Abstract
A clade of New World monkeys (NWMs) exhibits considerable diversity in both oxytocin (OT) ligand and oxytocin receptor (OTR) structure. Most notable is the variant Pro8-OT, with proline instead of leucine at the eighth position, resulting in a rigid bend in the peptide backbone. A higher proportion of species that express Pro8-OT also engage in biparental care and social monogamy. When marmosets (genus Callithrix), a biparental and monogamous Pro8-OT NWM species, are administered the ancestral Leu8-OT, there is no change in social behavior compared with saline treatment. However, when Pro8-OT is administered, marmosets’ sociosexual and prosocial behaviors are altered. The studies here tested the hypothesis that OTR binding affinities and OT-induced intracellular Ca2+ potencies would favor the native OT ligand in OTRs from four primate species, each representing a unique combination of ancestral lineage, breeding system, and native OT ligand: humans (Leu8-OT, monogamous, apes), macaques (Leu8-OT, nonmonogamous, Old World monkey), marmosets (Pro8-OT, monogamous, NWM), and titi monkeys (Leu8-OT, monogamous, NWM). OTRs were expressed in immortalized Chinese hamster ovary cells and tested for intact-cell binding affinities for Pro8-OT, Leu8-OT, and arginine vasopressin (AVP), as well as intracellular Ca2+ signaling after stimulation with Pro8-OT, Leu8-OT, and AVP. Contrary to our hypothesis, Pro8-OT bound at modestly higher affinities and stimulated calcium signaling at modestly higher potencies compared with Leu8-OT in all four primate OTRs. Thus, differences downstream from a ligand-receptor binding event are more likely to explain the different behavioral responses to these two ligands.
Introduction
Oxytocin (OT) is a nonapeptide neurohormone that is critical for mammalian parturition, lactation, and parental behavior (Ellendorff et al., 1982; Fuchs et al., 1982; McNeilly et al., 1983; Chan et al., 1996; Lee et al., 2009). OT binds and activates its canonical G protein–coupled receptor, the oxytocin receptor (OTR). Synthetic OT is used widely in clinical settings for inducing and accelerating labor. Because of its ability to modulate a wide variety of social behaviors (Lee et al., 2009), OT is currently being evaluated for its clinical use in disorders with a social component, such as autism spectrum disorder and schizophrenia (Bakermans-Kranenburg and van Ijzendoorn, 2013; Feifel et al., 2016; DeMayo et al., 2017; Parker et al., 2017). Considerable effort has been invested in engineering OT analogs and formulations for potential therapeutic use and to extend understanding of OT actions, particularly central nervous system and behavioral effects (Manning et al., 2012; Busnelli et al., 2013; Muttenthaler et al., 2017). Although these efforts with novel synthetic analogs have been reasonably successful, naturally occurring variants of the OT peptide could provide an alternate route to novel agents and therapies (Gruber et al., 2012).
The nonapeptide family of hormone ligands is ancient and is present in nearly all animal lineages (Beets et al., 2013; Lockard et al., 2017). OT-like ligands generally vary at the third, fourth, or eighth amino acid position (Gruber et al., 2012), and the amino acid at the eighth position strongly affects the activity of the peptide on its target organs (Sawyer and Manning, 1973; Manning et al., 2012; Muttenthaler et al., 2017). OT and the closely related nonapeptide arginine vasopressin (AVP) differ at amino acid positions 3 and 8 and have vastly different roles in mammalian physiology, even though the affinity of OT for OTRs is only 2-fold greater than the affinity of AVP for OTRs (Manning et al., 2012). Despite having only a 2-fold lower binding affinity for OTRs, AVP is over 30-fold less potent than OT for generating OTR responses (Manning et al., 2012). Variations among species in their nonapeptide receptors correlate with variations in their respective OT-like ligands, indicating ligand-receptor coevolution (Koehbach et al., 2013). The presence of OT-like peptides across diverse animal taxa suggests the universal importance of their functions, and their coevolution with their ligands suggests a tightly aligned signaling system for these functions.
Despite variation across Animalia taxa, the OT ligand is highly conserved within eutherian mammals (Wallis, 2012). Recently, a nonsynonymous nucleotide substitution in the OXT gene coding for OT was discovered in four species of New World monkeys (NWMs), resulting in a proline at amino acid position 8 (Pro8-OT) in place of the typical leucine (Leu8-OT) (Lee et al., 2011). Subsequent screening showed that the Pro8-OT variant is present in at least 20 NWM species (Ren et al., 2015; Vargas-Pinilla et al., 2015). Additional OT variants were also identified, for a total of six different forms of OT in NWMs, with at least one species from each NWM clade exhibiting an OT variant (Ren et al., 2015; Vargas-Pinilla et al., 2015). OTRs also vary in NWMs, particularly in the N terminus (Ren et al., 2015; Vargas-Pinilla et al., 2015), which is important for binding to the tail of the OT ligand (Postina et al., 1996; Gimpl and Fahrenholz, 2001), and there is strong evidence for OT-OTR coevolution (Koehbach et al., 2013; Ren et al., 2015; Vargas-Pinilla et al., 2015). Moreover, OXTR variation is associated with social monogamy among primates (Ren et al., 2015), and OT ligand variation at position 8 is associated with litter size within the family Cebidae (Vargas-Pinilla et al., 2015). Both native and non-native OT ligands modulate social behavior in NWMs expressing Pro8-OT (French et al., 2016), but the native Pro8-OT is more effective at modulating behavior than the ancestral Leu8-OT in the marmoset, a monogamous and biparental NWM (Cavanaugh et al., 2014; Mustoe et al., 2015, 2018). Together these findings indicate that both OT ligand variation and the corresponding variations in OTRs among NWMs contribute to functional outcomes.
