Abstract
The Ikzf1 locus encodes the lymphoid specific transcription factor Ikaros, which plays an essential role in both T and B cell differentiation, while deregulation or mutation of IKZF1/Ikzf1 is involved in leukemia. Tissue-specific and cell identity genes are usually associated with clusters of enhancers, also called super-enhancers, which are believed to ensure proper regulation of gene expression throughout cell development and differentiation. Several potential regulatory regions have been identified in close proximity of Ikzf1, however, the full extent of the regulatory landscape of the Ikzf1 locus is not yet established. In this study, we combined epigenomics and transcription factor binding along with high-throughput enhancer assay and 4C-seq to prioritize an enhancer element located 120 kb upstream of the Ikzf1 gene. We found that deletion of the E120 enhancer resulted in a significant reduction of Ikzf1 mRNA. However, the epigenetic landscape and 3D topology of the locus were only slightly affected, highlighting the complexity of the regulatory landscape regulating the Ikzf1 locus.
Introduction
Cell-type specific regulation of gene expression requires the activation of promoters by distal genomic elements defined as enhancers. The classical view of enhancer function is that they contribute to increasing the overall level of gene expression by inducing transcription from associated promoters [1]. Complex gene regulation is mediated by the association of clusters of enhancers, also called super-enhancers [2]. Whether the individual components (i.e. single enhancers) synergistically contribute to transcription regulation of their target genes or have distinct specialized functions has been a matter of debate [2–5].
With the increasing awareness of the important role of enhancers in normal development as well as in disease, there is strong scientific interest in identifying and characterizing these elements. However, few predicted enhancer elements have been shown to affect transcription of their endogenous genes or to alter phenotypes when disrupted, highlighting the need to integrate different epigenomic resources and functional assays to identify critical distal regulatory elements [6]. Although putative enhancers can be identified genome-wide based on chromatin accessibility or histone modifications [7], these approaches do not provide direct proof of enhancer function. Recent developments of functional high-throughput assays have enabled quantitative measurements of enhancer activity of thousands of regulatory elements in parallel, providing a straightforward approach to prioritize bona fide enhancers [8]. In particular, a common observation of high-throughput assays based on massively paralleled reporter assays [9–14] or CRISPR-based screens [15, 16] is that many predicted enhancer regions do not show enhancer activity in reporter assays or after CRISPR deletion. Therefore, it is crucial to experimentally assess whether genomic regions function as bona fide enhancers in living cells.
Ikaros is a lymphoid specific transcription factor that plays a major role in both T and B cell differentiation [17, 18]. During T cell differentiation Ikaros is required for proper gene regulation during the CD4-CD8- (double-negative; DN) to the CD4+CD8+ (double-positive; DP) transition (also called b-selection) mainly by recruiting chromatin repressors [19, 20] and silencing Notch1 target genes [20–23]. Importantly, Ikaros deregulation or mutation plays an important role in leukemia [24–33]. In mouse and human, Ikaros is encoded by the Ikzf1 gene and is known to harbor several transcript isoforms playing different regulatory roles [34–38]. Several potential regulatory regions have been identified in the proximity of the Ikzf1 locus, suggesting a complex network of regulatory elements are required to drive Ikaros expression during hematopoiesis and lymphocyte maturation [39–41]. To gain insight into the regulation of Ikzf1 locus in T cell precursors, we have integrated data from high-throughput reporter assays, chromatin modifications, binding of key T cell transcription factors as well as genomic interactions. We prioritized an enhancer located 120 kb upstream of Ikzf1 and studied the functional role of this regulatory element.
Material and methods
Cell culture
P5424 cell line [42] was kindly provided by Dr. Eugene Oltz, Washington, USA and was cultured as described previously [14]. Cells were passed every 2–3 days and routinely tested for mycoplasma contamination, and maintained in RPMI medium (Gibco) supplemented with 10% FBS (Gold, PAA) at 37 °C, 5% CO2. J1 mouse embryonic stem (ES) cells were grown on gamma-irradiated mouse embryonic fibroblast cells under standard conditions (4.5 g/L glucose-DMEN, 15% FCS, 0.1 mM non-essential amino acids, 0.1 mM beta-mercaptoethanol, 1 mM glutamine, 500 U/mL LIF, gentamicin), then passaged onto feeder-free 0.2% gelatin-coated plates for at least two passages to remove feeder cells before 4C.
Isolation of DN3 thymocytes
Thymuses from 4–5 weeks old c57/Bl6 mice were dissected and homogenized in cold PBE (PBS with 0.5% BSA and 2 mM EDTA), before incubation for 30 min at 4 °C with rat anti-CD4 and rat anti-CD8 anti-sera (gift from Susan Chan) and depletion of DP thymocytes with sheep anti-rat IgG magnetic beads (Invitrogen). 1x108 cells were resuspended in 300 μl PBE plus 3 μl anti-mouse CD4-FITC, 3 μl anti-mouse CD8a-FITC, 3 μl anti-mouse CD3e-FITC, 3 μl anti-mouse B220-FITC, 3 μl anti-mouse CD11b-FITC, 3 μl anti-mouse Ly-6G(Gr-1)-FITC, 3 μl anti-mouse NK1.1-FITC, 6 μl anti-mouse CD44-PE, and 6 μl anti-mouse CD25-PE (all eBioscience antibodies), and incubated on ice for 10 min before washing in PBE. DAPI was added to a final concentration of 100 ng/ml and live DN3 cells were purified by FACS (DAPI-negative, FITC-negative, APC-negative, PE-positive) before immediate fixation for 4C.
CRISPR/Cas9 genome editing
The targeted enhancer regions were defined by the peaks of CapStarr-seq and DNase-seq which bind the 6 TFs. Two gRNAs were designed at each end of the targeted region by CRISPR direct tool [43]. The gRNAs were cloned into the gRNA cloning vector (Addgene #41824) as previously described [44]. Two million cells were transfected with 3μg of each gRNA vector and 3μg of Cas9 vector (Addgene #41815) using the Neon transfection system (Life Technologies). After 3 days of transfection, the bulk transfected cells were plated in 96-well plates at the limiting dilution (0.5 cell per 100 μl per well) for clonal expansion. After 10–14 days, individual cell clones were screened for homozygous allele deletion by direct PCR using Phire Tissue Direct PCR Master Mix (Thermo Scientific) following the manufacturer’s protocol. Forward and reverse primers were designed bracketing the targeted regions allowing the detection of knockout and wild-type alleles. Clones with homologous allele deletion were considered if having at least one expected deletion band and no wild-type band. The gRNAs and primers are listed in the S1 Table.
Gene expression analysis
Total RNA was isolated using the RNeasy kit (Qiagen). RNA samples (1 μg) were reverse-transcribed into cDNA using Superscript VILO Master Mix (Thermo Scientific). The quantitative PCR was performed using power SYBR Master Mix (Thermo Scientific) on a QuantStudio 6 Flex Real-Time PCR System. Primer sequences are listed in S2 Table. Gene expression was normalized to that of Rpl32. The relative expression was calculated by delta Ct method and all the shown data reported from the fold change over the control. For each cell clone, the Student’s t-test was performed (unpaired, two-tailed, 95% confidence interval) from 3 biological replicates of independent cDNA preparations. Data are represented with standard deviation (s.d). For RT-qPCR, 1/20 of synthesized cDNA was used as template for one reaction; PCRs were performed with Phusion polymerase (Thermo Scientific), Tm = 60 °C, 35 cycles. RNA-seq from the P5424 cell line treated with either DMSO or PMA/ionomycin was published before [45] and was retrieved from GEO (GSE120655).
