Abstract
Both social and material rewards play a crucial role in daily life and function as strong incentives for various goal-directed behaviors. However, it remains unclear whether the incentive effects of social and material reward are supported by common or distinct neural circuits. Here, we have addressed this issue by quantitatively synthesizing and comparing neural signatures underlying social (21 contrasts, 207 foci, 696 subjects) and monetary (94 contrasts, 1083 foci, 2060 subjects) reward anticipation. We demonstrated that social and monetary reward anticipation engaged a common neural circuit consisting of the ventral tegmental area, ventral striatum, anterior insula, and supplementary motor area, which are intensively connected during both task and resting states. Functional decoding findings indicate that this generic neural pathway mediates positive value, motivational relevance, and action preparation during reward anticipation, which together motivate individuals to prepare well for the response to the upcoming target. Our findings support the common neural currency hypothesis by providing the first meta-analytic evidence to quantitatively show the common involvement of brain regions in both social and material reward anticipation.
Keywords: Social reward, Reward anticipation, Monetary incentive delay task, Meta-analysis, Functional decoding, Meta-analytic connectivity modeling, Resting-state functional connectivity
1. Introduction
As social beings, human behavior is strongly motivated by social rewards, i.e., desirable outcomes that are social in nature without material payoff. It is common for adults to purchase luxurious products to draw attention and admiration of others, adolescents to devote a lot of time in video games for higher virtual social status, and children to finish their homework with the expectation of being praised. Indeed, our anticipation of social rewards is such a powerful incentive that most of us (if not all) are motivated by it and employ it to motivate other people. Although the importance of social rewards in goal-directed behaviors has long been recognized (Fehr and Schmidt, 1999; Zajonc, 1965), investigations on neural underpinnings of social reward anticipation only emerged in the past decade. A key question addressed in recent neuroimaging studies is whether social rewards are represented in a specific neural circuit or in an analogue manner to the neural encoding of material rewards (e.g., money).
A growing body of studies has compared the neural signatures of social and material rewards with a variety of paradigms (Dvash et al., 2010; Izuma et al., 2008; Rilling et al., 2002; Spreckelmeyer et al., 2009; Tabibnia et al., 2008; Wake and Izuma, 2017; Zink et al., 2008). Among them, the monetary incentive delay (MID) task is one of the most reliable and widely-used paradigms (Knutson et al., 2001a, b; Knutson et al., 2003, 2000; Oldham et al., 2018; Rademacher et al., 2010; Spreckelmeyer et al., 2009; Wilson et al., 2018). In the classic MID, participants are first presented with an incentive cue (e.g., circle) indicating reward information of successfully responding to a target. The cue is followed by a short period of delay (i.e., anticipation phase), after which a target is presented, and participants need to respond to it. Finally, outcome is revealed based on participants’ performance (Knutson, Adams, Fong, & Hommer, 2001; Knutson et al., 2000). In this way, the MID paradigm offers several important advantages. First, this task allows for assessing neural signatures of anticipatory reward processing and separating them from those of consummatory reward processing (Knutson et al., 2001b; Rademacher et al., 2010). Second, the separation between anticipation and outcome phases enables elegant comparisons between anticipation of different types of rewards (e.g., money and smiling faces) by using simple shapes as cue stimuli that are well controlled for non-relevant attributes (e.g., physical properties, familiarity, and self-relevance).
Combining the MID task with functional magnetic resonance imaging (fMRI), previous studies have demonstrated consistent engagement of the ventral striatum (VS) in monetary reward anticipation. In particular, the VS plays a key role in encoding expected positive incentive value (Abler et al., 2006; Bjork et al., 2010a, 2008a; Knutson et al., 2001a; Mucci et al., 2015). Moreover, monetary reward anticipation further engages the anterior insula (AI) and dorsal anterior cingulate cortex/supplementary motor area (dACC/SMA), which are implicated in signaling salience of an upcoming event (Funayama et al., 2014; Kirk et al., 2015; Kollmann et al., 2017; Oldham et al., 2018; Wilson et al., 2018).
Building on previous findings, many recent studies have employed a social variant of the MID (here forth social incentive delay [SID]) task to examine neural signatures of social reward anticipation. In the SID task, social stimuli that are intrinsically rewarding, such as smiling faces, “thumbs-up” gestures, positive verbal messages, were employed as incentives (Goerlich et al., 2017; Kollmann et al., 2017; Spreckelmeyer et al., 2009). Evidence has shown that social reward anticipation recruits both overlapping and distinct neural circuits compared to monetary reward anticipation. On one hand, there is considerable evidence that both social and monetary reward anticipation frequently evoke the activity of VS (Barman et al., 2015; Carter et al., 2009; Kirsch et al., 2003; Rademacher et al., 2014; Spreckelmeyer et al., 2009), supporting the role of the VS as the generic mediator of reward prediction. On the other hand, several studies have shown that social reward anticipation also engages the temporoparietal junction (TPJ), dorsomedial prefrontal cortex (dmPFC), precuneus, and superior temporal gyrus (STG), brain regions that are critical for social-cognitive processes (Barman et al., 2015; Goerlich et al., 2017; Spreckelmeyer et al., 2013). Likewise, electroencephalographic evidence has shown that social and monetary incentive cues evoke differential event-related potential patterns at both early and late temporal stages (Flores et al., 2015). Moreover, neural activation induced by social and monetary reward anticipation are differentially modulated by age (Rademacher et al., 2013), sex (Greimel et al., 2018; Spreckelmeyer et al., 2009; Wang et al., 2017), and social proficiency (Gossen et al., 2014). Taken together, recent evidence suggests a hypothesis that social reward anticipation engages a more complex neural circuit than monetary reward anticipation, consisting of not only reward-related regions (e.g., VS) but also social-cognitive related regions (e.g., dmPFC, TPJ).
Our study examined this hypothesis with a meta-analytic approach to quantitatively synthesize and compare previous brain imaging findings on social and monetary reward anticipation. Meta-analysis is an increasingly popular method to overcome the heterogeneity and divergence of previous results and assess the consistency of previous findings (Gurevitch et al., 2018; Kotov et al., 2010; Müller et al., 2018). Specifically, we first utilized a coordinate-based meta-analysis to quantitatively synthesize previous neuroimaging findings on social and monetary reward anticipation. Second, we examined the correspondence between social and monetary reward anticipation to determine the common and distinct neural circuits underlying different types of rewards. Third, psychological functions of identified brain regions were decoded based on large-scale database, implementing a data-driven quantitative inference on mental processes associated with identified regions (Laird et al., 2009). Finally, the functional profiles of revealed brain regions were characterized by both task-dependent and task-independent functional connectivity. In short, these approaches aim to achieve a more fine-grained characterization of the neural signatures implicated in social and monetary reward anticipation.
2. Materials and methods
2.1. Meta-analysis
2.1.1. Literature search and selection
A systematic online database search was performed in May 2018 using PubMed, ISI Web of Science, and Google Scholar by entering various combinations of relevant search items (e.g., [“monetary incentive delay” OR “MID task” OR “anticipation of reward” OR “reward anticipation” OR “social incentive delay” OR “SID task” OR “incentive delay task”] AND [“functional magnetic resonance imaging” OR “fMRI” OR “positron emission tomography” OR “PET”]). In addition, we have explored several other sources, including: (i) the bibliography and citation indices of the pre-selected papers, and (ii) direct searches on the names of frequently occurring authors.
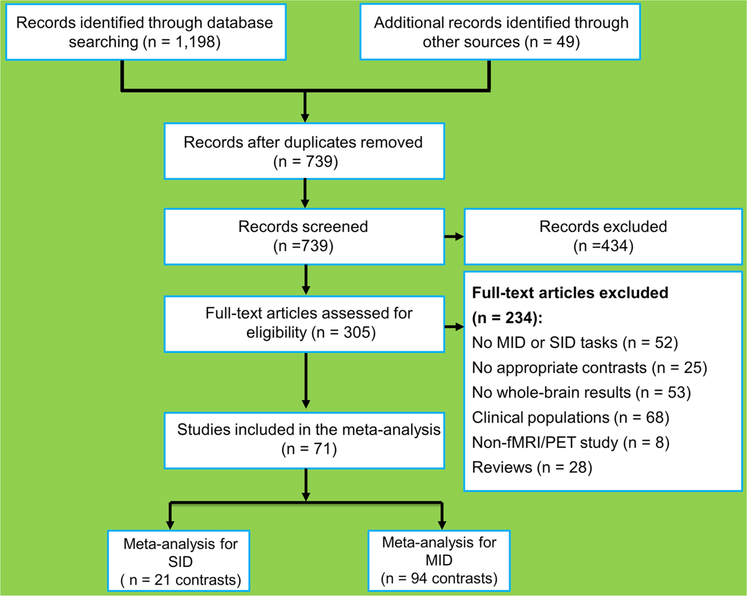
The identified papers were further assessed according to the following criteria (Fig. 1): (i) subjects were free from psychiatric or neurological diagnoses; (ii) the paper reported a SID task, a MID task, or both; (iii) fMRI or PET was used as the imaging modality; (iv) whole-brain general-linear-model-based analyses (rather than region of interest [ROI] analyses) were applied; (v) results were derived from a general linear model based on either a binary contrast (e.g., anticipation of reward > anticipation of no reward) or continuous parametric analyses; and (vi) activations were presented in a standardized stereotaxic space (Talairach or Montreal Neurological Institute, MNI). Note that for the papers reporting Talairach coordinates, a conversion to the MNI coordinates was employed using an icbm2tal algorithm (Lancaster et al., 2007). Filtering search results according to the inclusion/exclusion criteria yielded a total of 21 experiments (207 foci, 696 subjects) for the anticipation of social reward and 94 experiments (1083 foci, 2060 subjects) for the anticipation of monetary reward (Table 1). Notably, the sample in Gossen et al. study (2014: n = 35) overlapped with that in Goerlich et al. (2017: n = 45). Accordingly, we conducted a robust test by excluding the data by Gossen et al. (2014) from the meta-analysis, and the results were essentially same as those of the primary analysis (Fig. S1 and S2).
Fig. 1.
Flow chart of the study selection process for the meta-analysis. SID, social incentive delay task; MID, monetary incentive delay task; fMRI, functional magnetic resonance imaging; PET, positron emission tomography.
Table 1.
Summary of studies included for the meta-analysis of social and monetary reward.