Based on the findings summarized above, we hypothesized that the binding affinities and signaling potencies of primate OT variants are different for different OTR variants, with each receptor variant preferring the ligand variant from the same species. The studies presented here test this hypothesis by measuring binding affinities and signaling potencies for Leu8, Pro8, and AVP at the OTRs from four primate species, each representing a unique combination of ancestral lineage, breeding system, and native OT ligand.
Materials and Methods
OTR Transfection and Cell Culture.
Chinese hamster ovary (CHO; female origin) cells were purchased from American Type Culture Collection (Manassas, VA) and cultured at 37°C with 5% CO2 using Ham’s F-12 medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 µg/ml streptomycin. Human, marmoset, and macaque OTR plasmids (Table 1) were purchased from GenScript (Piscataway, NJ) in a pcDNA3.1+ vector. The titi monkey plasmid was generated by amplifying and ligating the coding region of the titi OXTR from genomic titi monkey DNA (flanked with BamHI and XhoI restriction sites) and ligating it into a T vector (pMD19). Competent Escherichia coli were transformed using this vector, plated onto Luria-Bertani/ampicillin/isopropyl β-d-1-thiogalactopyranoside/X-gal plates, and incubated overnight at 37°C. White colonies were selected, then plasmid DNA was purified and sequenced. Sequence-confirmed plasmids were then digested with BamHI and XhoI and ligated into a pcDNA3.1+ vector. CHO cells were then transfected using TurboFect (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions and kept under selective pressure using 400 µg/ml G418 antibiotic. Individual clonal lines were generated by plating batch-transfected cells at approximately 10 cells/ml (1 cell/100 µl) into 96-well plates and then selecting wells for screening that originated from a single colony. Clonal lines were screened using an intact cell 125I-ornithine vasotocin analog (OVTA) binding assay and selected for similar receptor expression across species, defined as total radioligand binding. All experiments were done in a single clone per species, except for the marmoset, in which two clones were used.
TABLE 1.
Representative primate species OTRs
| Group | Human | Macaque | Marmoset | Titi Monkey |
|---|---|---|---|---|
| Lineage (family) | Old World (Hominidae) | Old World (Cercopithidae) | New World (Callitrichidae) | New World (Pithecidae) |
| Breeding system | Monogamous | Polygamous | Monogamous | Monogamous |
| Native OT ligand | Leu8-OT | Leu8-OT | Pro8-OT | Leu8-OT |
Intact Cell Saturation Binding Assays.
CHO cells expressing primate OTRs were plated at 150,000 cells/ml (15,000 cells/well per 100 µl) into 96-well plates and grown to 80%–90% confluence. On the day of assay, growth medium was aspirated and cells were quickly washed once with 100 µl ice-cold high glucose HEPES-buffered Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin (HGH-BSA) and then placed on ice. Then 50 µl ice-cold 125I-OVTA (PerkinElmer, Waltham, MA) in doubling concentrations from about 15 to 2000 pM was added in triplicate (technical replicates) to all wells and incubated for 3 hours on ice. At the end of the assay, an aliquot of the binding medium was collected to quantify free radioligand directly, eliminating any concerns about differential depletion of ligand due to differential receptor expression levels. Cells were then washed four times with 100 µl ice-cold HGH-BSA, solubilized with 100 µl 0.2 N NaOH, and counted on a gamma counter. Nonspecific binding was defined as 125I-OVTA binding occurring in the presence of excess competitor (10−4 M Leu8-OT). Binding affinity (Kd) for 125I-OVTA was determined using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) to fit the specific bound versus free ligand data to a single-site binding equation. These assays were done at least three times on 3 different days using fresh aliquots of 125I-OVTA and competitor, and Kd values were averaged across three biologic replicates (five biologic replicates for marmoset).
Intact Cell Competition Binding Assays.
