Abstract
Salmonella spp. is one of the worldwide leading causes of food-borne illnesses for which the inclusion of probiotics or organic acids in animal feeds can be useful control methods. Experimental models are utilized to test the efficacy of strategies against pathogens, but they exhibit limitations which may preclude finding sensible evaluation parameters. The objective of this work is to evaluate the efficacy of 2 different feed additives; a Bacillus licheniformis based probiotic and a protected sodium butyrate (SB) salt, using an experimental model of salmonellosis and, second, to explore if behavior analysis can be used as a sensible evaluation tool for additives evaluation. A total of 78 piglets weaned at 24 d, 8.3 kg BW, were used. Seventy-two were placed in 3 rooms of 8 pens (3 animals/pen) with evenly distributed treatments (n = 8): CON, control group with plain diet; PRO, plain diet with 1 kg/t of Proporc (109 cfu of B. licheniformis/kg of feed), and BUT, plain diet with 3 kg/t of Gustor BP70 (2.1 g of partially protected SB salt/kg of feed). Remaining piglets (n = 6) were separated and used as a challenge negative control. The experiment lasted 16 d. After 1 wk of adaptation, animals were challenged with 5 × 108 cfu of Salmonella Typhimurium. One pig per pen was euthanized and sampled at d 4 and 8 post-inoculation (PI). There were no significant differences among treatments for ADFI, ADG, G:F, rectal temperature, fecal consistency, pH, ammonia, short-chain fatty acids and lactic acid concentrations, cytokine TNF-α, Pig-MAP acute-phase proteins and histological parameters. However, both products were equally able to reduce colonization and shedding of Salmonella (P = 0.016 for PRO and BUT vs. CON). In addition, PRO treatment had a positive effect on behavioral displays, particularly exploring (P < 0.05 vs. CON), feeding (P < 0.05 vs. CON and BUT) and other active behaviors (P < 0.05 vs. CON and BUT) in the morning period (0830 to 1030 h). In the afternoon (1400 to 1600 h), the challenge effect was most significant. Pigs were less active after the challenge (P < 0.001), with a decrease in positive contacts (P = 0.004), exploration (P < 0.001) and feeding behaviors (P < 0.001) on d 3 PI, in comparison with before the challenge. Accordingly, many lying conducts increased at d 3 PI (P < 0.05). In conclusion, both treatments had positive effects against Salmonella, and behavior analysis appears to be a sensible tool to be considered.
Keywords: Bacillus licheniformis, behavior, feed additives, piglets, Salmonella Typhimurium, sodium butyrate
INTRODUCTION
Salmonella spp. is one of the worldwide leading causes of food-borne illnesses, contaminated pork being a significant source for human salmonellosis (EFSA, 2013; CDC, 2014). Control methods against this pathogen are needed (Andres and Davies, 2015) and probiotics and organic acids have been demonstrated to be potentially useful.
Bacillus species are widely used as probiotics in animal feeds (Cutting, 2011), with proven efficacy in vivo with porcine experimental models of salmonellosis (Spiehs et al., 2008; Walsh et al., 2012a; Ahmed et al., 2014). Alternatively, supplementation with organic acids or their salts has also been proposed as a possible tool to combat Salmonella in pigs (Creus et al., 2007) and butyrate's antimicrobial effects have been proven in vivo (Boyen et al., 2008).
To prove the efficacy of an in-feed additive against Salmonella is not easy. The best scenario would be to demonstrate its activity under natural conditions of disease, but this implies working with a high number of animals, considering that the fecal shedders may be low and intermittent (Scherer et al., 2008). Alternatively experimental models are used, although they also exhibit limitations for which sensible parameters are needed. Recently, it has been described how sickness behavior is a coordinated and adaptive response to illness (Weary et al., 2009) and in particular how Salmonella infections in pigs promote changes in animal behavior (Rostagno et al., 2011; Ahmed et al., 2015). This background suggests that behavior may be a good tool to be included in trials, potentially able to respond to treatment effects in animal health.
The objective of this work is, therefore, first to evaluate the efficacy of 2 different feed additives, a Bacillus licheniformis based probiotic and a partially protected sodium butyrate (SB) salt, using an experimental model of salmonellosis in pigs and, second, to explore if behavior analysis can be used as a sensible tool to evaluate in feed additives.
MATERIALS AND METHODS
The experiment was performed at the Experimental Unit of the Universitat Autònoma de Barcelona (UAB) and received prior approval (permit no. CEAAH1619) from the Animal and Human Experimental Ethical Committee of this institution. The treatment, management, housing, husbandry and slaughtering conditions conformed to European Union Guidelines (Directive 2010/63/EU, 2010).
Animals and Housing
The trial was conducted as a Level 2 High Risk Biosecurity Procedure, with appropriate training of the personnel involved. A total of 78 male piglets (Large White × Landrace) from a high-sanitary-status farm and from mothers serologically negative to Salmonella were used. Animals were weaned at 24 (± 4) days of age, 8.3 (± 0.32) kg BW on average, and were transported to the UAB facilities. From these animals, 72 were placed in 3 rooms of 8 pens each (24 pens, 3 animals per pen) taking initial BW into account for a similar average BW within pens. Each pen (2 m2) had a feeder and a water nipple to provide feed and water for ad libitum consumption. The weaning rooms were equipped with automatic heating, forced ventilation and an individual heat-light per pen. The experiment was conducted during the spring season (May), with an average room temperature of 28°C (± 4°C). The experimental treatments were distributed evenly among the 3 rooms. Regarding the 6 remaining piglets, they were allocated in 3 pens of a separate room (2 pigs per pen) being used as a negative control (NC) for the challenge model.
Experimental Products and Diets
All feed additives are commercially available and were supplied by Norel SA (Madrid, Spain): a partially protected SB based feed additive: 70% of active ingredient; 40% free and 30% protected with vegetable fats (Gustor BP70), designed with the objective of having active principle available all along the gastrointestinal tract (Mallo et al., 2012) and a probiotic with 109 cfu/g of Bacillus licheniformis (Proporc).
Diets (Table 1) were formulated to satisfy the nutrient requirement standards for pigs (NRC, 2012). All diets were manufactured in the same batch and treatments were included in a second mixture, on top, following the manufacturer's recommended dosages. There were 3 experimental diets: CON, control group with a plain diet without additives; PRO, a plain diet supplemented with 1 kg/t of Proporc (equivalent to 109 cfu of Bacillus licheniformis/kg of feed); and BUT, a plain diet supplemented with 3 kg/t of Gustor BP70 (equivalent to 2.1 g of partially protected SB salt/kg of feed).
Table 1.
Ingredient and nutrient composition of the experimental diets as-fed basis, g/kg
| Ingredients | Inclusion, g/kg |
|---|---|
| Maize | 280.8 |
| Wheat | 170.0 |
| Barley 2 row | 150.0 |
| Extruded soybean | 122.4 |
| Sweet wheypowder (cattle) | 100.0 |
| Fishmeal | 50.0 |
| Soybean meal 44 | 50.0 |
| Wheypowder 50% fat | 30.3 |
| Monocalcium phosphate | 21.3 |
| Calcium carbonate (CaCO3) | 8.2 |
| L-Lysine HCL | 4.5 |
| Vitamin-Mineral Premix1 | 4.0 |
| Sodium chloride (marine salt) | 3.0 |
| DL-Methionine 99 | 2.4 |
| L-Threonine | 2.3 |
| L-Triptophane | 0.9 |
| Analyzed composition | |
| DM | 898.5 |
| CP | 170.5 |
| CF | 51.1 |
| NDF | 100.5 |
| ADF | 35.8 |
| Ash | 64.2 |
Provided per kilogram of complete diet: 10,200 IU vitamin A, 2,100 IU vitamin D3, 39.9 mg vitamin E, 3 mg vitamin K3, 2 mg vitamin B1, 2.3 mg vitamin B2, 3 mg vitamin B6, 0.025 mg vitamin B12, 20 mg calcium panthotenate, 60 mg nicotinic acid, 0.1 mg biotin, 0.5 mg folic acid, 150 mg Fe, 156 mg Cu, 0.5 mg Co, 120 mg Zn, 49.8 mg Mn, 2 mg I, 0.3 mg Se.
Bacterial Strain
The bacterial strain used in the present study was a Salmonella Typhimurium var. Monophasic (formula: 4,5,12:i:-, resistance profile: ACSSuT-Ge, Fagotype: U302) that was isolated from a salmonellosis outbreak (mainly enteric and with sporadic septicemia) of fattening pigs in Spain, and was provided by the Infectious Diseases Laboratory (Ref. 301/99) of the UAB. The oral inoculum was prepared by 24 h incubation at 37°C in buffered peptone water (BPW; Thermo Fisher Scientific, Oxoid, Hampshire, UK) and diluted (1:20) with sterile phosphate buffered saline (PBS; Sigma-Aldrich, Madrid, Spain) to reach a final concentration of 2.5 × 108 cfu/ml.
Experimental Procedure
The duration of the study was 16 d, in which performance and clinical data were evaluated. Body weight was recorded on d 1, 8, 12, and 16, while feed consumption was recorded at d 1, 8, 11, 13, and 16. The ADG, ADFI and G:F were calculated by pen.