| Study | Task | Methodology | N(male) | Age(sd) | Software | Tesla |
|---|---|---|---|---|---|---|
| Abler et al. (2005) | monetary incentive delay task | fMRI | 12(6) | 21–33 | BrainVoyager 4.9 | 1.5 |
| Adcock et al. (2006) | monetary incentive delay task | fMRI | 12(9) | 18–35 | SPM2 | 3 |
| Arrondo et al. (2015) | monetary incentive delay task | fMRI | 21(17) | 34.33(10.11) | FSL | 3 |
| Balodis et al. (2012) | monetary incentive delay task | fMRI | 14(10) | 37.1(11.3) | SPM5 | 3 |
| Barman et al. (2015) | monetary incentive delay task/social incentive delay task | fMRI | 63(32) | 24.56 | SPM8 | 3 |
| Beck et al. (2009) | monetary incentive delay task | fMRI | 19(19) | 41.68(8.97) | SPM5 | 1.5 |
| Behan et al. (2015) | monetary incentive delay task | fMRI | 20(9) | 23.05 | AFNI | 3 |
| Bjork et al. (2004) | monetary incentive delay task | fMRI | 24(12) | 19.27 | AFNI | 3 |
| Bjork et al. (2008a) | monetary incentive delay task | fMRI | 13(8) | 13.8(0.4) | AFNI | 3 |
| Bjork et al. (2008b) | monetary incentive delay task | fMRI | 23(12) | 32.0(8.0) | AFNI | 3 |
| Bjork et al. (2010b) | monetary incentive delay task | fMRI | 48(24) | 22.05 | AFNI | 3 |
| Bjork et al. (2010a) | monetary incentive delay task | fMRI | 12(9) | 15.3(1.4) | AFNI | 3 |
| Bjork et al. (2012) | monetary incentive delay task | fMRI | 23(12) | 30.1(5.9) | AFNI | 3 |
| Bustamante et al. (2014) | monetary incentive delay task | fMRI | 18(18) | 37.44(8.15) | SPM8 | 1.5 |
| Carl et al. (2016) | monetary incentive delay task | fMRI | 31(8.8) | 20(6) | FSL 5.0.1 | 3 |
| Carter et al. (2009) | monetary incentive delay task/social incentive delay task | fMRI | 17(N) | 24 | FEAT(FSL) | 3 |
| Costumero et al. (2013) | monetary incentive delay task | fMRI | 44(44) | 23.4(4.1) | SPM5 | 1.5 |
| Cremers et al. (2015) | social incentive delay task | fMRI | 20(11) | 27.7(7.7) | FSL Version 4.1.3 | 3 |
| Damiano et al. (2014) | monetary incentive delay task | fMRI | 31(14) | 23.58(3.15) | FEAT(FSL) | 3 |
| Dillon et al. (2010) | monetary incentive delay task | fMRI | 32(18) | 21.68(3.35) | FS-FAST and FreeSurfer | 1.5 |
| Enzi et al. (2012a) | monetary incentive delay task | fMRI | 19(10) | 29.6 | SPM5 | 3 |
| Enzi et al. (2012b) | monetary incentive delay task | fMRI | 15(8) | 34.73(8.29) | SPM5 | 1.5 |
| Figee et al. (2011) | monetary incentive delay task | fMRI | 19(6) | 32(6.6) | SPM2 | 1.5 |
| Funayama et al. (2014) | monetary incentive delay task | fMRI | 20(12) | 29.9 | SPM8 | 1.5 |
| Goerlich et al. (2017) | monetary incentive delay task/social incentive delay task | fMRI | 45(45) | 24.1(3.2) | SPM8 | 3 |
| Gossen et al. (2014) | monetary incentive delay task/social incentive delay task | fMRI | 35(35) | 24.17 | SPM8 | 3 |
| Herbort et al. (2016) | monetary incentive delay task | fMRI | 23(0) | 23.78(5.75) | SPM8 | 3 |
| Juckel et al. (2006) | monetary incentive delay task | fMRI | 10(10) | 31.7(8.4) | SPM2 | 1.5 |
| Juckel et al. (2012) | monetary incentive delay task | fMRI | 13(11) | 25.69(4.84) | SPM5 | 1.5 |
| Kappel et al. (2013) | monetary incentive delay task | fMRI | 30(28) | 20.13 | SPM8 | 3 |
| Kaufmann et al. (2013) | monetary incentive delay task | fMRI | 19(8) | 34.9(11.8) | SPM8 | 1.5 |
| Kirk et al. (2015) | monetary incentive delay task | fMRI | 44(20) | 36.5(9.7) | SPM8 | 3 |
| Kirsch et al. (2003) | monetary incentive delay task/social incentive delay task | fMRI | 27(3) | 23.3 | SPM99 | 1.5 |
| Knutson et al. (2001b) | monetary incentive delay task | fMRI | 9(2) | 26.45(5.85) | AFNI | 1.5 |
| Knutson et al. (2001a) | monetary incentive delay task | fMRI | 8(4) | 31 | AFNI | 1.5 |
| Knutson et al. (2003) | monetary incentive delay task | fMRI | 12(6) | 31 | AFNI | 1.5 |
| Knutson et al. (2008) | monetary incentive delay task | fMRI | 12(4) | 18–48 | AFNI | 1.5 |
| Kocsel et al. (2017) | monetary incentive delay task | fMRI | 37(15) | 25.92(4.18) | SPM12 | 3 |
| Kollmann et al. (2017) | monetary incentive delay task/social incentive delay task | fMRI | 41(20) | 40.2 | SPM8 | 3 |
| Maresh et al. (2014) | monetary incentive delay task | fMRI | 84(42) | 24.56(1.17) | FEAT6(FSL) | 3 |
| Mucci et al. (2015) | monetary incentive delay task | fMRI | 22(10) | 31.91(8.49) | SPM8 | 3 |
| Nawijn et al. (2017) | social incentive delay task | fMRI | 72(40) | 40.12 | SPM8 | 3 |
| Ossewaarde et al. (2011) | monetary incentive delay task | fMRI | 27(0) | 20.51 | SPM5 | 3 |
| Pfabigan et al. (2014) | monetary incentive delay task | fMRI | 25(12) | 23.8(3.6) | SPM8 | 3 |
| Plichta et al. (2013) | monetary incentive delay task | fMRI | 14(8) | 24(4.24) | SPM8 | 3 |
| Pujara et al. (2016) | monetary incentive delay task | fMRI | 14(9) | 62.6(3.9) | AFNI & FSL | 3 |
| Rademacher et al. (2010) | monetary incentive delay task/social incentive delay task | fMRI | 28(13) | 28.26 | SPM5 | 1.5 |
| Rademacher et al. (2014) | social incentive delay task | fMRI | 48(24) | 23.4 / 66.2 | SPM5 | 1.5 |
| Romanczuk-Seiferth et al. (2015) | monetary incentive delay task | fMRI | 17(17) | 37.41(11.76) | SPM8 | 3 |
| Samanez-Larkin et al. (2007) | monetary incentive delay task | fMRI | 24(12) | 23.75(2.05) / 72.92(5.50) | AFNI | 1.5 |
| Saji et al. (2013) | monetary incentive delay task | fMRI | 18(10) | 29.6(6.94) | SPM8 | 1.5 |
| Schlagenhauf et al. (2008) | monetary incentive delay task | fMRI | 10(9) | 31.8(8.7) | SPM2 | 1.5 |
| Schreiter et al. (2016) | monetary incentive delay task | fMRI | 20(8) | 41.45 (7.33) | SPM8 | 3 |
| Simon et al. (2010) | monetary incentive delay task | fMRI | 24(11) | 24.8(3.2) | SPM5 | 3 |
| Spreckelmeyer et al. (2009) | monetary incentive delay task/social incentive delay task | fMRI | 32(16) | 28.9 | SPM5 | 1.5 |
| Spreckelmeyer et al. (2013) | social incentive delay task | fMRI | 30(17) | 22.72 | SPM5 | 3 |
| Stark et al. (2011) | monetary incentive delay task/social incentive delay task | fMRI | 31(0) | 23(2.9) | SPM8 | 1.5 |
| Staudinger et al. (2011) | monetary incentive delay task | fMRI | 24(11) | 25.05(2.79) | SPM5 | 3 |
| Stoy et al. (2011) | monetary incentive delay task | fMRI | 12(12) | 28.08(6.2) | SPM5 | 1.5 |
| Stoy et al. (2012) | monetary incentive delay task | fMRI | 15(10) | 39.5(11.9) | SPM5 | 1.5 |
| Ströhle et al. (2008) | monetary incentive delay task | fMRI | 10(10) | 31.9(9.9) | SPM2 | 1.5 |
| Treadway et al. (2013) | monetary incentive delay task | fMRI | 38(20) | 22 | SPM5 | 3 |
| Van Duin et al. (2016) | monetary incentive delay task | fMRI | 12(8) | 29(9.6) | SPM8 | 3 |
| Weiland et al. (2014) | monetary incentive delay task | fMRI | 12(0) | 30.9(9) | SPM8 | 3 |
| Wittmann et al. (2005) | monetary incentive delay task | fMRI | 16(8) | 22.9(3) | SPM2 | 1.5 |
| Wrase et al. (2007b) | monetary incentive delay task | fMRI | 16(16) | 39.9(8.6) | SPM2 | 1.5 |
| Wrase et al. (2007a) | monetary incentive delay task | fMRI | 14(14) | 39.9(10.3) | SPM2 | 1.5 |
| Wu et al. (2014) | monetary incentive delay task | fMRI | 52(23) | 50(16.5) | AFNI | 1.5 |
| Yan et al. (2016a) | monetary incentive delay task | fMRI | 23(12) | 19.78(0.8) | SPM8 | 3 |
| Yan et al. (2016b) | monetary incentive delay task | fMRI | 22(11) | 19.78(0.8) | SPM8 | 3 |
| Yau et al. (2012) | monetary incentive delay task | fMRI | 20(12) | 20.1(1.3) | FSL 4.0 | 3 |
In light of recent studies on lesion network mapping (Darby et al., 2018a, b), we performed additional literature search and selection to include potentially relevant lesion studies. Specifically, we searched PubMed by entering the following keywords: (“monetary incentive delay” OR “MID task” OR “anticipation of reward” OR “expect of reward” OR “reward anticipation” OR “social incentive delay” OR “SID task” OR “incentive delay task”) AND (“MRI” OR “CT” OR “neuroimaging” OR “lesion”) AND (“damage” OR “stroke” OR “hemorrhage” OR “tumor” OR “lesion”). The search resulted in 10 papers in total, but none of them met the criteria to conduct following-up lesion network mapping.
2.1.2. Activation likelihood estimation (ALE) analysis
A coordinate-based meta-analysis of reported fMRI experiments was conducted, employing the ALE algorithm (in-house MATLAB scripts) (Eickhoff et al., 2009). ALE determines the convergence of foci reported from different functional (e.g., blood-oxygen-level dependent [BOLD] contrast imaging) or structural (e.g., voxel-based morphometry) neuroimaging experiments, with published foci in Talairach or MNI space (Laird et al., 2005; Turkeltaub et al., 2002). In addition, ALE interprets reported foci as spatial probability distributions, of which the widths are based on empirical estimates of the spatial uncertainty due to the between-subject and between-template variability of the neuroimaging data (Eickhoff et al., 2009). The ALE algorithm weighs the between-subject variability based on the number of subjects analyzed in the experiments, modeling larger sample sizes with smaller Gaussian distributions and, thus, presupposing more reliable approximations of the “true” activation for larger sample sizes (Eickhoff et al., 2009).
The union of the individual modulated activation maps, firstly created from the maximum probability associated with any one focus (always the closest one) for each voxel (Turkeltaub et al., 2002), was then calculated to obtain an ALE map across experiments. This ALE map was assessed against a null-distribution of random spatial association between the experiments using a non-linear histogram integration algorithm (Eickhoff et al., 2012, 2009). In addition, the average non-linear contribution of each experiment for each cluster was calculated from the fraction of the ALE values at the cluster, with and without the experiment in question (Eickhoff et al., 2017). Based on the calculated contribution, we employed two additional criteria to select significant clusters: (i) the contributions for one cluster were from at least two experiments so that the findings would not be merely driven by one single experiment; and (ii) the average contribution of the most dominant experiment (MDE) shall not exceed 50% and the average contribution of the two most dominant experiments (2MDEs) shall not exceed 80% (Eickhoff et al., 2017). All ALE maps were thresholded using a cluster-level family-wise error (cFWE) correction (P < 0.05) with a cluster-forming threshold of P < 0.001 using 10,000 permutations for correcting multiple comparisons (Eickhoff et al., 2017).
2.1.3. Conjunction and contrast analyses
After obtaining the consistent maxima separately for the anticipation of social reward and monetary reward, a conjunction analysis between them was conducted to assess the correspondence. This was implemented by using the conservative minimum statistic (Nichols et al., 2005), which is equivalent to identification of the intersection between two corrected ALE results. In addition, differences between the anticipation of social reward and monetary reward were tested by first performing separate ALE analyses for each task and computing the voxel-wise difference between the ensuing ALE maps (Eickhoff et al., 2011). Afterwards, all experiments contributing to either analysis were then pooled and randomly divided into two groups, whose sizes were the same as that in the two original sets of experiments, reflecting the contrasted ALE analyses (Langner et al., 2018). The ALE values for these two randomly assembled groups were calculated, and the differences between the ALE values were recorded for each voxel of the brain. Repeating this process for 25,000 times yielded a null-distribution of differences in ALE values between two tasks. The true difference in the ALE values was then tested against the voxel-wise null-distribution of label-exchangeability, and thresholded at a probability of P > 95% for true differences.
2.2. Task-based connectivity: meta-analytic connectivity modeling (MACM) analyses
To examine the co-activation patterns of the bilateral VS, ventral tegmental area (VTA), SMA, and left AI, which are commonly recruited by social and monetary reward anticipation (see the Results section), we conducted MACM analyses — with these regions as ROIs — using the BrainMap Database (http://www.brainmap.org/) (Laird et al., 2009). MACM delineates patterns of co-activation across thousands of studies using neuroimaging databases and produces data-driven functional connectivity maps based on pre-defined ROIs (Langner et al., 2014). For our analysis, only whole-brain neuroimaging studies reporting activation in standard stereotaxic space in a healthy population were included, while other studies investigating differences in age, sex, handedness, and training effects or clinical populations were excluded. First, whole-brain peak coordinates of all those studies from BrainMap were downloaded if the study reported at least one focus of activation within each ROI. Next, coordinates were analyzed using the ALE algorithm (as described above) to detect areas of convergence of coactivation with each seed. Finally, the ALE maps were thresholded using a cFWE correction (P < 0.05) with a cluster-forming threshold of P < 0.001 using 10,000 permutations for correcting multiple comparisons.
2.3. Task-free connectivity: resting-state functional connectivity (RSFC) analyses
To complement task-based connectivity derived from MACM analyses, whole-brain RSFC of the bilateral VS, VTA, SMA, and left AI as ROIs was assessed. The analysis was based on resting-state fMRI images of 192 healthy volunteers obtained from the enhanced Nathan Kline Institute-Rockland Sample (NKI-RS: http://fcon_1000.projects.nitrc.org/indi/enhanced/) (Nooner et al., 2012). The enhanced NKI-RS is a community-ascertained, lifespan sample in which age, ethnicity, and socioeconomic status are representative of the general population (Horn and Blankenburg, 2016) (see also supplementary methods for details). The enhanced NKI-RS dataset, similar to other open datasets such as Human Connectome Project (HCP, Van Essen et al., 2013), has been widely used in previous studies including those conducting meta-analysis (Krall et al., 2015; Wong et al., 2018). Notably, previous studies that employed both these databases have found that the results derived from these databases were highly similar (Fukushima et al., 2018). In the same vein, one of our recent studies showed that the RSFC results were essentially the same across different datasets (Bellucci et al., 2018), although these data were from (i) different sites and participant populations (Europe vs. China), (ii) different machines (7 T vs. 3 T), and (iii) different scanning parameters. Although validation using different datasets is an intriguing and important topic, this is beyond the scope of the current study.
Implementing a seed-based analysis, the functional connectivity (bivariate correction) between the average BOLD signals from given seed regions (bilateral VS, VTA, SMA, and left AI) and all other voxels in the brain was computed. The voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores and tested for consistency across subjects (see also supplementary methods for details).
2.4. Hierarchical clustering on resting-state functional connectivity of brain regions identified in the contrast analysis
To further reveal potential functions of those regions that were observed in the contrast analyses (i.e., social > monetary or monetary > social), we implemented hierarchical clustering based on RSFC of these regions. As such, we aimed to examine whether regions showing differential activation to social and monetary reward anticipation could also be distinguished by their RSFC patterns (Amft et al., 2015; Camilleri et al., 2018). RSFC analysis was conducted between all identified regions (see also supplementary methods for details). Using the singular value decomposition (SVD) function implemented in MATLAB/SPM8, the time-course of each seed (i.e., each identified region) was extracted per subject by computing the first eigenvariate of the time-series of all voxels within the seed. Employing the first eigenvariate is a common practice as this approach — compared to using mean time-series — is less likely to be influenced disproportionally by potential outliers when using large and inhomogeneous regional templates (Friston et al., 2006). However, both approaches (i.e., first eigenvariate and mean) will lead to reliable and highly similar results when ROIs are relatively small (as those in the current study) (see also Braun et al., 2012). To reduce spurious correlations, variance explained by the mean white matter and cerebral spinal fluid signal were removed from the time series, which was subsequently band-pass filtered to preserve the frequencies between 0.01 and 0.08 Hz. RSFC between all regions of the contrast analysis was computed using the FSLNets toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). Partial temporal correlations between time series data of all regions were computed to estimate pairwise functional connectivity (Marrelec et al., 2006). For each pairwise connection, Fisher’s Z-transformed functional connectivity values were submitted to one-sample t-tests. The resulting t values, reflecting connection strength as well as consistency across the sample, were z-transformed (i.e., into units of the standard normal distribution). This connectivity matrix was then fed into the Ward clustering, which is a commonly used method for hierarchical cluster analysis (Murtagh and Legendre, 2014).