CHO cells expressing primate OTRs were plated at 150,000 cells/ml (15,000 cells/well per 100 μl) into 96-well plates and grown to 80%–90% confluence. On the day of assay, growth medium was aspirated and cells were quickly washed once with 100 µl ice-cold HGH-BSA and then placed on ice. Then 50 µl of roughly 50,000 cpm ice-cold 125I-OVTA was added in triplicate (technical replicates) to all wells in the presence or absence of 10−11 to 10−5 M Pro8-OT (CYIQNCPPG-NH2; Anaspec, Fremont, CA), Leu8-OT (CYIQNCPLG-NH2; Anaspec), or AVP (CYFQNCPRG-NH2; Anaspec) and incubated for 3 hours on ice. At the end of the assay, an aliquot of the binding medium was collected to quantify free radioligand directly. Cells were then washed four times with 100 µl ice-cold HGH-BSA, solubilized with 100 µl 0.2 N NaOH, and counted on a gamma counter. Binding affinities (IC50) were determined by plotting bound 125I-OVTA versus competitor concentration. IC50 values were then calculated using the Cheng–Prusoff equation and each receptor’s affinity for 125I-OVTA to produce Ki values. These assays were done at least three times on 3 different days using fresh aliquots of 125I-OVTA and Leu8-OT, Pro8-OT, and AVP for three biologic replicates per clone.
Ca2+ Mobilization Assays.
CHO cells expressing primate OTRs were plated into 96-well plates and grown to 80%–90% confluence. On the day of assay, growth medium was aspirated and cells were incubated at 37°C with 100 µl Fluo-4 Direct dye mixed in Fluo-4 Direct Ca2+ Assay Buffer (Thermo Fisher Scientific) with 5 mM probenecid for 1 hour. At the end of 1 hour, baseline fluorescence was measured at 37°C followed by stimulated fluorescence in the presence or absence of 10−11 to 10−6 M Pro8-OT, Leu8-OT, or AVP (3 × technical replicates). Peak fluorescence minus baseline fluorescence was then plotted as a function of ligand concentration to determine EC50 values. These assays were done at least three times on 3 different days using fresh aliquots of Leu8-OT, Pro8-OT, and AVP for three biologic replicates per clone.
Data Analysis.
Binding affinities (Kd) for 125I-OVTA at each primate OTR were calculated by subtracting nonspecific binding and then plotting bound 125I-OVTA versus free 125I-OVTA.
Because concentrations of 125I-OVTA were not identical from experiment to experiment, technical replicates within each experiment (n = 3) were normalized and binding affinities (Ki) were calculated using the Cheng–Prusoff equation and the measured binding affinity for 125I-OVTA. Technical replicates were then averaged and used as biologic replicates (n = 3 per clone) to determine and compare Ki values for each ligand within species. A Bonferroni-corrected cutoff (P = 0.05 ÷ 3 = 0.0167) was used to determine statistically significant differences in Ki values.
Within-species differences in Ca2+ mobilization potency (EC50) were determined by normalizing and averaging each technical replicate (n = 3) and then using the biologic replicates (n = 3) to assess ligand comparisons (Pro8-OT vs. Leu8-OT, Pro8-OT vs. AVP, and Leu8-OT vs. AVP). A Bonferroni-corrected cutoff (P = 0.05 ÷ 3 = 0.0167) was used to determine statistically significant differences in Ki values.
All data were analyzed using the nonlinear least-squares curve-fitting capabilities of GraphPad Prism software.
Results
Saturation Binding Assays.
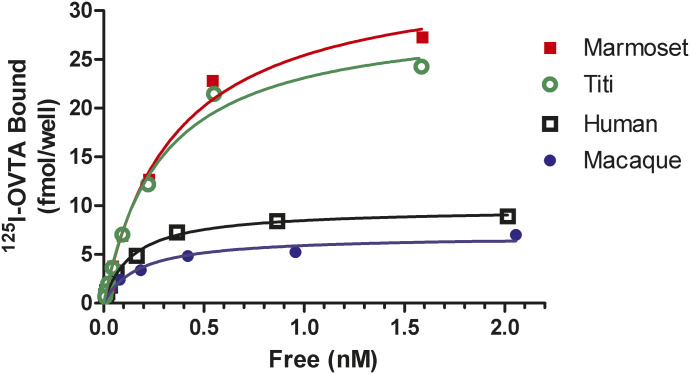
Saturation assays were performed on 96-well plates with 50 µl binding medium per well. Representative saturation curves for all receptors are shown in Fig. 1. All of the binding and signaling assays for the human receptor were conducted with a single clone with a Bmax value of 17 ± 6 fmol/well (n = 3). All assays for the macaque receptor were with a clone with a Bmax value of 12 ± 5 fmol/well (n = 3). All assays for the titi monkey receptor were with a clone with a Bmax value of 44 ± 12 fmol/well (n = 3). For the marmoset receptor, some assays were performed with a significantly higher expressing clone, R9, with a Bmax value of 91 ± 20 fmol/well (n = 2); additional experiments were performed with a clone with lower expression, R10, with a Bmax value of 33 ± 5 fmol/well (n = 4).
Fig. 1.