After 1 wk of adaptation to the diets (d 8), a single 2-mL dose (5 × 108 cfu) of Salmonella Typhimurium was administered to the challenged animals by oral gavage and a single 2-mL dose of sterile BPW to the non-challenged animals (challenge control group). Animals were checked daily for clinical signs to evaluate their status (i.e., dehydration, apathy and fecal score) after the Salmonella challenge, always by the same person. Fecal score was measured using a scale: 1 = solid and cloddy, 2 = soft with shape, 3 = very soft or viscous liquid and 4 = watery or with blood. Rectal temperature was assessed with a digital thermometer (Thermoval Rapid, Hartmann, Spain) on d 9 and 10 [1 and 2 post-inoculation (PI)]. Mortality rate was also recorded and no antibiotic treatment was administered to any of the animals of the experiment.
For microbiological analysis, on d 1 fecal samples were taken aseptically from 24 animals that were randomly selected from the total before distribution. Samples were taken after spontaneous defecation associated with the manipulation of the animal or by digital stimulation. On d 8, 9, 11, 13, and 15 (d 0, 1, 3, 5, and 7 PI), fecal samples were taken from the animal with the highest initial BW of each pen (N = 24).
At d 4 and 8 PI (Experimental d 12 and 16, respectively), 1 pig per pen was euthanized. On d 4 PI, the animal selected was the one with the intermediate initial BW, while on d 8 PI, the heaviest was selected. All NC animals were also euthanized at d 4 PI.
Animals were euthanized and sequentially sampled during the morning (between 0900 and 1200 h). Prior to euthanasia, a 10-mL sample of blood was obtained by venipuncture of the cranial vena cava using 10-mL tubes without anticoagulant (Aquisel, Madrid, Spain). Immediately after blood sampling, selected piglets received an intravenous lethal injection of sodium pentobarbital (200 mg/kg BW of Dolethal; Vetoquinol S.A., Madrid, Spain). Once dead, animals were bled, the abdomen was immediately opened and the whole gastrointestinal tract was excised.
Color range and consistency (on a scale ranging from 1 = liquid to 4 = semisolid) of intestinal contents, as well as the possible presence of fibrin, were recorded. Digesta (approximately 50 mL) from the ileum and proximal colon (considered to be 0.75 m from the ileocecal junction) was collected and homogenized. The pH of the contents was determined with a pH-meter calibrated on each day of use (Crison 52–32 electrode, Net Interlab, Barcelona, Spain) immediately after homogenization of the samples. Without delay, contents collected were subsampled and kept on ice all of the time. Colonic samples (1 g) were plated for Salmonella quantification the same day while samples for microbial counts of Bacillus licheniformis were kept refrigerated (4°C) and plated the following day. A set of ileal and colonic content samples were preserved in a H2SO4 solution (3 mL of content plus 3 mL of 0.2 N H2SO4) for ammonia (NH3) determination and were kept frozen at –20°C. An additional ileal and colonic sample set (approximately 20 g) was also frozen until analyzed for short-chain fatty acids (SCFA) and lactic acid.
For the histological study, 3-cm sections of the ileum were removed, opened longitudinally, washed thoroughly with sterile PBS and fixed by immersion in a 4% formaldehyde solution (Carlo-Erba Reagents, Sabadell, Spain).
Blood samples were centrifuged (3000 × g for 15 min at 4°C) after 4 h refrigeration, and the serum obtained was divided into different aliquots and stored at –20°C to evaluate immune response.
Analytical Procedures
Chemical analyses of the diets including DM, ash, CP and diethyl ether extract, were performed according to the Association of Official Agricultural Chemists standard procedures (AOAC International, 1995). NDF and ADF were determined according to the method of Van Soest et al. (1991).
Bacillus licheniformis was analyzed by traditional microbiology to determine probiotic colonization. Three grams of sample were diluted into 300 mL of sterile saline solution (0.9%) + Tween 80 (0.4%) and homogenized in a sterile mincer, resulting in a starting dilution 10–2 from the original sample. The diluted sample was homogenized by stirring at 10,000 rpm for 1 min, and afterward treated at 80°C for 1 min to keep only the spore forms. Further decimal dilutions were done (up to 10–6). Dilutions were plated in Triptic Soy Agar (Biokar Diagnostics, France), incubated for 48 h at 30°C and manual cell counting was performed.
In digesta, NH3 concentrations were determined with the aid of a gas-sensitive electrode (Hatch Co., Loveland, Colorado) combined with a digital voltmeter (Crison GLP 22, Crison Instruments, S.A., Barcelona, Spain). Three grams of acidified content were diluted (1:2) with 0.16M NaOH, after homogenization samples were centrifuged (1500 × g) for 10 min. The ammonia released was measured in the supernatants as different voltages in mV according to a procedure previously described in Hermes et al. (2009) that was adapted from Diebold et al. (2004). The SCFA and lactic acid analyses were performed by gas chromatography. The samples were submitted to an acid-base treatment followed by an ether extraction and derivatization with N-(tertbutyldimethylsilyl)-N-methyl-trifluoroacetamide plus 1% tert-butyldimethylchlorosilane agent, using the method of Richardson et al. (1989), modified by Jensen et al. (1995). For Salmonella bacteria counts, all samples were transferred (1:10) to BPW. Quantitative assessment was made by seeding the serial dilutions 10–2, 10–4, and 10–6 of the samples in Xylose-Lactose-Tergitol-4 (XLT4) plates (Merck, Madrid, Spain). The qualitative assessment was made by incubating samples in BPW (37°C, 24 h), transferring them to Rappaport-Vassiliadis enrichment broth (Thermo Fisher Scientific, Oxoid, Hampshire, UK) for a second incubation (42°C, 48 h) and seeding them in XLT4 plates to observe H2S positive colonies.
Tissue samples for morphological measures were dehydrated and embedded in paraffin wax, sectioned to a 4-µm thickness and stained with hematoxylin and eosin. Measurements of 10 different villous-crypt complexes per sample were performed with a light microscope (BHS, Olympus, Barcelona Spain) using the technique described in Nofrarías et al. (2006). Serum concentrations of Tumor Necrosis Factor-α (TNF-α) were determined by Quantikine Porcine TNF-α kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Pig major acute-phase protein (Pig-MAP) concentration was determined by a sandwich-type ELISA (Pig MAP Kit ELISA, Pig CHAMP Pro Europe S.A., Segovia, Spain) as described in Saco et al. (2011). Serological antibodies of Salmonella were tested by ELISA Salmonella Herdcheck (Idexx laboratories, Hoofddorp, Netherlands), and the cut-off for positivity was established at optic density ≥ 40%.
Behavior Analysis
Behavioral measures were assessed applying the scan-sampling methodology found in the Welfare Quality Assessment protocol for pigs (Welfare Quality, 2009) complemented with parameters observed in Escobar et al. (2007) and Temple et al. (2011). Active (positive + negative + exploration + feeding + drinking + walking + others) and inactive (lying laterally or ventrally and with or without contact with littermates) behaviors were recorded. An explanation of the recorded behaviors during the scan sampling is given in Table 2.
Table 2.
Summary of recorded behaviors during the scan-sampling
| Behaviors | Explanation |
|---|---|
| Active behavior | |
| Negative social behavior | Aggressive behavior, including biting or any social behavior with a response from the disturbed animal. |
| Positive social behavior | Sniffing, nosing, licking and moving gently away from the animal without an aggressive or flight reaction from this individual. |
| Exploration of the pen | Sniffing, nosing, licking all features of the pen. |
| Feeding | Pig with the head in the feeder. |
| Drinking | Pig with the mouth at the nipple. |
| Walking | Two steps were minimum requirements to configure walking. |
| Other | Other active behaviors not cited (defecation, urination, air sniffing, etc.). |
| Inactive behavior | |
| Lying laterally without contact | Resting lying laterally without contact or with less than half of their body in contact with other pen mates. |
| Lying ventrally without contact | Resting lying ventrally without contact or with less than half of their body in contact with other pen mates. |
| Lying laterally with contact | Resting lying laterally with more than half of their body in contact with other pen mates. |
| Lying ventrally with contact | Resting lying ventrally with more than half of their body in contact with other pen mates. |
Two observers, who were blind to treatments, performed the direct visual observations using a focal sampling technique (Lehner, 1998). To minimize differences between observers and to standardize the behavioral observations, the observers followed an identical previous training. Behaviors were recorded during 2 periods (0830 to 1030 h and 1400 to 1600 h) in a 14 h light phase (7.30 h to 21.30 h) on d 2 and 1 before the inoculation (Experimental d 6 and 7) and d 1, 2, and 3 PI (Experimental d 9, 10, and 11). Behaviors recorded before and after the oral challenge were compared to determine the challenge effect. Space data collection was performed together with behavior analysis using scan-sampling to evaluate the preference of pigs to use different areas of the pen: water nipple, heat-light and feeder area.
During the observation period, the frequency of times engaged in each behavior was registered at 2-min sampling intervals. Each pen received 20 scans every day in the morning and the afternoon period. Throughout the experimental period a total of 4,777 scans were made.
