Abstract
The two known isoforms of IL-15 contain either a long signal peptide (LSP) or a short signal peptide (SSP), and are produced by alternatively spliced transcripts. It has been proposed that SSP IL-15 remains exclusively intracellular, and its function is unclear. In this study, we show that, similar to LSP IL-15, the SSP IL-15 is stabilized and secreted efficiently upon coexpression of IL-15Rα. Coinjection of SSP IL-15- and IL-15Rα-expressing plasmids into mice resulted in increased plasma levels of bioactive heterodimeric IL-15 and mobilization and expansion of NK and T cells. Therefore, SSP IL-15 is secreted and bioactive when produced as a heterodimer with IL-15Rα in the same cell. The apparent t1/2 of this heterodimer is lower compared with LSP IL-15/IL-15Rα, due to different intracellular processing. Coexpression of both LSP IL-15 and SSP IL-15 in the presence of IL-15Rα results in lower levels of bioactive IL-15, indicating that LSP and SSP IL-15 compete for the binding to IL-15Rα when expressed in the same cell. Because the SSP IL-15 interaction to IL-15Rα leads to a complex with lower apparent stability, SSP IL-15 functions as competitive inhibitor of LSP IL-15. The data suggest that usage of alternative splicing is an additional level of control of IL-15 activity. Expression of both SSP and LSP forms of IL-15 appears to be conserved in many mammals, suggesting that SSP may be important for expressing a form of IL-15 with lower magnitude or duration of biological effects.
Interleukin-15 is a pleiotropic cytokine of the four α-helix bundle family. IL-15 mRNA is expressed by different cell types, but it has been concluded that many primary cells that express IL-15 mRNA do not release detectable amounts of this cytokine. The discrepancy between IL-15 transcription and IL-15 secretion is explained by the fact that IL-15 production is controlled at the levels of transcription, translation, protein trafficking, and stability (1–8). All these limiting steps contribute to the tightly controlled activity of IL-15.
IL-15 pre-mRNA is alternatively spliced to produce two distinct isoforms of pre-IL-15, which differ only in the length of their signal peptides, the 48-aa long signal peptide (LSP)3 or the 21-aa short signal peptide (SSP; see Fig. 1A) (6, 8–11). SSP appears to affect both stability and localization of SSP IL-15 because lower levels of the SSP isoform were detected when the two isoforms were expressed from similar vectors (5, 8, 12). Although the mature cytokine portion after cleavage of the corresponding signal peptide is identical, the two isoforms of IL-15 show different cellular localization. The SSP IL-15 remains exclusively cell associated (8, 12, 13), whereas the LSP IL-15 is localized in the endoplasmic reticulum and Golgi apparatus and can be detected in the supernatant of transfected cells, even though secretion is reported to be inefficient. The combination of two approaches, namely mRNA optimization (14) of the IL-15 coding sequences and substitution of the signal peptide with other secretory signals, resulted in greatly improved expression and secretion of bioactive IL-15 (2, 5, 14).
FIGURE 1.
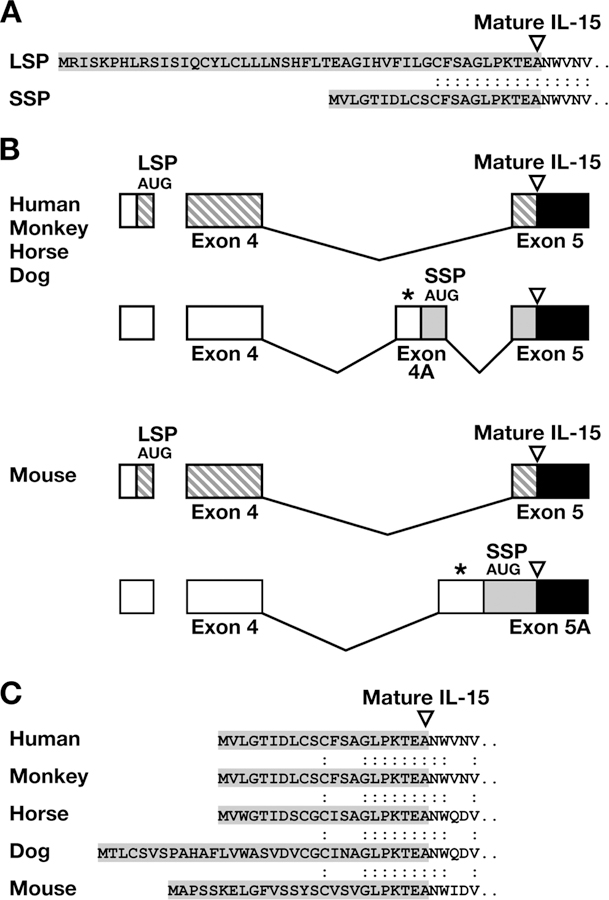
LSP and SSP IL-15 alternatively spliced mRNA is conserved in several mammals. A, Alignment of the amino acid sequences of the LSP and SSP of human IL-15. B, Structural organization of the two alternatively spliced transcripts of human, monkey, horse, dog, and mouse IL-15. Exons are named differently in the mouse. The regions encoding LSP IL-15 prepeptide are shown as  , whereas regions encoding SSP IL-15 prepeptide are shown as
, whereas regions encoding SSP IL-15 prepeptide are shown as  . The corresponding AUGs for LSP and SSP IL-15 are indicated. *, Indicates stop codons in frame with LSP AUG. Coding regions of the mature IL-15 are indicated as
. The corresponding AUGs for LSP and SSP IL-15 are indicated. *, Indicates stop codons in frame with LSP AUG. Coding regions of the mature IL-15 are indicated as  . C, Alignment of amino acid sequences of SSP IL-15 of different species.