Representative saturation assays for 125I-OVTA binding to OTRs from each of the four species. Cells on 96-well plates were incubated on ice in 50 µl binding medium with the indicated concentrations of 125I-OVTA for 3 hours, and specific binding was then quantified. Data are from a single experiment with all four receptors tested side by side in triplicate. Values for this experiment are in good agreement with the average values in the Results and Table 1: humans, Bmax = 9.7 fmol/well and Kd = 0.12 nM; macaques, Bmax = 6.9 fmol/well and Kd = 0.144 nM; marmosets (R10), Bmax = 34 fmol/well and Kd = 0.30 nM; and titi monkeys, Bmax = 30 fmol/well and Kd = 0.24 nM.
Saturation binding analyses revealed only relatively small differences in binding affinities for the radioligand 125I-OVTA among the four species, ranging from 161 to 481 pM (Table 2). The human and macaque OTRs exhibited very similar affinities that were somewhat higher than those for the titi monkey and marmoset, with the marmoset exhibiting the lowest affinity.
TABLE 2.
Binding affinities for ligands at various primate OTRs
Data are presented as means ± S.E.M. and means ± log S.E.M. for Kd and Ki values, respectively.
| OTR | n | 125I-OVTA Kd |
Ki |
||
|---|---|---|---|---|---|
| Pro8-OT | Leu8-OT | AVP | |||
| nM | |||||
| Human | 3 | 0.161 ± 0.019 | 22.78 ± 0.10a | 71.84 ± 0.10 | 541.1 ± 0.07a,b |
| Macaque | 3 | 0.199 ± 0.036 | 43.36 ± 0.07 | 74.99 ± 0.08 | 474.8 ± 0.10a,b |
| Marmoset | 5, 6 | 0.481 ± 0.041 | 81.31 ± 0.11 | 170.2 ± 0.10 | 1093 ± 0.13a,b |
| Titi monkey | 3 | 0.289 ± 0.031 | 146.9 ± 0.11a | 894.5 ± 0.12 | 1924 ± 0.16b |
Indicates a significant within-species difference compared with Leu8-OT using a Bonferroni-corrected cutoff of P < 0.0167.
Indicates a significant within-species difference compared with Pro8-OT using a Bonferroni-corrected cutoff of P < 0.0167.
Competition Binding Assays with OT Variants and AVP.
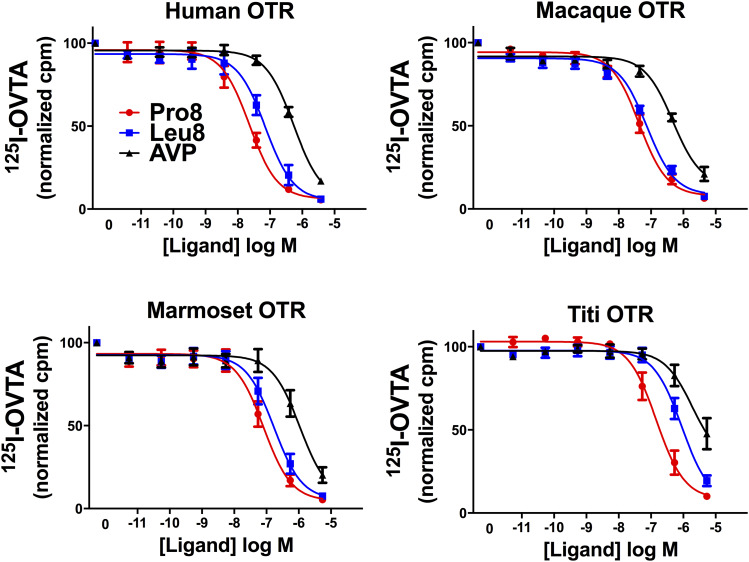
In competition binding assays, Pro8-OT exhibited a higher binding affinity than Leu8-OT for all four species, with a 1.5-fold difference for macaques, a 2-fold difference for marmosets, a 3-fold difference for humans, and over 6-fold difference for titi monkeys. Only for titi monkeys and humans was the difference in binding affinity statistically significant [F(1, 42) > 12.1, P < 0.016]. For the human OTR, the difference was due to greater affinity for Pro8-OT; for the titi OTR, the difference was due to lower affinity for Leu8-OT, rather than higher affinity for Pro8-OT compared with the other species (Fig. 2; Table 2). For both OT variants, the absolute binding affinities were 3- to 5-fold higher for humans, macaques, and marmosets than for titi monkeys.
Fig. 2.
Competition curves for Pro8-OT and Leu8-OT for each primate species OTR. Increasing concentrations of competitor ligand (Pro8-OT, Leu8-OT, or AVP) were added to a constant concentration of 125I-OVTA in intact CHO cells expressing one of four primate OTRs. All values are expressed as the percentage of the maximal binding in the absence of OT or AVP.