Statistical Analysis
The experiment was conceived as a complete randomized design that included 3 treatments (CON, PRO and BUT). Results are expressed as means with their standard errors unless otherwise stated. The general linear and mixed models of SAS (SAS Inst. Inc., Cary, NC) were used to analyze the effect of experimental treatments except on microbiological data, where frequencies of positive animals were analyzed as contingency tables with Fisher's exact test.
For behavioral records, no differences were found between d –2 and –1 in relation to infection, so these data were pooled and named as –1. The time spent in different behaviors was expressed in proportion of the total number of observations (active + inactive animals) and transformed using a square-root transformation.
When treatment effects were established, treatment means were separated using the probability of differences function adjusted by Tukey–Kramer. The pen was considered as the experimental unit for analysis, and random effect was used to account for variation between pens. The α-level used for the determination of significance for all of the analysis was P = 0.05. The statistical trend was also considered for P < 0.10.
RESULTS
Adaptation to Diets and Challenge Response
In general, the animals used in the study showed a good state of health at the beginning of the trial, none of the animals seeded Salmonella spp. on their arrival and no signs of diarrhea throughout the first week of adaptation to the diets were registered.
Bacillus licheniformis was only recovered in countable numbers from animals in the PRO group, with a mean concentration of 1.8 ± 0.47 × 105 cfu/g for ileal content and 5.0 ± 1.15 × 105 cfu/g for colonic content.
Regarding the experimental model of salmonellosis, the NC group was compared to the challenged CON group at d 4 PI. After the challenge, animals showed a mild course of diarrhea, and the NC group showed numerically lower fecal scores, in comparison to the infected CON group (1.9 ± 0.44 vs. 2.3 ± 0.27, P = 0.225). Although none of the challenged groups reached fever levels, the NC group had a lower body temperature than did the challenged CON, specially manifested at 24 h PI (39.1°C ± 0.11°C vs. 38.5°C ± 0.17°C, P = 0.037). As expected, none of the NC animals seeded Salmonella in feces. Although no significant differences were recorded in TNF-α serological concentrations, Pig-Map tended to be significantly lower in the NC group, in comparison to the CON group (0.63 ± 0.158 mg/dl vs. 1.07 ± 0.137 mg/dl, P = 0.060).
Histological parameters did not differ except concerning a tendency for the NC group for higher villous height in the ileum, in comparison to the CON group (287 ± 16.7 µm vs. 248 ± 14.5 µm; P = 0.100). Moreover, no significant differences were found between these 2 groups regarding microbial activity (pH, ammonia, SCFA and lactic acid concentration; data not shown).
Effects on Performance, Intestinal Environment and Immune Response
ADFI, ADG, and G:F mean values for the different challenged groups are shown in Table 3. No significant differences were detected in animal performance parameters related to the use of the in-feed additives. No relevant changes were seen either in rectal temperature, fecal, ileal and colonic consistency or presence of fibrin (data not shown).
Table 3.
Performance of piglets fed the experimental diets and orally challenged with Salmonella Typhimurium at d 8
| Treatments1 | |||||
|---|---|---|---|---|---|
| Item | CON | PRO | BUT | SEM2 | P-value |
| BW, kg | |||||
| Initial | 8.3 | 8.2 | 8.3 | 0.05 | 0.172 |
| Final | 11.0 | 10.2 | 10.6 | 0.69 | 0.725 |
| ADFI, g/d | |||||
| Pre-inoculation3 | 191 | 185 | 184 | 16.2 | 0.941 |
| Post-inoculation4 | 360 | 336 | 385 | 46.1 | 0.759 |
| ADG, g/d | |||||
| Pre-inoculation | 67.5 | 38.0 | 37.5 | 30.28 | 0.734 |
| Post-inoculation | 243 | 198 | 208 | 54.30 | 0.835 |
| G:F | |||||
| Overall5 | 0.62 | 0.48 | 0.46 | 0.136 | 0.668 |
Treatments: CON, plain diet without additives; PRO, plain diet supplemented with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet supplemented with 3 kg/t of Gustor BP70 (2.1g of partially protected sodium butyrate salt/kg of feed).
Pooled SEM; n = 8/treatment.
Experimental d 0 to 7.
Experimental d 8 to 16 (0 to 8 PI).
Experimental d 1 to 16.
Fecal prevalence of Salmonella spp. in feces (or colon digesta for d 8 PI) after the oral inoculation is shown in Fig. 1. Significant differences among groups were seen on d 0 (P = 0.033) and 8 PI (P = 0.043). When analyzing the Salmonella prevalence in the complete experimental period, both treatments succeeded in reducing the number of positive animals significantly (P = 0.016 for PRO and BUT) in relation to the control ones.
Figure 1.
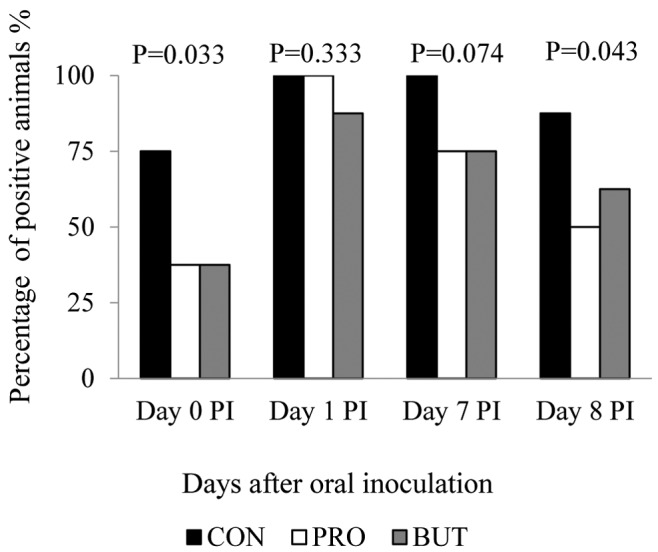
Percentage of Salmonella shedders along the post-inoculation (PI) period. Percentage of animals (n = 8) that showed Salmonella in feces at d 0, 1, 7 post-inoculation (PI) or colon digesta at d 8 PI. P-values obtained by Fisher's exact test. Treatments: CON, plain diet without additives; PRO, plain diet with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet with 3 kg/t of Gustor BP70 (2.1 g of partially protected sodium butyrate salt/kg of feed).
Despite the fact that most of the tested animals were positive in feces for Salmonella along the study, only 8% of the animals reached quantitative levels (> 103cfu/g). No significant differences were observed among experimental groups in the frequency of quantifiable shedders or in the amount of Salmonella found in feces in the quantifiable animals (data not shown).
Table 4 shows pH values and fermentation products in the colon. No significant differences were found in pH values, ammonia, SCFA or lactic acid concentrations between treatments. Regarding the molar proportions of SCFA, no significant differences between treatments were found (data not shown), although at d 4 PI the molar proportion of butyric acid was numerically higher in the BUT group (14.0% ± 1.69%, 10.8% ± 1.69%, and 16.0% ± 1.80% for CON, PRO, and BUT, respectively; P = 0.121).
Table 4.
Colonic pH values, ammonia concentration and fermentation products for d 4 and 8 post-inoculation (PI)
| Item | Days PI | Treatment1 | SEM2 | P-value | ||
|---|---|---|---|---|---|---|
| CON | PRO | BUT | ||||
| pH | 4 | 5.89 | 6.13 | 6.09 | 0.120 | 0.327 |
| 8 | 6.04 | 5.90 | 6.01 | 0.086 | 0.478 | |
| NH3,mM | 4 | 19.5 | 19.8 | 19.4 | 1.99 | 0.990 |
| 8 | 55.2 | 46.2 | 50.9 | 4.93 | 0.447 | |
| Lactic Acid, mmol/kg | 4 | 7.45 | 4.64 | 0.99 | 2.458 | 0.225 |
| 8 | 1.75 | 2.49 | 0.94 | 0.904 | 0.492 | |
| Total VFA, mmol/kg | 4 | 119.3 | 104.4 | 119.2 | 8.71 | 0.405 |
| 8 | 129.6 | 115.8 | 129.4 | 7.85 | 0.381 | |
Treatments: CON, plain diet without additives; PRO, plain diet supplemented with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet supplemented with 3 kg/t of Gustor BP70 (2.1g of partially protected sodium butyrate salt/kg of feed).
Pooled SEM; n = 8/treatment.
All of the animals euthanized remained serologically negative to Salmonella along the study. No change was detected in the mean levels of pro-inflammatory cytokine TNF-α (91.1 ± 7.08 pg/ml, 98.2 ± 7.08 pg/ml and 97.6 ± 7.08 pg/ml for CON, PRO and BUT, respectively; P = 0.532) nor of acute phase protein Pig-MAP (1.81 ± 0.175 mg/dl, 2.09 ± 0.175 mg/dl and 1.92 ± 0.175 mg/dl for CON, PRO, and BUT, respectively; P = 0.737).
The results of histological analysis revealed no significant differences among the experimental groups despite a trend for BUT treatment on d 4 PI to increase crypt depth, when compared with the CON group (P = 0.104). Histological measurements at d 4 PI are shown in Table 5.
Table 5.