The key concept of Ward/hierarchical clustering is to group the initial elements (regions) in a stepwise fashion such that the elements within a cluster have features that are as homogeneous as possible, while different clusters are distinct from each other as far as possible. This is achieved through an agglomerative approach in which clusters initially formed by individual regions are subsequently merged according to their similarity using standardized Euclidean distances and Ward’s incremental sum of squares method (Eickhoff et al., 2011; Timm and Kruse, 2002). This hierarchical approach then reveals cliques of all identified regions at different level of granularity based on resting-state connectivity. In particular, the distance between two clusters is defined as the increase in variance for the cluster being merged (Ward, 1963). Based on this understanding, Ward clustering forms clusters by minimizing the total within-cluster variation, which is measured as the sum of squares of the Euclidean distances between each point in the cluster and the cluster centroid. In each step, the two clusters with the lowest intracluster variation would be joined (Danielsson et al., 1999). According to Sharma and Sharma (1996), Ward’s method is superior in giving a larger amount of correct classified observations.
2.5. Functional decoding
Based on the BrainMap database (http://www.brainmap.org/), functional decoding was implemented for each region identified in the conjunction analysis and for each subcluster of brain regions derived from contrast analysis (see also the Results section). In particular, we performed the functional characterization based on the behavioral domain meta-data categories available for each neuroimaging experiment included in the BrainMap database (Turner and Laird, 2012) (see http://brainmap.org/scribe/). We determined the individual functional profile corresponding to each region/subcluster by using forward and reverse inference approaches. Forward inference refers to the probability of identifying activity in a region/subcluster, given the knowledge of the psychological process, whereas reverse inference refers to the probability of a psychological process being present, given the knowledge of a particular region/subcluster (for details, see also Bzdok et al., 2013; Chen et al., 2018; Genon et al., 2017). Significance was obtained at P < 0.05, corrected for multiple comparisons using the false discovery rate (FDR) method.
2.6. Validation analysis
We implemented additional analyses to validate findings derived from conventional ALE meta-analysis. First, we implemented a leave-one-experiment-out (LOEO) analysis for the ALE meta-analyses on the anticipation of social reward or monetary reward. On each fold, one experiment was excluded, and the ALE meta-analysis was conducted on the remaining N-1 experiments. Afterwards, we conducted a conjunction analysis on the ALE results of all folds to identify the brain regions that were robustly engaged in social/monetary reward anticipation. These analyses were employed to validate our main ALE meta-analysis findings on social or monetary reward anticipation.
Second, the unbalanced number of experiments between social and monetary reward anticipation (21 vs. 94) could lead to differences in statistical power for the experiments to be compared. To address this issue, we implemented an additional re-subsampling approach for the conjunction and contrast analyses. The re-subsampling was repeated for 1000 rounds. During each round, 19 experiments were randomly selected from datasets of social and monetary reward anticipation. The conjunction and contrast analyses were then conducted for the selected experiments of each dataset. Afterwards, conjunction and contrast results of all rounds were averaged to represent the re-subsampling findings. These analyses were employed to validate our main conjunction and contrast findings.
Third, the ALE approach treats different experiments within a paper as distinct experiments (Laird et al., 2009). This approach (i) ensures that all relevant information in a paper is represented in the meta-analysis, and (ii) avoids selection of a single experiment based on subjective and inconsistent criteria across publications. However, an issue with this approach is that multiple experiments from a single study might be related. Although this issue could be partly addressed by the LOEO and resampling methods mentioned above, we further implemented a supplementary analysis by combining different experiments from the same paper into a single experiment. As such, multiple experiments from a single paper would not independently influence the results of meta-analyses (Turkeltaub et al., 2012). Combining within-paper experiments resulted in 18 experiments for social reward anticipation and 75 experiments for monetary reward anticipation.
All ALE maps were thresholded using a cFWE correction (P < 0.05) with a cluster-forming threshold of P < 0.001 using 10,000 permutations for correcting multiple comparisons.
3. Results
3.1. ALE findings
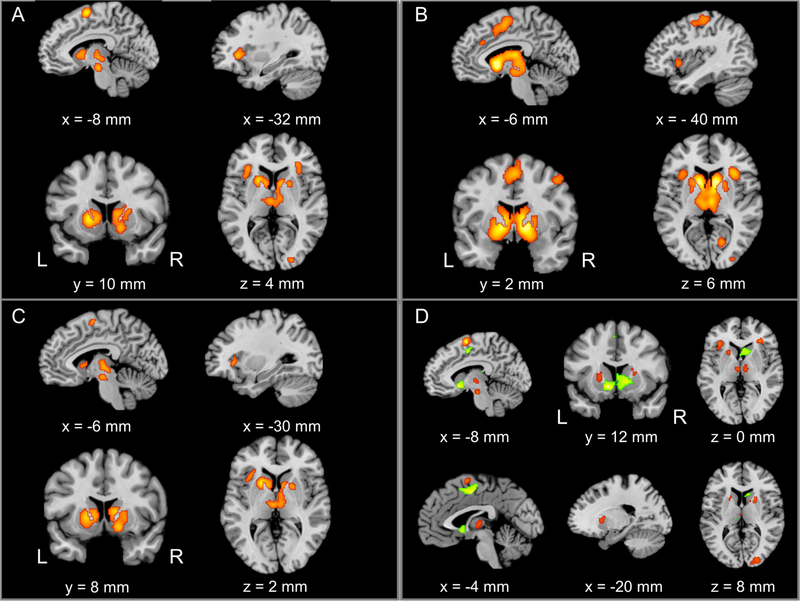
For social reward anticipation, the ALE meta-analysis revealed significant convergence of activity in the bilateral VS, dACC/SMA, AI, VTA, and middle occipital gyrus (MOG) (Fig. 2a and Table 2). Twelve out of 21 contrasts (reward vs. no reward) contributed to the cluster in the left VS (MDE = 11.6%; 2MDE = 22.92%, Table 3). Fifteen out of 21 contrasts contributed to the cluster in the right VS (MDE = 16.02%; 2MDE = 28.61%, Table 3). Seven out of 21 contrasts contributed to the cluster in the dACC/SMA (MDE = 18.46%; 2MDE = 35.75%, Table 3). Five out of 21 contrasts contributed to the cluster in the left AI (MDE = 31.64%; 2MDE = 60.69%, Table 3). Seven out of 21 contrasts contributed to the cluster in the right AI (MDE = 25.99%; 2MDE = 49.93%, Table 3). Seven out of 21 contrasts contributed to the cluster in the midbrain (MDE = 28.92%; 2MDE = 56.83%, Table 3). Seven out of 21 contrasts contributed to the cluster in the MOG (MDE = 26.94%; 2MDE = 47.77%, Table 3).
Fig. 2.
Significant clusters from the main coordinate-based activation likelihood estimation meta-analysis (cluster-level family-wise error correction [P < 0.05] with a cluster-forming threshold of P < 0.001 using 10,000 permutations) for social reward anticipation, monetary reward anticipation, and their conjunction and contrasts. Consistent maximum for: (A) social reward anticipatation; (B) monetary reward anticipation; (C) the conjunction of social and monetary reward anticipation; (D) the contrasts of social and monetary reward anticipation. Brain regions showing higher activation in the anticipation of social reward are illustrated in red, whereas regions showing higher activation in the anticipation of monetary reward are illustrated in green. L, left; R, right.
Table 2.
ALE meta-analysis results for anticipation of social reward, monetary reward, their conjunction and contrasts.
| Laterality | Brain Regions | Brodmann Area | MNI Coordinates |
peak Z score | Cluster Size (mm3) | |||
|---|---|---|---|---|---|---|---|---|
| (mm) |
||||||||
| x | y | z | ||||||
| Social reward anticipation | ||||||||
| L | VS | / | −18 | 12 | 0 | 7.43 | 3672 | |
| R | VS | / | 16 | 6 | −10 | 6.14 | 6872 | |
| L/R | dACC/SMA | 6 | −8 | 0 | 64 | 7.03 | 2608 | |
| L | anterior insula | 13/47 | −32 | 26 | 0 | 5.47 | 1208 | |
| R | anterior insula | 13/47 | 30 | 34 | 0 | 4.89 | 768 | |
| L | ventral tegmental area | / | −8 | −18 | −18 | 5.25 | 736 | |
| R | middle occipital gyrus | 18/19 | 26 | −92 | 8 | 4.6 | 960 | |
| Monetary reward anticipation | ||||||||
| L/R | VS | / | −12 | 10 | −2 | 8.5 | 46296 | |
| L/R | dACC | 32/24 | 0 | 32 | 26 | 5.01 | 872 | |
| L/R | dACC/SMA | 6/32/24/8 | 0 | −2 | 54 | 6.7 | 7560 | |
| L | postcentral gyrus | 4/6/3 | −40 | −22 | 56 | 4.74 | 1824 | |
| R | precentral gyrus | 6 | 46 | 0 | 46 | 4.93 | 1096 | |
| R | middle occipital gyrus | 18/19 | 32 | −88 | −6 | 5.03 | 1608 | |
| R | cuneus | 31/23/17 | 16 | −70 | 10 | 5.93 | 1440 | |
| Conjunction | ||||||||
| L | VS | / | −18 | 12 | 0 | 7.43 | 3664 | |
| R | VS | / | 16 | 6 | −10 | 6.14 | 6136 | |
| L/R | SMA | 6 | −6 | −2 | 60 | 4.66 | 616 | |
| L | anterior insula | 13/47 | −30 | 24 | 0 | 3.97 | 608 | |
| L | ventral tegmental area | / | −6 | −16 | −16 | 4.57 | 480 | |
| Social reward > Monetary reward | ||||||||
| R | dorsal caudate | / | 18 | 8 | 12 | 2.75 | 96 | |
| L/R | SMA | 6 | −8 | 0 | 64 | 7.03 | 2344 | |
| L | dorsal anterior insula | 47/13/45 | −36 | 28 | 2 | 3.02 | 720 | |
| R | dorsal anterior insula | / | 34 | 32 | 4 | 3.06 | 416 | |
| L | putamen | / | −18 | 12 | 4 | 2.31 | 320 | |
| R | putamen | / | 22 | 6 | 10 | 2.62 | 240 | |
| L | thalamus | / | −4 | −14 | 4 | 2.42 | 536 | |
| R | thalamus | / | 8 | −10 | −2 | 3.14 | 592 | |
| L | midbrain | / | −6 | −18 | −22 | 2.67 | 368 | |
| R | middle occipital gyrus | 18 | 26 | −90 | 8 | 2.67 | 840 | |
| Monetary reward > Social reward | ||||||||
| L | VS | / | −8 | 12 | −10 | 3.24 | 4464 | |
| R | dorsal caudate | / | 12 | −2 | 16 | 1.93 | 88 | |
| L/R | dACC | 6/24/32 | 0 | −4 | 48 | 3.2 | 1872 | |
| R | ventral anterior insula | 47/13 | 28 | 20 | −8 | 1.98 | 72 | |
| L | thalamus | / | −10 | −24 | 12 | 2.01 | 112 | |
VS, ventral striatum; dACC, dorsal anterior cingulate cortex; SMA, supplementary motor area.
Table 3.
Average contribution of each experimental contrast for significant clusters identified for the meta-analysis of social reward anticipation.
| Cluster Name. | Study | N | Task | Contrast | No. of foci | Average contribution (%) |
|---|---|---|---|---|---|---|
| left VS | Spreckelmeyer et al. (2009) | 32 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 11.6 |
| Rademacher et al. (2014) | 48 | social incentive delay task | social reward > no reward | 8 | 11.32 | |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 11 | 11.21 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | social reward > no reward | 9 | 10.99 | |
| Carter et al. (2009) | 17 | social incentive delay task | social reward > no reward | 10 | 9.83 | |
| Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 8.96 | |
| Rademacher et al. (2010) | 28 | social incentive delay task | social reward > no reward | 13 | 7.62 | |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 3 | 6.93 | |
| Gossen et al. (2014) | 15 | social incentive delay task | social reward > no reward | 25 | 5.99 | |
| Cremers et al. (2015) | 40 | social incentive delay task | social reward > no reward | 4 | 5.91 | |
| Kollmann et al. (2017) | 41 | social incentive delay task | social reward > no reward | 11 | 4.83 | |
| Kirsch et al. (2003) | 27 | social incentive delay task | social reward > no reward | 18 | 4.76 | |
| right VS | Spreckelmeyer et al. (2013) | 30 | social incentive delay task | high social reward > low social reward | 20 | 16.02 |
| Gossen et al. (2014) | 15 | social incentive delay task | social reward > no reward | 25 | 12.59 | |
| Gossen et al. (2014) | 35 | social incentive delay task | social reward > no reward | 10 | 11.34 | |
| Carter et al.(2009) | 17 | social incentive delay task | social reward > no reward | 10 | 10.61 | |
| Kirsch et al. (2003) | 27 | social incentive delay task | social reward > no reward | 18 | 8.51 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | social reward > no reward | 9 | 8.45 | |
| Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 7.64 | |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 11 | 6.43 | |
| Rademacher et al. (2014) | 48 | social incentive delay task | social reward > no reward | 8 | 4.92 | |
| Kollmann et al. (2017) | 41 | social incentive delay task | social reward > no reward | 11 | 3.22 | |
| Spreckelmeyer et al. (2013) | 30 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 3.2 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 1.99 | |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 3 | 1.88 | |
| Cremers et al. (2015) | 40 | social incentive delay task | social reward > no reward | 4 | 1.8 | |
| Rademacher et al. (2010) | 28 | social incentive delay task | social reward > no reward | 13 | 1.4 | |
| dACC/SMA | Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 18.46 |
| Gossen et al. (2014) | 35 | social incentive delay task | social reward > no reward | 10 | 17.29 | |
| Gossen et al. (2014) | 20 | social incentive delay task | social reward > no reward | 11 | 16.6 | |
| Spreckelmeyer et al. (2013) | 30 | social incentive delay task | high social reward > low social reward | 20 | 15.93 | |
| Gossen et al. (2014) | 15 | social incentive delay task | social reward > no reward | 25 | 14.7 | |
| Kollmann et al. (2017) | 41 | social incentive delay task | social reward > no reward | 11 | 9.36 | |
| Nawijn et al. (2017) | 72 | social incentive delay task | social reward > no reward | 5 | 7.67 | |
| anterior insula | Gossen et al. (2014) | 35 | social incentive delay task | social reward > no reward | 10 | 31.64 |
| Gossen et al. (2014) | 20 | social incentive delay task | social reward > no reward | 11 | 29.05 | |
| Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 20.11 | |
| Kollmann et al. (2017) | 41 | social incentive delay task | social reward > no reward | 11 | 17.73 | |
| Kirsch et al. (2003) | 27 | social incentive delay task | social reward > no reward | 18 | 1.41 | |
| anterior insula | Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 25.99 |
| Rademacher et al. (2010) | 28 | social incentive delay task | social reward > no reward | 13 | 23.94 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 17.6 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | social reward > no reward | 9 | 17.6 | |
| Kirsch et al. (2003) | 27 | social incentive delay task | social reward > no reward | 18 | 10.34 | |
| Carter et al.(2009) | 17 | social incentive delay task | social reward > no reward | 10 | 4.13 | |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 11 | 0.4 | |
| ventral tegmental area | Goerlich et al. (2017) | 45 | social incentive delay task | social reward > no reward | 20 | 28.92 |
| Gossen et al. (2014) | 35 | social incentive delay task | social reward > no reward | 10 | 27.91 | |
| Gossen et al. (2014) | 15 | social incentive delay task | social reward > no reward | 25 | 25.44 | |
| Spreckelmeyer et al. (2013) | 30 | social incentive delay task | high social reward > low social reward | 20 | 15.41 | |
| Carter et al.(2009) | 17 | social incentive delay task | social reward > no reward | 10 | 1.34 | |
| Spreckelmeyer et al. (2013) | 30 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 0.57 | |
| Kirsch et al. (2003) | 27 | social incentive delay task | social reward > no reward | 18 | 0.4 | |
| middle occipital gyrus | Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 11 | 26.94 |
| Spreckelmeyer et al. (2009) | 16 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 3 | 20.83 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | social reward > no reward | 9 | 19.86 | |
| Spreckelmeyer et al. (2009) | 32 | social incentive delay task | brain regions showing proportional activation to increasing anticipated reward | 6 | 18.46 | |
| Spreckelmeyer et al. (2013) | 30 | social incentive delay task | high social reward > low social reward | 20 | 13.14 | |
| Rademacher et al. (2010) | 28 | social incentive delay task | social reward > no reward | 13 | 0.52 | |
| Goerlich et al. (2017) | 45 | Monetary incentive delay task/social incentive delay task | social reward > monetary reward | 6 | 0.18 |
The ALE meta-analysis on monetary reward anticipation revealed significant convergence of activity in the VS, dACC, dACC/SMA, postcentral gyrus, precentral gyrus, MOG, and cuneus (Fig. 2b and Table 2). Eighty-eight out of 94 contrasts (reward vs. no reward) contributed to the cluster in the VS (MDE = 3.25%; 2MDE = 6.39%, Table 4). Thirteen out of 94 contrasts contributed to the cluster in the dACC (MDE = 14.84%; 2MDE = 27.55%, Table 4). Forty-two out of 94 contrasts contributed to the cluster in the dACC/SMA (MDE = 7.01%; 2MDE = 12.82%, Table 4). Twenty-one out of 94 contrasts contributed to the cluster in the postcentral gyrus (MDE = 8.01%; 2MDE = 15.83%, Table 4). Fourteen out of 94 contrasts contributed to the first cluster in the precentral gyrus (MDE = 13.73%; 2MDE = 27.33%, Table 4). Twenty-two out of 94 contrasts contributed to the second cluster in the MOG (MDE = 11.65%; 2MDE = 21.81%, Table 4). Twenty-one out of 94 contrasts contributed to the cluster in the cuneus (MDE = 10.2%; 2MDE = 9.89%, Table 4).