. C, Alignment of amino acid sequences of SSP IL-15 of different species.
SSP IL-15 transcript was first identified in a human lung cancer cell line, in human lymphocyte cell lines (6, 9, 10), and in a human testicular cDNA library (8). Analysis of different tissues reported high expression of SSP IL-15 mRNA in heart, thymus, and appendix. Weak expression was also seen in gall bladder, pancreas, and testis (7, 8). A variety of human lymphocyte cell lines revealed the presence of both SSP and LSP IL-15 mRNA. Lymphoma-derived L591 and HuT102 cells expressed mostly the LSP IL-15 isoform; thymocyte-derived Cole cell line expressed mostly SSP IL-15 isoform (6). The pattern of expression of SSP and LSP IL-15 is different in different tissues, suggesting differential regulation of the two IL-15 isoforms.
Several experiments have suggested that the simultaneous expression of IL-15Rα in the same cell is necessary for the production and secretion of IL-15 under physiological conditions (15–24). We have previously shown that intracellular interaction between IL-15 and IL-15Rα results in the generation of a stable complex that can trans-locate to the cell membrane, be cleaved, and released as bioactive heterodimeric cytokine (23, 25). Thus, the reported requirement for expression of IL-15 and IL-15Rα in the same cells reflects the necessity for an intracellular complex formation for efficient secretion. Therefore, IL-15Rα can be viewed as part of the heterodimeric IL-15 cytokine rather than part of the receptor. Recent results suggest that the IL-15/IL-15Rα complex, either on the surface of the cells or as a soluble heterodimer, is the most active form of IL-15 (3, 25–30).
In the present work, we tested expression vectors encoding the SSP IL-15 isoform in the presence or absence of IL-15Rα. Our results show that, similar to LSP IL-15, coexpression of IL-15Rα leads also to stabilization and secretion of SSP IL-15, previously proposed to remain exclusively intracellular. We further show that the secreted SSP IL-15 in the heterodimeric form with IL-15Rα is bioactive. These results are important for the understanding of the complex regulation and function of IL-15.
Materials and Methods
DNA plasmids
The parent vector used for the generation of all constructs, pCMVkan, contains the human CMV promoter, the bovine growth hormone (BGH) polyadenylation site, and the kanamycin-resistance gene (31, 32). The RNA-optimized expression vectors for the human LSP IL-15 and for the human IL-15Rα have been described elsewhere (14, 25). An additional expression plasmid producing the RNA-optimized isoform of SSP IL-15 was also constructed. The C-terminal hemagglutinin (HA)- and FLAG-tagged forms of SSP IL-15 and LSP IL-15 were generated for the immunofluorescence studies. Tagged plasmids express bioactive forms of IL-15. For the in vivo studies, highly purified, endotoxin-free DNA plasmids were produced using Qiagen EndoFree Giga kit.
In vitro transient transfection and protein analysis
Expression of IL-15 from the different plasmids was tested after transient transfection of human 293 cells using the calcium phosphate coprecipitation technique. Cells were transfected using 0.1 μg of either the SSP IL-15 or the LSP IL-15 expression plasmids alone or in combination with 0.1 μg of the IL-15Rα expression plasmid. Cotransfection of 0.05 μg of the GFP expression vector pFRED143 (33) served as internal control. GFP levels of the cell extracts were measured using the SpectraMax Gemini EM fluorometer, giving less than 50% variability between different samples. After 48 h, culture supernatants were collected and cells were harvested and lysed in 0.5% Triton X-100 buffer. Protein stability analysis was performed by treating the transfected cells with 25 μg/ml cycloheximide. Culture supernatants and cells were harvested from 0 to 80 min after treatment. Human IL-15 protein levels were measured by ELISA using the human IL-15 Quantikine colorimetric kit (R&D Systems). This ELISA measures human IL-15 in both monomeric form and also as a complex with the IL-15Rα. Protein degradation was studied by treating the transfected cells with the mix of proteasome inhibitors (lactacystein (5 μM), MG132 (5 μM), and epoxomycin (1 μM)). After 18 h, cells were harvested and IL-15 expression was tested by Western blot, using the polyclonal goat anti-human IL-15 Ab (AF315; R&D Systems).
Localization of IL-15/IL-15Rα complexes
Twenty-four hours after transfection, human 293 cells were harvested, stained with PE-conjugated anti-human IL-15 (IC247IP; R&D Systems), and analyzed by flow cytometry using the LSR (BD Biosciences). For confocal microscopy, Hela cells were transfected by Superfect (Invitrogen) with either 0.5 μg of SSP IL-15 HA or 0.1 μg of LSP IL-15 FLAG plasmids in the presence or absence of 1 μg of the IL-15Rα plasmid. Forty-eight hours later, the cells were either fixed and permeabilized or directly surface stained using either rabbit anti-HA (1:500; Sigma-Aldrich) or mouse anti-FLAG (1/2000 dilution; Sigma-Aldrich), and visualized using Alexa-594-labeled goat anti-rabbit IgG and Alexa-488-labeled goat anti-mouse IgG (1/500 dilution; Molecular Probes, Invitrogen), respectively.