Histological determinations in ileum on d 4 PI
| Treatments1 | |||||
|---|---|---|---|---|---|
| Determinations | CON | PRO | BUT | SEM2 | P-value |
| Villous height, μm | 248 | 291 | 275 | 18.6 | 0.267 |
| Crypt Depth, μm | 203 | 238 | 251 | 15.9 | 0.107 |
| Villus:Crypt Ratio | 1.24 | 1.25 | 1.13 | 0.101 | 0.682 |
| IEL3,/100 µm | 1.10 | 0.94 | 1.30 | 0.224 | 0.535 |
| GC4,/100 µm | 1.19 | 0.92 | 1.23 | 0.170 | 0.384 |
| Mitosis5,/100 µm | 0.33 | 0.30 | 0.29 | 0.053 | 0.832 |
Treatments: CON, plain diet without additives; PRO, plain diet supplemented with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet supplemented with 3 kg/t of Gustor BP70 (2.1g of partially protected SB salt/kg of feed).
Pooled SEM; n = 8/treatment.
IEL = Villous intraepithelial lymphocytes;
GC = Villous goblet cells/100 µm;
Number of mitosis in crypts.
Effects on Behavior Analysis
Table 6 shows the mean frequencies of behaviors and use of spaces recorded during the scans in the morning and afternoon. Changes between these 2 periods were found, the sum of active behaviors being 51.5% in the morning vs. 29.3% in the afternoon. Differences in pig expression of inactive behaviors between morning and afternoon were recorded too, with the lateral and ventral lying consuming about 70% of the time in the afternoon vs. 48% in the morning. Regarding the use of spaces, the use of the feeder area was higher in the morning (41.8% for the morning vs. 31.4% for the afternoon) and of the light area in the afternoon (53.8% for the morning vs. 64.1% for the afternoon). The water nipples area use was similar in both periods. Due to the different pattern found in behaviors between morning and afternoon periods (0830 to 1030 h and 1400 to 1600 h), possible changes related to treatments were analyzed separately.
Table 6.
General descriptive statistics of behaviors and use of the space (pooled values of d –2, –1, +1. +2 and +3 post-inoculation) recorded during morning (0830 to 1030 h) and afternoon (1400 to 1600 h)1
| Observations | Morning | Afternoon |
|---|---|---|
| Behaviors, % | ||
| Positive | 4.2 ± 0.44 | 2.6 ± 0.32 |
| Negative | 2.0 ± 0.29 | 0.5 ± 0.18 |
| Exploration | 5.5 ± 0.55 | 2.2 ± 0.26 |
| Feeding | 30.7 ± 1.82 | 18.4 ± 1.79 |
| Drinking | 2.3 ± 0.22 | 1.7 ± 0.22 |
| Walking | 1.6 ± 0.18 | 1.2 ± 0.14 |
| Others | 5.2 ± 0.42 | 2.7 ± 0.31 |
| Lying laterally without contact | 2.3 ± 0.44 | 3.4 ± 0.63 |
| Lying ventrally without contact | 5.5 ± 0.60 | 7.0 ± 0.77 |
| Lying laterally with contact | 11.5 ± 1.32 | 20.6 ± 1.72 |
| Lying ventrally with contact | 28.7 ± 2.16 | 39.2 ± 2.39 |
| Use of the space, % | ||
| Feeder area | 41.8 ± 2.16 | 31.4 ± 2.50 |
| Light area | 53.8 ± 2.29 | 64.1 ± 2.62 |
| Drinker area | 4.4 ± 0.38 | 4.8 ± 0.43 |
Means values ± SEM of untransformed data (n = 120).
Behaviors in the Morning:
The general expression of pig behaviors in the morning period is summarized in Table 7.
Table 7.
Pig behavior expressions in the morning (0830 to 1030h) according to dietary treatment and days in relation to challenge1
| Behavior expressions | Days in relation to challenge | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| -12 | 1 | 2 | 3 | Total | TRT3 | DAY4 | TRT × DAY | |
| Positive contacts: | ||||||||
| CON | 1.96 c | 1.59 c | 5.95 a,b | 0.77 c | 2.22 | 0.142 | 0.038 | 0.025 |
| PRO | 8.29 a | 2.02 b,c | 3.88 a,b,c | 2.13 b,c | 3.72 | |||
| BUT | 2.28 b,c | 1.82 b,c | 1.42 c | 2.34 b,c | 1.96 | |||
| Total | 3.72A | 1.82B | 3.50A,B | 1.66B | ||||
| Negative contacts: | ||||||||
| CON | 0.61 | 0.58 | 3.39 | 0.67 | 1.10 | 0.571 | 0.080 | 0.563 |
| PRO | 0.86 | 0.06 | 2.04 | 1.02 | 0.81 | |||
| BUT | 1.04 | 0.30 | 0.61 | 0.52 | 0.59 | |||
| Total | 0.83 | 0.27 | 1.82 | 0.72 | ||||
| Exploration: | ||||||||
| CON | 2.13 | 2.69 | 4.49 | 1.42 | 2.56 B | 0.050 | 0.347 | 0.265 |
| PRO | 8.94 | 2.56 | 4.93 | 5.71 | 5.29 A | |||
| BUT | 4.41 | 3.65 | 1.72 | 2.19 | 2.89 A,B | |||
| Total | 4.80 | 2.96 | 3.53 | 2.86 | ||||
| Feeding: | ||||||||
| CON | 11.49 | 26.63 | 28.30 | 25.00 | 22.28B | 0.003 | 0.030 | 0.850 |
| PRO | 29.05 | 33.87 | 55.06 | 34.81 | 37.58A | |||
| BUT | 19.45 | 20.52 | 30.47 | 19.18 | 22.18B | |||
| Total | 19.27 B | 26.73 A,B | 37.09 A | 25.91 A,B | ||||
| Others: | ||||||||
| CON | 3.06 | 4.71 | 3.46 | 1.23 | 2.96 B | 0.039 | 0.192 | 0.966 |
| PRO | 5.95 | 5.90 | 6.60 | 3.72 | 5.48 A | |||
| BUT | 3.84 | 2.86 | 4.24 | 2.28 | 3.28 B | |||
| Total | 4.20 | 4.41 | 4.67 | 2.28 | ||||
| Lying laterally without contact: | ||||||||
| CON | 0.86 | 1.00 | 0.81 | 1.06 | 0.92 | 0.821 | 0.448 | 0.967 |
| PRO | 1.44 | 0.41 | 0.46 | 1.77 | 0.92 | |||
| BUT | 1.08 | 0.37 | 0.21 | 1.02 | 0.61 | |||
| Total | 1.12 | 0.56 | 0.46 | 1.25 | ||||
| Lying ventrally without contact: | ||||||||
| CON | 1.80 | 3.96 | 4.41 | 6.66 | 4.00 | 0.937 | 0.001 | 0.887 |
| PRO | 2.02 | 1.99 | 2.92 | 9.80 | 3.69 | |||
| BUT | 2.28 | 4.45 | 3.24 | 7.56 | 4.16 | |||
| Total | 2.02 B | 3.39 B | 3.50 B | 7.95 A | ||||
| Lying laterally with contact: | ||||||||
| CON | 12.46 | 4.97 | 7.90 | 9.55 | 8.53 A | < 0.001 | 0.258 | 0.946 |
| PRO | 3.88 | 2.37 | 0.64 | 1.00 | 1.77 B | |||
| BUT | 13.03 | 4.93 | 9.00 | 12.18 | 9.49 A | |||
| Total | 9.24 | 4.00 | 4.84 | 6.40 | ||||
| Lying ventrally with contact: | ||||||||
| CON | 31.47 | 38.81 | 25.30a | 24.21 | 29.70 A | 0.008 | 0.249 | 0.683 |
| PRO | 10.11 | 21.44 | 5.52b | 21.90 | 13.76 B | |||
| BUT | 17.64 | 29.92 | 26.52a | 30.69 | 25.91 A | |||
| Total | 18.75 | 29.59 | 17.47 | 25.40 | ||||
LSmeans within rows without common letters differ by the Means-Tukey adjustment test (P < 0.05).
LSmeans within columns without common letters differ by the Means-Tukey adjustment test (P < 0.05).
Values expressed are a proportion of total number of observations (active + inactive). For the statistical analysis, data were previously transformed using square root transformation. Drinking and walking behaviors are not shown, as they were not modified by any experimental treatment.
No differences were found between d –2 and –1 before the inoculation, so these data were pooled and named as –1.
Treatment effect. Treatments: CON, plain diet without additives; PRO, plain diet supplemented with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet supplemented with 3 kg/t of Gustor BP70 (2.1g of partially protected sodium butyrate salt/kg of feed).
Inoculation effect (measured as difference between days before (–1) and after (1, 2, 3) the challenge).
Challenge effects (evaluated as day effect) were less evident in the morning than in the afternoon. No interaction between day and treatment was found for any variable except for positive contacts (P = 0.025). Despite this, a general significant time effect could be seen during the morning on the time spent feeding, with values that increased on d 2 PI (P = 0.004), and also in the time lying ventrally without contact, that also increased on d 3 PI (P < 0.001).