Table 4.
Average contribution of each experimental contrast for significant clusters identified for the meta-analysis of monetary reward anticipation.
| Cluster Name. | Study | N | Task | Contrast | No. of foci | Average contribution (%) |
|---|---|---|---|---|---|---|
| VS | Adcock et al. (2006) | 12 | monetary incentive delay task | monetary reward > no reward | 17 | 3.25 |
| Adcock et al. (2006) | 12 | monetary incentive delay task | monetary reward > no reward | 17 | 3.14 | |
| Bjork et al. (2004) | 12 | monetary incentive delay task | monetary reward > no reward | 13 | 2.9 | |
| Treadway et al. (2013) | 38 | monetary incentive delay task | monetary reward > no reward | 13 | 2.55 | |
| Herbort et al. (2016) | 23 | monetary incentive delay task | monetary reward > no reward | 25 | 2.5 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 21 | 2.38 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task | monetary reward > no reward | 26 | 2.2 | |
| Enzi et al. (2012a) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 2.17 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task/social incentive delay task | monetary reward > social reward | 12 | 2.13 | |
| Saji et al. (2013) | 18 | monetary incentive delay task | monetary reward > no reward | 22 | 2.07 | |
| Wu et al. (2014) | 52 | monetary incentive delay task | monetary reward > no reward | 20 | 2.05 | |
| Kirk et al. (2015) | 44 | monetary incentive delay task | monetary reward > no reward | 15 | 1.91 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 1.85 | |
| Stark et al. (2011) | 31 | monetary incentive delay task | monetary reward > no reward | 14 | 1.82 | |
| Knutson et al. (2001b) | 9 | monetary incentive delay task | monetary reward > no reward | 13 | 1.68 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 43 | 1.61 | |
| Wittmann et al. (2005) | 16 | monetary incentive delay task | monetary reward > no reward | 22 | 1.6 | |
| Bjork et al. (2004) | 12 | monetary incentive delay task | monetary reward > no reward | 8 | 1.56 | |
| Knutson et al. (2008) | 12 | monetary incentive delay task | monetary reward > no reward | 8 | 1.54 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 1.47 | |
| Wrase et al. (2007a) | 14 | monetary incentive delay task | monetary reward > no reward | 18 | 1.46 | |
| Knutson et al. (2001a) | 8 | monetary incentive delay task | monetary reward > no reward | 10 | 1.4 | |
| Enzi et al. (2012b) | 15 | monetary incentive delay task | monetary reward > no reward | 9 | 1.39 | |
| Beck et al. (2009) | 19 | monetary incentive delay task | monetary reward > no reward | 6 | 1.39 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 13 | 1.36 | |
| Damiano et al. (2014) | 31 | monetary incentive delay task | monetary reward > no reward | 15 | 1.35 | |
| Costumero et al. (2013) | 44 | monetary incentive delay task | monetary reward > monetary punishment | 18 | 1.34 | |
| Juckel et al. (2012) | 13 | monetary incentive delay task | monetary reward > no reward | 18 | 1.32 | |
| Maresh et al. (2014) | 84 | monetary incentive delay task | monetary reward > no reward | 18 | 1.32 | |
| Knutson et al. (2001a) | 8 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 1.31 | |
| Knutson et al. (2003) | 12 | monetary incentive delay task | monetary reward > no reward | 10 | 1.29 | |
| Bjork et al. (2010b) | 12 | monetary incentive delay task | monetary reward > no reward | 7 | 1.28 | |
| Juckel et al. (2006) | 10 | monetary incentive delay task | monetary reward > no reward | 9 | 1.26 | |
| Rademacher et al. (2010) | 28 | monetary incentive delay task | monetary reward > no reward | 36 | 1.23 | |
| Bjork et al. (2008b) | 23 | monetary incentive delay task | monetary reward > no reward | 17 | 1.22 | |
| Kappel et al. (2013) | 10 | monetary incentive delay task | monetary reward > no reward | 13 | 1.19 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 10 | 1.19 | |
| Carl et al. (2016) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 1.17 | |
| Arrondo et al. (2015) | 21 | monetary incentive delay task | monetary reward > no reward | 29 | 1.13 | |
| Pfabigan et al. (2014) | 25 | monetary incentive delay task | monetary reward > no reward | 12 | 1.13 | |
| Carter et al. (2009) | 17 | monetary incentive delay task | monetary reward > no reward | 10 | 1.12 | |
| Ströhle et al. (2008) | 10 | monetary incentive delay task | monetary reward > no reward | 7 | 1.04 | |
| Yau et al. (2012) | 20 | monetary incentive delay task | monetary reward > no reward | 6 | 1.03 | |
| Costumero et al. (2013) | 44 | monetary incentive delay task | monetary reward > no reward | 16 | 1.02 | |
| Yan et al. (2016a) | 23 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 15 | 0.99 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 12 | 0.98 | |
| Figee et al. (2011) | 19 | monetary incentive delay task | monetary reward > no reward | 7 | 0.97 | |
| Spreckelmeyer et al. (2009) | 32 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 7 | 0.96 | |
| Staudinger et al. (2011) | 24 | monetary incentive delay task | high monetary reward > low monetary reward | 6 | 0.91 | |
| Romanczuk-Seiferth et al. (2015) | 17 | monetary incentive delay task | monetary reward > no reward | 22 | 0.89 | |
| Simon et al. (2010) | 24 | monetary incentive delay task | monetary reward > no reward | 6 | 0.87 | |
| Plichta et al. (2013) | 14 | monetary incentive delay task | monetary reward > no reward | 6 | 0.87 | |
| Spreckelmeyer et al. (2009) | 32 | monetary incentive delay task | monetary reward > no reward | 8 | 0.87 | |
| Stoy et al. (2012) | 15 | monetary incentive delay task | monetary reward > no reward | 6 | 0.86 | |
| Funayama et al. (2014) | 20 | monetary incentive delay task | monetary reward > no reward | 17 | 0.85 | |
| Schlagenhauf et al. (2008) | 10 | monetary incentive delay task | monetary reward > no reward | 12 | 0.84 | |
| Kappel et al. (2013) | 20 | monetary incentive delay task | monetary reward > no reward | 6 | 0.83 | |
| Kollmann et al. (2017) | 41 | monetary incentive delay task | monetary reward > no reward | 17 | 0.82 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 8 | 0.82 | |
| Stoy et al.(2011) | 12 | monetary incentive delay task | monetary reward > no reward | 6 | 0.82 | |
| Kocsel et al. (2017) | 37 | monetary incentive delay task | monetary reward > no reward | 10 | 0.76 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 7 | 0.75 | |
| Abler et al. (2005) | 12 | monetary incentive delay task | monetary reward anticipation > target + outcome | 3 | 0.74 | |
| Schlagenhauf et al. (2008) | 10 | monetary incentive delay task | monetary reward > no reward | 9 | 0.74 | |
| Abler et al. (2005) | 12 | monetary incentive delay task | high monetary reward > low monetary reward | 4 | 0.73 | |
| Behan et al. (2015) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 0.71 | |
| Dillon et al. (2010) | 32 | monetary incentive delay task | monetary reward > no reward | 7 | 0.71 | |
| Yan et al. (2016a) | 23 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 6 | 0.7 | |
| Kappel et al. (2013) | 20 | monetary incentive delay task | monetary reward > no reward | 7 | 0.66 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 0.63 | |
| Ossewaarde et al. (2011) | 27 | monetary incentive delay task | monetary reward > no reward | 12 | 0.62 | |
| Gossen et al. (2014) | 20 | monetary incentive delay task | monetary reward > no reward | 8 | 0.62 | |
| Gossen et al. (2014) | 15 | monetary incentive delay task | monetary reward > no reward | 7 | 0.53 | |
| Kaufmann et al. (2013) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 0.51 | |
| Kaufmann et al. (2013) | 19 | monetary incentive delay task | monetary reward > monetary punishment | 3 | 0.48 | |
| Stoy et al. (2012) | 15 | monetary incentive delay task | monetary reward > no reward | 3 | 0.42 | |
| Wrase et al. (2007b) | 16 | monetary incentive delay task | monetary reward > no reward | 2 | 0.4 | |
| Schreiter et al. (2016) | 20 | monetary incentive delay task | monetary reward > no reward | 8 | 0.38 | |
| Barman et al. (2015) | 63 | monetary incentive delay task/social incentive delay task | monetary reward > social reward | 2 | 0.37 | |
| Pujara et al. (2016) | 14 | monetary incentive delay task | monetary reward > no reward | 6 | 0.32 | |
| Bustamante et al. (2014) | 18 | monetary incentive delay task | monetary reward > no reward | 3 | 0.28 | |
| Mucci et al. (2015) | 22 | monetary incentive delay task | monetary reward > no reward | 11 | 0.27 | |
| Weiland et al. (2014) | 12 | monetary incentive delay task | monetary reward > no reward | 4 | 0.26 | |
| Abler et al. (2005) | 12 | monetary incentive delay task | monetary reward > no reward | 1 | 0.25 | |
| Bjork et al. (2008a) | 13 | monetary incentive delay task | monetary reward > no reward | 1 | 0.2 | |
| Costumero et al. (2013) | 44 | monetary incentive delay task | high monetary reward > low monetary reward | 2 | 0.19 | |
| Simon et al. (2010) | 24 | monetary incentive delay task | monetary reward > no reward | 1 | 0.15 | |
| Balodis et al. (2012) | 14 | monetary incentive delay task | monetary reward > no reward | 4 | 0.14 | |
| dACC | Treadway et al. (2013) | 38 | monetary incentive delay task | monetary reward > no reward | 13 | 14.84 |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 12.71 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | monetary reward > no reward | 17 | 11.91 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 11.91 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | monetary reward > no reward | 17 | 11.27 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 9.47 | |
| Arrondo et al. (2015) | 21 | monetary incentive delay task | monetary reward > no reward | 29 | 7.85 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 12 | 7.35 | |
| Knutson et al. (2001a) | 8 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 5.06 | |
| Rademacher et al. (2010) | 28 | monetary incentive delay task | monetary reward > no reward | 36 | 2.85 | |
| Enzi et al. (2012a) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 2.3 | |
| Simon et al. (2010) | 24 | monetary incentive delay task | monetary reward > no reward | 6 | 1.29 | |
| Carter et al. (2009) | 17 | monetary incentive delay task/social incentive delay task | monetary reward > social reward | 7 | 0.88 | |
| dACC/SMA | Maresh et al. (2014) | 84 | monetary incentive delay task | monetary reward > no reward | 18 | 7.01 |
| Goerlich et al. (2017) | 45 | monetary incentive delay task | monetary reward > no reward | 4 | 5.81 | |
| Costumero et al. (2013) | 44 | monetary incentive delay task | monetary reward > no reward | 16 | 5.13 | |
| Knutson et al. (2001a) | 8 | monetary incentive delay task | monetary reward > no reward | 10 | 4.32 | |
| Funayama et al. (2014) | 20 | monetary incentive delay task | monetary reward > no reward | 17 | 3.97 | |
| Knutson et al. (2001b) | 9 | monetary incentive delay task | monetary reward > no reward | 13 | 3.96 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task | monetary reward > no reward | 26 | 3.63 | |
| Plichta et al. (2013) | 14 | monetary incentive delay task | monetary reward > no reward | 6 | 2.78 | |
| Herbort et al. (2016) | 23 | monetary incentive delay task | monetary reward > no reward | 25 | 2.75 | |
| Gossen et al. (2014) | 35 | monetary incentive delay task | monetary reward > no reward | 3 | 2.74 | |
| Ossewaarde et al. (2011) | 27 | monetary incentive delay task | monetary reward > no reward | 12 | 2.72 | |
| Knutson et al. (2001a) | 8 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 2.67 | |
| Kappel et al. (2013) | 20 | monetary incentive delay task | monetary reward > no reward | 7 | 2.59 | |