RNA analysis
After transfection of 293 cells, cytoplasmic polyadenylated mRNA was isolated and analyzed, as described previously (34, 35). RNA was transferred onto Duralon-UV membranes (Stratagene) using the standard capillary transfer method and hybridized using the QuikHyb hybridization solution (Stratagene), according to the manufacturer’s instructions. A DNA probe for the BGH polyadenylation region was synthesized using the Prime-It II Random Primer Labeling kit (Stratagene) and was used to detect IL-15 and IL-15Rα. A probe specific for cellular GAPDH mRNA was used to detect this mRNA in the same blots as internal control.
IL-15 expression and function in vivo upon hydrodynamic DNA delivery
Six-week-old female BALB/c mice were obtained from Charles River Laboratories. Hydrodynamic injection of the IL-15 and IL-15Rα expression plasmids into mice was performed, as previously described (36–38). Briefly, the IL-15 plasmids alone or in combination with IL-15Rα plasmid in 1.6 ml of sterile 0.9% NaCl were injected into mice through the tail vein within 7 s using a 27.5-gauge needle. Mice were bled at days 1 and 3 after injection, and the plasma levels of IL-15 were measured using human IL-15 chemiluminescent immunoassay (QuantiGlo; R&D Systems). This ELISA measures human IL-15 in both monomeric form and also as a complex with the IL-15Rα, whereas it does not cross-react with mouse IL-15. Three days after injection, mice were sacrificed, and spleen and lungs were collected and analyzed. For splenocyte purification, spleens were gently squeezed through a 100-μm cell strainer (Thomas Instruments) and washed in RPMI 1640 (Life Technologies) to remove any remaining organ stroma. The cells were resuspended in RPMI 1640 containing 10% FCS and counted using Acridine Orange (Molecular Probes)/ethidium bromide (Fisher Scientific) dye. To isolate lymphocytes from lungs, the tissues were minced and incubated with 200 U/ml collagenase (Sigma-Aldrich) and 30 U/ml DNase (Roche) for 1 h at 37°C, and then single cells were collected and resuspended in complete RPMI 1640 with 10% FCS. For phenotyping, the cells were incubated with the following mix of directly conjugated anti-mouse Abs (BD Pharmingen): CD3 allophycocyanin Cy7, CD4 PerCP, CD8 PECy7, CD44 allophycocyanin, CD49b FITC, and CD62L PE. Before flow cytometry, the samples were stained with 4′,6′-diamidino-2-phenylindole to exclude dead cells. The samples were acquired in a LSR II flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (Tree Star).
Statistical analysis
The p values for all of the in vivo analyses were determined by unpaired Student’s t test. Correlation of serum IL-15 levels with IL-15 biological effects in vivo was determined by nonlinear regression curve fit.
Results
Coexpression of IL-15Rα increased the production of SSP IL-15
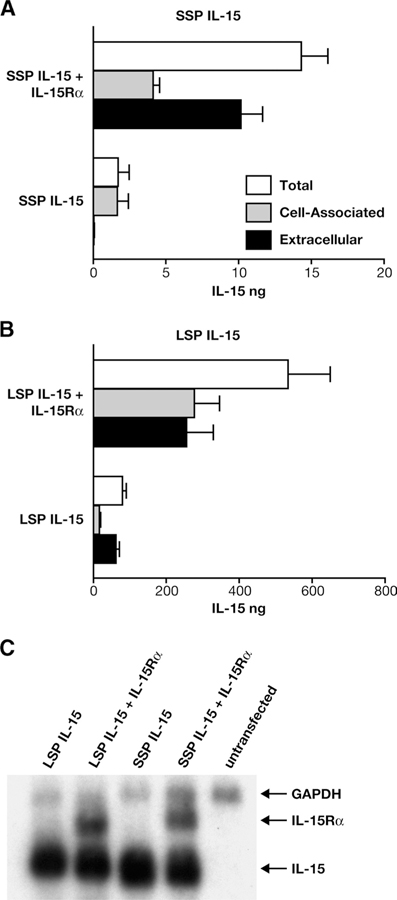
Human IL-15 contains a LSP of 48 aa, which has been implicated in its inefficient secretion. In addition, an alternative form of IL-15, containing a SSP of 21 aa, is generated by alternative splicing of the primary transcript (Fig. 1A) (4, 6, 8). Alternative splicing leading to two IL-15 isoforms appears to be conserved in several mammals, suggesting a conserved functional requirement for the two isoforms (Fig. 1, B and C). To study the two different IL-15 forms, we generated optimized expression vectors for mammalian cells having the different leader peptides LSP and SSP. We have previously shown that coexpression of LSP IL-15 with IL-15Rα leads to intracellular complex formation, stabilization, and increased secretion of both molecules (25). To test more specifically whether this effect was applicable to the SSP isoform of IL-15, we compared SSP IL-15 expression to LSP IL-15 in the presence or absence of IL-15Rα-producing plasmid upon transient transfection of human 293 cells. Production of both SSP IL-15 (Fig. 2A) and LSP IL-15 (Fig. 2B) was increased 5-fold by IL-15Rα coexpression. In agreement with previous reports, we also found ~40-fold lower IL-15 levels produced from the SSP IL-15 plasmid compared with LSP IL-15, most likely due to the difference in the leader peptide (5, 8). Interestingly, we found that this difference was maintained in the presence of the IL-15Rα (Fig. 2). In the case of LSP IL-15, upon cotransfection with IL-15Rα, approximately half of the produced IL-15 was cell associated, and half was secreted in the supernatant (Fig. 2B). Note that the cell-associated material in the presence of IL-15Rα is concentrated on the plasma membrane (see below; Fig. 3). In the case of SSP IL-15, we noticed a remarkable switch in the cell-associated vs extracellular fractions, resulting in the secretion of ~70% of the total produced IL-15 in the presence of IL-15Rα (Fig. 2A). These results show that the SSP isoform of IL-15, previously thought to be exclusively intracellular, is efficiently produced and secreted upon coexpression with IL-15Rα.