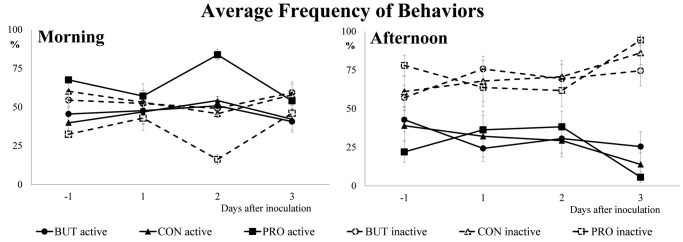
Regarding the effect of the treatments on morning behaviors, pigs supplemented with PRO spent more time feeding (P = 0.003 for CON and P = 0.002 for BUT), exploring the pen (P = 0.026 for CON and P = 0.054 for BUT) and with other active behaviors (P = 0.018 for CON and P = 0.040 for BUT). Furthermore, the total time lying ventrally or laterally with contact was significantly lower in pigs fed the PRO diet, in comparison with others treatments (P = 0.004 and P = 0.017 vs. CON, P < 0.001 and P < 0.001 vs. BUT; for total time lying ventrally and laterally, respectively). These effects were seen not only after, but also during the days previous to the challenge. When the data for different lying behaviors were analyzed within the total resting time, there was no significant effect of treatments nor challenge or interaction between factors for time spent lying ventrally and lying laterally. Considering all the active or inactive behaviors together, the occurrences of active as well as inactive ones were significantly higher and lower in the PRO group's treatments (P = 0.008 and P = 0.006 vs. CON, P < 0.001 and P < 0.001 vs. BUT in active and inactive behaviors). A graphic display of the animals in active or inactive behaviors can be seen in Fig. 2.
Figure 2.
Frequency of animals with active and inactive behaviors in the morning (0830 to 1030h) and afternoon (1400 to 1600 h), before and after the oral inoculation of the pathogen, for the different dietary treatments. Each data point represents a mean value (n = 8). Treatments: CON, plain diet without additives; PRO, plain diet with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet with 3 kg/t of Gustor BP70 (2.1g of partially protected sodium butyrate salt/kg of feed). Active = positive + negative + exploration + feeding + drinking + walking + others. Inactive = lying laterally or ventrally, with or without contact with pen mates.
In relation to the use of space (data not shown), pigs supplemented with PRO used the feeding area more frequently (30.7%, 36.1% and 50.7% for CON, BUT and PRO, respectively; P < 0.001 for PRO vs. CON and P = 0.012 for PRO vs. BUT) and the lying area less time (57.3%, 53.1% and 36.7% for CON, BUT and PRO, respectively; P < 0.001 for PRO vs. CON and BUT) than with the other treatments. No effect of treatments and challenge on the frequency of pigs in the drinker area was observed.
Behavior in the Afternoon:
In contrast to the morning, in the afternoon the factor with most effect on pig behavior was the challenge (days in relation to the challenge). As can be seen in Fig. 2, pigs were more active before the pathogen inoculation in comparison with d 3 PI (P < 0.001).
Table 8 summarizes the expression of behaviors of pigs in the afternoon. Because treatments had little effect on behavior at this time of the day, only the mean values for the different days are presented. The only difference found related to treatments was a less frequent behavior of lying laterally without contact for the animals on the PRO and BUT, in comparison to the CON groups (3.49%, 0.90% and 1.08% for CON, BUT and PRO, respectively; P < 0.001 for PRO vs. CON and BUT).
Table 8.
Expression of behaviors of pigs in the afternoon (1400 to 1600 h) according to days in relation to challenge1,2
| Days in relation to challenge | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Observations | -13 | +1 | +2 | +3 | TRT4 | DAY5 | TRT × DAY |
| Positive contacts | 1.61A | 1.25A | 1.90A | 0.23B | 0.632 | 0.015 | 0.894 |
| Exploration | 1.99A | 0.85B,C | 1.06A,B | 0.18C | 0.681 | 0.002 | 0.740 |
| Feeding | 15.60A | 11.29A | 13.18A | 2.96B | 0.786 | 0.003 | 0.159 |
| Others | 1.74 | 1.12 | 2.22 | 0.45 | 0.648 | 0.063 | 0.817 |
| Lying laterally without contact | 0.62B | 0.74B | 3.35A | 2.76A | 0.040 | 0.002 | 0.497 |
| Lying ventrally without contact | 2.66B | 2.89B | 7.95A | 7.73A | 0.835 | < 0.001 | 0.875 |
| Lying laterally with contact | 21.16A | 8.70B | 13.84A | 13.69A | 0.528 | 0.038 | 0.731 |
| Lying ventrally with contact | 30.14B | 37.58AB | 27.35B | 45.02A | 0.399 | 0.035 | 0.541 |
Means within rows without common letters differ by the Means-Tukey adjustment test (P < 0.05).
Data are mean (n = 8) expressed in proportion of total number of observations (active + inactive). For the statistical analysis, data were previously transformed using square root transformation.
Drinking and walking behaviors are not shown, as they were not modified by any experimental treatment.
No differences were found between d –2 and –1 before the inoculation, so these data were pooled and named as –1.
Treatment effect. Treatments: CON, plain diet without additives; PRO, plain diet supplemented with 1 kg/t of Proporc (109 cfu/kg of feed of Bacillus licheniformis); BUT, plain diet supplemented with 3 kg/t of Gustor BP70 (2.1g of partially protected sodium butyrate salt/kg of feed).
Inoculation effect (measured as difference in days before (–1) and after (1, 2, 3) the infection).
However, many significant differences were found related to the experimental days. Positive contacts, exploration and feeding behaviors (P = 0.004, P < 0.001 and P < 0.001, respectively) were less frequent on d 3 PI, in comparison to the period before the inoculation. Lying laterally without contact and lying ventrally without contact increased on d 2 and 3 PI, in comparison with before inoculation (P < 0.050). Lying ventrally with contact also increased after inoculation but only on d 3 PI (P = 0.016).
In relation to the use of different areas (data not shown), in the afternoon the use of the feeder space decreased significantly (33.4% on d –1; 16.6% on d 1 and 9.6% on d 3 PI; P = 0.016 for d –1 vs. 1 PI and P < 0.001 for d –1 vs. 3 PI) and the use of the lying area with light increased significantly (54.6% on d –1 and 71.7% on d 3 PI; P = 0.040) after the challenge. No significant differences were found concerning the use of the drinker area related to the experimental treatments.
DISCUSSION
The Salmonella Typhimurium oral challenge resulted in an effective infection, as nearly all challenged animals excreted Salmonella after the oral inoculation, with the NC animals remaining negative until the end of the trial.
Experimental models of salmonellosis in pigs found in the literature have very variable responses. Whereas some authors report models with mild infectuous outcomes (Fraser et al., 2007; Szabó et al., 2009; Walsh et al., 2012a, 2012b), others report acute clinical responses (Balaji et al., 2000; Spiehs et al., 2008), and even responses that surpassed treatment capacity (Casey et al., 2007). These differences may be primarily attributed to different challenge dosages and different virulence of the strains. In our case, a mild outcome was planned to evaluate a treatment administered as a feed additive, and we succeeded by achieving minor clinical signs. The animals never stopped eating, and some even became negative in feces at the end of the trial.
Unexpectedly, after the first adaptation week, some animals from the challenged groups started to excrete Salmonella in feces (Fig. 1) at low levels (below countable numbers < 103 cfu/g). It should be considered that in the 3 rooms in which the challenged groups were allocated, there had previously been another trial with Salmonella (same strain) and although the facilities were cleaned and disinfected, it could be possible that an ambient load could have remained. Low concentrations of Salmonella in the environment (102 to 103 cfu) have been reported as being able to infect the exposed animals (Hurd et al., 2001; Boughton et al., 2007). The fact that all fecal samples were negative for Salmonella on their arrival, and that all euthanized piglets remained sero-negative at the end of the study, reaffirms that these animals were not previously exposed to the pathogen in the farm of origin (Nielsen et al., 1995). Moreover, the NC group allocated in a room not previously used for Salmonella challenges remained negative along all the study.
In-feed Additive Effects in the Animal´s Response to Salmonella
The inclusion of the Bacillus licheniformis probiotic in the feed did not show significant effects on performance parameters. Other authors, however, have demonstrated positive effects of different Bacillus spp. based probiotics on performance (Alexopoulos et al., 2004; Link and Kováč, 2006). A possible explanation for these positive results could be the production of enzymes by the germinated spores in the gut, like proteases, lipases, or amylases, that might help the digestion of nutrients from feed (Link and Kováč, 2006). Nevertheless, under challenge conditions, these positive effects are probably precluded by the limited number of replicates and the high individual variation in the clinical response. Similar results have been obtained by other authors testing different Bacillus spp. based probiotics under challenge conditions who were not able to find differences in performance (Dänicke & Döll, 2010; Walsh et al., 2012a,b).
Our results demonstrate, as in Leser et al. (2008), the good viability of the Bacillus spp. bacteria in the gut. Actually, the cfu concentration found in the gut (between 2 and 5 105 cfu/g) would reflect that most of the administered spores were viable, considering that the in-feed doses were of 106 cfu/g and that dry-matter content of digesta usually vary by around 10% to 20%. Some authors have even demonstrated, in murine models, higher number of spores excreted in the feces than in the original inoculum, suggesting the ability of the administered spores to germinate and sporulate in the gut (Hoa et al., 2001).