| Figee et al. (2011) | 19 | monetary incentive delay task | monetary reward > no reward | 7 | 2.57 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 13 | 2.49 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 2.49 | |
| Wittmann et al. (2005) | 16 | monetary incentive delay task | monetary reward > no reward | 22 | 2.46 | |
| Dillon et al. (2010) | 32 | monetary incentive delay task | monetary reward > no reward | 7 | 2.38 | |
| Kollmann et al. (2017) | 41 | monetary incentive delay task | monetary reward > no reward | 17 | 2.21 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 2.21 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 10 | 2.2 | |
| Kirk et al. (2015) | 44 | monetary incentive delay task | monetary reward > no reward | 15 | 2.19 | |
| Kappel et al. (2013) | 20 | monetary incentive delay task | monetary reward > no reward | 6 | 2.16 | |
| Knutson et al. (2003) | 12 | monetary incentive delay task | monetary reward > no reward | 10 | 2.14 | |
| Behan et al. (2015) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 2.08 | |
| Damiano et al. (2014) | 31 | monetary incentive delay task | monetary reward > no reward | 15 | 2.08 | |
| Bjork et al. (2008b) | 23 | monetary incentive delay task | monetary reward > no reward | 17 | 1.96 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 43 | 1.85 | |
| Gossen et al. (2014) | 20 | monetary incentive delay task | monetary reward > no reward | 8 | 1.83 | |
| Kaufmann et al. (2013) | 19 | monetary incentive delay task | monetary reward > monetary punishment | 3 | 1.8 | |
| Staudinger et al. (2011) | 24 | monetary incentive delay task | high monetary reward > low monetary reward | 6 | 1.66 | |
| Kaufmann et al. (2013) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 1.57 | |
| Stark et al. (2011) | 31 | monetary incentive delay task | monetary reward > no reward | 14 | 1.5 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 12 | 1.5 | |
| Saji et al. (2013) | 18 | monetary incentive delay task | monetary reward > no reward | 22 | 1.26 | |
| Pfabigan et al. (2014) | 25 | monetary incentive delay task | monetary reward > no reward | 12 | 1.18 | |
| Enzi et al. (2012a) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 1.15 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task/social incentive delay task | monetary reward > social reward | 26 | 0.96 | |
| Kappel et al. (2013) | 10 | monetary incentive delay task | monetary reward > no reward | 13 | 0.86 | |
| Arrondo et al. (2015) | 21 | monetary incentive delay task | monetary reward > no reward | 29 | 0.64 | |
| Romanczuk-Seiferth et al. (2015) | 17 | monetary incentive delay task | monetary reward > no reward | 22 | 0.32 | |
| Rademacher et al. (2010) | 28 | monetary incentive delay task | monetary reward > no reward | 36 | 0.04 | |
| postcentral gyrus | Costumero et al. (2013) | 44 | monetary incentive delay task | monetary reward > no reward | 16 | 8.01 |
| Stark et al. (2011) | 31 | monetary incentive delay task | monetary reward > no reward | 14 | 7.82 | |
| Knutson et al. (2001b) | 9 | monetary incentive delay task | monetary reward > no reward | 13 | 7.61 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 7.56 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 43 | 7.42 | |
| Kappel et al. (2013) | 20 | monetary incentive delay task | monetary reward > no reward | 6 | 6.95 | |
| Behan et al. (2015) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 6.91 | |
| Kollmann et al. (2017) | 41 | monetary incentive delay task | monetary reward > no reward | 17 | 6.77 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 10 | 6.38 | |
| Pfabigan et al. (2014) | 25 | monetary incentive delay task | monetary reward > no reward | 12 | 6.25 | |
| Romanczuk-Seiferth et al. (2015) | 17 | monetary incentive delay task | monetary reward > no reward | 22 | 5.07 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task | monetary reward > no reward | 26 | 4.12 | |
| Arrondo et al. (2015) | 21 | monetary incentive delay task | monetary reward > no reward | 29 | 3.68 | |
| Herbort et al. (2016) | 23 | monetary incentive delay task | monetary reward > no reward | 25 | 3.65 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 13 | 3.54 | |
| Carl et al. (2016) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 2.59 | |
| Wittmann et al. (2005) | 16 | monetary incentive delay task | monetary reward > no reward | 22 | 1.87 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 1.63 | |
| Bjork et al. (2008b) | 23 | monetary incentive delay task | monetary reward > no reward | 17 | 0.87 | |
| Ossewaarde et al. (2011) | 27 | monetary incentive delay task | monetary reward > no reward | 12 | 0.79 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 43 | 0.31 | |
| precentral gyrus | Herbort et al. (2016) | 23 | monetary incentive delay task | monetary reward > no reward | 25 | 13.73 |
| Arrondo et al. (2015) | 21 | monetary incentive delay task | monetary reward > no reward | 29 | 13.6 | |
| Kollmann et al. (2017) | 41 | monetary incentive delay task | monetary reward > no reward | 17 | 12.89 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task | monetary reward > no reward | 26 | 12.54 | |
| Damiano et al. (2014) | 31 | monetary incentive delay task | monetary reward > no reward | 15 | 10.78 | |
| Kirsch et al. (2003) | 27 | monetary incentive delay task/social incentive delay task | monetary reward > social reward | 12 | 10.04 | |
| Saji et al. (2013) | 18 | monetary incentive delay task | monetary reward > no reward | 22 | 9.2 | |
| Kaufmann et al. (2013) | 19 | monetary incentive delay task | monetary reward > no reward | 15 | 6.63 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 6.6 | |
| Dillon et al. (2010) | 32 | monetary incentive delay task | monetary reward > no reward | 7 | 1.45 | |
| Wittmann et al. (2005) | 16 | monetary incentive delay task | monetary reward > no reward | 22 | 0.86 | |
| Pfabigan et al. (2014) | 25 | monetary incentive delay task | monetary reward > no reward | 12 | 0.75 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 0.69 | |
| Rademacher et al. (2010) | 28 | monetary incentive delay task | monetary reward > no reward | 36 | 0.12 | |
| middle occipital gyrus | Kocsel et al. (2017) | 37 | monetary incentive delay task | monetary reward > no reward | 10 | 11.65 |
| Kirsch et al. (2003) | 27 | monetary incentive delay task | monetary reward > no reward | 26 | 10.16 | |
| Bjork et al. (2012) | 23 | monetary incentive delay task | monetary reward > no reward | 19 | 9.41 | |
| Juckel et al. (2012) | 13 | monetary incentive delay task | monetary reward > no reward | 18 | 8.04 | |
| Funayama et al. (2014) | 20 | monetary incentive delay task | monetary reward > no reward | 17 | 7.96 | |
| Van Duin et al. (2016) | 12 | monetary incentive delay task | monetary reward > no reward | 13 | 7.65 | |
| Yan et al. (2016b) | 22 | monetary incentive delay task | monetary reward > no reward | 33 | 7.65 | |
| Pfabigan et al. (2014) | 25 | monetary incentive delay task | monetary reward > no reward | 12 | 7.11 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 21 | 6.88 | |
| Maresh et al. (2014) | 84 | monetary incentive delay task | monetary reward > no reward | 18 | 4.36 | |
| Stoy et al. (2011) | 12 | monetary incentive delay task | monetary reward > no reward | 6 | 3.96 | |
| Dillon et al. (2010) | 32 | monetary incentive delay task | monetary reward > no reward | 7 | 3.63 | |
| Spreckelmeyer et al. (2009) | 32 | monetary incentive delay task | monetary reward > no reward | 8 | 3.23 | |
| Weiland et al. (2014) | 12 | monetary incentive delay task | monetary reward > no reward | 4 | 3.11 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 12 | 2.02 | |
| Behan et al. (2015) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 0.95 | |
| Kollmann et al. (2017) | 41 | monetary incentive delay task | monetary reward > no reward | 17 | 0.74 | |
| Damiano et al. (2014) | 31 | monetary incentive delay task | monetary reward > no reward | 15 | 0.32 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 8 | 0.27 | |
| Abler et al. (2005) | 12 | monetary incentive delay task | high monetary reward > low monetary reward | 4 | 0.24 | |
| Carter et al. (2009) | 17 | monetary incentive delay task | monetary reward > no reward | 10 | 0.21 | |
| Kappel et al. (2013) | 10 | monetary incentive delay task | monetary reward > no reward | 13 | 0.17 | |
| cuneus | Damiano et al. (2014) | 31 | monetary incentive delay task | monetary reward > no reward | 15 | 10.2 |
| Romanczuk-Seiferth et al. (2015) | 17 | monetary incentive delay task | monetary reward > no reward | 11 | 9.89 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 8 | 9.08 | |
| Spreckelmeyer et al. (2009) | 32 | monetary incentive delay task | monetary reward > no reward | 8 | 8.69 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | monetary reward > no reward | 17 | 8.57 | |
| Saji et al. (2013) | 18 | monetary incentive delay task | monetary reward > no reward | 22 | 8.27 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 13 | 8.14 | |
| Spreckelmeyer et al. (2009) | 16 | monetary incentive delay task | brain regions showing proportional activation to increasing anticipated monetary reward | 12 | 8.04 | |
| Adcock et al. (2006) | 12 | monetary incentive delay task | high monetary reward > low monetary reward | 12 | 7.42 | |
| Enzi et al. (2012b) | 15 | monetary incentive delay task | monetary reward > no reward | 9 | 6.74 | |
| Carl et al. (2016) | 20 | monetary incentive delay task | monetary reward > no reward | 15 | 6.06 | |
| Costumero et al. (2013) | 44 | monetary incentive delay task | monetary reward > no reward | 16 | 4.28 | |
| Bjork et al. (2008b) | 23 | monetary incentive delay task | monetary reward > no reward | 17 | 2.51 | |
| Samanez-Larkin et al. (2007) | 12 | monetary incentive delay task | monetary reward > no reward | 43 | 0.33 | |
| Bjork et al. (2004) | 12 | monetary incentive delay task | monetary reward > no reward | 13 | 0.32 | |
| Stoy et al. (2012) | 15 | monetary incentive delay task | monetary reward > no reward | 6 | 0.31 | |
| Kocsel et al. (2017) | 37 | monetary incentive delay task | monetary reward > no reward | 10 | 0.29 | |
| Carter et al. (2009) | 17 | monetary incentive delay task | monetary reward > no reward | 10 | 0.23 | |
| Stoy et al. (2012) | 15 | monetary incentive delay task | monetary reward > no reward | 3 | 0.19 | |
| Juckel et al. (2012) | 13 | monetary incentive delay task | monetary reward > no reward | 18 | 0.16 | |
| Bjork et al. (2010a) | 24 | monetary incentive delay task | monetary reward > no reward | 10 | 0.15 |
3.2. Conjunction findings
3.2.1. Conjunction analysis
The conjunction analysis revealed a common activation maximum in the bilateral VS, VTA, SMA, and left AI, for both social and monetary reward anticipation (Fig. 2c and Table 2).
3.2.2. Quantitative functional profiling of conjunction regions
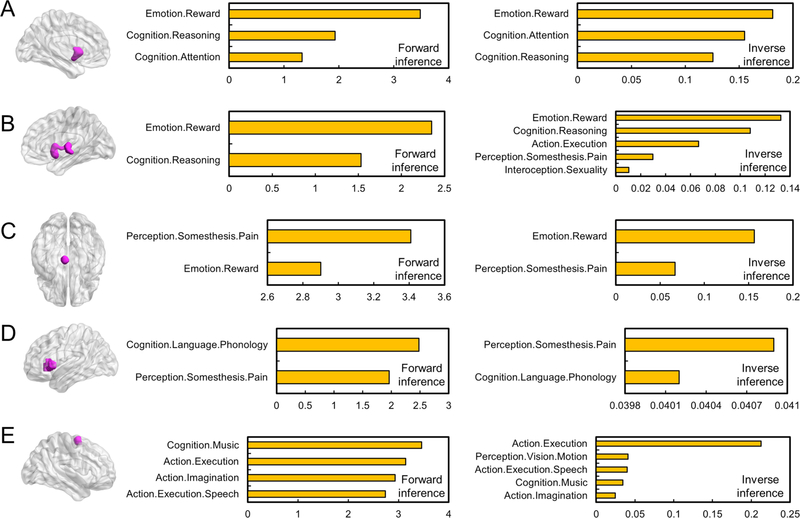
Results regarding functional decoding for each subcluster are illustrated in Fig. 3. For the left VS (Fig. 3A), forward and reverse inference alike indicated an association with reward, reasoning, and attention. For the right VS (Fig. 3B), an association with reward, reasoning, action execution, pain, and sexuality was revealed. For the VTA (Fig. 3C), the decoding analysis revealed an association with reward and pain. For the left AI (Fig. 3D), an association with phonology and pain was indicated. For the SMA (Fig. 3E), the results indicated an association with music, action execution, action imagination, and visual motion.
Fig. 3.
Quantitative forward and reverse inference on each region identified in the conjunction analysis. Quantitative forward and reverse inference for: (A) left VS; (B) right VS; (C) VTA; (D) left AI; and (E) SMA.
