Abstract
The heart is critically dependent on mitochondrial respiration for energy supply. Ischemia decreases oxygen availability, with catastrophic consequences for cellular energy systems. After a few minutes of ischemia, the mitochondrial respiratory chain halts, ATP levels drop and ion gradients across cell membranes collapse. Activation of cellular proteases and generation of reactive oxygen species by mitochondria during ischemia alter mitochondrial membrane permeability, causing mitochondrial swelling and fragmentation and eventually cell death. The mitochondria, therefore, are important targets of cardioprotection against ischemic injury. We have previously shown that ixazomib (IXA), a proteasome inhibitor used for treating multiple myeloma, effectively reduced the size of the infarct produced by global ischemia in isolated rat hearts and prevented degradation of the sarcoplasmic reticulum calcium release channel RyR2. The aim of this work was to further characterize the protective effect of IXA by determining its effect on mitochondrial morphology and function after ischemia. We also quantified the effect of IXA on levels of mitofusin-2, a protein involved in maintaining mitochondrial morphology and mitochondria-SR communication. We found that mitochondria were significantly preserved and functional parameters such as oxygen consumption, the ability to generate a membrane potential, and glutathione content were improved in mitochondria isolated from hearts perfused with IXA prior to ischemia. IXA also blocked the release of cytochrome c observed in ischemia and significantly preserved mitofusin-2 integrity. These beneficial effects resulted in a significant decrease in the left ventricular end diastolic pressure upon reperfusion and a smaller infarct in isolated hearts.
Introduction
The search for protective measures against cardiac ischemia/reperfusion injury has been a matter of active research for the last 30 years. Therapeutic interventions at the onset of reperfusion can limit the damage produced by ischemia, but outcomes after reperfusion remain critically dependent on the degree and duration of ischemia [1]. Cardiac tissue is highly dependent on mitochondrial oxidative phosphorylation for energy production, and when oxygen availability is low, the mitochondrial respiratory rate falls, ATP levels drop, and whole-cell homeostasis is impaired. Alteration of ionic gradients across mitochondrial membranes causes loss of membrane potential, swelling and disorganization of cristae, fragmentation of mitochondria and the release of molecules that eventually produce cell death [2,3]. Therefore, therapeutic measures to prevent or delay mitochondrial damage during ischemia would increase the resistance of the heart to ischemic injury and would undoubtedly be an advantage in those cases where myocardial ischemia can be programmed in advance, such as heart surgeries or organ transplantation.
Many proteins are degraded during ischemia by the proteolytic action of the 20S proteasome, including ryanodine receptors (RyR2), the calcium release channels located in the sarcoplasmic reticulum (SR). RyR2 are rapidly oxidized and degraded during myocardial ischemia, significantly impacting cardiac performance [4,5]. The 20S proteasome has three main proteolytic activities: chymotrypsin-like (CT-like), caspase-like and trypsin-like activities. In a recent work, we showed that after 30 minutes of global ischemia in isolated rat hearts, CT-like activity increases by 60%, while caspase-like and trypsin-like catalytic activities remain unchanged [6]. Inhibition of CT-like activity with ixazomib (IXA), a proteasome inhibitor currently used in patients with multiple myeloma [7], prevents RyR2 degradation during ischemia and significantly improves cell survival after ischemia/reperfusion [6]. In the heart, the SR and mitochondria are physically connected, forming microdomains that allow for the transfer of calcium from the SR to the mitochondria so that mitochondrial energy production can satisfy energy requirements [8,9]. Several proteins are involved in the organization of SR-mitochondria microdomains, including RyR2 and mitofusin-2 (Mfn2), a GTPase localized to the microdomains known as mitochondrial associated membranes, that include the SR [10,11]. Mfn2 also regulates mitochondrial fusion [12] and respiratory chain function by maintaining mitochondrial levels of coenzyme Q [13]. Mitochondrial function is therefore critically dependent on Mfn2 integrity. Under stress conditions, such as ischemia, Mfn2 is phosphorylated and degraded by the proteasome [14]. As a consequence of the degradation of this and other proteins, mitochondria undergo fragmentation and degradation. The effect of IXA on post-ischemia mitochondrial function has not been investigated before and since this inhibitor effectively protected RyR2, one of the proteins involved in the SR-mitochondrial association, we aimed to determine whether Mfn2 degradation can be prevented by IXA; and to evaluate the effect of the drug on the functional and morphologic changes to the mitochondria that occur during ischemia but before reperfusion in isolated rat hearts.
Materials and methods
Male Sprague-Dawley (SD) rats (250–300 g) were obtained from the animal facility of the University of Chile, School of Medicine. All study procedures were approved by the Institutional Ethics Committee of the School of Medicine (Protocol CBA #541 FMUCH) and performed according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH, 2011).