FIGURE 2.
IL-15Rα stabilizes both LSP and SSP IL-15. A, Human 293 cells were transfected with 0.1 μg of SSP IL-15 DNA alone or with 0.1 μg of IL-15Rα-expressing plasmid. Cell-associated and extracellular IL-15 was measured by ELISA 2 days after transfection. Bars indicate mean values of IL-15 production; SD of three independent transfections is shown. B, Human 293 cells were transiently transfected with 0.1 μg of LSP IL-15 DNA alone or with 0.1 μg of IL-15Rα-expressing plasmids. Cell-associated and extracellular IL-15 was measured by ELISA 2 days after transfection. Bars indicate mean values of IL-15 production; SD of three independent transfections is shown. C, Alternative splicing to generate SSP and LSP mRNA does not alter IL-15 mRNA levels. Cytoplasmic RNA was isolated from cells transfected with 0.1 μg of either LSP IL-15 or SSP IL-15 DNA alone or in the presence of the IL-15Rα-expressing plasmid. Northern blot analysis was performed with probes detecting the IL-15 and IL-15Rα. A GAPDH probe was included to detect cellular GAPDH mRNA as a loading standard.
FIGURE 3.
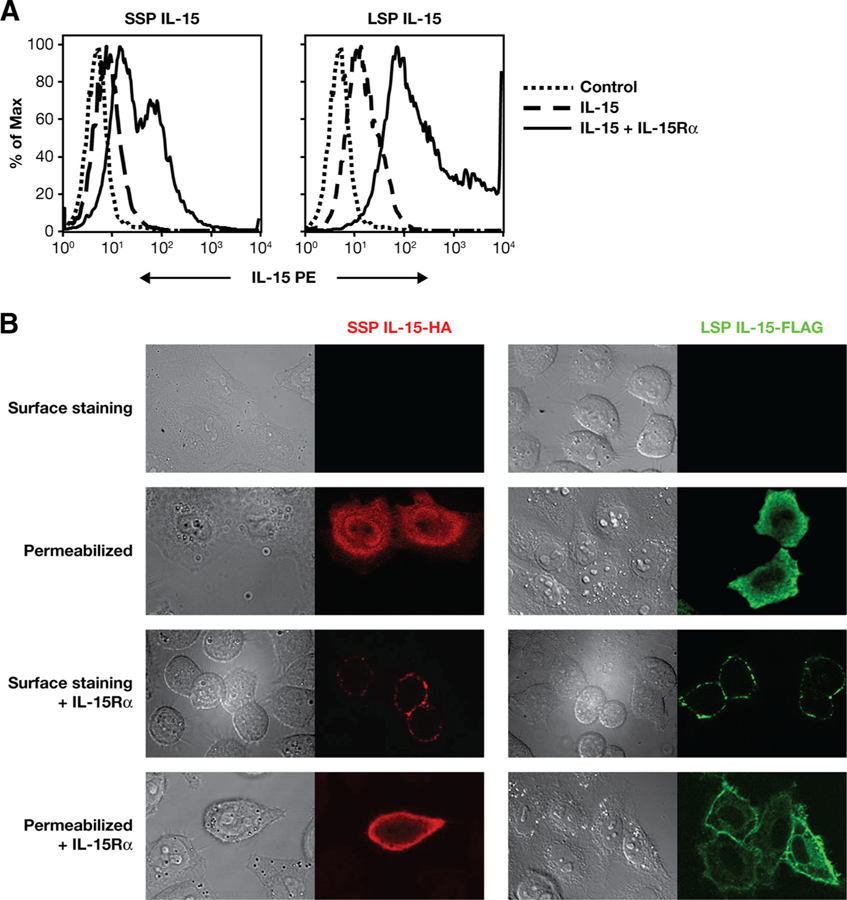
Expression of IL-15/IL-15Rα complex at the cell surface. A, Human 293 cells were transfected with plasmids expressing SSP IL-15 or LSP IL-15 alone or in combination with IL-15Rα. The cells were analyzed for IL-15 surface expression by flow cytometry after staining with PE-labeled anti-IL-15 Ab (IC247IP; R&D Systems). Cells transfected with bluescript plasmid and stained with the same Ab served as negative control. B, Confocal microscopy images of Hela cells transfected with either the SSP IL-15-HA- or LSP IL-15-FLAG-expressing plasmids in presence or absence of IL-15Rα. Cells were either permeabilized using 3.7% solution of formaldehyde or left intact for surface staining. After fixation, the cells were stained with either a rabbit polyclonal anti-HA Ab or mouse anti-FLAG mAb and visualized using Alexa 594-conjugated anti-rabbit IgG or Alexa 488-conjugated anti-mouse IgG, respectively.