PMA/ionomycin induction
1 x 106 cell/ ml of P5424 cells (wt and ΔIkE120) were stimulated for 6 hours with 20 μg/ml of PMA plus 0.5 μg/ml of ionomycin in 6-well plate of 3 independent experiments. Then, total RNA was prepared from non-stimulated or stimulated cells using the RNeasy kit (Qiagen) as recommended by the manufacturer.
Chromatin immunoprecipitation-sequencing (ChIP-seq)
Total 40 x106 of wt and ΔIkE120P5424 cells were crosslinked in 1% formaldehyde for 10 min at 20 °C, followed by quenching with glycine at a final concentration of 250 mM. Pelleted cells were washed twice with ice-cold PBS, and then re-suspended in lysis buffer (20 mM Hepes PH 7.6, 1% SDS, 1X PIC) at final cell concentration of 15 x 106 cells/ml. Chromatin was sonicated with Bioruptor (Diagenode) to an average length of 200–400 bp (5 pulses of 30 sec ON and 30 sec OFF). An aliquot of sonicated cell lysate equivalent to 0.5 x 106 cells was diluted with SDS-free dilution buffer (1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris pH 8.0, 167 mM NaCl) for single immunoprecipitation. Specific antibody to H3K27ac (C15410196; Diagenode) (1 μg per ChIP) and proteinase inhibitor cocktail were added to the lysate and rotated overnight at 4 °C. On the next day, Protein A magnetic beads (Invitrogen) were washed twice with dilution buffer (0.15% SDS, 1% Triton X-100,1.2 mM EDTA, 16.7 mM Tris pH 8.0, 167 mM NaCl and 0.1% BSA) and added to the lysate and rotated 1 hour at 4 °C. Then, beads were washed with each of the following buffers: once with Wash Buffer 1 (2 mM EDTA, 20 mM Tris pH 8.0, 1% Triton X-100, 01% SDS, 150 mM NaCl), twice with Wash Buffer 2 (2 mM EDTA, 20 mM Tris pH 8.0, 1% Triton X-100, 0.1% SDS, 500 mM NaCl), twice with Wash Buffer 3 (1 mM EDTA, 10 mM Tris pH 8.0). Finally, beads were eluted in Elution buffer (1% SDS, 0.1 M NaHCo3) and rotated at RT for 20 min. Eluted materials were then added with 0.2 M NaCl, 0.1 mg/ml of proteinase K and incubated overnight at 65 °C reverse cross-linking, along with the untreated input (10% of the starting material). The next day, DNA was purified with QIAquick PCR Purification Kit (Qiagen) and eluted in 30 μl of water. At least 1 ng of ChIP was used for library preparation. Libraries for ChIPs against H3K27ac was prepared according to Illumina ChIP-Seq protocol and sequenced on a Nextseq500 (Illumina) according to the manufacturer’s instructions. ChIP-seq was processed as described previously [14] and RPKM-normalized using deepTools bamCoverage [46] before visualization as heatmaps with deepTools plotHeatmap and as genome tracks with the IGV genome browser [47]. Differential H3K27ac signal between wt and ΔIkE120 cells was generated with the IGV genome browser.
CapStarr-seq
CapStarr-seq data in P5424 (two replicates) and NIH3T3 cell lines with a selected set of DHSs were previously published [14] and processed data retrieved from GEO (GSE60029). As described previously, the enhancer activity was computed by calculating the ratio (fold change; FC) of FPKMs (Fragment Per Kilobase per Million mapped reads) between the CapStarr-seq signal over the plasmid library (input). DHSs with a FC between 1.5 and 3 were labelled as ‘weak enhancer’ and DHSs with a FC equal or higher than 3 were labelled as ‘strong enhancer’. To visualize the CapStarr-seq signal per individual cloned fragments we generated a bed file with an RGB color code proportional to the enhancer activity.
Conservation of Ikzf1 enhancers
Mammalian conservation of Ikzf1 enhancers and coordinates of human orthologues regions were assessed using the UCSC genome browser [48]. ChIP-seq data for H3K27ac in developing human thymocytes were obtained from the Blueprint consortium ([49]; http://dcc.blueprint-epigenome.eu; S3 Table).
Analyses of ImmGen dataset
ATAC-Seq normalized signals for cell types of hematopoietic lineages were retrieved from the ImmGen project [50, 51] as bigwig files (GSE100738). Their coverage within IkE120 locus (chr11:11,564,323–11,564,574, mm10) was extracted using deepTools multiBigwigSummary (http://doi.org/10.1093/nar/gkw257) and compared to Ikzf1 DeSeq2 normalized RNA-Seq signal of corresponding cell types obtained from the ImmGen portail (www.immgen.org).
Hi-C and virtual 4C
Raw Hi-C data from primary DP thymocytes were taken from Hu et al. [52] and processed with FAN-C [53], entailing iterative mapping to the mm9 genome assembly with bowtie2, filtering self-ligation events and PCR duplicates, binning the data to 10 kb bins and balancing the chromosome-wide matrices with the Knight-Ruiz method. TAD boundaries were identified by computing insulation scores [54] with windows of 100 kb (10 bins), normalizing to chromosome-wide averages of insulation scores, then filtering the local minima with the delta vector calculated for the three bins flanking the computed one, and with the difference of the minima and maxima of the delta vector being at least 0.7. Virtual 4C plots were made by plotting the values for one “row” of the normalized Hi-C matrix (corresponding to the interactions of different bins with one specific bin, set to either the Ikzf1 promoter or the E120 enhancer) against the genomic coordinate of the interacting bin.