Experimental protocol
Hearts were excised under deep anesthesia with pentobarbital (80 mg/kg, intraperitoneal) and rapidly perfused at 37°C via the ascending aorta at a constant flow of 10–14 mL/minute, using a peristaltic infusion pump with Krebs-Henseleit solution containing (in mmol/L): 128.3 NaCl, 4.7 KCl, 1.35 CaCl2, 1.1 MgSO4, 20.2 NaHCO3, 0.4 NaH2PO4, and 11.1 glucose, at a pH of 7.4, equilibrated with a gas mixture of 95% O2/5% CO2. A latex balloon was inserted into the left ventricle and connected to a pressure transducer to measure hemodynamic parameters.
Experimental groups. Control group (N = 15): Hearts were perfused with Krebs buffer solution for 50 minutes. Ischemia group (N = 15): hearts were stabilized for 20 minutes with Krebs buffer solution, followed by 30 minutes of global ischemia at 37°C. Control group with IXA (N = 9): Hearts were stabilized for 10 minutes with Krebs buffer solution, followed by 10 minutes of perfusion with Krebs plus the proteasome inhibitor ixazomib (IXA, 0.10 μmol/L), and then 30 minutes of perfusion with Krebs buffer. Ischemia group with IXA (N = 9): Hearts were stabilized for 10 minutes with Krebs buffer solution, followed by 10 minutes of perfusion with IXA, and then 30 minutes of global ischemia.
Immediately after ischemia, or the equivalent time in controls, heart ventricles were snap-frozen in liquid nitrogen, reduced to powder under liquid N2, and kept under argon atmosphere at -80°C.
Infarct size measurement
Hearts subjected to ischemia in the absence (N = 10) or in the presence of IXA (N = 10) were reperfused with Krebs solution for 60 minutes and then perfused with triphenyltetrazolium chloride (TTC, Sigma-Aldrich, St. Louis, MO) to measure the infarct size as described previously [15].
Proteasome activity
Whole-ventricle homogenates were prepared from frozen tissue, and chymotrypsin-like activity of the proteasome was determined as described previously [6,16].
Quantification of mitochondrial DNA
Total DNA from ventricular tissue was obtained using DNAzol® (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The homogenate was centrifuged at 10,000 x g for 10 minutes at 4ºC. Ethanol was added to the supernatant to precipitate total DNA. Mitochondrial DNA (mtDNA) was quantified by detecting D-loop with the PCR primers, F: 5’GGT TCT TAC TTC AGG GCC ATC A-3’ and R: 5’-GAT TAG ACC CGT TAC CAT CGA GAT-3’. β-actin, used as a housekeeping gene, was detected with specific primers: F: 5’-GGG ATG TTT GCT CCA ACC AA-3’ and R: 5’-GCG CTT TTG ACT CAA GGA TTT AA-3’. The 2-ΔΔCt method was used to calculate relative transcript abundance.
Preparation of mitochondria
Frozen ventricles were homogenized with a Dounce glass Teflon homogenizer with 6 volumes of buffer solution consisting of (in mmol/L): 225 mannitol, 75 sucrose, 0.5 EGTA-K, 30 TRIS-HCl, at a pH 7.4, with 0.5% BSA. The crude mitochondrial fraction was isolated by differential centrifugation according the procedure described by Wieckowski [17]. The supernatant of the mitochondrial fraction was saved to measure cytochrome c content by Western blot.
Oxygen consumption
Mitochondrial fractions were incubated in a buffer containing (in mmol/L): 225 mannitol, 75 sucrose, 0.5 EGTA-K, 30 TRIS-HCl, at pH 7.4, with 0.5% BSA supplemented with 25 mmol/L succinate and 1.65 mmol/L ADP and the fluorescent probe MitoXpress a 37ºC. Samples were covered with a layer of mineral oil, according to the manufacturer's recommendations (MitoXpress ®, catalog # 600800, Cayman Chemical, MI-USA) and the light excited at 380 nm and emitted at 650 nm was measured in a plate reader. The results were normalized with respect to controls and expressed in arbitrary units of fluorescence/minute/mg of protein.
Mitochondrial membrane potential
Mitochondrial fractions were incubated in a solution containing 110 mM KCl, 10 mM ATP, 10 mM MgCl2, 10 mM sodium succinate, 1 mM EGTA and 20 mM MOPS, pH 7.5 and the fluorescent probe JC-1 (Catalog Number J4519, Sigma-Aldrich, St. Louis, MO) according manufacturer´s recommendations. JC-1 aggregates in polarized mitochondria and changes its fluorescent emission. Fluorescence was excited at 490 and the ratio of emitted light at 590/540 nm was measured. The results were expressed in arbitrary units of fluorescence/mg protein.