The different signal peptides do not alter IL-15 mRNA level
To exclude the possibility that the different protein levels of LSP IL-15 and SSP IL-15 resulted from different mRNA levels, we performed Northern blot analyses (Fig. 2C). LSP IL-15- and SSP IL-15-producing plasmids were expressed in human 293 cells in the presence or absence of IL-15Rα. The cytoplasmic transcripts were visualized using a probe for the BGH portion, which detects the mRNAs for both IL-15 isoforms and IL-15Rα expressed in our vectors. As a loading control, the GAPDH mRNAs were probed on the same membrane. No significant differences in the IL-15 mRNA levels were found between LSP IL-15- and SSP IL-15-transfected cells. The presence of IL-15Rα did not affect the SSP IL-15 mRNA accumulation. As expected from previous studies (14, 25), the coexpression of IL-15Rα did not alter the LSP IL-15 mRNA levels. These results show that protein trafficking and stability rather than mRNA stability are responsible for the difference in the production of LSP IL-15 and SSP IL-15 in either absence or presence of IL-15Rα.
SSP IL-15 localization in the presence of IL-15Rα
To investigate the role of IL-15Rα in the intracellular trafficking of SSP IL-15, we performed flow cytometric and confocal microscopy analysis in cells transfected with IL-15 expression plasmids alone or in combination with IL-15Rα expression plasmid (Fig. 3). Flow cytometric analysis of cells transfected with either SSP or LSP IL-15 expression vectors in the presence or absence of IL-15Rα expression plasmid revealed high levels of IL-15 at the cell surface only when IL-15Rα was coexpressed (Fig. 3A). Transfected Hela cells were also examined for SSP IL-15 and LSP IL-15 localization in the presence or absence of IL-15Rα by confocal microscopy (Fig. 3B). The use of DNAs expressing C-terminally tagged SSP IL-15-HA and LSP IL-15-FLAG allowed for the sensitive visualization of the two IL-15 isoforms. Due to the lower expression of IL-15 SSP, 5 times more of the SSP IL-15-encoding plasmid than of the LSP IL-15-encoding plasmid was used in the transfections. In the absence of IL-15Rα, SSP IL-15 and LSP IL-15 did not localize at the plasma membrane and had a diffusely cytoplasmic distribution. In addition, SSP IL-15 localized also in the nuclei of some transfected cells, whereas LSP IL-15 is excluded from this compartment, as previously reported (8) (Fig. 3B). The coexpression of IL-15Rα resulted in a dramatic distribution shift of both molecules, because both SSP and LSP IL-15 colocalized predominantly at the plasma membrane in the presence of IL-15Rα (Fig. 3B). Taken together, these results show that SSP IL-15 reaches the plasma membrane and is secreted only in a complex with IL-15Rα.
IL-15Rα coexpression increased the t1/2 of both SSP and LSP IL-15 isoforms
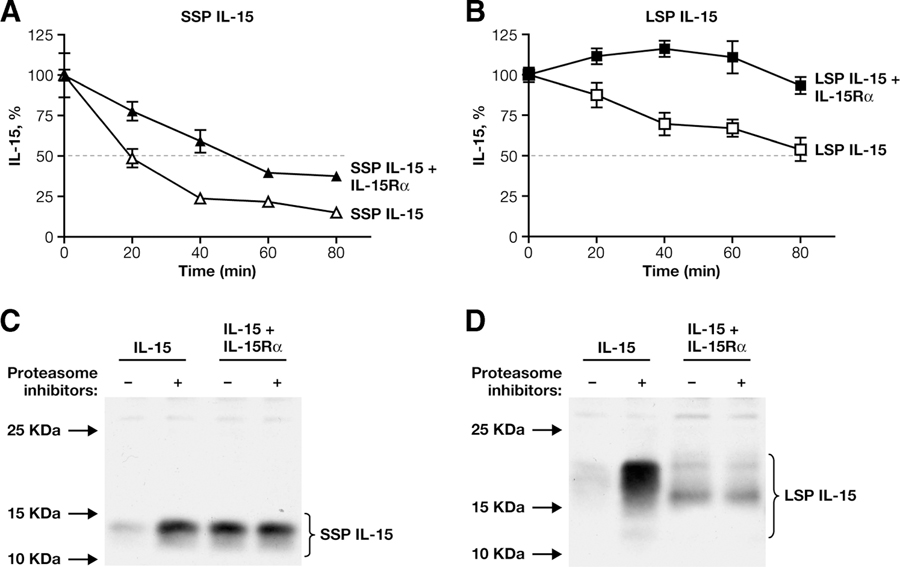
To evaluate and compare the in vitro t1/2 of both isoforms of IL-15 in absence or presence of IL-15Rα, we transfected human 293 cells with the vectors expressing these molecules and inhibited protein synthesis after 24 h by the addition of cycloheximide. Measurement of total IL-15 levels during the first 80 min after cycloheximide addition revealed that SSP IL-15 has a t1/2 of 20 min (Fig. 4A, ∆) and it is less stable than LSP IL-15 that shows a t1/2 of ~80 min (Fig. 4B, □). This difference can account for the difference in the IL-15 total protein production after transient transfection of SSP IL-15 and LSP IL-15 DNAs in 293 cells (Fig. 2). Coexpression of IL-15Rα leads to a great increase in the stability of both SSP and LSP IL-15 (Fig. 4, A and B, ▲ and ■, respectively). Interestingly, even in the presence of IL-15Rα, SSP IL-15/IL-15Rα complex has a t1/2 of 50 min, which is lower than LSP IL-15 expressed alone. These findings suggest that SSP IL-15 may have some distinct biological functions compared with LSP IL-15.
FIGURE 4.
