise) values. (Mann–Whitney U-test), **p < 0.01, ***p < 0.001; aMean difference compared to pre-exercise levels of the physically active individuals.
Abbreviations: suPAR, soluble urokinase plasminogen activator receptor, a biomarker of immune activation; CD163, a biomarker of monocyte-macrophage activation; IL, interleukin; TNF-α, tumor necrosis factor-alpha; TGF-β, transforming growth factor-beta.
Comparisons of the relative increases in the mediators of inflammation in relation to the type of the exercise (based on the percentage change from the pre-race values) are summarized in Table 3. While the increases in blood leukocytes following the different types of exercise were similar, markedly more pronounced responses in those with more intense activity were found to occur in serum TNF-α (p < 0.01), IL-8 (p < 0.01) and CD163 (p < 0.05) (Table 3). In addition, striking increases in serum calprotectin, IL-6 and TGF-β were observed in the individual who completed the most extreme and prolonged exercise (24-h skiing) (Table 3).
Table 3.
Relative Changes (% from Pre-Exercise Levels) in Mediators of Inflammation After Various Types of Exertion
| Marathon Run | Half-Marathon Run | 24-h Skiing | |
|---|---|---|---|
| Calprotectin | +342 | +304 | +1427 |
| suPAR | +53 | +20 | +51 |
| CD163 | +71 * | +9 | +4 |
| IL-6 | +687 | +561 | +2178 |
| IL-8 | +252 ** | +18 | +251 |
| IL-10 | +228 | +95 | +40 |
| TNF-α | +160 ** | –28 | +140 |
| TGF-β | –24 | –3 | +721 |
| Leukocytes | +128 | +148 | +132 |
Notes: Relative change significantly different between marathon run and half-marathon run (Mann–Whitney U-test), *p < 0.05, **p < 0.01.
Abbreviations: suPAR, soluble urokinase plasminogen activator receptor, a biomarker of immune activation; CD163, a biomarker of monocyte-macrophage activation; IL, interleukin; TNF-α, tumor necrosis factor-alpha; TGF-β, transforming growth factor-beta.
The changes in serum calprotectin (3-h post-exercise and adjusted for baseline levels), correlated with those of IL-6 (rs = 0.720, p < 0.01), IL-10 (rs = 0.615, p < 0.05), TNF-α (rs = 0.594, p < 0.05), suPAR (rs = 0.587, p < 0.05) and blood leukocytes (rs = 0.762, p < 0.01). Comparisons of the relative changes in the mediators of inflammation and biochemical indices of rhabdomyolysis or other possible adverse health effects of extreme exercise indicated significant correlations between the change in TNF-α and creatine kinase (CK) (rs = 0.667, p < 0.05), aspartate aminotransferase (AST) (rs = 0.733, p < 0.05) and C-reactive protein (CRP) (rs = 0.670, p < 0.05). The changes in IL-8 also correlated with those of TNF-α (rs = 0.883, p < 0.001), CK (rs = 0.633, p < 0.05), AST (rs = 0.733, p < 0.05) and CD163 (rs = 0.700, p < 0.05).
Discussion
Our study comparing serum calprotectin, a marker of neutrophil activation, with other biomarkers of inflammation after extreme exercise demonstrates distinct characteristics in the patterns of exercise-induced immune responses. The data also indicates differences in the magnitude of the responses in proportion to the intensity and duration of the exercise.
Although the beneficial health effects of physical activity in proper amounts have been well established, extreme physical exertion may also trigger adverse consequences on immune function.1–7,26–28 The present data indicates that serum calprotectin, IL-6 and IL-10 respond to an acute bout of physical exercise in a highly sensitive manner. The findings of increased IL-6 and IL-10 in both marathon and half-marathon finishers is in accordance with recent findings by Nielsen and coworkers from Norway.6 Post-exercise increases in serum calprotectin also coincide with increased leukocyte counts suggesting that calprotectin could also serve as a biomarker for myeloid reactions when analyzing the body´s immune response to exercise. Calprotectin release may indicate exercise-induced activation of neutrophil-driven inflammation, which have previously been linked with anti-inflammatory processes and scavenging of tissue-damaging reactive oxygen species.16,18 A switch of the immune system towards either pro- or anti-inflammatory consequences in association with extreme physical exertion may be influenced by multiple possible precipitating factors, such as changes in the extracellular pH during high-intensity exercise17 or the status of regular exercise training.28 Calprotectin could play a pivotal regulatory role in controlling the inflammatory cascades and leukocyte trafficking to yield more coordinated immune reactions.17–19,29 Thus, it could also contribute to the elicitation of an appropriate inflammatory response and prevention of tissue damage.18,19,29
Previous studies comparing multiple serum cytokines in response to acute bouts of exercise have yielded variable findings.3,4,6,8,12 While some studies have found increases in IL-6, IL-8 and TNF-α levels, other studies have shown relatively stable levels of these biomarkers. The intensity, type, duration and familiarity of the exercise may all play a role in explaining such divergent findings.4,28 Studies on the relationships between physical activity and the status of inflammation have indicated that even rather short sessions of regular exercise can reduce inflammation through downregulation of TNF-α production via beta-adrenergic activation.30 While IL-6 levels after acute exercise appear to increase more than other cytokines,30,31 moderate weekly physical activity is generally associated with lower levels of IL-6.3,8,32 However, the baseline levels of IL-6 in the current group of physically active individuals did not differ significantly from those observed in the samples taken at rest from the subjects with low or sedentary activity.