The ability to stimulate the immune response has been largely attributed to Bacillus spp. probiotics (Duc et al., 2004), although very little information is available on the particular strain we tested. In our study, pro-inflammatory cytokine TNF-α and acute-phase protein Pig-MAP were not affected by the probiotic treatment, similar to Walsh et al. (2012a), who did not find any differences in serum TNF-α levels after the administration of a Bacillus licheniformis and B. subtilis combination. However, the absence of effects in these 2 broad indexes does not discard the possible effects of this probiotic on the immune response, as Bacillus spp. stimulation effects have been widely reported (Arena et al., 2006; Skjolaas et al., 2007; Walsh et al., 2012a).
In relation to Salmonella shedding, a significant reduction in the percentage of animals positive to Salmonella was seen when considering the overall PI period for the PRO group. Other authors have also described the potential of Bacillus spp. to reduce bacterial loads of Salmonella or E. coli after a challenge (Ahmed et al., 2014). In contrast, Spiehs et al. (2008) and Walsh et al. (2012a) did not see any effective reduction in Salmonella shedding using a B. licheniformis and B. subtilis combination in piglets. Hence, it is important to bear in mind that the effect of probiotic bacteria can depend on the particular strain tested.
Regarding the inclusion of sodium butyrate in pig diets, the BUT treatment did not significantly influence performance. This lack of effects could also be due to the reasons stated above related to the limited number of replicates in this kind of controlled disease models. There are many authors who have found performance improvements in non-challenge trials with post-weaning piglets (Kotunia et al., 2004; Manzanilla et al., 2006; Lu et al., 2008; Piva et al., 2010), although others reported no performance differences (Biagi and Piva, 2007; Mallo et al., 2012; Fang et al., 2014).
Inconsistencies found in the bibliography may be due to, among other factors, the form of presentations of the butyric acid. In some studies it is used as a free acid, but commonly it is used as its salt form (sodium or calcium salts with different solubility) to make handling easier. Also, these butyric salts can be protected with a fat coating or be combined with glycerol in esters of butyric acid, trying to delay its absorption in the gastrointestinal tract and its release along the intestine (Mallo et al., 2012). The butyrate source used in this experiment was a partially protected sodium butyrate salt that was expected to be released slowly in the gut and reach distal sections, but no significant differences in butyric acid concentrations were seen in the colon.
Different effects on the animals have been potentially attributed to butyrate. It is an important energy source for intestinal epithelial cells and plays a role in the maintenance of colonic homeostasis. Among other functions, butyrate exerts potent effects throughout inhibiting inflammation and reinforcing various components of the defense barrier (Hamer et al., 2008). In response to a Salmonella challenge, it could be expected that the inclusion of butyrate in the diets would have better preserved the integrity of the intestinal architecture. In this regard, Jerzsele et al. (2012) and Chamba et al. (2014), who tested the same source of butyrate in challenged and non-challenged trials on broilers, found positive effects on the villus and crypt architecture (such as an increase in villus length, or villus:crypt ratio). In our study, although we were not able to demonstrate significant modifications, we found a trend in the ileal crypt depth to be increased at d 4 PI.
Other positive effects of butyrate could be related to its ability to be transformed into butyric acid (combined with a hydrogen ion) and potentially influence the intestinal environment with antimicrobial activity. In our study, BUT treatment did improve Salmonella shedding, in consonance to Fernández-Rubio et al. (2009), who tested the same butyrate source and found a significant reduction of Salmonella Enteritidis in a broiler challenge model. These results agree with those of many other authors who demonstrated the benefits of sodium butyrate in challenges with Salmonella in vitro (Gantois et al., 2006; Boyen et al., 2008), in poultry (Van Immerseel et al., 2005) and in piglet models (Boyen et al., 2008). This bactericidal effect could be attributed to the undissociated form of newly formed butyric acid, which can penetrate a bacterial cell wall and dissociate to H+ and anions inside the cell, lowering intracellular pH and resulting in energy deficiency and osmotic problems in the microbial organism (Gálfi and Bokori, 1990; Warnecke and Gill, 2005).
In summary, both additives exerted a similar positive effect on the shedding of Salmonella, with a higher number of pigs that turned negative in feces for the pathogen at the end of the study, as compared with the control. It is also interesting to point out the differences seen for both experimental treatments after the first week of adaptation, when the animals were presumably exposed to a low environmental load of the pathogen (see comment above). In this case, the percentage of positive animals were reduced from 75 to 37.5%, suggesting that both additives would be specially effective when animals are exposed to low doses of Salmonella during prolonged times, the most frequent event in practical conditions.
Behavior Analysis for In-Feed Additive Evaluation
In this assay animal behavior was registered during the morning and afternoon. From the results shown, the best observational period to detect differences related to the diets resulted in being the morning, whereas the afternoon mostly showed effects due to the Salmonella challenge, assuming that changes observed between days are related to the infective process.
Sickness behavior, as reviewed by Weary et al. (2009), is an adaptive response of the animals to illness and some particular behaviors could be valid indicators to identify disease and discomfort of the animals. It is hypothesized that behaviors most likely to decline are those that provide long-term fitness benefits (such as playing), as animals divert resources to those functions of critical short-term survival such as maintaining body temperature. In our experiment, we were able to detect sickness behavior in the afternoon, when animals were more motivated to perform extra activities other than feeding, and these activities underwent a gradual reduction that reached statistical significance at d 3 PI. Similar effects were also described by Rostagno et al. (2011), who hypothesized that infection would cause the pigs to feel sick, and thus be less willing to express investigative behavior. Moreover, Escobar et al. (2007) observed that resting animals may assume postures that conserve heat when sick; they found pigs infected with the porcine reproductive and respiratory syndrome virus spent more time in ventral decubitus. Even though their challenge differs from ours, this sickness behavior in ill pigs was also confirmed in our experiment by an increment of pigs lying ventrally on days post-infection. Likewise, Rostagno et al. (2011) found that infected pigs with Salmonella spent more time in ventral recumbence to conserve body heat by lying on their feet and not exposing their underside.
Regarding the morning behaviors, feeding activity was prominent and, in accordance with Feddes et al. (1989), appeared to be driven by light changes. After the dark resting period, pigs were more motivated to feeding because they were hungry. In our study, the lights were turned on at 0730 h and turned off at 2130 h so in mornings the behavior of the pigs was recorded near the peak of feeding activity. In this regard, the use of the feeding area was also more frequent in the morning and the light-resting area in the afternoon. Food is an essential item and therefore the demand for it is inelastic, in other words, it would be the last behavior that the piglet would decrease when it gets sick (Matthews and Ladewig, 1994; Weary et al., 2009). We hypothesize that demand for food masked the effects in sickness behavior in the morning, and for this reason the effects of inoculation could not be perceived easily. We did not find any significant effects in the expression of active or inactive behaviors during this period.
Regarding the behaviors that were modified by the diets, pigs fed the probiotic diet showed positive, significant effects on exploring the pen, feeding and other active behaviors in the morning. Some authors have seen a correlation between feeding activity and food intake (Soltan, M. A., & Said, 2008; Ahmed et al., 2015) although in our experiment no increase in feed intake was seen in the PRO group. Our results also showed that lying with contact was less frequent in pigs fed with PRO. In accordance to Escobar et al. (2007), these results would suggest that supplementation brought some benefits for pigs because lying in contact with a pen mate is a strategy to achieve heat conservation for producing the beneficial febrile response in illness situations. These improvements were not only seen after the Salmonella challenge but also during the days previous to the inoculation (nonsignificant interaction of treatment × day). It must be remembered that weaning itself is a challenge for the piglets (Heo et al., 2013), and our results suggest that the probiotic diet may also have positive effects in the weaning response.
Up until now, probiotics have been attributed several functions when fighting against pathogens, namely: reducing nutrients to pathogenic bacteria by competition in the gut; competitive exclusion in binding sites on the intestinal epithelium, and producing bacteriocines or stimulating the immune system (Cho et al., 2011). Nonetheless, our results may suggest that other pathways could also be activated by probiotics. The gut-brain axis is emerging as an exciting concept where intestinal bacteria might influence nerve and brain function, and ultimately behavior (Cryan and O'Mahony, 2011). There is recent evidence that probiotics can reduce behaviors associated with stress, anxiety, and depression by improving the role of GABA, a prevalent neurotransmitter in the brain. Stilling et al. (2014) reported some biochemical and behavioral parameters (including anxiety, sociability, hypothalamic-pituitary-adrenal axis, and tryptophan metabolism) that could be reversed in germ-free mice by re-colonization with a conventional microbiota or probiotic treatment. Moreover, other effects of probiotics on brain functions have been reported. Bravo et al. (2011), feeding mice with Lactobacillus rhamnosus, showed changed levels of signaling chemicals in the brain as well as increased numbers of receptors associated with learning, memory and emotional control. In humans, Messaoudi et al. (2011) showed how a combination of Lactobacillus helveticus and Bifidobacterium longum improved health scores designed to assess mental health, and chronically administered in rats, significantly reduced anxiety-like behaviors. In view of our data, we cannot determine the causes of the behavioral changes related to the probiotic treatment because other factors such as an up-regulation of the immune response or a decrease in pathogenic pressure could also contribute to diminish sickness stress. However, the lack of behavioral effects in the BUT group, which also reduced Salmonella shedding, indicate that probiotics may act by other mechanisms.