A and B, Determination of IL-15 t1/2 by cycloheximide treatment. Human 293 cells were transfected with SSP IL-15 (A)- or LSP IL-15 (B)-expressing vectors either in the absence or presence of IL-15Rα DNA. The total amount of IL-15 in the cells and medium was measured by ELISA at the indicated times after addition of 25 μg/ml cycloheximide to inhibit protein synthesis. The level of IL-15 at the start of drug treatment was set as 100%. C and D, IL-15 degradation by the proteasome. A total of 106 human 293 cells was transfected with SSP IL-15 (C)- or LSP IL-15 (D)-expressing vectors either in absence or presence of IL-15Rα DNA. The cells were treated with a mix of proteasome inhibitors (lactacystein (5 μM), MG132 (5 μM), and epoxomycin (1 μM)). IL-15 production was analyzed by Western immunoblot of cell extracts after 18 h, using a goat anti-IL-15 Ab (R&D Systems; AF315). One of 35 of SSP IL-15-transfected cells and 1 of 70 of LSP IL-15-transfected cells were loaded per lane in the experiment shown. A GFP expression plasmid was included in the transfection, and GFP was used as a loading standard. The relative GFP values for the transfections in the figure were, for each lane: C, 30, 36, 35, and 32; D, 35, 30, 32, and 35.
IL-15Rα inhibits IL-15 degradation through the proteasome
To analyze the mechanisms involved in IL-15 degradation in the presence and absence of IL-15Rα, transfected 293 cells expressing either SSP IL-15 (Fig. 4C) or LSP IL-15 (Fig. 4D) alone or in the presence of IL-15Rα were treated with a mix of three different proteasome inhibitors for 18 h, and IL-15 levels were assessed by Western immunoblots. Blocking of proteasomes resulted in a great increase in both SSP and LSP IL-15 intracellular accumulation, suggesting that, in the absence of IL-15Rα, a large portion of IL-15 is rapidly degraded immediately after synthesis through the proteasome (Fig. 4, C and D, left lanes), as previously suggested (3, 4, 25, 30). In contrast, upon cotransfection with IL-15Rα-expressing DNA (Fig. 4, C and D, right lanes), no significant differences in the amount of either SSP or LSP IL-15 were found between untreated and treated samples. These data are consistent with a model in which IL-15 is degraded in the proteasome after retrograde transport in the cytoplasm. IL-15Rα binding to IL-15 early after synthesis prevents the rapid movement of unbound, unstable IL-15 to the proteasome degradation pathway and promotes trafficking of the complex to the cell surface.
In vivo bioactivity comparison of SSP IL-15 and LSP IL-15 in combination with IL-15Rα
It has been previously shown that IL-15Rα is essential for the IL-15 bioactivity in vivo (15–20, 22, 25–28). We investigated whether coexpression of IL-15Rα can also promote the bioactivity of SSP IL-15 in vivo, and whether LSP IL-15 and SSP IL-15 can exert different functions in the presence of IL-15Rα in vivo. Mice were injected hydrodynamically with the optimized plasmids encoding SSP IL-15 or LSP IL-15 either in the absence or in the presence of IL-15Rα-expressing plasmid. A GFP expression plasmid was injected as negative control. Mice were bled at days 1 and 3 after injection to measure IL-15 levels in plasma. Fig. 5 shows that LSP IL-15 plasmid produced peak levels of 728 pg of human IL-15/ml plasma (SD = 352, n = 5), whereas the SSP IL-15 plasmid produced lower, but detectable levels of 88 pg/ml plasma (SD = 42, n = 5). Coinjection of IL-15Rα-expressing DNA resulted in an increase of ~20- and ~100-fold in the amount of IL-15 in the plasma for SSP and LSP IL-15 isoforms, respectively. We noticed that the plasma IL-15 level after injection of LSP IL-15 plasmid by itself and the SSP IL-15 plus IL-15Rα DNAs were similar, suggesting that even in the presence of IL-15Rα, SSP IL-15 is a less stable molecule than LSP IL-15. This conclusion agrees with the results of the cycloheximide experiment (see Fig. 4, A and B).
FIGURE 5.
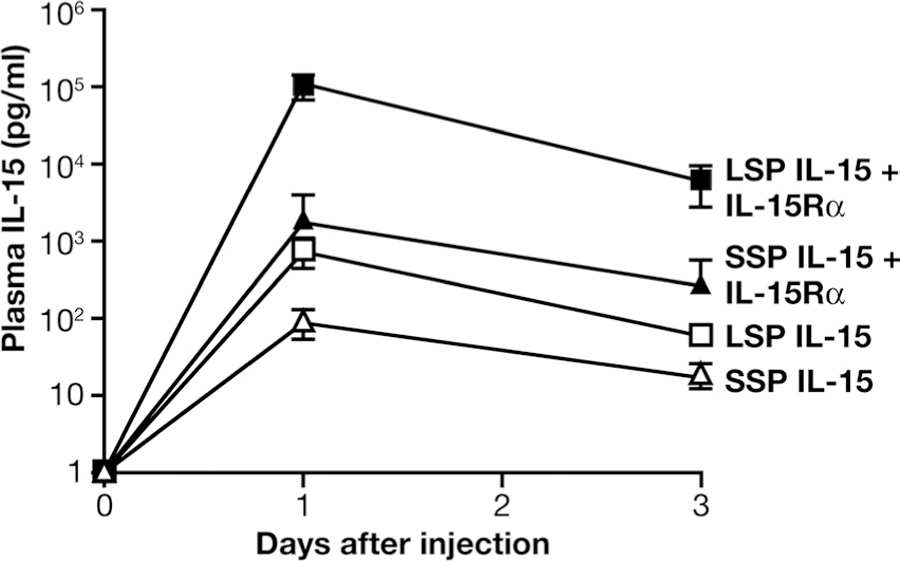
Comparison of plasma levels of human IL-15 after hydrodynamic DNA delivery of SSP or LSP IL-15 DNA in mice. Hydrodynamic DNA delivery using 0.5 μg of IL-15-expressing plasmid (SSP or LSP), in the absence or presence of 1.5 μg of IL-15Rα plasmid (five mice per group). Blood samples were collected at days 1 and 3 postinjection, and plasma IL-15 levels were determined by ELISA (Quantiglo; R&D Systems). The mean SD of IL-15 plasma levels for each group of animals is shown.