Our findings indicating striking increases in the levels of IL-6, IL-8, TNF-α and calprotectin following the most extreme physical exertion suggests that the intensity and duration of the acute exercise has a major impact on the magnitude of the post-exercise inflammatory responses. In particular, the increases in IL-8 and TNF-α, which are typical pro-inflammatory mediators associated with tissue damage and oxidative stress,33–35 were greatest in those with the most intense activity. The exercise-intensity dependent IL-6 responses might be explained in part by its release from muscle tissue.14,30,36,37 This view is also supported by the finding that the highest individual IL-6 levels occurred in the subject who completed 24 hours of cross-country skiing: unlike running, skiing involves much of the body musculature for propulsion and eccentric muscle activity.25 Interestingly, IL-6 signaling has previously been suggested to play a role in the autocrine and paracrine benefits of physical activity as well as the overall health benefits related to regular exercise.11,14,37 While IL-6 has been usually regarded as a pro-inflammatory cytokine,36 it may also stimulate the production of anti-inflammatory cytokines.4,6,11,14 IL-6 secretion also appears to be associated with the regulation of the acute phase response, hematopoiesis and tissue regeneration.36 The relatively parallel changes observed here in pro- (IL-6, IL-8, TNF-α) and anti-inflammatory (IL-10, TGF-β) pathways following strenuous physical activity is also in accordance with the view that a coordinated balance between pro- and anti-inflammatory cascades is needed to counteract exercise-induced inflammatory threat and tissue damage.4,6,12 Both IL-6 and IL-10 can stimulate the Th2 pathway, activate anti-inflammatory cascades and inhibit TNF-α.35,37
Long-term strenuous exercise was also found here to be associated with significant increases in certain serum proteins, which have previously been established as indicators of harmful responses and disease risks in various inflammatory conditions. suPAR is elevated in patients with severe systemic inflammation and the elevated levels of this biomarker show an association with poorer outcomes in such conditions.20–22,38–41 CD163 is present on macrophages and circulating monocytes and has also been implicated as an independent risk marker of progression in inflammatory diseases.23,42 In light of exercise-induced changes in CD163, it is of interest to note that CD163 has been shown to play a role in innate immune defense by sequestering hemoglobin-bound iron and scavenging oxidative stress-induced by-products of hemoglobin.24,42
Further studies appear warranted to examine whether the use of current biomarkers could be of value in the follow-up of immune function in physically active individuals engaged in frequent episodes of strenuous exercise. It may be assumed that skewed balances in the ratios of pro- and anti-inflammatory cytokines correlate with unfavorable over-activation of the sympathetic nervous system, production of reactive oxygen species and oxidative stress.30,43,44 The extent of pro-inflammatory changes may also be important in determining the status of individual susceptibility to coincidentally occurring pathogens.4,6 In the present study, the post-exercise changes in serum creatine kinase (CK), a laboratory indicator of muscle damage, were found to correlate with increases in IL-8 and TNF-α. Given the key role of TNF-α in low-grade inflammation in various pathological conditions, future studies are warranted to examine whether the generation of a pro-inflammatory milieu and excess TNF-α production could also play a pathogenic role in the development of adverse health effects of high-intensity physical exercise, including exertional rhabdomyolysis and cardiac or renal injuries.45–47 Extreme endurance exercise can also impair gastrointestinal integrity and function, which is associated with both local and systemic inflammation, endotoxemia and increased levels of proinflammatory cytokines.27
Additional research is also needed to address the questions of how and to what extent physical activity may lead to a reduction of inflammation and oxidative stress as recently proposed by several lines of evidence.5,43,48 At an individual level, adherence to a healthy diet may also be a significant contributor to the status of inflammation and oxidative stress.27 In the current study, data on the dietary status of the participants were not collected so its possible influence could not be evaluated. It should further be noted that the inflammatory responses related to physical activity and associated changes in biomarkers may occur in an age-dependent manner.8,47 Since exercise training has been suggested as a tool to ameliorate aging-related immunosenescence3,8,49 future studies are warranted to examine the potential role of physical exercise as part of therapeutic and preventive strategies in various age-categorized populations.