Binding affinities for AVP were assessed alongside the two OT variants for all of the receptors. Compared with Pro8-OT and Leu8-OT, respectively, binding affinity for AVP was 20- and 8-fold lower for humans, 11- and 6-fold lower for macaques, and 13- and 6-fold lower for marmosets, but 13- and only 2-fold lower for titi monkeys. In fact, the affinity of the titi OTR for Leu8-OT was not significantly higher than that for AVP [F(1, 42) = 2.07, P = 0.157]. However, the rank order of potencies was the same for all species, with affinities for Pro8 > Leu8 > AVP.
Ca2+ Signaling Assays.
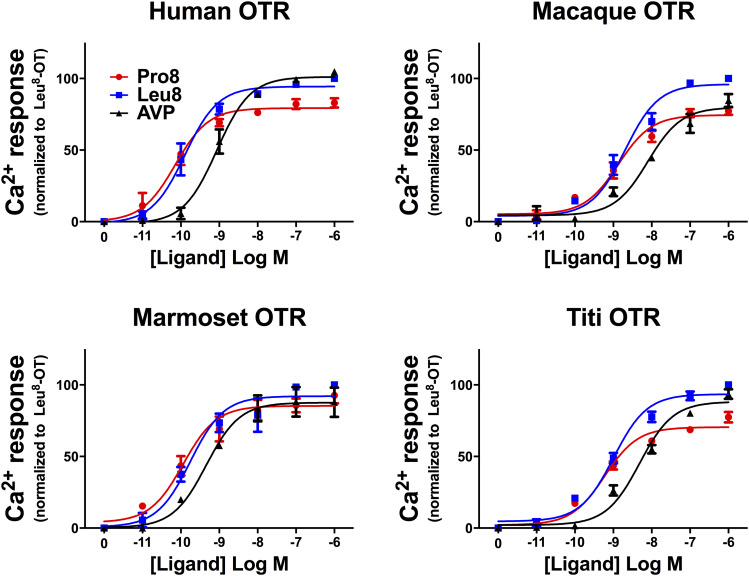
In Ca2+ mobilization assays, the rank order of potencies was the same for all species and with the same pattern as for binding, Pro8 > Leu8 > AVP; however, the magnitude of the differences was smaller for signaling than for binding. Pro8 and Leu8-OT were roughly equipotent for all species, with only 1.5-fold greater potency of Pro8-OT versus Leu8-OT for humans, macaques, marmosets, and titi monkeys (Fig. 3; Table 3). Pro8-OT consistently exhibited a slightly lower maximal response than Leu8 for all species except the marmoset.
Fig. 3.
Intracellular Ca2+ increases for each primate species OTR. Increasing concentrations of Pro8,-OT, Leu8-OT, or AVP were used to stimulate intracellular Ca2+ mobilization in CHO cells expressing one of four primate OTRs. All values are expressed as the percentage of the maximal response to 10−6 M Leu8-OT for each species.
TABLE 3.
Ca2+ mobilization potencies for ligands at various primate OTRs
Data are presented as means ± log S.E.M.
| OTR (n = 3) | Ca2+ EC50 |
||
|---|---|---|---|
| Pro8-OT | Leu8-OT | AVP | |
| nM | |||
| Human | 0.072 ± 0.12 | 0.127 ± 0.10 | 0.864 ± 0.07a,b |
| Macaque | 1.341 ± 0.11 | 2.025 ± 0.11 | 7.981 ± 0.12a,b |
| Marmoset | 0.115 ± 0.15 | 0.176 ± 0.14 | 0.459 ± 0.14b |
| Titi monkey | 0.595 ± 0.09 | 1.010 ± 0.09 | 4.821 ± 0.09a,b |
Indicates a significant within-species difference compared with Leu8-OT using a Bonferroni-corrected cutoff of P < 0.0167.
Indicates a significant within-species difference compared with Pro8-OT using a Bonferroni-corrected cutoff of P < 0.0167.
Ca2+ mobilization potencies for AVP were assessed alongside the two OT variants for all of the receptors. Compared with Pro8-OT and Leu8-OT, respectively, potency for AVP was 12- and 7-fold lower for humans, 6- and 4-fold lower for macaques, 5- and 2-fold lower for marmosets, and 8- and 5-fold lower in titi monkeys. The absolute potencies for Pro8-OT and Leu8-OT for humans and marmosets were similar, but the potency of AVP for the marmoset receptor was nearly 2-fold higher than it was for the human receptor. Potencies for each ligand across species were higher for human and marmoset OTRs compared with the macaque and titi monkey.
Ca2+ mobilization potencies relative to binding affinities were also computed as a metric of coupling efficiency (Table 4). In general, efficiencies within species were similar, with signaling EC50 values exhibiting potencies over 2 log units higher than the binding affinities for all three ligands. The macaque OTR was the least efficient, signaling at potencies less than 2 log units higher than the binding affinity for all three ligands. Notably, in all species except the titi monkey, AVP was equally or more efficient at mobilizing Ca2+ than Pro8-OT or Leu8-OT, per unit of binding affinity.
TABLE 4.