Glutathione content
Total glutathione (GSH + GSSG) concentration was determined in isolated mitochondria as described by Griffith [18].
Western blot analysis
Proteins were separated by electrophoresis in polyacrylamide gel (8 or 15% gels), transferred to PVDF membranes (Bio-Rad), and immunoblotted. The primary antibodies used were: anti-Mfn2 (ab50483, lot# GR297107-2, Abcam, Cambridge, UK), anti-cytochrome c (C5723, lot# 038K1427, Sigma Chemicals Co., St. Louis, MO), anti-GAPDH (G9547, lot# 026M4836V, Sigma Chemicals Co., St. Louis, MO). Antigen-antibody reactions were detected by ECL (Amersham Biosciences), and blots were quantified using Image Lab software. Results were normalized to GADPH. Protein concentration was determined using the bicinchoninic acid assay (Pierce BCA Protein Assay Kit; Thermo Scientific, Rockford, IL).
Transmission Electron Microscope (TEM)
Immediately after the experiment, the ischemic hearts were perfused for 30 minutes with a solution of 3% glutaraldehyde in 0.1 mol/L sodium phosphate buffer, at a pH of 7.3, and treated with 1% osmium tetroxide. Samples were dehydrated in ethanol solutions of increasing concentrations and finally placed in EPON resin. Sections, 60-nm thick, were cut from the tissue and mounted on electron microscopy grids. Images were acquired using a transmission electron microscope at 80 kV (Philips Tecnai 12, at the Advanced Microscopy Facility, Pontificia Universidad Católica) or in a high-resolution scanning electron microscope (Inspect F50, FEI, at the Microscopy Facility of the Facultad de Ciencias Químicas, Universidad de Chile) at 10kV with a STEM detector.
Statistical analysis
Data are expressed as mean ± SEM. Data were analyzed using Kruskal-Wallis test followed by Dunn´s test. When comparing two groups Student’s t-test was used. Differences were considered significant at p<0.05.
Results
Inhibition of CT-like activity of the proteasome prevents mitofusin-2 degradation during ischemia
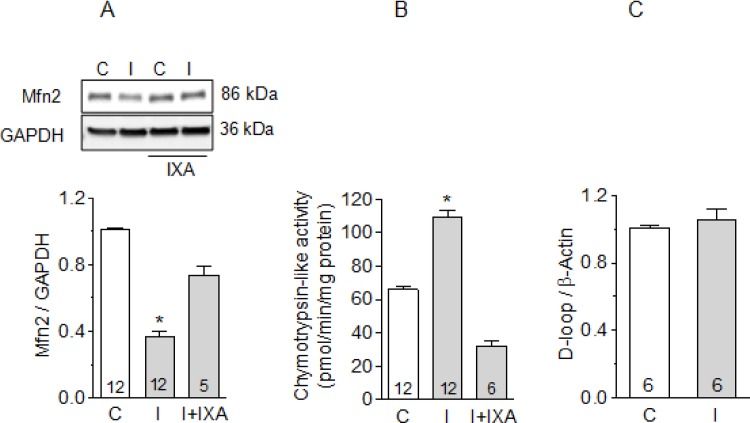
We found that Mfn2 was highly sensitive to ischemia, decreasing by 60% after 30 minutes of global ischemia in isolated hearts. This decrease was prevented when the hearts were previously perfused with IXA (Fig 1A). As shown previously, the increase in proteasomal CT-like activity observed after ischemia was also inhibited by IXA (Fig 1B), suggesting that Mfn2 was degraded by the proteasome. This effect is similar to the previously-reported inhibition of RyR2 degradation by IXA during ischemia [6]. We did not observe changes in mitochondrial DNA after 30 minutes of ischemia (Fig 1C), suggesting that mitochondrial mass did not change during this period. Likewise, we found no degradation of other mitochondrial proteins such as Mitofusin-1 or Fis-1, both localized to the outer mitochondrial membrane, nor in OPA-1 or COX IV, both integral proteins of the inner mitochondrial membrane or in Cyclofilin D, a mitochondrial matrix protein, suggesting that the degradation of Mfn2 is not the result of the generalized degradation of proteins after ischemia (S1 Fig).
Fig 1. Effect of ixazomib on mitofusin-2 content and proteasome activity after ischemia.