4C-seq
Cell preparations were fixed with 2% formaldehyde in their respective culture medium for 10 min at 23°C. The fixation was quenched with cold glycine at a final concentration of 125 mM, then cells were washed with PBS and permeabilized on ice for 1 h with 10 mM Tris-HCl, pH 8, 100 mM NaCl, 0.1% NP-40 and protease inhibitors. Nuclei were resuspended in DpnII restriction buffer at 10 million nuclei/mL concentration, and 5 million nuclei aliquots were further permeabilized by treatment for either 1 h with 0.4% SDS at 37°C (ES cells), or for 20 min with 0.7% SDS at 65°C, then for 40 min at 37°C (DN3 and P5424 cells). The SDS was then neutralized by incubating for a further 1h with either 2.6% (ES) or 3.3% (DN3 and P5424) Triton-X100 at 37°C. Nuclei were digested overnight with 1000 U DpnII at 37°C, then washed twice by centrifuging and resuspending in T4 DNA ligase buffer. In situ ligation was performed in 400 μL T4 DNA ligase buffer with 20,000 U T4 DNA ligase overnight at 16°C. DNA was purified by reverse cross-linking with an overnight incubation at 65°C with proteinase K, followed by RNase A digestion, phenol/chloroform extraction and isopropanol precipitation. The DNA was digested with 5 U/μg Csp6I at 37°C overnight, then re-purified by phenol/chloroform extraction and isopropanol precipitation. The DNA was then circularized by ligation with 200 U/μg T4 DNA ligase under dilute conditions (5 ng/μL DNA), and purified by phenol/chloroform extraction and isopropanol precipitation. 50 ng aliquots of this DNA were used as template for PCR with a bait-specific primer located 1.2 kb upstream of E1L (chr11: 11,583,929–11,583,952) and containing Illumina adapter termini (optimal PCR conditions available on request). PCR reactions were pooled, primers removed by washing with 1.8x AMPure XP beads, then quantified on a Bioanalyzer (Agilent) before sequencing with a HiSeq 4000 (Illumina). All bait sequence (including and downstream of the primer sequence, up to but not including the GATC DpnII site) are trimmed by the demultiplexing Sabre tool (https://github.com/najoshi/sabre), allowing two mismatches, before mapping to the mm9 genome with Bowtie [55]. Mapped reads were processed and visualized by 4See [56]. Interactions were called by peakC [57] with a window size of 21.
Results and discussion
Prioritization of an Ikzf1 enhancer
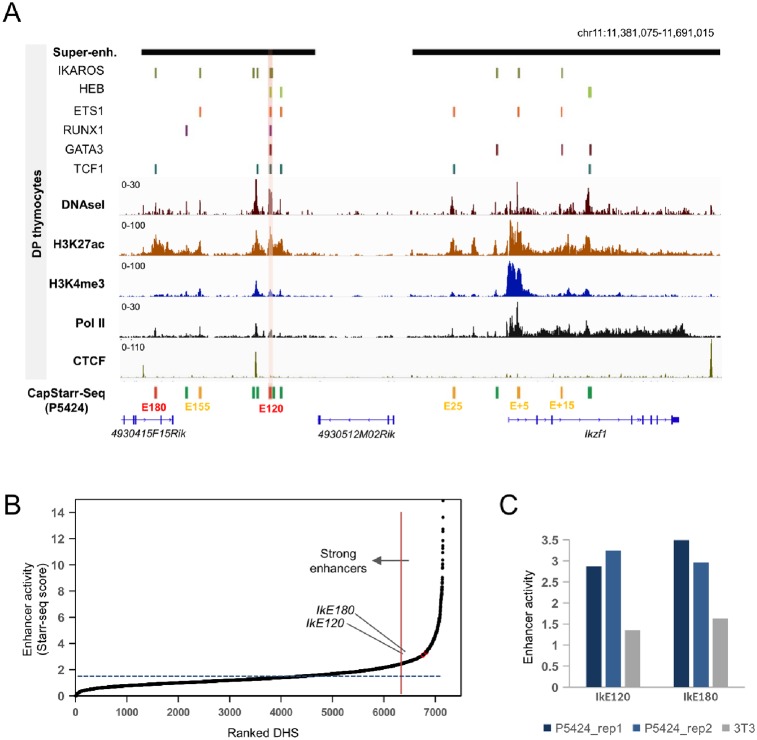
In primary DP thymocytes, Ikzf1 is associated with two clusters of enhancers or super-enhancers (Fig 1A). We identified thirteen DNAse I hypersensitive sites (DHSs) within the two super-enhancers in DP thymocytes (Fig 1A; S4 Table). To prioritize functional Ikzf1 enhancers, we used our previously generated data from a CapStarr-seq high-throughput reporter assay [14] performed in the P5424 cell line [42], which roughly reflects T cell precursors [45]. In this assay, DHSs from primary DP thymocytes were assessed for enhancer activity [14]. Enhancer activity was calculated by the fold change (FC) of CapStarr-seq over the input signals for each DHS. As defined previously [14], active enhancers were classified as weak (1.5 < FC < 3) or strong (FC > 3). We found that 6 out of 13 Ikzf1 associated DHSs displayed significant enhancer activity (Fig 1A). Of these, the weak enhancer located 15 kb downstream of the Ikzf1 promoter (IkE+15) overlapped with a previously described enhancer [39, 41]. The two strongest enhancers (average FC higher than 3) were located 180 kb (hereafter IkE180) and 120 kb (hereafter IkE120) upstream of Ikzf1 and have not been previously identified. These two strong enhancers overlapped the Ikzf1 upstream super-enhancer and were classified within the top 5% of the most active DHSs present in primary DP thymocytes (ranked 360 and 405 out of 7,152 tested DHSs) (Fig 1B). Neither of the two enhancers was active in the fibroblast-derived NIH-3T3 cell line (Fig 1C). Analyses of H3K27ac ChIP-seq data from human developing thymocytes [58], suggested that the orthologous regions of IkE180 and IkE120 enhancers are also actives at certain stages of thymic T cell differentiation (S1 Fig).
Fig 1. Prioritization of Ikzf1 enhancers.
(A) Epigenomic profiles of the Ikzf1 locus showing ChIP-Seq signals for H3K4me1, H3K27ac and Pol II, DNAseI-seq, Super-enhancers and peaks of the indicated lymphoid transcription factors in mouse primary DP thymocytes. The enhancer activity of DHS regions as assessed by CapStarr-seq in P5424 cells (green: inactive; orange: weak; red: strong; merged from two replicates) is also shown. A strong enhancer associated with six transcription factors is highlighted. Coordinates of DHS and datasets are listed in S3 and S4 Tables, respectively. (B) Ranked DHSs from primary DP thymocytes in the function of enhancer activity assessed by CapStarr-seq in the P5424 cell line (merge of two replicates). The vertical line indicates the top 5% of the most active enhancer. The IkE120 enhancer is highlighted. (C) Enhancer activity of two Ikzf1 enhancers assessed by CapStarr-seq in the P5424 and NIH3T3 cell lines.
We next explored whether the putative enhancers were bound by lymphoid specific transcription factors in primary DP thymocytes, using previously published ChIP-seq data for six transcription factors [14, 22, 59–61](S3 Table). Strikingly, the IkE120 enhancer was the only one to be bound by all tested lymphoid transcription factors, including Ikaros itself (Fig 1A). Interestingly, it was also located between two CTCF sites flanking the Ikzf1 locus (Fig 1A), a known hallmark of “architectural” chromatin loops [62].