Nonetheless, the detection of so many effects was a surprising result and the concept of social facilitation should also be considered to interpret treatment effects in behavior, as the pig is a highly social animal and social facilitation is a common feature in its behavior (Hsia and Wood-Gush, 1984; Weary et al., 2008). According to Clayton (1976), social facilitation is an increase in the frequency or intensity of responses, or initiation of particular responses already in an animal's repertoire when shown in the presence of others engaged in the same behavior. Therefore, a slight difference in health in some pigs produced by treatments, even if not perceptible by clinical signs monitored, could be amplified in behavior expression by social facilitation. We suggest that this line of research is important to further characterize the animal response mechanism. Encountering sensible evaluation tools, especially in challenge trials, is a way of diminishing the cost of the experiment and improving welfare by applying one of the basic principles of the 3 r's, reduction of the animals used.
On the other hand, to our knowledge, this is the first time that methodical observation has been performed to evaluate sodium butyrate in relation to animal behavior. In our study, although the treatment did exert a positive effect on the Salmonella shedding, we were not able to show behavioral changes promoted by this additive in a Salmonella challenge context.
To wrap up the behavioral analysis, the PRO treatment influenced the behavior and use of space positively. In the light of our results, behavior analysis may be a useful tool to appreciate significant changes in parameters studied by using a low number of animals.
conclusions
Both products evaluated had a favorable effect against Salmonella Typhimurium, as they were equally able to reduce the colonization and shedding of Salmonella Typhimurium in piglets. In addition Bacillus licheniformis had a positive significant effect on some behavioral displays, particularly those related to exploring the pen, feeding and other active behaviors in the morning period. Behavior analysis appears as a sensible tool to be considered in feed additive research, being able to detect slight improvements in the response of the animals and offering complementary information regarding other possible mechanisms of action of these additives.
Footnotes
This research project was funded by Norel SA (Madrid, Spain).
LITERATURE CITED
- Ahmed S., Hoon J., Hong-Seok M., Chul-Ju Y.. 2014. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Am. J. Ment. Retard. 8:96–104. [Google Scholar]
- Ahmed S., Mun H., Yoe H., Yang C.. 2015. Monitoring of behavior using a video-recording system for recognition of Salmonella infection in experimentally infected growing pigs. Animal 9:115–121. doi:10.1017/S1751731114002213 [DOI] [PubMed] [Google Scholar]
- Alexopoulos C., Georgoulakis I. E., Tzivara A., Kyriakis C. S., Govaris A., Kyriakis S. C.. 2004. Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 51:306–312. doi:10.1111/j.1439-0442.2004.00637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres V. M., Davies R. H.. 2015. Biosecurity Measures to Control Salmonella and Other Infectious Agents in Pig Farms: A Review. Compr. Rev. Food Sci. Food Saf. 14:317–335. doi:10.1111/1541-4337.12137 [Google Scholar]
- AOAC International 1995. Official methods of analysis of AOAC International. 15th ed. Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Arena A., Maugeri T. L., Pavone B., Iannello D., Gugliandolo C., Bisignano G.. 2006. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 6:8–13. doi:10.1016/j.intimp.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Balaji R., Wright K. J., Hill C. M., Dritz S. S., Knoppel E. L., Minton J. E.. 2000. Acute phase responses of pigs challenged orally with Salmonella Typhimurium. J. Anim. Sci. 78:1885–1891. doi:10.2527/2000.7871885x [DOI] [PubMed] [Google Scholar]
- Biagi G., Piva A.. 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 85(5):1184–1191. doi:10.2527/jas.2006-378 [DOI] [PubMed] [Google Scholar]
- Boughton C., Egan J., Kelly G., Markey B., Leonard N.. 2007. Rapid infection of pigs following exposure to environments contaminated with different levels of Salmonella typhimurium. Foodborne Pathog. Dis. 4:33–40. doi:10.1089/fpd.2006.58 [DOI] [PubMed] [Google Scholar]
- Boyen F., Haesebrouck F., Vanparys A., Volf J., Mahu M., Van Immerseel F., Rychlik I., Dewulf J., Ducatelle R., Pasmans F.. 2008. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 132:319–327. doi:10.1016/j.vetmic.2008.05.008 [DOI] [PubMed] [Google Scholar]
- Bravo J. A., Forsythe P., Chew M. V., Escaravage E., Savignac H. M., Dinan T. G., Bienenstock J., Cryan J. F.. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 108:16050–16055. doi:10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. G., Gardiner G. E., Casey G., Bradshaw B., Lawlor P. G., Lynch P. B., Leonard F. C., Stanton C., Ross R. P., Fitzgerald G. F., Hill C.. 2007. A Five-Strain Probiotic Combination Reduces Pathogen Shedding and Alleviates Disease Signs in Pigs Challenged with Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 73:1858–1863. doi:10.1128/AEM.01840-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2014. National Salmonella Surveillance Annual Report, 2012. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- Chamba F., Puyalto M., Ortiz A., Torrealba H., Mallo J.J., Riboty R.. 2014. Effect of Partially Protected Sodium Butyrate on Performance, Digestive Organs, Intestinal Villi and E. coli Development in Broiler Chickens. Int. J. Poult. Sci. 13:390–396. doi:10.3923/ijps.2014.390.396 [Google Scholar]
- Cho J., Zhao P., Kim I.. 2011. Probiotics as a Dietary Additive for Pigs: A Review. J. Anim. Vet. Adv. 10(16):2127–2134. doi:10.3923/javaa.2011.2127.2134 [Google Scholar]
- Clayton D. 1976. The effects of pre-test conditions on social facilitation of drinking in ducks. Anim. Behav. 24:125–134. doi:10.1016/S0003-3472(76)80105-4 [Google Scholar]
- Creus E., Pérez J. F., Peralta B., Baucells F., Mateu E.. 2007. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health 54:314–319. doi:10.1111/j.1863-2378.2007.01069.x [DOI] [PubMed] [Google Scholar]
- Cryan J. F., O'Mahony S. M.. 2011. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol. Motil. 23:187–192. doi:10.1111/j.1365-2982.2010.01664.x [DOI] [PubMed] [Google Scholar]
- Cutting S. M. 2011. Bacillus probiotics. Food Microbiol. 28:214–220. doi:10.1016/j.fm.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Dänicke S., Döll S.. 2010. A probiotic feed additive containing spores of Bacillus subtilis and B. licheniformis does not prevent absorption and toxic effects of the Fusarium toxin deoxynivalenol in piglets. Food Chem. Toxicol. 48:152–158. doi:10.1016/j.fct.2009.09.032 [DOI] [PubMed] [Google Scholar]
- Diebold G., Mosenthin R., Piepho H.-P., Sauer W. C.. 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi:10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Directive 2010/63/EU 2010. on the protection of animals used for scientific purposes. European Parliament and of the Council. Official Journal.