(A) Mitofusin-2 content in control hearts, after 30 minutes of ischemia, or after ischemia in hearts previously perfused with IXA. Representative Western blots are shown at the top. (B) Chymotrypsin-like activity of the proteasome in control hearts, after ischemia, or after ischemia in hearts previously perfused with IXA. (C) mtDNA (D-loop) to nDNA (β-actin) by real-time quantitative PCR in control or ischemic hearts. Labels: C, control hearts, I: ischemic hearts. Bars show the average ± SEM of the number of hearts shown in each bar. *p<0.05 vs. all other conditions. #p<0.05 vs. C; Kruskal-Wallis test followed by Dunn`s test.
Ixazomib preserves mitochondrial function during ischemia
To evaluate the effect of IXA on the functional status of the mitochondria, we measured oxygen consumption and the capacity of isolated mitochondria to generate a membrane potential in the presence of respiratory substrates and cofactors. Oxygen consumption by mitochondria isolated from ischemic hearts was reduced to less than 10% of control levels but showed only a 40% reduction when ischemia was performed in the presence of IXA (Fig 2A). To evaluate the ability to generate a potential difference across the inner mitochondrial membrane we measured the accumulation of the lipophilic cationic dye JC-1. Mitochondria isolated from ischemic hearts accumulated significantly less JC-1 suggesting that they produced a smaller membrane potential difference than controls (Fig 2B). IXA significantly prevented the decrease in JC-1 accumulation observed after ischemia (Fig 2B). We also measured cytochrome c (cyt c) levels in the supernatant of the mitochondrial fraction as another measure of mitochondrial dysfunction. We observed a significant increase in cyt c content in the soluble fraction of ischemic hearts (Fig 2C). This effect was completely prevented by IXA, confirming the protective effect of the inhibitor on the mitochondria.
Fig 2. Mitochondrial function after ischemia is preserved by ixazomib.
(A) Oxygen consumption, (B) JC-1 fluorescence ratio 590/540, (C) cytosolic cyt c and (D) mitochondrial glutathione content in mitochondria isolated from control hearts or after 30 minutes of ischemia with or without previous perfusion with IXA. Labels C: control, I: ischemia. Oxygen consumption and Cyt c were expressed relative to controls. Number of measurements in different hearts is shown in each bar. *p<0.05. Kruskal-Wallis test followed by Dunn`s test.
An important cause of ischemia-induced mitochondrial dysfunction is the generation of ROS, which oxidize lipids and proteins. Mitochondria maintain their own pool of glutathione [19], which is involved in the protection of thiol groups, detoxification of peroxides, and other redox reactions in the mitochondrial matrix. Mitochondrial glutathione content is an indirect index of ROS generation. We found that the glutathione (GSH + GSSG) content in isolated mitochondria was greatly decreased after ischemia and this was largely prevented by IXA (Fig 2D). IXA did not modify any of the above-mentioned mitochondrial parameters when infused into control hearts. Taken together, these results suggest that IXA protects mitochondria from the damage produced by ischemia.
Ixazomib attenuates the morphological changes to the mitochondria produced by ischemia
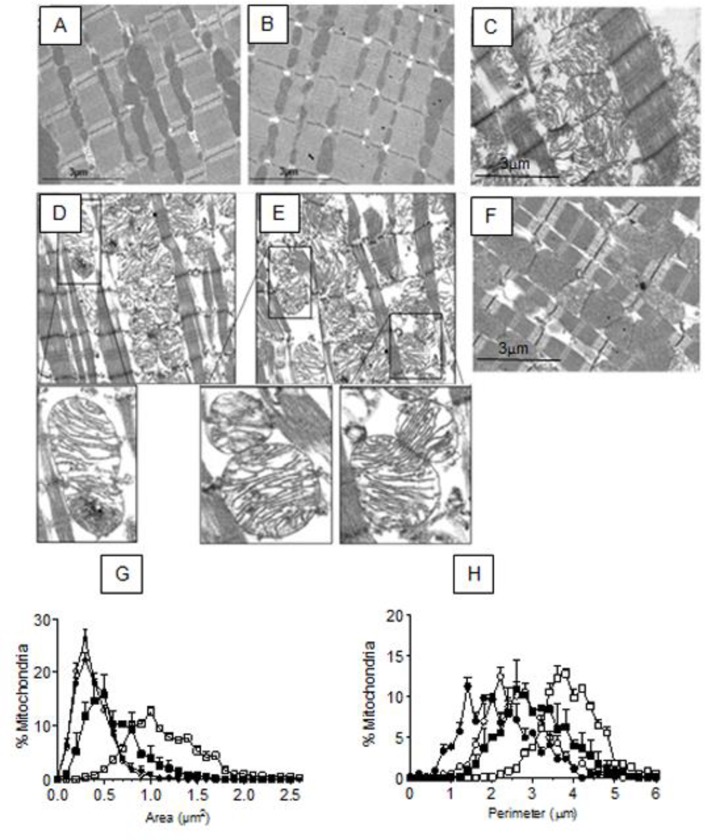
The results shown above suggest that IXA protects the mitochondria during ischemia. To test this observation at a morphological level, we analyzed transmission electron microscopy images of cardiac tissue subjected to ischemia in the presence or absence of the drug. A longitudinal section of a control heart not subjected to ischemia, in which interfibrillar mitochondria arranged between sarcomeres can be appreciated, is shown in Fig 3A. IXA did not alter the mitochondrial ultrastructure under control conditions (Fig 3B). Fig 3C shows a section of cardiac tissue after 30 minutes of global ischemia. Ischemia provoked significant tissue damage, as demonstrated by disorganized sarcomeres and swollen mitochondria with disorganized cristae and a less electron-dense matrix (Fig 3C). Higher resolution images of particularly swollen mitochondria (increased area and lower electron density) accompanied by aberrant cristae in terms of transversal continuity and frequency are shown in Fig 3D and 3E. These changes were markedly reduced by the presence of IXA during ischemia (Fig 3F) indicating that proteasome inhibition protected the mitochondrial structure. Average mitochondrial morphological parameters after ischemia in the presence and absence of IXA are shown in Table 1. In summary, ischemia increased mitochondrial area (Fig 3G, Table 1) and perimeter (Fig 3H, Table 1) due to swelling and both indices were significantly decreased when ischemia was performed in the presence of IXA.