Coupling efficiencies for ligands at various primate OTRs
| OTR | Potency/Affinity Ratio −Log(Ca2+ IC50/Ki) |
||
|---|---|---|---|
| Pro8-OT | Leu8-OT | AVP | |
| Human | 2.51 | 2.75 | 2.80 |
| Macaque | 1.51 | 1.57 | 1.77 |
| Marmoset | 2.85 | 3.00 | 3.38 |
| Titi monkey | 2.39 | 2.95 | 2.60 |
Discussion
The studies here tested the hypothesis that the coevolution between Pro8-OT and OTRs in NWMs (Ren et al., 2015; Vargas-Pinilla et al., 2015) would confer greater selectivity in binding and signaling for Pro8-OT over the ancestral Leu8-OT at receptors from Pro8-OT–expressing species, and conversely higher selectivity for Leu8-OT at receptors from species expressing Leu8-OT. The binding and signaling data in this study show that this hypothesis is at best only partially supported. For the marmoset OTR, the species-native Pro8-OT bound with only modestly higher affinity and induced Ca2+ mobilization with higher potency than Leu8-OT. In humans and titi monkeys, the species–non-native ligand Pro8-OT also bound with higher affinity than the species-native ligand Leu8-OT. For receptors from all three Leu8-OT–expressing species, the two ligands were equipotent at mobilizing Ca2+. The higher binding affinity for Pro8-OT for all of the species, including those whose native hormone is Leu8-OT, was unexpected and not consistent with our hypothesis of binding affinities for each species correlating with their native ligand. One explanation for the observed preference for Pro8-OT over Leu8-OT in all species may be that the flexible (Kotelchuck et al., 1972; Brewster et al., 1973) tail of Leu8-OT can orient into a conformation that is similar to the more rigid structure of Pro8-OT (Lee et al., 2011) for only a smaller percentage of ligand-receptor interactions than Pro8-OT, and that the optimal conformation for Leu8-OT is one that is similar to the structure of Pro8-OT. The lack of significant preferences for the endogenous ligand in terms of signaling potencies was similarly unexpected. Thus, differences in other factors, perhaps downstream of the initial receptor binding and activation steps, are now the more likely explanations for the differential behavioral responses to OT in Leu8-OT– versus Pro8-OT–expressing species.
The ability of AVP to bind and activate primate OTRs was also tested, because AVP binds and activates OTRs, and AVP is known to affect social behavior in primates, including titi monkeys and marmosets (Caldwell et al., 2008; Jarcho et al., 2011; Taylor and French, 2015; Taylor et al., 2017). In all species except the titi monkey, AVP bound with much lower affinity to the OTR than Pro8-OT or Leu8-OT, and in all species AVP had lower potency for mobilizing Ca2+ than Pro8-OT or Leu8-OT. These results show that the coevolution of Pro8-OT and the OTR in marmosets has not altered selectivity for AVP versus the two OT variants.
To our knowledge, this study is the first description of NWM OT ligand variant binding in nonhuman primates, and the Ca2+ mobilization data inform the recent research investigating OTR signaling and NWM ligand variants in rodent models. Parreiras-E-Silva et al. (2017) found no differences in Ca2+ signaling at the human OTR between Pro8-OT, Leu8-OT, or the additional NWM OT variant Val3Pro8-OT. Our data also partially replicate those obtained by our collaborators (Pierce et al., 2016, unpublished observations), who found no difference in Ca2+ signaling between Pro8-OT and Leu8-OT at the human OTR but that Pro8-OT was more efficacious than Leu8-OT at inducing Ca2+ mobilization at the marmoset OTR. Taken together, these data indicate that the substitution of proline in place of leucine does not inhibit the G protein–coupled activity in species that express the ancestral Leu8-OT. Perhaps more importantly, the Pro8 substitution confers equal or greater potency for Ca2+ mobilization in a species in which Pro8-OT is the native ligand.
These binding and signaling data provide a functional link between the genetic surveys of the OT system in NWMs and the growing body of work comparing the behavioral effects of intranasal treatment with Pro8-OT and Leu8-OT in marmosets. Pro8-OT, but not Leu8-OT, enhances a variety of pairmate-directed social approach behaviors (Cavanaugh et al., 2014, 2018). Moreover, Pro8-OT increases the amount of social behavior that an OT-treated marmoset receives from its mate and reduces sociosexual behavior directed toward individuals other than the pairmate (Cavanaugh et al., 2014; Mustoe et al., 2015). Leu8-OT does affect some social behavior in marmosets, but Leu8-OT never enhances a social behavior that Pro8-OT does not also enhance (Mustoe et al., 2018). The binding and signaling data support these behavioral findings. Pro8-OT not only bound to the marmoset OTR with greater affinity but was also modestly more potent at stimulating Ca2+ mobilization. Although it is unlikely that this difference in signaling between Pro8-OT and Leu8-OT is the only contributing factor to the behavioral differences between treatment with Pro8-OT and Leu8-OT in marmosets, it is likely at least one contributing factor.