3.2.3. Functional connectivity profiles
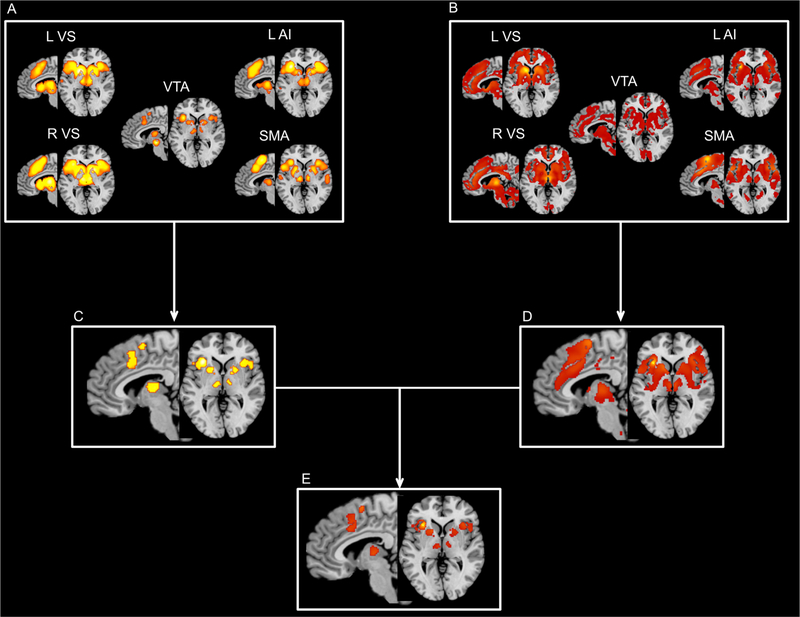
These overlapping regions were commonly connected to brain regions important for reward and salience processing during both resting and task states (Fig. 4), including VS, AI, SMA, and dACC.
Fig. 4.
Results for the task-based meta-analytic connectivity modeling (MACM) analyses, task-free resting-state functional connectivity (RSFC) analyses, and their conjunctions for the regions commonly involved in the social and monetary reward anticipation. (A) task-based connectivity; (B) task-free connectivity; (C) conjunction across all MACM maps; (D) conjunction across all RSFC maps; (E) conjunction across all MACM and RSFC maps. L, left; R, right; VS, ventral striatum; AI, anterior insula; VTA, ventral tegmental area; SMA, supplementary motor area.
3.3. Contrast findings
3.3.1. Contrast analysis
Results of contrast analysis (social vs. monetary; Table 2) showed that the dorsal caudate, SMA, dorsal AI, putamen, thalamus, midbrain, and MOG were more activated for social reward anticipation than monetary reward anticipation (Fig. 2D). In contrast, the VS, dorsal caudate, dACC, right ventral AI, and left thalamus were more activated for monetary reward anticipation than social reward anticipation (Fig. 2D). Notably, however, brain regions revealed in two directions of contrast analyses were adjacent to each other.
3.3.2. Clustering on RSFC between regions identified in the contrast analysis
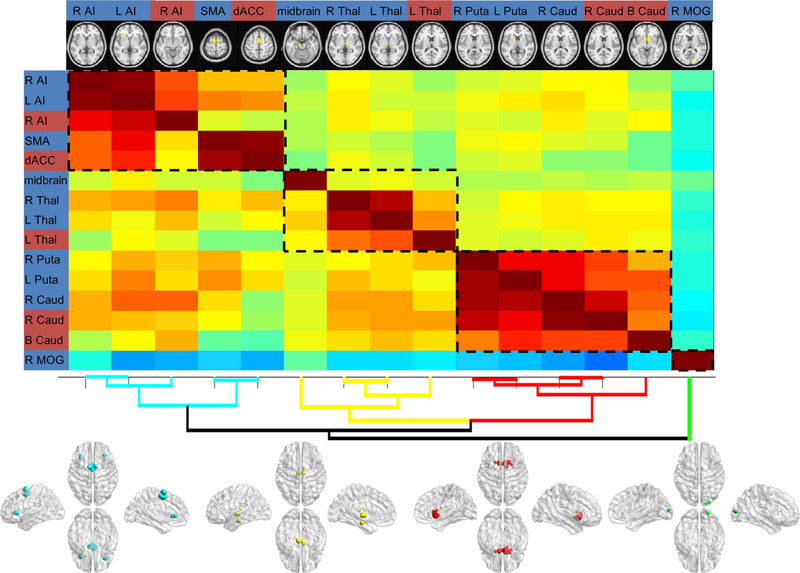
The hierarchical clustering based on RSFC patterns between all regions identified in the contrast analysis (social vs. monetary) grouped these regions into subclusters with strong within-cluster similarity (Fig. 5). Importantly, this data-driven grouping did not separate regions activated more in social reward anticipation from those activated more in monetary reward anticipation. Instead, most subclusters (3 of 4) included regions from both directions of contrast analyses. Specifically, cluster 1 consisted of the bilateral AI, SMA, and dACC (three regions from the contrast of social > monetary and two regions from the contrast of monetary > social); cluster 2 consisted of the bilateral midbrain and thalamus (three regions from the contrast of social > monetary and one region from the contrast of monetary > social); cluster 3 comprised of the bilateral caudate and putamen (three regions from the contrast of social > monetary and two regions from the contrast of monetary > social); finally, cluster 4 included the MOG (one region from the contrast of social > monetary). These results indicate that the regions derived from two directions of contrast analyses (social > monetary or monetary > social) could not be separated according to their functional connectivity profiles. Instead, these regions belonged to similar subclusters.
Fig. 5.
Clustering on functional connectivity between regions identified in the contrast analyses. Full (below the diagonal) and partial (above the diagonal) correlation matrices were illustrated, with warmer colors denoting positive correlations and cooler colors denoting negative correlations. The location of each region was displayed at the top of each column. The background color of region label indicates the contrast of each region (blue = social > monetary; red = monetary > social). The spatial map of each subcluster is illustrated at the bottom. L, left; R, right; AI, anterior insula; SMA, supplementary motor area; dACC, dorsal anterior cingulate cortex; Thal, thalamus; Puta, putamen; Caud, caudate; MOG, middle occipital gyrus.
3.3.3. Quantitative functional profiling of subclusters
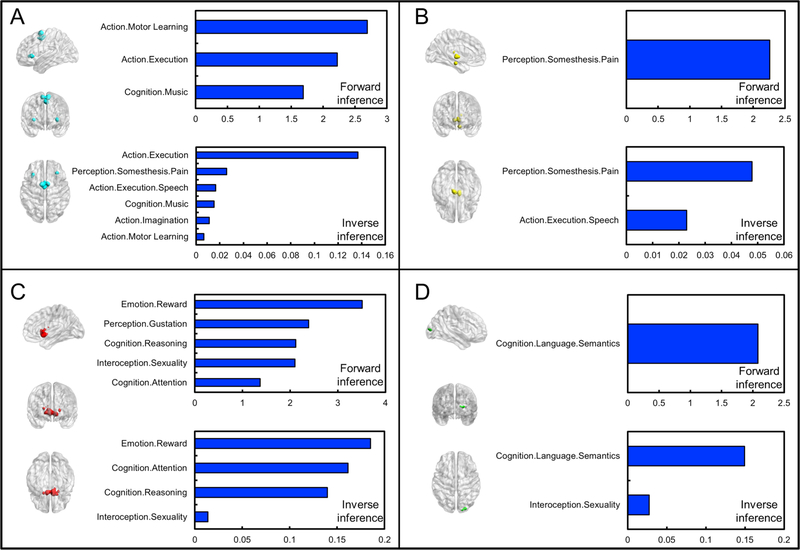
Results of functional decoding for each subcluster are illustrated in Fig. 6. For subcluster 1 (Fig. 6A), forward and reverse inference alike indicated a significant association of the subcluster with action (especially learning and execution) and music-related cognition. Reverse inference additionally revealed an association with pain, speech action, and action imagination. For subcluster 2 (Fig. 6B), both forward and reverse inference indicated an association with pain perception, while reverse inference also indicated an association with speech action. For subcluster 3 (Fig. 6C), an association of this subcluster was found with reward, reasoning, attention, and sexuality in both forward and reverse inference. Forward inference further revealed an association with gustation. For subcluster 4 (Fig. 6D), forward and reverse inference identified an association with semantics, and reverse inference further revealed an association with sexuality.
Fig. 6.
Quantitative forward and reverse inference on each subcluster identified in the hierarchical clustering. Quantitative forward and reverse inference for: (A) subcluster 1; (B) subcluster 2; (C) subcluster 3; and (D) subcluster 4.
3.4. Validation analyses
3.4.1. LOEO findings of social reward anticipation
Consistent activation maxima for social reward anticipation were found in the bilateral caudate/putamen, thalamus, and dACC/SMA (Fig. S3 and Table S1). That is to say, the results of the LOEO approach corroborated the findings of the standard ALE meta-analysis.
3.4.2. LOEO findings of monetary reward anticipation
The LOEOALE meta-analysis on monetary reward anticipation revealed consistent maxima in the bilateral caudate, dACC/SMA, posterior cingulate cortex, precentral gyrus, cuneus, MOG, cerebellum, left middle frontal gyrus, and inferior parietal lobule (Fig. S4 and Table S1). These results confirmed the main findings derived from conventional ALE meta-analysis.
3.4.3. Re-subsampling findings
The re-subsampling analyses revealed results largely overlapping with those of main conjunction and contrast analyses (Fig. S5–S7).
3.4.4. Within-paper experiments combined
This analysis revealed essentially the same findings as those from main ALE meta-analyses, conjunction, and contrast analyses (Figure S8–S12 and Tables S2–S4), thus further validating our findings.
4. Discussion
The power of social incentives on human goal-directed behaviors has been a long-standing topic in multiple disciplines (Fehr and Schmidt, 1999; Zajonc, 1965), with well-known examples showing that the mere presence of others could facilitate individual performance (Zajonc, 1965). With the advent of human brain imaging techniques (e.g., fMRI), the past decade has witnessed a surge of interest in delineating neural underpinning of social reward processing (Báez-Mendoza and Schultz, 2013; Ruff and Fehr, 2014). Neuroscience studies have employed various experimental designs and stimuli, and have identified heterogeneous findings, making it difficult to capture a clear picture of social reward circuitry in human brain. In this regard, the SID/MID task represents an ideal paradigm to unveil neural signatures of social reward anticipation and to compare with those of material reward anticipation, since these tasks share all elements but the nature of the reward.
Based on previous fMRI studies employing MID/SID task, this study quantitatively synthesized and compared neuroimaging findings on social and monetary reward anticipation. Our results identified a convergence of reported activation foci in neural circuits important in reward and salience processing for both social and monetary reward anticipation. Specifically, consistent involvements of the VS, VTA, AI, and SMA were identified in the anticipation of both social and monetary rewards. These meta-analytic findings were validated with additional validation analyses (e.g., LOEO). Further, our findings also identified several regions showing differences between social and monetary reward anticipation. However, social-reward sensitive and monetary-reward sensitive regions were adjacent to each other, and clustering findings indicated that these regions belonged to four subclusters, such that regions within each subcluster exhibited similar functional connectivity profiles. These findings together suggest that anticipation of social and monetary rewards engages an overlapping neural circuit in human brain, mainly consisting of the VS, VTA, AI, and SMA.
Among these regions, the VTA and VS are key regions in the dopaminergic system. The VTA is a core node of the dopaminergic projection system that produces dopamine and transmits it to other regions (e.g., VS) in the mesocorticolimbic neurocircuitry (Kalivas, 1993). Both single-unit recording and human brain imaging studies have detected the activity of VTA in response to reward-predicting cues and during anticipation of reward (Haber and Knutson, 2010; Ikemoto, 2010; Schultz et al., 1997). The VS is a primary target region of dopaminergic projections from the VTA, such that reward-related neural responses in the VS are well linked with dopamine release in the VTA (Buckholtz et al., 2010; Schott et al., 2008). Similar to the VTA, VS is consistently activated during anticipation of reward (Knutson and Cooper, 2005; Oldham et al., 2018; Plichta and Scheres, 2014). Notably, VS activity is proportional with magnitude of anticipated reward (Bjork et al., 2004, 2008a; Knutson et al., 2001a) and correlates with self-reported pleasure induced by the upcoming reward (Bjork et al., 2008a; Knutson et al., 2001a). Moreover, although there is evidence showing that the VS is engaged by anticipation of both reward and loss (Carter et al., 2009; Oldham et al., 2018), this region usually exhibits stronger activity during reward compared to loss anticipation (Carter et al., 2009; Cooper and Knutson, 2008; Juckel et al., 2006). Finally, our functional decoding findings indicate that the VS and VTA are most strongly associated with reward processing. Taken together, these findings suggest that the VS works together with VTA to code for expected positive incentive value during reward anticipation.
Notably, our findings indicate that the positive valence conveyed by the activity of VS and VTA might generalize across material and social rewards. In line with our findings, many studies have shown that VS and VTA are frequently engaged by reward processing and reward-related behaviors in the social domain (Báez-Mendoza and Schultz, 2013), such as social status (Zink et al., 2008), social acceptance (Izuma et al., 2008), fairness (Tabibnia et al., 2008), and cooperation (Rilling et al., 2002). Likewise, there is also evidence showing that the VS and VTA are involved in social learning (Diaconescu et al., 2017; Klucharev et al., 2009), such as learning the trustworthiness of others (Fareri et al., 2012; Krueger et al., 2007). These findings, thus, support the common-currency hypothesis, holding that a common neural network is engaged by a variety of reward processing (Levy and Glimcher, 2012; McNamara and Houston, 1986; Ruff and Fehr, 2014). In other words, the activity of VS and VTA may reflect a generic mediator of positive valence associated with expected rewards.
The AI constitutes a key node in the salience network implicated in detecting the motivational salience of events (Seeley et al., 2007). Specifically, the AI receives input from multiple regions such as the VTA, VS, and amygdala (Cauda et al., 2012) and integrates this information to represent the reward or loss saliency of the stimuli (Critchley et al., 2013; Pizzagalli et al., 2009). Accordingly, the AI is consistently involved in the anticipation of both reward and loss (Bjork et al., 2010b; Enzi et al., 2012a, b; Funayama et al., 2014; Van Duin et al., 2016). In addition, the AI has been frequently involved in detecting the salience of social stimuli or events (Feng et al., 2015; Luo et al., 2018). For instance, the activity of AI is induced by both positive and negative facial expressions (Chen et al., 2009), social interactions that are both better or worse than expectations (Feng et al., 2018; Xiang et al., 2013; Yu et al., 2014), or behaviors/opinions deviated from group norms (Wu et al., 2016). Considering the role of AI in both social and nonsocial context, it is plausible that this region may act as a universal mediator of motivational salience. In line with this conjecture, our functional decoding results suggest that the AI is mainly involved in cognition and pain processing that engage in the detection of salience.