Fig 3. Effect of ixazomib on mitochondrial morphology in ischemic hearts.
Transmission electron microscope images of (A) a control heart, (B) a control heart perfused with IXA, (C) a heart subjected to ischemia. (D and E) are images of an ischemic heart captured with a high-resolution scanning electron microscopy. Insets correspond to amplifications of the indicated zones in (D) and (E) showing mitochondrial cristae aberrations such as transversal discontinuity. (F) a heart subjected to ischemia in the presence of IXA (G) Frequency distribution of mitochondrial area and (H) perimeter of mitochondria in each experimental group. Symbols: C, white circles; C+IXA, black circles; I, white squares; I + IXA, black squares. Data on (G) and (H) are the average of 600–800 mitochondria.
Table 1. Mitochondrial morphological parameters.
| Control | Ischemia | Control + IXA | Ischemia + IXA | |
|---|---|---|---|---|
| Area (μm2) | 0.374±0.004 | 1.161±0.034* | 0.396±0.025 | 0.602±0.106# |
| Perimeter (μm) | 2.648±0.081 | 3.938±0.058* | 2.753±0.092 | 3.076±0.287# |
| Circularity | 0.664±0.028 | 0.912±0.005* | 0.658±0.019 | 0.743±0.046# |
The values correspond to the averages ± the standard deviation. 600–800 mitochondria were counted in three different hearts of each experimental group.
*p<0.05 vs Control
# p<0.05 vs ischemia, Kruskal-Wallis test followed by Dunn`s test.
Inhibition of proteasome CT-like activity attenuates the increase in left ventricular end diastolic pressure and reduces infarct size at the end of reperfusion
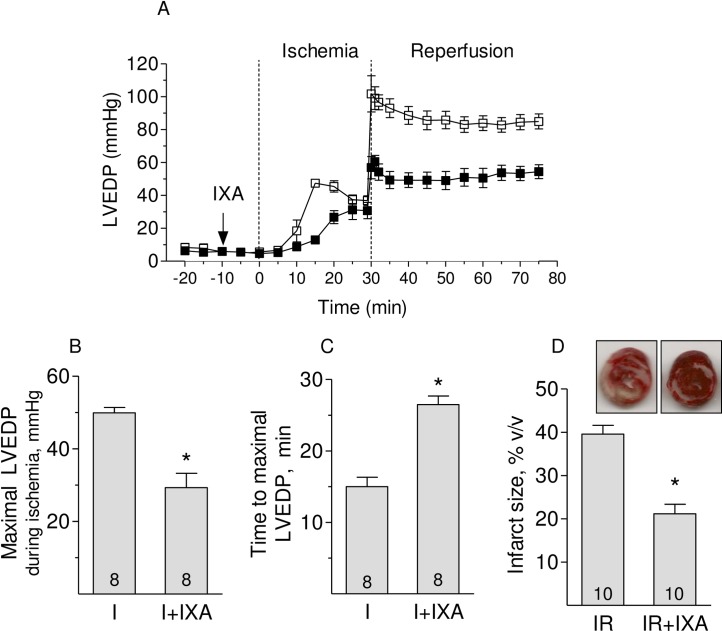
Isolated hearts subjected to global ischemia stop beating in a few minutes and experience a progressive increase in the left ventricular end-diastolic pressure (LVEDP) starting at around 10 minutes of ischemia (Fig 4A). Reperfusion strongly exacerbated the increase in LVEDP that remained elevated during the following hour of reperfusion (Fig 4A). In the presence of IXA, the increase in LVEDP during ischemia was smaller and developed later, indicating that the myocardium remained viable for a longer period (Fig 4A). Likewise, LVEDP after one hour of reperfusion was lower in hearts treated with IXA (Fig 4A).These effects are summarized in Fig 4B and 4C. The reductions in these variables imply a decrease in tissue damage during ischemia, resulting in a significantly lower infarct size in the presence of IXA (Fig 4D).