These binding and signaling data also shed new light on the clade-wise surveys of the OXT, OXTR, and AVP V1a receptor (AVPR1A) genes in NWMs. First and foremost, our data help to explain the finding that social monogamy and the OTR coevolved in NWMs (Ren et al., 2015; Vargas-Pinilla et al., 2015), with Pro8-OT binding and Ca2+signaling both enhanced in the socially monogamous species that expresses Pro8-OT natively. Moreover, Ca2+ signaling was not reduced by the substitution of proline for leucine at the eighth position in OTRs from Leu8-OT species. This suggests that the OTR is permissive for this substitution, providing a potential mechanism for the coevolution of Pro8-OT and OTRs in NWMs (Ren et al., 2015; Vargas-Pinilla et al., 2015). The single nucleotide substitution that produced Pro8-OT may have had modest consequences for neurotransmission, and thus the OTR may have evolved to accommodate this substitution. There is also a relationship between social monogamy and variation in the AVPR1A gene. Interestingly, in one of the only Leu8-OT NWMs that exhibits social monogamy (the titi monkey), Leu8-OT and AVP bound the OTR with similar affinity. These genetic and signaling data suggest that interrogation of the NWM AVP receptors (V1aR, V1bR, V2R) may provide new insights into nonapeptide signaling in primates, and these studies are currently in progress.
Alongside the work of Parreiras-E-Silva et al. (2017), this article constitutes a “first look” at the characteristics of the marmoset and titi monkey OTRs when bound to the Pro8-OT variant. As such, we only explored two facets of GPCR function: ligand binding and Ca2+ signaling. Other characteristics of these receptors, such as differential coupling to specific Gα subunits or bias for G proteins versus β-arrestin, are beyond the scope of this project but are nonetheless interesting future directions. The OTR is capable of coupling to a variety of Gα subunits resulting in a variety of cellular outcomes [see Gimpl and Fahrenholz (2001) and Mustoe et al. (2018) for detailed reviews], and there is a need for and a value to the production and characterization of ligands that are functionally selective at the OTR as tools to target specific signaling cascades. Indeed, even relatively small modifications to the OT ligand can alter the functional selectivity at the OTR, causing it to couple to different Gα subunits (Busnelli et al., 2012), and it is already known that Pro8-OT is less efficacious than Leu8-OT at promoting β-arrestin recruitment and internalization at the human receptor (Parreiras-E-Silva et al., 2017). It is possible that Pro8-OT and Leu8-OT may differentially promote coupling to specific Gα subunits or bias signaling via G proteins versus β-arrestin in marmosets and titi monkeys as well, and these experiments may provide more insight into the evolution of this system in NWMs. Another interesting possibility is that the OTRs may form dimers with various other GPCRs, and the Leu8-OT and Pro8-OT variants might exhibit selectivity for binding or activating one of these dimers versus another. Such dimer selectivity would not be detected in these assays with only the OTR expressed. Thus, multiple possible explanations for the ligand variation and its correlations with GPCR signaling and behavior remain to be explored.
A final potentially important outcome of these studies is that the higher binding affinity of Pro8-OT versus Leu8-OT at OTRs from all species, including humans, should presumably make Pro8-OT a better ligand for future binding studies, in either a radiolabeled or fluorescently tagged form. The 3-fold higher binding affinity would allow the use of 3-fold lower concentrations of the ligand to achieve the same fractional receptor occupancy, thus decreasing the amount of ligand required and the corresponding cost and usage of the ligand. The tighter binding of the Pro8-OT variant to the OTR may be useful in other contexts as well.
Acknowledgments
We thank Dr. Dongren Ren for constructing the titi monkey plasmid and Dr. Emily Harrison and Dr. Sara Freeman for assistance during the initial stages of this project. We also thank Dr. Thomas Murray, Dr. Marsha Pierce, and Dr. Aaryn Mustoe for input during the planning of this project.
Abbreviations
- AVP
arginine-8-vasopressin
- CHO
Chinese hamster ovary
- HGH-BSA
high glucose HEPES-buffered Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin
- NWM
New World monkey
- OT
oxytocin
- OTR
oxytocin receptor
- OVTA
ornithine vasotocin analog
Authorship Contributions
Participated in research design: Taylor, Schulte, French, Toews.
Conducted experiments: Taylor, Schulte.
Performed data analysis: Taylor, Schulte, Toews.
Wrote or contributed to the writing of the manuscript: Taylor, Schulte, French, Toews.
Footnotes
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R01HD089147] and the University of Nebraska at Omaha [Graduate Research and Creative Activity Award (“Functional Characteristics of Four Primate Oxytocin Receptors: Relationships to Biparental Care and Social Monogamy”)].