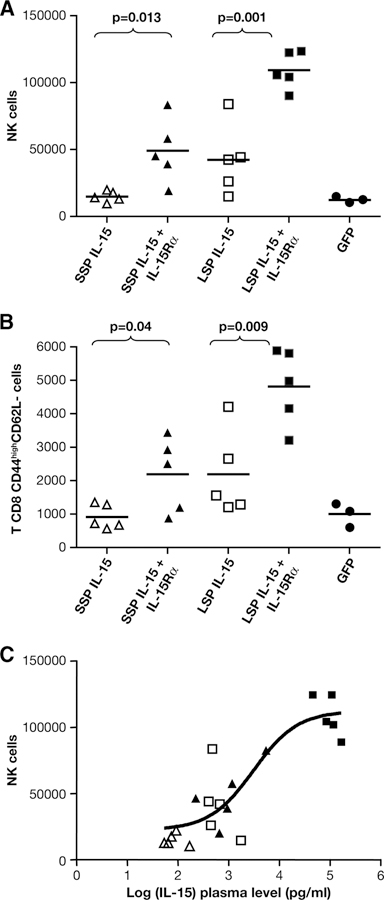
To study the bioactivity of IL-15, the mice were sacrificed at day 3 after injection, spleens and lungs were harvested, and the lymphocytes contained in these organs were analyzed by multiparametric flow cytometry. We detected a significant ~3-fold increase in the frequency of NK cells (Fig. 6A) and ~2-fold increase in the frequency of CD8+ CD44highCD62L− cells (Fig. 6B) in the lungs of animals receiving either SSP IL-15 or LSP IL-15 DNAs in combination with IL-15Rα DNA, in comparison with mice receiving the corresponding IL-15 expression plasmids alone. An increased frequency of NK cells and CD8+ CD44highCD62L− cells was also found in spleen (data not shown). These results suggested that both SSP IL-15/IL-15Rα and LSP IL-15/IL-15Rα heterodimeric complexes promoted expansion and/or mobilization of IL-15-dependent cellular subsets to the peripheral organs, where they can exert their functions. In addition, there is good correlation between the peak IL-15 plasma values and the NK frequency in lung (r2 = 0.78; Fig. 6C).
FIGURE 6.
Bioactivity comparison of LSP and SSP IL-15 DNA in mice. Mice (five animals per group) were injected hydrodynamically into the tail vein using 0.5 μg of the human IL-15 plasmids (SSP or LSP) either in the absence or presence of 1.5 μg of human IL-15Rα plasmid. Control mice were injected with a GFP plasmid (33). The mice were sacrificed at day 3 postinjection, and lung cells were analyzed using multicolor FACS. A, Frequency of lung cells identified as CD3− CD49b+ cells. B, Frequency of lung CD8 T cells with an effector memory phenotype (CD3+ CD8+ CD62L− CD44high). Values of p obtained using Student’s t test are shown. C, Correlation between the peak IL-15 plasma values and the NK frequency in lung. Data were fitted to a sigmoidal dose-response curve using Prism software package; correlation coefficient was r2 = 0.78.
Thus, coexpression of IL-15Rα is necessary for the secretion and bioactivity of both known physiological isoforms of IL-15. Once IL-15 is secreted in the serum, no qualitative difference was found between the LSP and SSP IL-15/IL-15Rα-dependent actions, and IL-15 effects correlate only with IL-15 plasma level, regardless of the isoform of IL-15 produced. Even in the presence of IL-15Rα, the apparent stability of SSP IL-15 is lower than LSP IL-15, resulting in lower secretion and steady-state levels of SSP IL-15/IL-15Rα. This may be a mechanism to produce lower levels of this powerful cytokine in specific tissues, such as the thymus (see Discussion).
Coexpressed SSP IL-15 and LSP IL-15 compete for binding to IL-15Rα
SSP IL-15 has been proposed to have regulatory functions (8, 12, 13, 39, 40). To test whether the coexpression of SSP and LSP IL-15 influences production and bioactivity, we injected mice via hydrodynamic delivery with a fixed amount of LSP IL-15 and IL-15Rα plasmids (0.1 μg) in the absence or presence of increasing amount of SSP IL-15 plasmid (from 0.1 to 10 μg), and compared cytokine plasma levels at 1 and 3 days post-delivery. Coexpression of LSP IL-15 and SSP IL-15 in complex with IL-15Rα in the same cells resulted in lower level of plasma IL-15 at both days 1 and 3 after DNA delivery (Fig. 7A). In addition, increasing ratio of delivered SSP/LSP IL-15 DNAs resulted in inferior bioactivity and progressive reduction of spleen size at day 3 after DNA delivery (Fig. 7B). Reduction in spleen size ranged from 10% in group receiving same amount of SSP and LSP IL-15 DNAs to 40% in group receiving 10-fold more of SSP IL-15 DNA. Spleen size is a reliable biomarker for IL-15 function (25, 26) and correlated very well with frequency of NK in spleen and lung and with IL-15 plasma levels (data not shown). This experiment suggested that, when coexpressed in the same cell, LSP IL-15 and SSP IL-15 compete for binding to IL-15Rα. Although SSP IL-15 forms also a bioactive complex with IL-15Rα, the kinetics and intracellular stability of this complex are different, resulting in a net decrease of bioactive IL-15 in the plasma. Similar results demonstrating competition for binding to the IL-15Rα were obtained in tissue culture experiments, after transfections of cells with equal amounts of LSP IL-15 and IL-15Rα (0.1 μg) DNAs in absence or presence of increasing amount of SSP IL-15 DNA (range: 0.05–1 μg) (Fig. 7C). Inclusion of SSP IL-15 resulted in a decrease of total IL-15. This is because of the SSP IL-15 complex formation to IL-15Rα, which has a higher chance to be degraded, as shown in Fig. 4. Thus, SSP IL-15 acts as competitive inhibitor for LSP IL-15, suggesting that usage of alternative splicing can be considered an additional step of regulation of IL-15 activity.