Fig 4. Effect of Ixazomib on left ventricular end-diastolic pressure and on the infarct size.
(A) Left ventricular end-diastolic pressure (LVEDP) in isolated hearts during global ischemia and reperfusion in the presence (black squares) or absence (white squares) of IXA. (B) Maximal LVEDP and (C) time to maximal LVEDP during ischemia in the presence or absence of IXA were determined from records similar to the examples shown in (A) (D) Infarct size in the presence or absence of IXA. Number of hearts is shown in each bar. *p<0.05 Student’s t test.
Discussion
This work extends our prior findings on the protective effects of IXA during myocardial ischemia [6]. The previously-observed decrease in infarct size in the presence of IXA prompted us to study the effect of this inhibitor on mitochondrial morphology and function during ischemia. Due to its elevated energy requirements, the heart is highly dependent on mitochondrial respiration, and practically all of the ATP required by the heart is generated by oxidative phosphorylation. Interruption of the blood supply causes a rapid drop in ATP production, with a consequent decline in contractile force and loss of cellular homeostasis. Oxygen deprivation provokes time-dependent mitochondrial damage that eventually becomes irreversible and produces cell death. Therefore, mitochondria are important targets for cardioprotection. We specifically analyzed the effect of IXA on Mfn2 as this protein plays a critical role not only in mitochondrial dynamics, but also in communication between the mitochondria and sarcoplasmic reticulum [9], as well as in other aspects of cell metabolism [20]. IXA was very effective in reducing mitochondrial damage during ischemia, as indicated by the preservation of mitochondrial morphology and improvement in mitochondrial function indices such as oxygen consumption, membrane potential, and glutathione content. Importantly, IXA blocked the release of cyt c from the mitochondria into the cytosol, which otherwise would initiate activation of the intrinsic apoptosis pathway [21], contributing to tissue death. The loss of cyt c would also contribute to the decrease in ATP generation during ischemia, even before oxygen is depleted. Moreover, cytc is a ROS scavenger. Loss of cyt c, therefore, would further contribute to mitochondrial damage, as suggested by the decrease in mitochondrial GSH levels. Consistently, IXA delayed the onset and decreased the amplitude of LVEDP, resulting in a significant reduction in infarct size.
The role of Mfn2 in mitochondrial structure and function in adult hearts is complex and not yet fully understood. IXA not only prevented the degradation of Mfn2 during ischemia but also, as we showed before, of RyR2 [6]. Since both proteins are present in SR-mitochondria associated membranes, these results suggest that the preservation of this interaction is important for cell survival under stress. In addition to the above-mentioned involvement in mitochondrial metabolism and SR-mitochondria communication, Mfn2 also protects neurons from apoptotic death following ischemia/reperfusion by modulating mitophagy, a process needed to rid the cells of damaged mitochondria [22]. Certainly, Mfn2 is unlikely to be the only protein preserved by proteasome inhibition during ischemia, but our data suggests that avoiding its degradation has an important role in the protection conferred by IXA.
The first attempts to inhibit the proteasome as a therapeutic measure against ischemia/reperfusion injury were highly controversial. Beneficial [23,24] or deleterious [25,26] effects were observed in various models of ischemia using different inhibitors and concentrations. We [6] and others [27,28] have shown that the degree of proteasome inhibition is critical to the protective effect. As shown in our previous work, the IXA concentrations used here only inhibit CT-like activity, without affecting proteasomal activities of caspase or trypsin-like activity. Inhibition of caspase-like activity associated with higher concentrations of IXA may cause undesired effects [6].
Most studies on ischemia/reperfusion injury focus on the injury produced at reperfusion, since ischemia typically occurs without prior warning. Nevertheless, irreversible damage and cell death begin during ischemia, and our results emphasize the need to protect the mitochondria during the ischemic period itself. Pharmacological approaches to prevent mitochondrial damage have yet to be translated into clinically-relevant therapeutic measures against myocardial ischemia. One of the reasons for this lack of success may be that many of the relevant experimental drugs are not currently approved for human use. Proteasome inhibition by IXA may be a feasible way to protect the heart from ischemic damage in cases where it is known in advance that ischemia will occur (cardiac surgery with extracorporeal circulation, for instance), as the drug is currently used in patients with multiple myeloma [29]. Furthermore, the IXA concentrations used in this study are similar to plasma concentrations of IXA in multiple myeloma patients [30], suggesting that a significant protective effect against myocardial ischemia can be achieved with pharmacological concentrations of the drug.