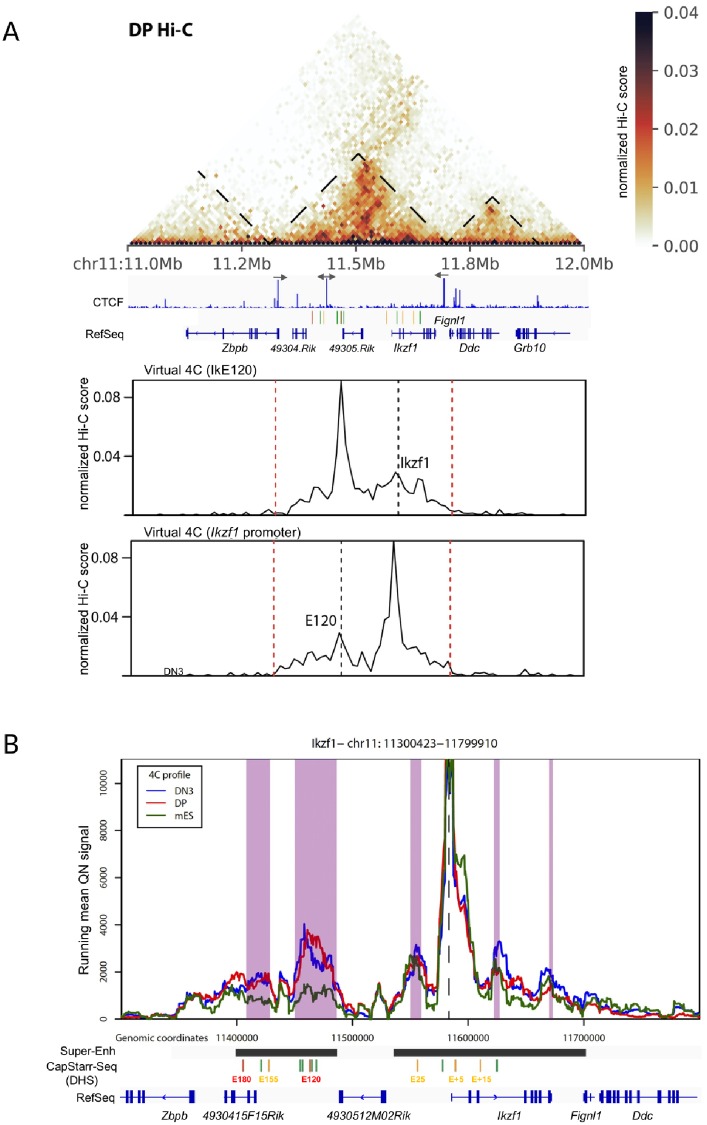
To assess whether the identified enhancers directly interact with the Ikzf1 promoter, we initially analyzed published Hi-C data from primary DP thymocytes [52]. Identified enhancers were all embedded in the same Topological Associated Domain (TAD) as the Ikzf1 locus, and flanked by convergent CTCF sites (Fig 2A). Virtual circularized chromosome conformation capture (4C) plots suggested that IkE120 and Ikzf1 promoter preferentially interact together (Fig 2A, bottom panels). To directly demonstrate the interaction between IkE120 and Ikzf1 promoter we analyzed published 4C-sequencing (4C-seq) experiments in primary DP thymocytes [56] along with newly generated 4C-seq data in primary CD44+CD25+ DN thymocytes (DN3) and non-expressing embryonic stem cells (ES), using the Ikzf1 promoter as the viewpoint (Fig 2B). The Ikzf1 promoter specifically interacted with the upstream super-enhancer in thymic cells but not ES cells, and displayed a strong interaction with the IkE120 enhancer.
Fig 2. 3D topology of the Ikzf1 locus.
(A) Hi-C view of primary DP thymocytes around the Ikzf1 locus (top panel). TAD boundaries are shown. The orientation of the main CTCF peaks in primary DP thymocytes is displayed. Virtual 4C plots corresponding to the Hi-C interactions with the Ikzf1 promoter or the E120 enhancer are shown in the bottom panels. (B) 4C-seq analysis of Ikzf1 promoter interactions. Running mean (window of 21 fragments), quantile normalized 4C-seq profiles are shown from the Ikzf1 promoter bait (dotted line) for primary DN3 (blue) and DP (red) thymocytes and mouse ES cells (green). Locations of genes and the six regions with enhancer activity in CapStarr-seq are shown below the plot. Conserved called interactions with thymic cells are highlighted in purple.
To further interrogate how IkE120 enhancer relates to Ikzf1 expression, we compared Ikzf1 expression (RNA-seq) and chromatin accessibility (ATAC) at IkE120 enhancer using a comprehensive resource of hematopoietic cells from the ImmGen consortium [50, 51] (S2A Fig; representative examples are shown in S2B Fig). The IkE120 enhancer displayed the highest ATAC-seq signal in a subset of hematopoietic cells expressing moderated levels of Ikzf1 expression, including T and B cell precursors and hematopoietic stem cells (HSC). In contrast, hematopoietic cells expressing high levels of Ikzf1, such as NK, γδ and mature CD4+ T cells, displayed a weak ATAC-seq signal at IkE120. Stroma cells that did not express Ikzf1 were not associated with ATAC-seq peak at IkE120. These observations suggest that the IkE120 enhancer might play a preferential role in the expression of the Ikzf1 gene in lymphoid precursors.
In conclusion, the IkE120 enhancer, displayed one of the strongest enhancer activities within the Ikzf1 locus, was found to be associated with key lymphoid transcription factors and directly interacted with the Ikzf1 promoter. While the regulatory elements within the Ikzf1-overlapping super-enhancer have been extensively studied [39, 41], the upstream super-enhancer harboring the IkE120 enhancer has remained unexplored. We, therefore, decided to further explore the functional role of this enhancer within its endogenous context.
Deletion of the Ikzf1 enhancer IkE120
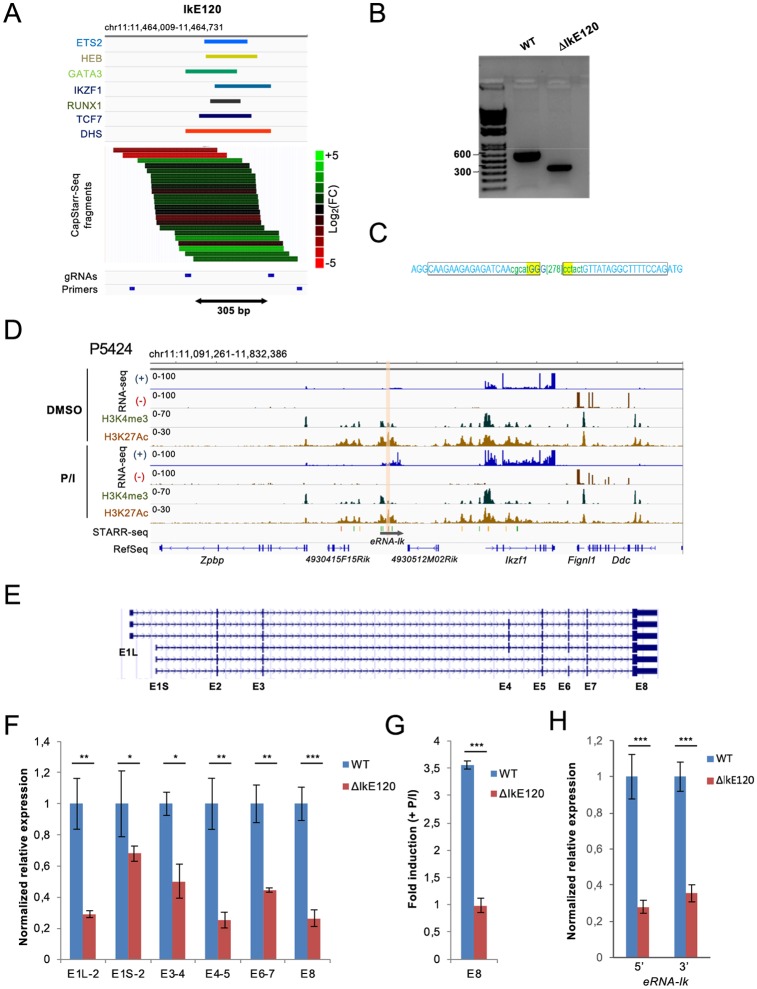
We used CRISPR/Cas9 technology to delete the IkE120 genomic region in the P5424 cell line, encompassing 305 bp covering the DHS site and the six transcription factor binding sites (ΔIkE120) (Fig 3A). Homozygous deletion of IkE120 was assessed by qualitative PCR and Sanger sequencing (Fig 3B and 3C). Note that the P5424 cell line was a valid model to study the endogenous IkE120 enhancer as the enhancer was highly enriched in H3K27ac in these cells and was associated with enhancer RNA (eRNA) expression (Fig 3D). In particular, expression of both Ikzf1 and associated eRNA can be induced by the treatment of P5424 cells with PMA/ionomycin, which partially mimics T cell differentiation and β-selection [45](Fig 3D and S3 Fig).