The SMA plays a critical role in movement control (Picard and Strick, 1996), such as the preparation and execution of externally-triggered movement (Cunnington et al., 2002). Specifically, the SMA contributes to planning and elaboration of actions and action sequences by mediating between medial limbic cortex and primary motor cortex (Goldberg, 1985). Thus, the involvement of the SMA has been widely observed in various cognitive tasks that require motor operations, including word production and response inhibition (Alario et al., 2006; Hu et al., 2014). Indeed, our functional characterization findings indicate that the SMA is associated with action execution in a variety of tasks. Notably, the SMA activity is modulated by reward expectancy, such that the saccades associated with upcoming reward evoke bursting activity in the SMA neurons, whereas the activity diminishes when the saccades and reward are no longer associated (Campos et al., 2005). In the context of SID/MID task, the activity of the SMA might reflect the preparation of action in response to reward cues.
In short, our meta-analytic study identified a series of brain regions in response to both social and material incentive cues, which mediates positive value, motivational salience, and action preparation during reward anticipation. In light of previous research on the brain reward network in humans (Doya, 2008; Frank et al., 2009), we propose a domain-general neural circuitry responsible for encoding social and monetary reward anticipation. First, the presentation of reward cue (either social or material) evokes expectancy of a positive outcome, which induces dopamine release and neural activity in the VTA. Changes in dopamine release are projected to the VS, where activity represents positive value and/or motivational salience of expected positive outcome. Furthermore, the AI integrates information from the VS, VTA, and other regions to represent motivational relevance of the reward cue. Finally, the positive value encoded in the VS/VTA and motivational significance encoded in the AI motivate individuals to better prepare for the adequate action, manifested as neural activity in the SMA, to enhance the chance of obtaining actual reward. This conjecture is consistent with functional profiles of these regions, such that they are connected with each other and with other regions in the brain associated with reward and salience processing. However, it should be noted that the above hypothesis of how brain regions interact is tentative, and empirical studies combining SID/MID task with effective connectivity analysis are needed to address this issue.
In addition to common neural circuits recruited by both social and monetary reward anticipation, previous findings suggest that social reward anticipation specifically engages the brain regions important for social-cognitive processes, such as the STG, TPJ, and dmPFC (Barman et al., 2015; Goerlich et al., 2017; Spreckelmeyer et al., 2013). However, neither the current main ALE analysis nor contrast analyses (social vs. monetary) revealed consistent involvement of these “social brain regions” in the anticipation of social reward. Instead, our contrast analyses revealed that the brain regions showing differences in social and monetary reward anticipation were adjacent to each other and to those regions consistently engaged across both domains (i.e., conjunction analysis). Furthermore, hierarchical clustering findings indicate that social-sensitive and monetary-sensitive regions exhibit similar functional profiles within four subclusters. Lastly, functional decoding results indicate that these subclusters are associated with similar functions with those of conjunction regions, including action, salience, and reward. These findings, thus, provide convergent evidence that social and monetary reward anticipation engage similar regions but only to different extents.
However, this conclusion should be considered with caution due to several reasons. First, the current common neural circuit was revealed with SID/MID, which represents a simplified but elegant paradigm to specifically probe anticipation processes. Therefore, one may not generalize the current conclusion to other tasks involving complex decision-making. Indeed, by using more complicated task designs associated with social interaction, previous studies have detected the involvement of social brain regions, such as the dmPFC and TPJ (Carter et al., 2012; Feng et al., 2016). Second, it is noteworthy that adjacent or overlapping brain activation does not necessarily imply identical functions for all neurons within these regions (Haxby et al., 2001). Follow-up studies are needed to examine the functions of the adjacent/overlapping regions with more fine-grained techniques (e.g., multivoxel pattern analysis) or specifically designed task paradigms (e.g., repetition suppression paradigm), which allow for comparison of the neural patterns of adjacent/overlapping regions at the sub-voxel level (Norman et al., 2006; Peelen and Downing, 2007; Wake and Izuma, 2017). Likewise, the independent component analysis (ICA) might be employed to investigate whether social and monetary reward anticipation engage networks that are similar in terms of both spatial distribution and neural response function. For example, Zhang and Li (2012) using ICA identified sub-networks for error processing, attentional monitoring, and response inhibition, which otherwise seemingly engage a common cognitive control network. Similarly, Xu et al. (2013) revealed with ICA that multiple networks commonly engaged by attention and working memory exhibit distinct neural response functions.
Several limitations related to the current study should be noted. First, the limited number of studies in the social domain did not allow us to synthesize and compare neural mechanisms associated with consummatory reward or anticipatory and consummatory loss. Preliminary evidence has shown that social and material reward consumption engages distinct neural circuits (Rademacher et al., 2010), which requires systematic investigation in future meta-analytic studies with more publications on social reward. Second, the current study did not examine aberrant brain functions of reward anticipation across mental disorders, which are closely related to dysfunctions of reward-related processing (Hanewald et al., 2017; Husain and Roiser, 2018; Knutson and Heinz, 2015). Future studies in this line might reveal common neural circuit disruptions in reward anticipation across psychiatric disorders. Finally, as noted in the Materials and Methods section, a recently validated lesion network mapping approach investigates whether cognitive and behavioral abnormalities are a result of dysfunction of brain regions connected to a lesion location besides the lesion itself (Darby et al., 2018a, b). This technique may allow us to make causal inference about the functional networks underlying reward anticipation, an important topic to consider with growing number of lesion studies of reward processing in the future.
To sum up, the current results indicate that social and monetary reward anticipation engage a common neural circuit consisting of the VTA, VS, AI, and SMA, regions connected with each other during both task and resting states (see also Lin et al., 2011; Rademacher et al., 2015). This generic neural pathway might mediate positive value, motivational relevance, and action preparation during reward anticipation, which together motivate individuals to prepare well for the response to the upcoming target. Therefore, our findings provide the first meta-analytic evidence to quantitatively show the common involvement of brain regions in both social and non-social reward anticipation. These results are consistent with the common-currency hypothesis that a shared neural circuit is responsible for encoding different types of rewards (Berridge and Kringelbach, 2013). The development of a common reward neural circuit makes it possible for disparate categories of rewards to be compared in a common currency of utility (Berridge and Robinson, 2003; Lebreton et al., 2009), and thus facilitates decision-making between incommensurable options (e.g., love vs. money) when necessary.
Supplementary Material
Acknowledgments
This work was supported by the National Postdoctoral Program for Innovative Talents (BX201600019), the China Postdoctoral Science Foundation (2017M610055), the National Natural Science Foundation of China (31500920, 31470979, 31571124, 31530031), Natural Science Foundation of Shenzhen University (85303-00000275), Shenzhen Peacock Program (827-000235 and KQTD2015033016104926), the Major Program of the Chinese National Social Science Foundation (17ZDA324), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain”, and the European Union’s Horizon 2020 Research and Innovation Programme (7202070, HBP SGA1). R.G. and his wife thank their pet hamster “Rice” (2017–2019).
Footnotes
Conflict of interest
The authors are unaware of any conflicts of interest, financial or otherwise.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2019.02.017.
References
- Abler B, Walter H, Erk S, 2005. Neural correlates of frustration. Neuroreport 16, 669–672. [DOI] [PubMed] [Google Scholar]
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M, 2006. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage 31, 790–795. [DOI] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD, 2006. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50, 507–517. [DOI] [PubMed] [Google Scholar]
- Alario F-X, Chainay H, Lehericy S, Cohen L, 2006. The role of the supplementary motor area (SMA) in word production. Brain Res. 1076, 129–143. [DOI] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB, 2015. Definition and characterization of an extended social-affective default network. Brain Struct. Funct 220, 1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, Robbins TW, Fletcher PC, Murray GK, 2015. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front. Psychol 6, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W, 2013. The role of the striatum in social behavior. Front. Neurosci 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN, 2012. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol. Psychiatry 71, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman A, Richter S, Soch J, Deibele A, Richter A, Assmann A, Wüstenberg T, Walter H, Seidenbecher CI, Schott BH, 2015. Gender-specific modulation of neural mechanisms underlying social reward processing by Autism Quotient. Soc. Cogn. Affect. Neurosci 10, 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, 2009. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry 66, 734–742. [DOI] [PubMed] [Google Scholar]
- Behan B, Stone A, Garavan H, 2015. Right prefrontal and ventral striatum interactions underlying impulsive choice and impulsive responding. Hum. Brain Mapp 36, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci G, Feng C, Camilleri J, Eickhoff SB, Krueger F, 2018. The role of the anterior insula in social norm compliance and enforcement: evidence from coordinate-based and functional connectivity meta-analyses. Neurosci. Biobehav. Rev 92, 378–389. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML, 2013. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr. Opin. Neurobiol 23, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 2003. Parsing reward. Trends Neurosci. 26, 507–513. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW, 2004. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 24, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW, 2008a. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction 103, 1308–1319. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW, 2008b. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage 42, 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW, 2010a. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry 51, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW, 2010b. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One 5, e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW, 2012. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum. Brain Mapp 33, 2174–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, Sauer C, Haddad L, Grimm O, Mier D, Mohnke S, Heinz A, Erk S, 2012. Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage 59, 1404–1412. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, 2010. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci 13, 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JC, Barrós-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N, Llopis JJ, Ávila C, 2014. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict. Biol 19, 885–894. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB, 2013. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage 81, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri J, Müller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, Eickhoff SB, 2018. Definition and characterization of an extended multiple-demand network. Neuroimage 165, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M, Breznen B, Bernheim K, Andersen RA, 2005. Supplementary motor area encodes reward expectancy in eye-movement tasks. J. Neurophysiol 94, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Dichter GS, 2016. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J. Affect. Disord 203, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Macinnes JJ, Huettel SA, Adcock RA, 2009. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front. Behav. Neurosci 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Bowling DL, Reeck C, Huettel SA, 2012. A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science 337, 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A, 2012. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage 62, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Dammers J, Boers F, Leiberg S, Edgar JC, Roberts TP, Mathiak K, 2009. The temporal dynamics of insula activity to disgust and happy facial expressions: a magnetoencephalography study. Neuroimage 47, 1921–1928. [DOI] [PubMed] [Google Scholar]
- Chen T, Becker B, Camilleri J, Wang L, Yu S, Eickhoff SB, Feng C, 2018. A domain-general brain network underlying emotional and cognitive interference processing: evidence from coordinate-based and functional connectivity meta-analyses. Brain Struct. Funct 223, 3813–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC, Knutson B, 2008. Valence and salience contribute to nucleus accumbens activation. Neuroimage 39, 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costumero V, Barrós-Loscertales A, Bustamante JC, Ventura-Campos N, Fuentes P, Ávila C, 2013. Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur. J. Neurosci 38, 2399–2407. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Veer IM, Spinhoven P, Rombouts SA, Roelofs K, 2015. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front. Behav. Neurosci 8, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Eccles J, Garfinkel SN, 2013. Interaction between cognition, emotion, and the autonomic nervous system Handbook of Clinical Neurology. Elsevier, pp. 59–77. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E, 2002. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 15, 373–385. [DOI] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Dunlap K, Burrus CJ, Mosner MG, Kozink RV, McLaurin RE, O’Dhaniel A, Carter RM, Huettel SA, 2014. Association between the oxytocin receptor (OXTR) gene and mesolimbic responses to rewards. Mol. Autism 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson Á, Cato I, Carman R, Rahm L, 1999. Spatial clustering of metals in the sediments of the Skagerrak/Kattegat. Appl. Geochem 14, 689–706. [Google Scholar]
- Darby RR, Horn A, Cushman F, Fox MD, 2018a. Lesion network localization of criminal behavior. Proc. Natl. Acad. Sci 115, 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Joutsa J, Burke MJ, Fox MD, 2018b. Lesion network localization of free will. Proc. Natl. Acad. Sci 115, 10792–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu AO, Mathys C, Weber LA, Kasper L, Mauer J, Stephan KE, 2017. Hierarchical prediction errors in midbrain and septum during social learning. Soc. Cogn. Affect. Neurosci 12, 618–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Bogdan R, Fagerness J, Holmes AJ, Perlis RH, Pizzagalli DA, 2010. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum. Brain Mapp 31, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K, 2008. Modulators of decision making. Nat. Neurosci 11, 410–416. [DOI] [PubMed] [Google Scholar]
- Dvash J, Gilam G, Ben-Ze’ev A, Hendler T, Shamay-Tsoory SG, 2010. The envious brain: the neural basis of social comparison. Hum. Brain Mapp 31, 1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT, 2011. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT, 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT, 2017. Implementation errors in the GingerALE Software: description and recommendations. Hum. Brain Mapp 38, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi B, Duncan NW, Kaufmann J, Tempelmann C, Wiebking C, Northoff G, 2012a. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex-a combined fMRI-MRS study. Neuroscience 227, 102–109. [DOI] [PubMed] [Google Scholar]
- Enzi B, Edel M-A, Lissek S, Peters S, Hoffmann R, Nicolas V, Tegenthoff M, Juckel G, Saft C, 2012b. Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: a functional magnetic resonance study. Exp. Neurol 235, 256–264. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Chang LJ, Delgado MR, 2012. Effects of direct social experience on trust decisions and neural reward circuitry. Front. Neurosci 6, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Schmidt KM, 1999. A theory of fairness, competition, and cooperation. Q. J. Econ 114, 817–868. [Google Scholar]
- Feng C, Luo YJ, Krueger F, 2015. Neural signatures of fairness-related normative decision making in the ultimatum game: a coordinate-based meta-analysis. Hum. Brain Mapp 36, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Deshpande G, Liu C, Gu R, Luo YJ, Krueger F, 2016. Diffusion of responsibility attenuates altruistic punishment: a functional magnetic resonance imaging effective connectivity study. Hum. Brain Mapp 37, 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Feng X, Wang L, Wang L, Gu R, Ni A, Deshpande G, Li Z, Luo Y-J, 2018. The neural signatures of egocentric bias in normative decision-making. Brain Imaging Behav published online. [DOI] [PubMed] [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D, 2011. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol. Psychiatry 69, 867–874. [DOI] [PubMed] [Google Scholar]