Limitation of the study
We acknowledge the following limitations that should be addressed in future studies: a) ROS formation was not directly assessed during ischemia. This determination is challenging in Langendorff perfused hearts and it was not possible to perform under our protocol of ischemia. B) It would have been also desirable to determine the extent of mitochondrial fusion and fission in hearts treated with IXA. However, there are methodological restrictions to effectively measure mitochondrial fusion or fission in the heart. c) The effect of IXA on changes in gene expression (mRNA levels) associated with cardiac energetics was not investigated in the present work. It would be important to determine changes in mRNA levels associated with cardiac energetics within the first 30 min as previously reported in other studies [31, 32].
Conclusion
Given its low toxicity and adequate tolerance [33], ixazomib may be a useful drug to protect cardiac mitochondria in cases of programmed ischemia.
Supporting information
Proteins were quantified by Western blots in whole heart homogenates from control or ischemic hearts. The antibodies used were the following: anti Mitofusin 1 (cat # ab104274, Abcam, Cambridge, UK; anti FIS-1 (cat # PA5-22142, Thermo Scientific, Waltham, MA, USA); anti OPA-1 (cat # sc-367890, Santa Cruz Biotechnology, Dallas, TX, USA); COX IV (cat # 4844, Cell Signaling Technologies, Danvers, MA, USA); Cyclophilin D (cat # AP1035, Merck, Burlington, MA, USA). Protein content was normalized by the content of GAPDH (cat # G9547, Sigma Chemicals Co., St. Louis, MO). Bars show Mean ± S.E.M values of western blots like those shown on top, obtained in 4 different hearts.
(TIF)
Data Availability
All relevant data are within the paper.
Funding Statement
Fondo Nacional de Investigación Cientifica y Tecnológica (Fondecyt) Grants 1110257 (PD), 1130407 (GS), 11170962 (IO), and 1180613 (ZP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92–100. 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P (2007) Mitochondria and cardioprotection. Heart Fail Rev 12: 249–260. 10.1007/s10741-007-9028-z [DOI] [PubMed] [Google Scholar]
- 3.Camara AK, Bienengraeber M, Stowe DF (2011) Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front Physiol 2: 13 10.3389/fphys.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domenech RJ, Sanchez G, Donoso P, Parra V, Macho P (2003) Effect of tachycardia on myocardial sarcoplasmic reticulum and Ca2+ dynamics: a mechanism for preconditioning? J Mol Cell Cardiol 35: 1429–1437. 10.1016/j.yjmcc.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Becerra R, Roman B, Di Carlo MN, Mariangelo JI, Salas M, et al. (2016) Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart. Am J Physiol Heart Circ Physiol 311: H713–724. 10.1152/ajpheart.00142.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez G, Berrios D, Olmedo I, Pezoa J, Riquelme JA, et al. (2016) Activation of Chymotrypsin-Like Activity of the Proteasome during Ischemia Induces Myocardial Dysfunction and Death. PLoS One 11: e0161068 10.1371/journal.pone.0161068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson PG, Zweegman S, O'Donnell EK, Laubach JP, Raje N, et al. (2018) Ixazomib for the treatment of multiple myeloma. Expert Opin Pharmacother 19: 1949–1968. 10.1080/14656566.2018.1528229 [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D (2010) The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res 88: 30–39. 10.1093/cvr/cvq225 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, et al. (2012) Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res 111: 863–875. 10.1161/CIRCRESAHA.112.266585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohlhaas M, Maack C (2013) Calcium release microdomains and mitochondria. Cardiovasc Res 98: 259–268. 10.1093/cvr/cvt032 [DOI] [PubMed] [Google Scholar]
- 11.de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610. 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, et al. (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200. 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, et al. (2015) Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J Cell Biol 208: 429–442. 10.1083/jcb.201411100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, et al. (2012) Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell 47: 547–557. 10.1016/j.molcel.2012.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, et al. (2007) CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res 73: 689–698. 10.1016/j.cardiores.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Lima CF, Rattan SI (2010) Determination of proteasomal activities. Methods Mol Biol 648: 183–192. 10.1007/978-1-60761-756-3_12 [DOI] [PubMed] [Google Scholar]