Fig 3. Deletion of the IkE120 enhancer.
(A) Genomic tracks showing the binding peaks of the indicated transcription factors overlapping the IkE120 enhancer in primary DP thymocytes as well as the enhancer activity of individual clones assessed by the CapStarr-seq assay in P5424 cells. The color scale indicates the enhancer activity as a Log2 fold change of the CapStarr-seq signal over the input. The two sgRNAs used to delete the enhancer and primers to detect the deletion are also shown. (B) PCR analyses of IkE120 deletion in the P5424 cell line. (C) Sanger sequencing results from deletion junctions amplified from the genomic DNA of the targeted ΔIkE120 clone. The rectangles represent the position of the sgRNA. The deleted region is indicated in the bracket. (D) Genomic tracks for RNA-seq and ChIP-seq around the Ikzf1 locus in P5424 cells stimulated or not with PMA/ionomycin (P/I). The IkE120 enhancer is highlighted. The scale of the RNA-seq tracks has been adjusted to visualize the non-coding transcripts overlapping the IkE120 enhancer (a screenshot with unmodified scales for the Ikzf1 gene is shown in S3 Fig) (E) UCSC genome browser showing the transcripts isoforms of the Ikzf1 gene found in RefSeq. (F) RT-qPCR analyses of Ikzf1 expression at the indicated exon-exon junctions in wt and ΔIkE120 P5424 cells. Values represent relative expression as compared with wt samples. (G) Fold induction of Ikzf1 expression (exon 8) after treatment with PMA and ionomycin of P5424 cells. (H) Relative expression of the non-coding transcripts (eRNA) overlapping the IkE120 enhancer in ΔIkE120 cells as compared with wt P5424 samples. Two sets of primers surrounding the IkE120 deleted region were used. In panels F-H, each point represents the means of three independent experiments normalized by the Rpl32 housekeeping gene. Statistical significance was assessed by Student’s t-test (unpaired, two-tailed) from 3 biological replicates (***P < 0.001, **P < 0.01, *P < 0.1). Error bars represent standard deviation.
Based on RefSeq annotation, the Ikzf1 locus harbors 6 transcript isoforms (Fig 3E), which might play different regulatory functions [34–38]. We assessed the effect of Ik120 deletion on different exon-exon junctions encompassing all annotated Ikzf1 transcripts by reverse transcription quantitative PCR (RT-qPCR) analyses in wild-type (wt) and ΔIkE120 P5424 cells (Fig 3F). The expression of the common 3’ UTR Exon 8 (E8) was decreased four-fold in the ΔIkE120 clone with respect to wt cells. Same results were observed for transcripts encompassing exons E4-E5, while those encompassing E3-E4 and E6-E7 were decreased only two-fold in the ΔIkE120 cells (Fig 3F). We also assessed promoter usages by quantifying the transcripts initiating from either E1L or E1S (Fig 3F). Transcripts originating from both promoters were significantly reduced, although the most upstream promoter appeared to be more affected (Fig 3F).
The deletion of the Ik120 enhancer completely inhibited the upregulation of Ikzf1 by PMA/ionomycin treatment (Fig 3G). The Ik120 enhancer is associated with an eRNA transcript (hereafter eRNA-Ik), whose expression is correlated with Ikzf1 induction in P5424 cells and during the DN to DP transition [45](Fig 3D). As expected, the expression of the eRNA-Ik transcript was strongly reduced in ΔIkE120 cells (Fig 3H).
In conclusion, the Ik120 enhancer appears to similarly regulate the different Ikzf1 isoforms and is particularly required for the induction of the Ikzf1 gene after cell stimulation.
Deletion of IkE120 affects local epigenomic profiles
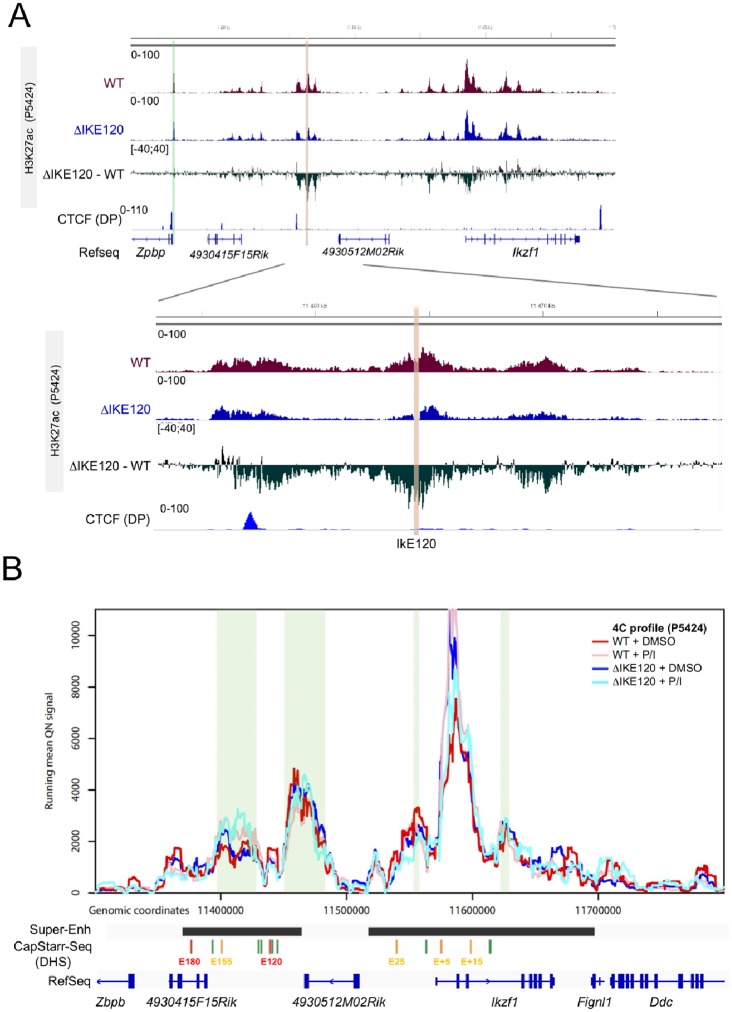
To assess whether IkE120 deletion affects the epigenomic profile of the Ikzf1 locus we performed ChIP-seq experiments to assess H3K27ac profiles. As shown in Fig 4A, the deletion of IkE120 resulted in decreased levels of H3K27ac around the deleted enhancer region and to a lesser extent around the Ikzf1 promoter, while H3K27ac at the promoter of the neighbor Zpbp gene was not affected. Besides, IkE120 deletion did not result in global changes of H3K27ac at gene promoters (S4 Fig). We next performed 4C-seq experiments using the Ikzf1 promoter as a viewpoint in wt and ΔIkE120 P5424 cells in normal and stimulated conditions (Fig 4B). The genomic interactions observed in wt and mutant P5424 cells were very similar to the interactions observed in primary thymocytes (see Fig 2). Furthermore, no differences were observed between the wt and mutant cells, suggesting that IkE120 is not absolutely required for the establishment of the genomic interaction between the 5’ super-enhancer and the Ikzf1 promoter (Fig 4B). Curiously, the promoter-IkE120 interaction is equivalent in normal and stimulated P5424 cells, whereas the interaction with IkE180 appears to increase slightly on Ikzf1 upregulation during stimulation. Such findings are consistent with previous studies suggesting that some promoter-enhancer interactions are concomitant with transcriptional induction whereas others are formed prior to gene expression regulation [63]. Despite an overall reduction in Ikzf1 expression and a complete loss of response to stimulation on IkE120 deletion, these topology dynamics are unchanged by the deletion. Overall, the IkE120 enhancer does not have a wide-spread influence on H3K27 acetylation and 3D topology of the locus, but rather contribute to localized epigenetic marking. This suggest that others regulatory elements within the 5’ super-enhancer are required to ensure the interaction with the Ikzf1 promoters. Such role might be played by the DHS bound by CTCF upstream of the IkE120 enhancer (Figs 1A and 2A), which also corresponds to the 5’ border of influence of IkE120 on H3K27ac (Fig 4A).