- Flores A, Münte TF, Doñamayor N, 2015. Event-related EEG responses to anticipation and delivery of monetary and social reward. Biol. Psychol 109, 10–19. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Cohen MX, Sanfey AG, 2009. Multiple systems in decision making: a neurocomputational perspective. Curr. Dir. Psychol. Sci 18, 73–77. [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN, 2006. A critique of functional localisers. Neuroimage 30, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Betzel RF, He Y, de Reus MA, van den Heuvel MP, Zuo X-N, Sporns O, 2018. Fluctuations between high-and low-modularity topology in time-resolved functional connectivity. NeuroImage 180, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama T, Ikeda Y, Tateno A, Takahashi H, Okubo Y, Fukayama H, Suzuki H, 2014. Modafinil augments brain activation associated with reward anticipation in the nucleus accumbens. Psychopharmacology 231, 3217–3228. [DOI] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Müller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, 2017. The right dorsal premotor mosaic: organization, functions, and connectivity. Cereb. Cortex 27, 2095–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich KS, Votinov M, Lammertz SE, Winkler L, Spreckelmeyer KN, Habel U, Gründer G, Gossen A, 2017. Effects of alexithymia and empathy on the neural processing of social and monetary rewards. Brain Struct. Funct 222, 2235–2250. [DOI] [PubMed] [Google Scholar]
- Goldberg G, 1985. Supplementary motor area structure and function: review and hypotheses. Behav. Brain Sci 8, 567–588. [Google Scholar]
- Gossen A, Groppe SE, Winkler L, Kohls G, Herrington J, Schultz RT, Gründer G, Spreckelmeyer KN, 2014. Neural evidence for an association between social proficiency and sensitivity to social reward. Soc. Cogn. Affect. Neurosci 9, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Bakos S, Landes I, Tollner T, Bartling J, Kohls G, Schulte-Korne G, 2018. Sex differences in the neural underpinnings of social and monetary incentive processing during adolescence. Cogn. Affect. Behav. Neurosci 18, 296–312. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Koricheva J, Nakagawa S, Stewart G, 2018. Meta-analysis and the science of research synthesis. Nature 555, 175. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewald B, Behrens F, Gruppe H, Sammer G, Gallhofer B, Krach S, Iffland J, 2017. Anticipation of social and monetary rewards in schizophrenia. J. Psychiatry 20, 1–7. [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P, 2001. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430. [DOI] [PubMed] [Google Scholar]
- Herbort MC, Soch J, Wüstenberg T, Krauel K, Pujara M, Koenigs M, Gallinat J, Walter H, Roepke S, Schott BH, 2016. A negative relationship between ventral striatal loss anticipation response and impulsivity in borderline personality disorder. Neuroimage Clin. 12, 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Blankenburg F, 2016. Toward a standardized structural-functional group connectome in MNI space. Neuroimage 124, 310–322. [DOI] [PubMed] [Google Scholar]
- Hu S, Tseng YC, Winkler AD, Li CSR, 2014. Neural bases of individual variation in decision time. Hum. Brain Mapp 35, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Roiser JP, 2018. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci 19, 470–484. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, 2010. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci. Biobehav. Rev 35, 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N, 2008. Processing of social and monetary rewards in the human striatum. Neuron 58, 284–294. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, Wrase J, Heinz A, 2006. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29, 409–416. [DOI] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgürdal S, Gudlowski Y, Knutson B, Wrase J, Brüne M, Heinz A, 2012. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology 66, 50–56. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, 1993. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res. Rev 18, 75–113. [DOI] [PubMed] [Google Scholar]
- Kappel V, Koch A, Lorenz RC, Brühl R, Renneberg B, Lehmkuhl U, Salbach-Andrae H, Beck A, 2013. CID: a valid incentive delay paradigm for children. J. Neural Transm 120, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Beucke J, Preuße F, Endrass T, Schlagenhauf F, Heinz A, Juckel G, Kathmann N, 2013. Medial prefrontal brain activation to anticipated reward and loss in obsessive-compulsive disorder. Neuroimage Clin. 2, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Brown KW, Downar J, 2015. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc. Cogn. Affect. Neurosci 10, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D, 2003. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage 20, 1086–1095. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Hytonen K, Rijpkema M, Smidts A, Fernández G, 2009. Reinforcement learning signal predicts social conformity. Neuron 61, 140–151. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC, 2005. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol 18, 411–417. [DOI] [PubMed] [Google Scholar]
- Knutson B, Heinz A, 2015. Probing psychiatric symptoms with the monetary incentive delay task. Biol. Psychiatry 77, 418–420. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D, 2000. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12, 20–27. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D, 2001a. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 21 RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001b. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D, 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH, 2008. Neural responses to monetary incentives in major depression. Biol. Psychiatry 63, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsel N, Szabó E, Galambos A, Édes A, Pap D, Elliott R, Kozák LR, Bagdy G, Juhász G, Kokonyei G, 2017. Trait rumination influences neural correlates of the anticipation but not the consumption phase of reward processing. Front. Behav. Neurosci 11, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann B, Scholz V, Linke J, Kirsch P, Wessa M, 2017. Reward anticipation revisited-evidence from an fMRI study in euthymic bipolar I patients and healthy first-degree relatives. J. Affect. Disord 219, 178–186. [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D, 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull 136, 768. [DOI] [PubMed] [Google Scholar]
- Krall S, Rottschy C, Oberwelland E, Bzdok D, Fox PT, Eickhoff SB, Fink GR, Konrad K, 2015. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct 220, 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, Heinecke A, Grafman J, 2007. Neural correlates of trust. Proc. Natl. Acad. Sci 104, 20084–20089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT, 2005. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp 25, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT, 2009. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinform 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT, 2007. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp 28, 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Rottschy C, Laird AR, Fox PT, Eickhoff SB, 2014. Meta-analytic connectivity modeling revisited: controlling for activation base rates. NeuroImage 99, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Leiberg S, Hoffstaedter F, Eickhoff SB, 2018. Towards a human self-regulation system: common and distinct neural signatures of emotional and behavioural control. Neurosci. Biobehav. Rev 90, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M, 2009. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron 64, 431–439. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW, 2012. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol 22, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A, 2011. Social and monetary reward learning engage overlapping neural substrates. Soc. Cogn. Affect. Neurosci 7, 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Eickhoff SB, Hétu S, Feng C, 2018. Social comparison in the brain: a coordinate-based meta-analysis of functional brain imaging studies on the downward and upward comparisons. Hum. Brain Mapp 39, 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh EL, Allen JP, Coan JA, 2014. Increased default mode network activity in socially anxious individuals during reward processing. Biol. Mood Anxiety Disord 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G, Krainik A, Duffau H, Pélégrini-Issac M, Lehéricy S, Doyon J, Benali H, 2006. Partial correlation for functional brain interactivity investigation in functional MRI. Neuroimage 32, 228–237. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Houston AI, 1986. The common currency for behavioral decisions. Am. Nat 127, 358–378. [Google Scholar]
- Mucci A, Dima D, Soricelli A, Volpe U, Bucci P, Frangou S, Prinster A, Salvatore M, Galderisi S, Maj M, 2015. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med 45, 1765–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, 2018. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev 84, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh F, Legendre P, 2014. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? J. Classif 31, 274–295. [Google Scholar]
- Nawijn L, van Zuiden M, Koch S, Frijling J, Veltman D, Olff M, 2017. Intranasal oxytocin increases neural responses to social reward in post-traumatic stress disorder. Soc. Cogn. Affect. Neurosci 12, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B, 2005. Valid conjunction inference with the minimum statistic. Neuroimage 25, 653–660. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, 2012. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neurosci 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV, 2006. Beyond mind-reading: multivoxel pattern analysis of fMRI data. Trends Cogn. Sci 10, 424–430. [DOI] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, Lorenzetti V, 2018. The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum. Brain Mapp 39, 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernández G, Hermans EJ, 2011. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 55, 345–352. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE, 2007. The neural basis of visual body perception. Nat. Rev. Neurosci 8, 636–648. [DOI] [PubMed] [Google Scholar]
- Pfabigan DM, Seidel E-M, Sladky R, Hahn A, Paul K, Grahl A, Küblbock M, Kraus C, Hummer A, Kranz GS, 2014. P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage 96, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL, 1996. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex 6, 342–353. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M, 2009. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 166, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Scheres A, 2014. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev 38, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Wolf I, Hohmann S, Baumeister S, Boecker R, Schwarz AJ, Zangl M, Mier D, Diener C, Meyer P, 2013. Simultaneous EEG and fMRI reveals a causally connected subcortical-cortical network during reward anticipation. J. Neurosci 33, 14526–14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M, 2016. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J. Neurosci 36, 5047–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN, 2010. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49, 3276–3285. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Gründer G, Spreckelmeyer KN, 2013. Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc. Cogn. Affect. Neurosci 9, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Gründer G, Spreckelmeyer KN, 2014. Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc. Cogn. Affect. Neurosci 9, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Schulte-Rüther M, Hanewald B, Lammertz S, 2015. Reward: From Basic Reinforcers to Anticipation of Social Cues, Social Behavior From Rodents to Humans. Springer, pp. 207–221. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD, 2002. A neural basis for social cooperation. Neuron 35, 395–405. [DOI] [PubMed] [Google Scholar]
- Romanczuk-Seiferth N, Koehler S, Dreesen C, Wüstenberg T, Heinz A, 2015. Pathological gambling and alcohol dependence: neural disturbances in reward and loss avoidance processing. Addict. Biol 20, 557–569. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Fehr E, 2014. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci 15, 549–562. [DOI] [PubMed] [Google Scholar]
- Saji K, Ikeda Y, Kim W, Shingai Y, Tateno A, Takahashi H, Okubo Y, Fukayama H, Suzuki H, 2013. Acute NK1 receptor antagonist administration affects reward incentive anticipation processing in healthy volunteers. Int. J. Neuropsychopharmacol 16, 1461–1471. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B, 2007. Anticipation of monetary gain but not loss in healthy older adults. Nat. Neurosci 10, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A, 2008. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology 196, 673–684. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze H-J, Zilles K, 2008. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci 28,14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiter S, Spengler S, Willert A, Mohnke S, Herold D, Erk S, Romanczuk-Seiferth N, Quinlivan E, Hindi-Attar C, Banzhaf C, 2016. Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychol. Med 46, 3187–3198. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR, 1997. A neural substrate of prediction and reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sharma S, 1996. Applied Multivariate Techniques. John Wiley and Sons. [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H-C, Stippich C, Weisbrod M, Kaiser S, 2010. Neural reward processing is modulated by approach-and avoidance-related personality traits. Neuroimage 49, 1868–1874. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G, 2009. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci 4, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Rademacher L, Paulus FM, Gründer G, 2013. Neural activation during anticipation of opposite-sex and same-sex faces in heterosexual men and women. Neuroimage 66, 223–231. [DOI] [PubMed] [Google Scholar]
- Stark R, Bauer E, Merz C, Zimmermann M, Reuter M, Plichta Me, emsp14 al, Kirsch P, Lesch K, Fallgatter A, Vaitl D, 2011. ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia 49, 426–434. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H, 2011. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb. Cortex 21, 2578–2588. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Strohle A, 2011. Reward processing in male adults with childhood ADHD—a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology 215, 467–481. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, 2012. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J. Psychopharmacol 26, 677–688. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, 2008. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39, 966–972. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD, 2008. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry). Psychol. Sci 19, 339–347. [DOI] [PubMed] [Google Scholar]
- Timm H, Kruse R, 2002. A modification to improve possibilistic fuzzy cluster analysis. IEEE Int. Conf. Fuzzy Systems. [Google Scholar]
- Treadway MT, Buckholtz JW, Zald D, 2013. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front. Hum. Neurosci 7, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA, 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P, 2012. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Laird AR, 2012. The cognitive paradigm ontology: design and application. Neuroinformatics 10, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin ED, Goossens L, Hernaus D, da Silva Alves F, Schmitz N, Schruers K, Van Amelsvoort T, 2016. Neural correlates of reward processing in adults with 22q11 deletion syndrome. J. Neurodev. Disord 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, Consortium W-MH, 2013. The WU-Minn human connectome project: an overview. Neuroimage 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake SJ, Izuma K, 2017. A common neural code for social and monetary rewards in the human striatum. Soc. Cogn. Affect. Neurosci 12, 1558–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu T, Shi J, 2017. Development of monetary and social reward processes. Sci. Rep 7, 11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH, 1963. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc 58, 236–244. [Google Scholar]
- Weiland BJ, Heitzeg MM, Zald D, Cummiford C, Love T, Zucker RA, Zubieta J-K, 2014. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res. Neuroimaging 223, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP, Colizzi M, Bossong MG, Allen P, Kempton M, Abe N, Barros-Loscertales A, Bayer J, Beck A, Bjork J, 2018. The neural substrate of reward anticipation in health: a meta-analysis of fMRI findings in the monetary incentive delay task. Neuropsychol. Rev 28, 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E, 2005. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron 45, 459–467. [DOI] [PubMed] [Google Scholar]
- Wong TY, Sid A, Wensing T, Eickhoff SB, Habel U, Gur RC, Nickl-Jockschat T, 2018. Neural networks of aggression: ALE meta-analyses on trait and elicited aggression. Brain Struct. Funct publised online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A, 2007a. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage 36, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, 2007b. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35, 787–794. [DOI] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, Knutson B, 2014. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage 84, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Luo Y, Feng C, 2016. Neural signatures of social conformity: a coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev 71, 101–111. [DOI] [PubMed] [Google Scholar]
- Xiang T, Lohrenz T, Montague PR, 2013. Computational substrates of norms and their violations during social exchange. J. Neurosci 33, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang S, Calhoun VD, Monterosso J, Li C-SR, Worhunsky PD, Stevens M, Pearlson GD, Potenza MN, 2013. Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. Neuroimage 79, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Su L, Wang Y, Xu T, Yin D. z., Fan M. x., Deng C. p., Hu Y, Wang Z. x., Cheung EF, 2016a. Multivariate neural representations of value during reward anticipation and consummation in the human orbitofrontal cortex. Sci. Rep 6 srep29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang Y, Su L, Xu T, Yin D. z., Fan M.-x., Deng C. p., Wang Z. x., Lui SS, Cheung EF, 2016b. Differential mesolimbic and prefrontal alterations during reward anticipation and consummation in positive and negative schizotypy. Psychiatry Res. Neuroimaging 254, 127–136. [DOI] [PubMed] [Google Scholar]
- Yau W-YW, Zubieta J-K, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM, 2012. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci 32, 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Calder AJ, Mobbs D, 2014. Overlapping and distinct representations of advantageous and disadvantageous inequality. Hum. Brain Mapp 35, 3290–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB, 1965. Social facilitation. Science 149, 269–274. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li Cs.R., 2012. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum. Brain Mapp 33, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A, 2008. Know your place: neural processing of social hierarchy in humans. Neuron 58, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.