- 17.Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P (2009) Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4: 1582–1590. 10.1038/nprot.2009.151 [DOI] [PubMed] [Google Scholar]
- 18.Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212. 10.1016/0003-2697(80)90139-6 [DOI] [PubMed] [Google Scholar]
- 19.Calabrese G, Morgan B, Riemer J (2017) Mitochondrial Glutathione: Regulation and Functions. Antioxid Redox Signal 27: 1162–1177. 10.1089/ars.2017.7121 [DOI] [PubMed] [Google Scholar]
- 20.Chandhok G, Lazarou M, Neumann B (2018) Structure, function, and regulation of mitofusin-2 in health and disease. Biol Rev Camb Philos Soc 93: 933–949. 10.1111/brv.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632. 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- 22.Peng C, Rao W, Zhang L, Gao F, Hui H, et al. (2018) Mitofusin 2 Exerts a Protective Role in Ischemia Reperfusion Injury Through Increasing Autophagy. Cell Physiol Biochem 46: 2311–2324. 10.1159/000489621 [DOI] [PubMed] [Google Scholar]
- 23.Campbell B, Adams J, Shin YK, Lefer AM (1999) Cardioprotective effects of a novel proteasome inhibitor following ischemia and reperfusion in the isolated perfused rat heart. J Mol Cell Cardiol 31: 467–476. 10.1006/jmcc.1998.0880 [DOI] [PubMed] [Google Scholar]
- 24.Stansfield WE, Moss NC, Willis MS, Tang R, Selzman CH (2007) Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia-reperfusion injury. Ann Thorac Surg 84: 120–125. 10.1016/j.athoracsur.2007.02.049 [DOI] [PubMed] [Google Scholar]
- 25.Powell SR, Wang P, Katzeff H, Shringarpure R, Teoh C, et al. (2005) Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal 7: 538–546. 10.1089/ars.2005.7.538 [DOI] [PubMed] [Google Scholar]
- 26.Divald A, Powell SR (2006) Proteasome mediates removal of proteins oxidized during myocardial ischemia. Free Radic Biol Med 40: 156–164. 10.1016/j.freeradbiomed.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 27.Kloss A, Meiners S, Ludwig A, Dahlmann B (2010) Multiple cardiac proteasome subtypes differ in their susceptibility to proteasome inhibitors. Cardiovasc Res 85: 367–375. 10.1093/cvr/cvp217 [DOI] [PubMed] [Google Scholar]
- 28.Meiners S, Ludwig A, Stangl V, Stangl K (2008) Proteasome inhibitors: poisons and remedies. Med Res Rev 28: 309–327. 10.1002/med.20111 [DOI] [PubMed] [Google Scholar]
- 29.Gupta N, Hanley MJ, Xia C, Labotka R, Harvey RD, et al. (2019) Clinical Pharmacology of Ixazomib: The First Oral Proteasome Inhibitor. Clin Pharmacokinet 58: 431–449. 10.1007/s40262-018-0702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N, Hanley MJ, Venkatakrishnan K, Wang B, Sharma S, et al. (2016) The Effect of a High-Fat Meal on the Pharmacokinetics of Ixazomib, an Oral Proteasome Inhibitor, in Patients With Advanced Solid Tumors or Lymphoma. J Clin Pharmacol 56: 1288–1295. 10.1002/jcph.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu QP, Cao TJ, Zhang ZY, Liu W. (2004) Multiple gene differential expression patterns in human ischemic liver: safe limit of warm ischemic time. World J Gastroenterol 10:2130–2133. 10.3748/wjg.v10.i14.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra MS, Roncon-Albuquerque R Jr, Lourenço AP, Falcão-Pires I, Cibrão-Coutinho P et al. (2006). Remote myocardium gene expression after 30 and 120 min of ischaemia in the rat. Exp Physiol.91:473–480 10.1113/expphysiol.2005.032557 [DOI] [PubMed] [Google Scholar]
- 33.Shirley M (2016) Ixazomib: First Global Approval. Drugs 76: 405–411. 10.1007/s40265-016-0548-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proteins were quantified by Western blots in whole heart homogenates from control or ischemic hearts. The antibodies used were the following: anti Mitofusin 1 (cat # ab104274, Abcam, Cambridge, UK; anti FIS-1 (cat # PA5-22142, Thermo Scientific, Waltham, MA, USA); anti OPA-1 (cat # sc-367890, Santa Cruz Biotechnology, Dallas, TX, USA); COX IV (cat # 4844, Cell Signaling Technologies, Danvers, MA, USA); Cyclophilin D (cat # AP1035, Merck, Burlington, MA, USA). Protein content was normalized by the content of GAPDH (cat # G9547, Sigma Chemicals Co., St. Louis, MO). Bars show Mean ± S.E.M values of western blots like those shown on top, obtained in 4 different hearts.
(TIF)
Data Availability Statement
All relevant data are within the paper.