Conclusions
Taken together, the present data demonstrates significant changes in mediators of inflammation as a result of extreme physical exercise, these changes appear to become more pronounced in proportion to the intensity of the exercise. These findings help to provide a more comprehensive view on the relationships between extreme physical exertion and the status of inflammation. The observed changes may be associated with physiological inflammatory responses to exercise and early acute phase responses to muscle damage and oxidative stress. Our findings on alterations in the balance of pro- and anti-inflammatory mediators also support the view of a transient open window of altered immunity after extreme physical exertion, which could lead to increased susceptibility to infectious agents after completion of strenuous exercise.
Acknowledgments
The expert assistance of Ms Anni Halkola and Mrs Taana Sandman is gratefully acknowledged.
Funding Statement
This work was supported in part by Competitive State Research Financing of the Expert Responsibility area of Seinäjoki Central Hospital and University of Tampere, VTR 5300/3116 and by the Finnish Foundation for the Promotion of Laboratory Medicine.
Abbreviations
AST, aspartate aminotransferase; CD163, a biomarker of monocyte-macrophage activation; CK, creatine kinase; CRP, C-reactive protein; IL, interleukin; suPAR, soluble urokinase plasminogen activator receptor, a biomarker of immune activation; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest relevant to this article.
References
- 1.Sundberg CJ. Physical activity: what is already being done and how we can avert 1 million deaths annually in future. Br J Sports Med. 2016;50:319. doi: 10.1136/bjsports-2016-096026 [DOI] [PubMed] [Google Scholar]
- 2.Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend? Can J Cardiol. 2016;32(4):495–504. doi: 10.1016/j.cjca.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 3.Nieman DC, Wentz LM. The compelling link between physical activity and the body´s defence system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise – a systematic review. Front Physiol. 2020;10:1550. doi: 10.3389/fphys.2019.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen HG, Øktedalen O, Opstad P-E LT. Plasma cytokine profiles in long-term strenuous exercise. J Sports Med. 2016;7186137. doi: 10.1155/2016/7186137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnohr P, O’Keefe JH, Marott JL, Lange P, Jensen GB. Dose of jogging and long-term mortality: the Copenhagen city heart study. J Am Coll Cardiol. 2015;65:411–419. doi: 10.1016/j.jacc.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 8.Sellami M, Gasmi M, Denham J, et al. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187. doi: 10.3389/fimmu.2018.02187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernecker C, Scherr J, Schinner S, Braun S, Scherbaum WA, Halle M. Evidence for an exercise induced increase of TNF-alpha and IL-6 in marathon runners. Scand J Med Sci Sports. 2013;23:207–214. doi: 10.1111/j.1600-0838.2011.01372.x [DOI] [PubMed] [Google Scholar]
- 10.Frye CW, Mann S, Joseph JL, Hansen C, Sass B, Wakshlag JJ. Serum biochemistry and inflammatory cytokines in racing endurance sled dogs with and without rhabdomyolysis. Front Vet Sci. 2018;5:145. doi: 10.3389/fvets.2018.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–4148. doi: 10.1111/febs.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemelä M, Kangastupa P, Niemelä O, Bloigu R, Juvonen T. Acute changes in inflammatory biomarker levels in recreational runners participating in a marathon or half-marathon. Sports Med Open. 2016;2:21. doi: 10.1186/s40798-016-0045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans–effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–515. doi: 10.1007/s004210000312 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 15.Tauler P, Martínez S, Moreno C, Monjo M, Martínez P, Aguiló A. Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med Sci Sports Exerc. 2013;45:1269–1276. doi: 10.1249/MSS.0b013e3182857c8a [DOI] [PubMed] [Google Scholar]
- 16.Holmgaard DB, Mygind LH, Titlestad I, et al. Calprotectin–a marker of mortality in COPD? Results from a prospective cohort study. COPD. 2013;10:581–587. doi: 10.3109/15412555.2013.781580 [DOI] [PubMed] [Google Scholar]
- 17.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen B, Wolf M, Austermann J, et al. The alarmin Mrp8/14 as regulator of the adaptive immune response during allergic contact dermatitis. EMBO J. 2013;32:100–111. doi: 10.1038/emboj.2012.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 20.Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80:209–216. doi: 10.1002/jmv.21114 [DOI] [PubMed] [Google Scholar]