- Duc L. H., Hong H. A., Barbosa T. M., Henriques A. O., Cutting S. M.. 2004. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 70:2161–2171. doi:10.1128/AEM.70.4.2161-2171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA. (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control) 2013. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2011. EFSA Journal 2013. 11(4):3129 doi:10.2903/j.efsa.2013.3129 [Google Scholar]
- Escobar J., Van Alstine W. G., Baker D. H., Johnson R. W.. 2007. Behaviour of pigs with viral and bacterial pneumonia. Appl. Anim. Behav. Sci. 105:42–50. doi:10.1016/j.applanim.2006.06.005 [Google Scholar]
- Fang C. L., Sun H., Wu J., Niu H. H., Feng J.. 2014. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 98:680–685. doi:10.1111/jpn.12122 [DOI] [PubMed] [Google Scholar]
- Feddes J. J. R., Young B. A., DeShazer J. A.. 1989. Influence of temperature and light on feeding behaviour of pigs. Appl. Anim. Behav. Sci. 23:215–222. doi:10.1016/0168-1591(89)90112-3 [Google Scholar]
- Fernández-Rubio C., Ordóñez C., Abad-González J., Garcia-Gallego A., Honrubia M. P., Mallo J. J., Balaña-Fouce R.. 2009. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 88:943–948. doi:10.3382/ps.2008-00484 [DOI] [PubMed] [Google Scholar]
- Fraser J. N., Davis B. L., Skjolaas K. A., Burkey T. E., Dritz S. S., Johnson B. J., Minton J. E.. 2007. Effects of feeding Salmonella enterica serovar Typhimurium or serovar Choleraesuis on growth performance and circulating insulin-like growth factor-I, tumor necrosis factor-alpha, and interleukin-1beta in weaned pigs. J. Anim. Sci. 85:1161–1167. doi:10.2527/jas.2006-482 [DOI] [PubMed] [Google Scholar]
- Gálfi P., Bokori J.. 1990. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet. Hung. 38:3–17. [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Thompson A., Hinton J. C., Van Immerseel F., Hautefort I.. 2006. Butyrate Specifically Down-Regulates Salmonella Pathogenicity Island 1 Gene Expression Appl. Environ. Microbiol. Soc. 72:946–949. doi:10.1128/AEM.72.1.946-949.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H. M., Jonkers D., Venema K., Vanhoutvin S., Troost F. J., Brummer R.-J.. 2008. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27:104–119. doi:10.1111/j.1365-2036.2007.03562.x [DOI] [PubMed] [Google Scholar]
- Heo J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., Nyachoti C. M.. 2013. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl.) 97:207–237. doi:10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Hermes R. G., Molist F., Ywazaki M., Nofrarías M., Gomez de Segura A., Gasa J., Pérez J. F.. 2009. Effect of dietary level of protein and fiber on the productive performance and health status of piglets. J. Anim. Sci. 87:3569–3577. doi:10.2527/jas.2008-1241 [DOI] [PubMed] [Google Scholar]
- Hoa T. T., Duc L. H., Isticato R., Baccigalupi L., Ricca E., Van P. H., Cutting S. M.. 2001. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 67:3819–3823. doi:10.1128/AEM.67.9.3819-3823.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia L. C., Wood-Gush D. G. M.. 1984. Social facilitation in the feeding behaviour of pigs and the effect of rank. Appl. Anim. Ethol. 11:265–270. doi:10.1016/0304-3762(84)90033-6 [Google Scholar]
- Hurd H. S., Gailey J. K., McKean J. D., Rostagno M. H.. 2001. Rapid infection in market-weight swine following exposure to a Salmonella Typhimurium-contaminated environment. Am. J. Vet. Res. 62:1194–1197. doi:10.2460/ajvr.2001.62.1194 [DOI] [PubMed] [Google Scholar]
- Jensen M. T., Cox R. P., Jensen B. B.. 1995. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. 61:293–304. doi:10.1017/S1357729800013837 [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J. J., Galfi P.. 2012. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 91:837–843. doi:10.3382/ps.2011-01853 [DOI] [PubMed] [Google Scholar]
- Kotunia A., Woliński J., Laubitz D., Jurkowska M., Romé V., Guilloteau P., Zabielski R.. 2004. Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J. Physiol. Pharmacol. 55(Suppl 2):59–68. [PubMed] [Google Scholar]
- Lehner P. N. 1998. Handbook of Ethological Methods. C. U. Press, Colorado. [Google Scholar]
- Leser T. D., Knarreborg A., Worm J.. 2008. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 104:1025–1033. doi:10.1111/j.1365-2672.2007.03633.x [DOI] [PubMed] [Google Scholar]
- Link R., Kováč G.. 2006. The effect of probiotic BioPlus 2B on feed efficiency and metabolic parameters in swine. Biologia (Bratisl.) 61:783–787. doi:10.2478/s11756-006-0158-x [Google Scholar]
- Lu J., Zou X., Wang Y.. 2008. Effects of sodium butyrate on the growth performance, intestinal microflora and morphology of weanling pigs. J. Anim. Feed Sci. 17(4):568–578. doi:10.22358/jafs/66685/2008 [Google Scholar]
- Mallo J. J., Balfagón A., Gracia M. I., Honrubia P., Puyalto M.. 2012. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 90(Suppl 4):227–229. doi:10.2527/jas.53959 [DOI] [PubMed] [Google Scholar]
- Manzanilla E. G., Nofrarías M., Anguita M., Castillo M., Perez J. F., Martín-Orúe S. M., Kamel C., Gasa J.. 2006. Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 84:2743–2751. doi:10.2527/jas.2005-509 [DOI] [PubMed] [Google Scholar]
- Matthews L., Ladewig J.. 1994. Environmental requirements of pigs measured by behavioural demand functions. Anim. Behav. 47(3):713–719. doi:10.1006/anbe.1994.1096 [Google Scholar]
- Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.-F., Rougeot C., Pichelin M., Cazaubiel M., Cazaubiel J.-M.. 2011. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105:755–764. doi:10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Nielsen B., Baggesen D., Bager F., Haugegaard J., Lind P.. 1995. The serological response to Salmonella serovars typhimurium and infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet. Microbiol. 47:205–218. doi:10.1016/0378-1135(95)00113-1 [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 2012. Nutrient Requirements of Swine: Eleventh Revised Edition. Washington, DC: National Academic Press. [Google Scholar]
- Nofrarías M., Manzanilla E. G., Pujols J., Gibert X., Majó N., Segalés J., Gasa J.. 2006. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J. Anim. Sci. 84:2735–2742. doi:10.2527/jas.2005-414 [DOI] [PubMed] [Google Scholar]
- Piva A., Morlacchini M., Casadei G., Gatta P. P., Biagi G., Prandini A.. 2010. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 1:35–41. doi:10.4081/ijas.2002.35 [Google Scholar]
- Richardson A. J., Calder A. G., Stewart C. S., Smith A.. 1989. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 9:5–8. doi:10.1111/j.1472-765X.1989.tb00278.x [Google Scholar]
- Rostagno M. H., Eicher S. D., Lay D. C.. 2011. Immunological, physiological, and behavioral effects of Salmonella enterica carriage and shedding in experimentally infected finishing pigs. Foodborne Pathog. Dis. 8:623–630. doi:10.1089/fpd.2010.0735 [DOI] [PubMed] [Google Scholar]
- Saco Y., Fraile L., Giménez M., Alegre A., López-Jimenez R., Cortey M., Segalés J., Bassols A.. 2011. Serum acute phase proteins as biomarkers of pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Res. Vet. Sci. 91:52–57. doi:10.1016/j.rvsc.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Scherer K., Szabó I., Rosler U., Appel B., Hensel A., Nockler K.. 2008. Time Course of Infection with Salmonella Typhimurium and Its Influence on Fecal Shedding, Distribution in Inner Organs, and. J. Food Prot. 71:699–705. doi:10.4315/0362-028X-71.4.699 [DOI] [PubMed] [Google Scholar]
- Skjolaas K. A., Burkey T. E., Dritz S. S., Minton J. E.. 2007. Effects of Salmonella enterica serovar Typhimurium, or serovar Choleraesuis, Lactobacillus reuteri and Bacillus licheniformis on chemokine and cytokine expression in the swine jejunal epithelial cell line, IPEC-J2. Vet. Immunol. Immunopathol. 115:299–308. doi:10.1016/j.vetimm.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Soltan M. A., Said M.. 2008. Effect of probiotics and some spices as feed additives on the performance and behaviour of the Nile tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish 12:63–80. [Google Scholar]
- Spiehs M., Shurson G., Johnston L.. 2008. Effects of two direct-fed microbials on the ability of pigs to resist an infection with Salmonella enterica serovar Typhimurium. J. Swine Heal. Prod. 16:27–36. [Google Scholar]
- Stilling R. M., Dinan T. G., Cryan J. F.. 2014. Microbial genes, brain & behaviour- epigenetic regulation of the gut-brain axis. Genes Brain Behav. 13:69–86. [DOI] [PubMed] [Google Scholar]
- Szabó I., Wieler L. H., Tedin K., Scharek-Tedin L., Taras D., Hensel A., Appel B., Nöckler K.. 2009. Influence of a probiotic strain of Enterococcus faecium on Salmonella enterica serovar Typhimurium DT104 infection in a porcine animal infection model. Appl. Environ. Microbiol. 75:2621–2628. doi:10.1128/AEM.01515-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple D., Manteca X., Velarde A., Dalmau A.. 2011. Assessment of animal welfare through behavioural parameters in Iberian pigs in intensive and extensive conditions. Appl. Anim. Behav. Sci. 131(1–2):29–39. doi:10.1016/j.applanim.2011.01.013 [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., Ducatelle R.. 2005. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 84:1851–1856. doi:10.1093/ps/84.12.1851 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Rostagno M. H., Gardiner G. E., Sutton A. L., Richert B. T., Radcliffe J. S.. 2012a. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids: Part II. Effects on intestinal histology and active nutrient transport. J. Anim. Sci. 90:2599–2608. doi:10.2527/jas.2010-3599 [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Rostagno M. H., Gardiner G. E., Sutton A. L., Richert B. T., Radcliffe J. S.. 2012b. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids. Part I: Effects on growth performance, microbial populations, and immune status. J. Anim. Sci. 90:261–271. doi:10.2527/jas.2010-3598 [DOI] [PubMed] [Google Scholar]
- Warnecke T., Gill R. T.. 2005. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Fact. 4:25 doi:10.1186/1475-2859-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weary D. M., Jasper J., Hötzel M. J.. 2008. Understanding weaning distress. Appl. Anim. Behav. Sci. 110:24–41. doi:10.1016/j.applanim.2007.03.025 [Google Scholar]
- Weary D. M., Huzzey J. M., von Keyserlingk M. A. G.. 2009. Board-invited review: Using behavior to predict and identify ill health in animals. J. Anim. Sci. 87:770–7. doi:10.2527/jas.2008-1297 [DOI] [PubMed] [Google Scholar]
- Welfare Quality 2009. Welfare quality assessment protocol for pigs. Wageningen: Wageningen Academic Publishers; Lelystad, NL. [Google Scholar]