References
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. (2013) Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry 3:e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beets I, Temmerman L, Janssen T, Schoofs L. (2013) Ancient neuromodulation by vasopressin/oxytocin-related peptides. Worm 2:e24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AIR, Hruby VJ, Glasel JA, Tonelli AE. (1973) Proposed conformations of oxytocin and selected analogs in dimethyl sulfoxide as deduced from proton magnetic resonance studies. Biochemistry 12:5294–5304. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B. (2013) Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther 346:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, Chini B. (2012) Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem 287:3617–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, Young WS., 3rd (2008) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe A, French JA. (2018) Oxytocin regulates reunion affiliation with a pairmate following social separation in marmosets. Am J Primatol DOI: 10.1002/ajp.22750 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. (2014) Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology 49:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Wo NC, Manning M. (1996) The role of oxytocin receptors and vasopressin V1a receptors in uterine contractions in rats: implications for tocolytic therapy with oxytocin antagonists. Am J Obstet Gynecol 175:1331–1335. [DOI] [PubMed] [Google Scholar]
- DeMayo MM, Song YJC, Hickie IB, Guastella AJ. (2017) A review of the safety, efficacy and mechanisms of delivery of nasal oxytocin in children: therapeutic potential for autism and Prader-Willi syndrome, and recommendations for future research. Paediatr Drugs 19:391–410. [DOI] [PubMed] [Google Scholar]
- Ellendorff F, Forsling ML, Poulain DA. (1982) The milk ejection reflex in the pig. J Physiol 333:577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, MacDonald K. (2016) A review of oxytocin’s effects on the positive, negative, and cognitive domains of schizophrenia. Biol Psychiatry 79:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Taylor JH, Mustoe AC, Cavanaugh J. (2016) Neuropeptide diversity and the regulation of social behavior in New World primates. Front Neuroendocrinol 42:18–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernström MJ. (1982) Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science 215:1396–1398. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683. [DOI] [PubMed] [Google Scholar]
- Gruber CW, Koehbach J, Muttenthaler M. (2012) Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future Med Chem 4:1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. (2011) Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav 10:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. (2013) Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Trans 41:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck D, Scheraga HA, Walter R. (1972) Conformational energy studies of oxytocin and its cyclic moiety. Proc Natl Acad Sci USA 69:3629–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC Jr, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. (2011) A novel form of oxytocin in New World monkeys. Biol Lett 7:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani JH, Young WS., 3rd (2009) Oxytocin: the great facilitator of life. Prog Neurobiol 88:127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard MA, Ebert MS, Bargmann CI. (2017) Oxytocin mediated behavior in invertebrates: an evolutionary perspective. Dev Neurobiol 77:128–142. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. (2012) Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol 24:609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Robinson ICAF, Houston MJ, Howie PW. (1983) Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed) 286:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe A, Taylor JH, and French JA (2018) Oxytocin structure and function in New World monkeys: from pharmacology to behavior. Integr Zool DOI: 10.1111/1749-4877.12318 [published ahead of print]. [DOI] [PMC free article] [PubMed]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. (2015) Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Horm Behav 71:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttenthaler M, Andersson Å, Vetter I, Menon R, Busnelli M, Ragnarsson L, Bergmayr C, Arrowsmith S, Deuis JR, Chiu HS, et al. (2017) Subtle modifications to oxytocin produce ligands that retain potency and improved selectivity across species. Sci Signal 10:eaan3398. [DOI] [PMC free article] [PubMed]
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, et al. (2017) Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci USA 114:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreiras-E-Silva LT, Vargas-Pinilla P, Duarte DA, Longo D, Espinoza Pardo GV, Dulor Finkler A, Paixão-Côrtes VR, Paré P, Rovaris DL, Oliveira EB, et al. (2017) Functional New World monkey oxytocin forms elicit an altered signaling profile and promotes parental care in rats. Proc Natl Acad Sci USA 114:9044–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Kojro E, Fahrenholz F. (1996) Separate agonist and peptide antagonist binding sites of the oxytocin receptor defined by their transfer into the V2 vasopressin receptor. J Biol Chem 271:31593–31601. [DOI] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. (2015) Genetic diversity in oxytocin ligands and receptors in New World monkeys. PLoS One 10:e0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer WH, Manning M. (1973) Synthetic analogs of oxytocin and the vasopressins. Annu Rev Pharmacol 13:1–17. [DOI] [PubMed] [Google Scholar]
- Taylor JH, French JA. (2015) Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Horm Behav 75:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Intorre AA, French JA. (2017) Vasopressin and oxytocin reduce food sharing behavior in male, but not female marmosets in family groups. Front Endocrinol (Lausanne) 8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM de AG, Xavier A, Comas D, Pissinatti A, Sinigaglia M, Rigo MM, et al. (2015) Evolutionary pattern in the OXT-OXTR system in primates: coevolution and positive selection footprints. Proc Natl Acad Sci USA 112:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. (2012) Molecular evolution of the neurohypophysial hormone precursors in mammals: comparative genomics reveals novel mammalian oxytocin and vasopressin analogues. Gen Comp Endocrinol 179:313–318. [DOI] [PubMed] [Google Scholar]