- 21.Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. doi: 10.1186/cc10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.1155/2009/504294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1–13. doi: 10.3109/00365513.2011.626868 [DOI] [PubMed] [Google Scholar]
- 24.Buehler PW, Abraham B, Vallelian F, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466 [DOI] [PubMed] [Google Scholar]
- 25.Niemelä M, Juvonen J, Kangastupa P, Niemelä O, Juvonen T. Clinical and laboratory responses of cross-country skiing for a 24-h world record: case report. J Sports Sci Med. 2015;14:702–707. [PMC free article] [PubMed] [Google Scholar]
- 26.Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol (1985). 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016 [DOI] [PubMed] [Google Scholar]
- 27.Costa RJS, Snipe RMJ, Kitic CM, Gibson PR. Systematic review: exercise induced gastrointestinal syndrome – implications for health and intestinal disease. Aliment Pharmacol Ther. 2017;46:246–265. doi: 10.1111/apt.14157 [DOI] [PubMed] [Google Scholar]
- 28.Ahmethodzic A. The Effect of Strenuous Exercise on Circulating Cytokines [dissertation]. Halmstad University, School of Business, Engineering and Science; 2019; URN: urn: nbn:se:hh:diva-40652. [Google Scholar]
- 29.Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim Biophys Acta. 1998;1448:200–211. doi: 10.1016/S0167-4889(98)00144-X [DOI] [PubMed] [Google Scholar]
- 30.Dimitrov S, Hulteng E, Hong S. Inflammation and exercise: inhibition of monocytic TNF production by acute exercise via β2-adrenergic activation. Brain Behav Immun. 2017;61:60–68. doi: 10.1016/j.bbi.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–251. doi: 10.1136/bjsm.34.4.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons TJ, Sartini C, Welsh P, et al. Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med Sci Sports Exerc. 2017;49:459–465. doi: 10.1249/MSS.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142 [DOI] [PubMed] [Google Scholar]
- 34.Qazi BS, Tang K, Qazi A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int J Inflam. 2011;2011:908468. doi: 10.4061/2011/908468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidali M, Hietala J, Occhino G, et al. Immune responses against oxidative stress-derived antigens are associated with increased circulating tumor necrosis factor-alpha in heavy drinkers. Free Radic Biol Med. 2008;45:306–311. doi: 10.1016/j.freeradbiomed.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288–294. doi: 10.1158/2326-6066.CIR-14-0022 [DOI] [PubMed] [Google Scholar]
- 37.McGinnis GR, Ballmann C, Peters B, et al. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308:H1423–H1433. doi: 10.1152/ajpheart.00850.2014 [DOI] [PubMed] [Google Scholar]
- 38.Casagranda I, Vendramin C, Callegari T, et al. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern Emerg Med. 2015;10:725–730. doi: 10.1007/s11739-015-1268-7 [DOI] [PubMed] [Google Scholar]
- 39.Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x [DOI] [PubMed] [Google Scholar]
- 40.Eugen-Olsen J. suPAR - a future risk marker in bacteremia. J Intern Med. 2011;270:29–31. doi: 10.1111/j.1365-2796.2011.02372.x [DOI] [PubMed] [Google Scholar]
- 41.Rohde C, Polcwiartek C, Andersen E, Vang T, Nielsen J. Effect of a physical activity intervention on suPAR levels: a randomized controlled trial. J Sci Med Sport. 2018;21:286–290. doi: 10.1016/j.jsams.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 42.Rødgaard-Hansen S, St George A, Kazankov K, et al. Effects of lifestyle intervention on soluble CD163, a macrophage activation marker, in patients with non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 2017;77:498–504. doi: 10.1080/00365513.2017.1346823 [DOI] [PubMed] [Google Scholar]
- 43.Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039 [DOI] [PubMed] [Google Scholar]
- 44.Di Penta A, Moreno B, Reix S, et al. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One. 2013;8(2):e54722. doi: 10.1371/journal.pone.0054722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114(22):2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461 [DOI] [PubMed] [Google Scholar]
- 46.McCullough PA, Chinnaiyan KM, Gallagher MJ, et al. Changes in renal markers and acute kidney injury after marathon running. Nephrology (Carlton). 2011;16:194–199. doi: 10.1111/j.1440-1797.2010.01354.x [DOI] [PubMed] [Google Scholar]
- 47.Niemelä M, Kangastupa P, Niemelä O, Bloigu R, Juvonen T. Individual responses in biomarkers of health after marathon and half-marathon running: is age a factor in troponin changes? Scand J Clin Lab Invest. 2016;76:575–580. doi: 10.1080/00365513.2016.1225122 [DOI] [PubMed] [Google Scholar]
- 48.Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–1215. doi: 10.1002/hep.27544 [DOI] [PubMed] [Google Scholar]
- 49.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9 [DOI] [PubMed] [Google Scholar]