Fig 4. Epigenomic impact of IkE120 deletion.
(A) The H3K27ac ChIP-seq at the Ikzf1 locus (top) and around the IkE120 enhancer (bottom) in wt and ΔIkE120 P5424 cells are shown as individual tracks and as the differential signal between wt and ΔIkE120 cells. The genomic track of CTCF ChIP-seq in primary DP thymocytes is also shown. H3K27ac The IkE120 deleted region is highlighted in red and the promoter region of the neighbor Zpbp gene is highlighted in green. (B) Running mean (window of 21 fragments), quantile normalized 4C-seq profiles are shown from the Ikzf1 promoter bait for wt unstimulated (red), wt stimulated (pink), ΔIkE120 unstimulated (blue) and ΔIkE120 stimulated P5424 cells. Locations of genes and the six regions with enhancer activity in CapStarr-seq are shown below the plot. Conserved called interactions are highlighted in green.
Conclusion
Integrative analyses of high-throughput reporter assays, chromatin structure and, 3D topology identified a strong enhancer (IkE120) associated with the Ikzf1 gene. The deletion of the IkE120 enhancer using CRISPR/Cas9 technology demonstrated a critical role of this enhancer in controlling the expression of the Ikzf1 gene. However, the IkE120 enhancer has a modest impact on the chromatin structure and 3D topology of the locus, highlighting the complexity of the regulatory landscape regulating the Ikzf1 locus.
Supporting information
A) Conservation of Ikzf1 enhancers across mammalian species. Detailed view of the IkE120 enhancer conservation is indicated at the bottom panel. B) H3K27ac tracks at the indicated human T cell precursors and Hematopoietic Stem Cells (HSC). The position of the human orthologous regions of the Ikzf1 enhancers are indicated.
(PDF)
A) Comparison between Ikzf1 expression and IkE120 chromatin opening in the hematopoietic lineages as indicated. Normalized RNA-seq and ATAC-seq data was retrieved from the ImmGen portal (http://www.immgen.org). B) ATAC-seq signal around the IkE120 enhancer (Left panel; signal scale was set to 5) and Ikezf1 expression (right panel) at selected hematopoietic samples.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
The enhancer activity as assessed by CapStarr-seq in the P5424 cell line is indicated.
(PDF)
Lanes not included in the final figure were marked with an “X”. TrackIt 1 Kb Plus DNA Ladder (Thermo Fisher) was used as DNA ladder.
(PDF)
Acknowledgments
We thank the Transcriptomics and Genomics Marseille-Luminy (TGML) platform for sequencing the ChIP-seq samples and the Marseille-Luminy cell biology platform for the management of cell culture. Sequencing of 4C samples was performed by the IGBMC GenomEast platform. TGML and GenomEast platforms are member of the France Genomique consortium (ANR-10-INBS-0009).
Data Availability
ChIP-seq and 4C-seq data described in this study are available in GEO database under the accession number GSE147234 (http://www.ncbi.nlm.nih.gov/geo/).
Funding Statement
We thank the Transcriptomics and Genomics Marseille-Luminy (TGML) platform for sequencing the ChIP-seq samples and the Marseille-Luminy cell biology platform for the management of cell culture. Sequencing of 4C samples was performed by the IGBMC GenomEast platform. TGML and GenomEast platforms are member of the France Genomique consortium (ANR-10-INBS-0009). Work in the laboratory of S.S. was supported by recurrent funding from INSERM and Aix-Marseille University and specific grants from the Ligue contre le Cancer (Equipe Labellisée LIGUE 2018), the Agence Nationale pour la Recherche, ANR (ANR-17-CE12-0035; ANR-18-CE12-0019), Cancéropôle PACA, Institut National contre le Cancer (PLBIO018-031 INCA_12619), the Excellence Initiative of Aix-Marseille University - A*Midex, a French “Investissements d’Avenir” programme (ANR-11-IDEX-0001-02). Work in the lab of TS is supported by funds from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Starting Grant 678624 - CHROMTOPOLOGY), the ATIP-Avenir program, and the grant ANR-10-LABX-0030-INRT, a French State fund managed by the Agence Nationale de la Recherche under the frame program Investissements d’Avenir ANR-10-IDEX-0002-02. AMM is supported by funds from IDEX (University of Strasbourg) and the Institut National du Cancer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Plank JL, Dean A. Enhancer Function: Mechanistic and Genome-Wide Insights Come Together. Molecular cell. 2014;55(1):5–14. Epub 2014/07/06. 10.1016/j.molcel.2014.06.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pott S, Lieb JD. What are super-enhancers? Nature genetics. 2015;47(1):8–12. Epub 2014/12/31. 10.1038/ng.3167 . [DOI] [PubMed] [Google Scholar]
- 3.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A Phase Separation Model for Transcriptional Control. Cell. 2017;169(1):13–23. Epub 2017/03/25. 10.1016/j.cell.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki HI, Young RA, Sharp PA. Super-Enhancer-Mediated RNA Processing Revealed by Integrative MicroRNA Network Analysis. Cell. 2017;168(6):1000–14 e15. Epub 2017/03/12. 10.1016/j.cell.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevington SL, Cauchy P, Cockerill PN. Chromatin priming elements establish immunological memory in T cells without activating transcription: T cell memory is maintained by DNA elements which stably prime inducible genes without activating steady state transcription. BioEssays: news and reviews in molecular, cellular and developmental biology. 2017;39(2). Epub 2016/12/28. 10.1002/bies.201600184 . [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Ahituv N. Gene Regulatory Elements, Major Drivers of Human Disease. Annual review of genomics and human genetics. 2017. Epub 2017/04/13. 10.1146/annurev-genom-091416-035537 . [DOI] [PubMed] [Google Scholar]
- 7.Natoli G, Andrau JC. Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet. 2012;46:1–19. Epub 2012/08/22. 10.1146/annurev-genet-110711-155459 . [DOI] [PubMed] [Google Scholar]
- 8.Santiago-Algarra D, Dao LTM, Pradel L, Espana A, Spicuglia S. Recent advances in high-throughput approaches to dissect enhancer function. F1000Res. 2017;6:939 10.12688/f1000research.11581.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwasnieski JC, Fiore C, Chaudhari HG, Cohen BA. High-throughput functional testing of ENCODE segmentation predictions. Genome research. 2014;24(10):1595–602. 10.1101/gr.173518.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kheradpour P, Ernst J, Melnikov A, Rogov P, Wang L, Zhang X, et al. Systematic dissection of regulatory motifs in 2000 predicted human enhancers using a massively parallel reporter assay. Genome research. 2013;23(5):800–11. Epub 2013/03/21. 10.1101/gr.144899.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst J, Melnikov A, Zhang X, Wang L, Rogov P, Mikkelsen TS, et al. Genome-scale high-resolution mapping of activating and repressive nucleotides in regulatory regions. Nat Biotechnol. 2016;34(11):1180–90. 10.1038/nbt.3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, He L, Goggin SM, Saadat A, Wang L, Sinnott-Armstrong N, et al. High-resolution genome-wide functional dissection of transcriptional regulatory regions and nucleotides in human. Nat Commun. 2018;9(1):5380 Epub 2018/12/21. 10.1038/s41467-018-07746-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li QL, Wang DY, Ju LG, Yao J, Gao C, Lei PJ, et al. The hyper-activation of transcriptional enhancers in breast cancer. Clin Epigenetics. 2019;11(1):48 Epub 2019/03/15. 10.1186/s13148-019-0645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhille L, Griffon A, Maqbool MA, Zacarias-Cabeza J, Dao LTM, Fernandez N, et al. High-throughput and quantitative assessment of enhancer activity in mammals by CapStarr-seq. Nat Commun. 2015;6:6905 10.1038/ncomms7905 [DOI] [PubMed] [Google Scholar]
- 15.Fulco CP, Nasser J, Jones TR, Munson G, Bergman DT, Subramanian V, et al. Activity-by-contact model of enhancer-promoter regulation from thousands of CRISPR perturbations. Nature genetics. 2019;51(12):1664–9. Epub 2019/12/01. 10.1038/s41588-019-0538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasperini M, Hill AJ, McFaline-Figueroa JL, Martin B, Kim S, Zhang MD, et al. A Genome-wide Framework for Mapping Gene Regulation via Cellular Genetic Screens. Cell. 2019;176(1–2):377–90 e19. Epub 2019/01/08. 10.1016/j.cell.2018.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgopoulos K. The making of a lymphocyte: the choice among disparate cell fates and the IKAROS enigma. Genes Dev. 2017;31(5):439–50. Epub 2017/04/08. 10.1101/gad.297002.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heizmann B, Kastner P, Chan S. The Ikaros family in lymphocyte development. Curr Opin Immunol. 2018;51:14–23. Epub 2017/12/27. 10.1016/j.coi.2017.11.005 . [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–55. KIM99B. 10.1016/s1074-7613(00)80034-5 [DOI] [PubMed] [Google Scholar]
- 20.Kleinmann E, Geimer Le Lay AS, Sellars M, Kastner P, Chan S. Ikaros represses the transcriptional response to Notch signaling in T-cell development. Molecular and cellular biology. 2008;28(24):7465–75. KLEINMANN2008. 10.1128/MCB.00715-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sridharan R, Smale ST. Predominant Interaction of Both Ikaros and Helios with the NuRD Complex in Immature Thymocytes. Journal of Biological Chemistry. 2007;282(41):30227–38. SRI07. 10.1074/jbc.M702541200 [DOI] [PubMed] [Google Scholar]
- 22.Oravecz A, Apostolov A, Polak K, Jost B, Le Gras S, Chan S, et al. Ikaros mediates gene silencing in T cells through Polycomb repressive complex 2. Nat Commun. 2015;6:8823 Epub 2015/11/10. 10.1038/ncomms9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-del Arco P, Kashiwagi M, Jackson AF, Naito T, Zhang J, Liu F, et al. Alternative promoter usage at the Notch1 locus supports ligand-independent signaling in T cell development and leukemogenesis. Immunity. 2010;33(5):685–98. Epub 2010/11/26. 10.1016/j.immuni.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel G, Deau MC, Simand C, Caye-Eude A, Arfeuille C, Ittel A, et al. Large deletions of the 5’ region of IKZF1 lead to haploinsufficiency in B-cell precursor acute lymphoblastic leukaemia. Br J Haematol. 2019;186(5):e155–e9. Epub 2019/05/31. 10.1111/bjh.15994 . [DOI] [PubMed] [Google Scholar]
- 25.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–99. WIN95. 10.1016/0092-8674(95)90170-1 [DOI] [PubMed] [Google Scholar]
- 26.Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, et al. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nature immunology. 2013;14(10):1073–83. Epub 2013/09/10. 10.1038/ni.2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson L, Johansson B. Ikaros and leukaemia. Br J Haematol. 2015;169(4):479–91. 10.1111/bjh.13342 . [DOI] [PubMed] [Google Scholar]
- 28.Kastner P, Chan S. Role of Ikaros in T-cell acute lymphoblastic leukemia. World J Biol Chem. 2011;2(6):108–14. 10.4331/wjbc.v2.i6.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Jackson AF, Naito T, Dose M, Seavitt J, Liu F, et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nature immunology. 2012;13(1):86–94. Epub 2011/11/15. 10.1038/ni.2150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi I, Yoshida T, Jena N, Qi X, Zhang J, Van Etten RA, et al. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nature immunology. 2014;15(3):294–304. Epub 2014/02/11. 10.1038/ni.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churchman ML, Low J, Qu C, Paietta EM, Kasper LH, Chang Y, et al. Efficacy of Retinoids in IKZF1-Mutated BCR-ABL1 Acute Lymphoblastic Leukemia. Cancer cell. 2015;28(3):343–56. Epub 2015/09/01. 10.1016/j.ccell.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–4. Epub 2008/04/15. 10.1038/nature06866 . [DOI] [PubMed] [Google Scholar]
- 33.Churchman ML, Qian M, Te Kronnie G, Zhang R, Yang W, Zhang H, et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer cell. 2018;33(5):937–48 e8. Epub 2018/04/24. 10.1016/j.ccell.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellavia D, Mecarozzi M, Campese AF, Grazioli P, Talora C, Frati L, et al. Notch3 and the Notch3-upregulated RNA-binding protein HuD regulate Ikaros alternative splicing. The EMBO journal. 2007;26(6):1670–80. BELLAVIA2007A. 10.1038/sj.emboj.7601626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molnar A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Molecular and cellular biology. 1994;14:8292–303. MOL94. 10.1128/mcb.14.12.8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, et al. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J Immunol. 1996;156:585–92. MOL96. [PubMed] [Google Scholar]
- 37.Klug CA, Morrison SJ, Masek M, Hahm K, Smale ST, Weissman IL. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc Nat Acad Sci USA. 1998;95:657–62. KLU98. 10.1073/pnas.95.2.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Liu A, Georgopoulos K. Zing finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. The EMBO journal. 1996;15:5358–69. SUN96. [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufmann C, Yoshida T, Perotti EA, Landhuis E, Wu P, Georgopoulos K. A complex network of regulatory elements in Ikaros and their activity during hemo-lymphopoiesis. The EMBO journal. 2003;22(9):2211–23. Epub 2003/05/03. 10.1093/emboj/cdg186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perotti EA, Georgopoulos K, Yoshida T. An Ikaros Promoter Element with Dual Epigenetic and Transcriptional Activities. PloS one. 2015;10(7):e0131568 Epub 2015/07/03. 10.1371/journal.pone.0131568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida T, Landhuis E, Dose M, Hazan I, Zhang J, Naito T, et al. Transcriptional regulation of the Ikzf1 locus. Blood. 2013;122(18):3149–59. Epub 2013/09/05. 10.1182/blood-2013-01-474916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mombaerts P, Terhorst C, Jacks T, Tonegawa S, Sancho J. Characterization of immature thymocyte lines derived from T-cell receptor or recombination activating gene 1 and p53 double mutant mice. Proc Natl Acad Sci U S A. 1995;92(16):7420–4. Epub 1995/08/01. 10.1073/pnas.92.16.7420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics. 2015;31(7):1120–3. Epub 2014/11/22. 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. Epub 2013/01/05. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saadi W, Kermezli Y, Dao LTM, Mathieu E, Santiago-Algarra D, Manosalva I, et al. A critical regulator of Bcl2 revealed by systematic transcript discovery of lncRNAs associated with T-cell differentiation. Scientific Reports. 2019;9(1):4707 10.1038/s41598-019-41247-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic acids research. 2014;42(Web Server issue):W187–91. Epub 2014/05/07. 10.1093/nar/gku365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14(2):178–92. Epub 2012/04/21. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CM, Barber GP, Casper J, Clawson H, Diekhans M, Gonzalez JN, et al. UCSC Genome Browser enters 20th year. Nucleic acids research. 2020;48(D1):D756–D61. Epub 2019/11/07. 10.1093/nar/gkz1012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stunnenberg HG, Hirst M. The International Human Epigenome Consortium: A Blueprint for Scientific Collaboration and Discovery. Cell. 2016;167(5):1145–9. Epub 2016/11/20. 10.1016/j.cell.2016.11.007 . [DOI] [PubMed] [Google Scholar]
- 50.Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nature immunology. 2008;9(10):1091–4. Epub 2008/09/19. 10.1038/ni1008-1091 . [DOI] [PubMed] [Google Scholar]
- 51.Yoshida H, Lareau CA, Ramirez RN, Rose SA, Maier B, Wroblewska A, et al. The cis-Regulatory Atlas of the Mouse Immune System. Cell. 2019;176(4):897–912 e20. Epub 2019/01/29. 10.1016/j.cell.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu G, Cui K, Fang D, Hirose S, Wang X, Wangsa D, et al. Transformation of Accessible Chromatin and 3D Nucleome Underlies Lineage Commitment of Early T Cells. Immunity. 2018;48(2):227–42 e8. Epub 2018/02/22. 10.1016/j.immuni.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruse K, Hug CB, Vaquerizas JM. FAN-C: A Feature-rich Framework for the Analysis and Visualisation of C data. bioRxiv. 2020:2020.02.03.932517. 10.1101/2020.02.03.932517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crane E, Bian Q, McCord RP, Lajoie BR, Wheeler BS, Ralston EJ, et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature. 2015;523(7559):240–4. Epub 2015/06/02. 10.1038/nature14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25 Epub 2009/03/06. 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben Zouari Y, Platania A, Molitor AM, Sexton T. 4See: A Flexible Browser to Explore 4C Data. Front Genet. 2019;10:1372 Epub 2020/02/11. 10.3389/fgene.2019.01372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geeven G, Teunissen H, de Laat W, de Wit E. peakC: a flexible, non-parametric peak calling package for 4C and Capture-C data. Nucleic acids research. 2018;46(15):e91 Epub 2018/05/26. 10.1093/nar/gky443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams D, Altucci L, Antonarakis SE, Ballesteros J, Beck S, Bird A, et al. BLUEPRINT to decode the epigenetic signature written in blood. Nat Biotechnol. 2012;30(3):224–6. Epub 2012/03/09. 10.1038/nbt.2153 . [DOI] [PubMed] [Google Scholar]
- 59.Koch F, Fenouil R, Gut M, Cauchy P, Albert TK, Zacarias-Cabeza J, et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nature structural & molecular biology. 2011;18(8):956–63. Epub 2011/07/19. 10.1038/nsmb.2085 . [DOI] [PubMed] [Google Scholar]
- 60.Lepoivre C, Belhocine M, Bergon A, Griffon A, Yammine M, Vanhille L, et al. Divergent transcription is associated with promoters of transcriptional regulators. BMC genomics. 2013;14:914 Epub 2013/12/25. 10.1186/1471-2164-14-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35(2):299–311. Epub 2011/08/27. 10.1016/j.immuni.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–80. Epub 2014/12/17. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoenfelder S, Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nature reviews Genetics. 2019;20(8):437–55. Epub 2019/05/16. 10.1038/s41576-019-0128-0 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Conservation of Ikzf1 enhancers across mammalian species. Detailed view of the IkE120 enhancer conservation is indicated at the bottom panel. B) H3K27ac tracks at the indicated human T cell precursors and Hematopoietic Stem Cells (HSC). The position of the human orthologous regions of the Ikzf1 enhancers are indicated.
(PDF)
A) Comparison between Ikzf1 expression and IkE120 chromatin opening in the hematopoietic lineages as indicated. Normalized RNA-seq and ATAC-seq data was retrieved from the ImmGen portal (http://www.immgen.org). B) ATAC-seq signal around the IkE120 enhancer (Left panel; signal scale was set to 5) and Ikezf1 expression (right panel) at selected hematopoietic samples.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
The enhancer activity as assessed by CapStarr-seq in the P5424 cell line is indicated.
(PDF)
Lanes not included in the final figure were marked with an “X”. TrackIt 1 Kb Plus DNA Ladder (Thermo Fisher) was used as DNA ladder.
(PDF)
Data Availability Statement
ChIP-seq and 4C-seq data described in this study are available in GEO database under the accession number GSE147234 (http://www.ncbi.nlm.nih.gov/geo/).