FIGURE 7.
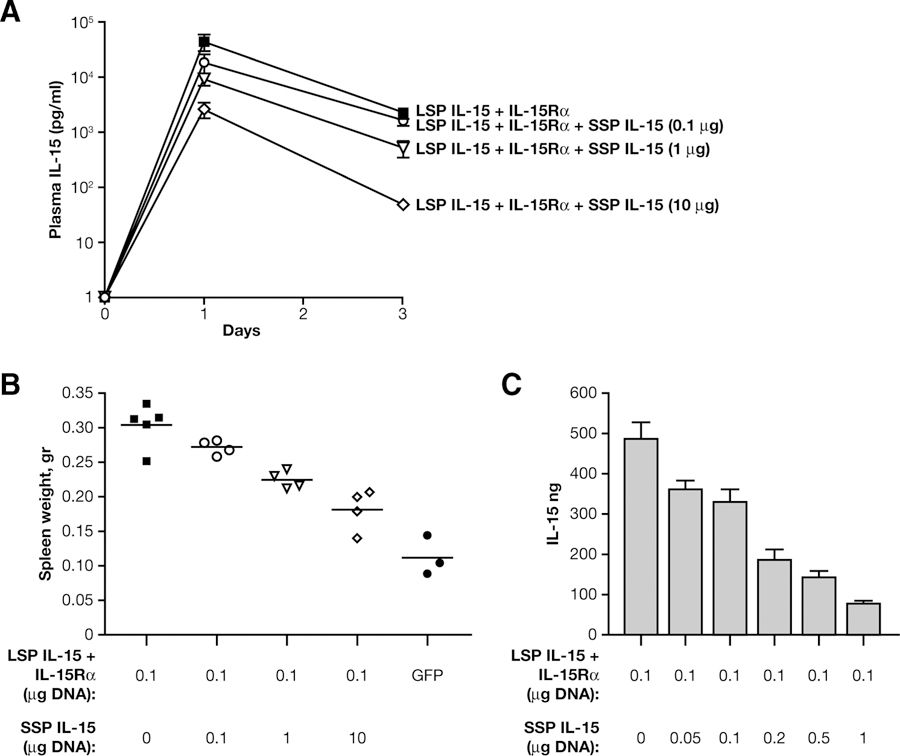
SSP IL-15 competes with LSP IL-15 for binding to IL-15Rα. Mice were hydrodynamically injected with 0.1 μg of LSP IL-15 expression plasmid in combination with 0.1 μg of IL-15Rα expression plasmid in the absence or presence of 0.1, 1, or 10 μg of SSP IL-15 DNA. A, IL-15 levels in plasma of injected mice were measured at days 1 and 3 by ELISA. B, Spleen weight was measured for the different groups (p < 0.001). C, In vitro transfection of 293 cells also shows competition of SSP and LSP IL-15 for binding to IL-15Rα. Cells were transfected with 0.1 μg of LSP IL-15 plasmid and 0.1 μg of IL-15Rα plasmid in the presence or absence of 0.05, 0.1, 0.2, 0.5, and 1 μg of SSP IL-15 plasmid. Total amount of produced cytokine was measured by ELISA.
Discussion
In this study, we compare expression and function of the two endogenously produced forms of IL-15, LSP and SSP, to further understand the function of SSP IL-15. The two isoforms are produced from the same transcript via alternative splicing and have identical amino acid sequence in the mature protein after the cleavage of the signal peptides, LSP or SSP. In agreement with previous reports (8, 12, 13), we found that SSP and LSP IL-15 expressed alone from optimized vectors have different localization and stability. Whereas the LSP IL-15 can be detected efficiently in the extracellular compartment, the SSP IL-15 is strictly found intracellularly and is detected both in the cytoplasm and the nucleus of DNA-transfected cells in vitro. IL-15 expressed from the SSP cDNA has a significantly reduced t1/2 compared with one expressed from LSP cDNA. We have previously shown that coexpression of IL-15Rα with LSP IL-15 increased the stability and extracellular levels of produced IL-15, via the formation of a heterodimer in the endoplasmic reticulum (25). We show in this study that IL-15Rα facilitates also the stability and secretion of SSP IL-15. Coexpressed IL-15Rα acts as an intracellular chaperone interacting with either form of IL-15 intracellularly and promoting their stability and progression through the secretory pathway. The heterodimeric cytokine is rapidly transported to the plasma membrane, where it is bioactive in trans and where it can be cleaved and released also in a soluble, bioactive form (3, 25, 30).
In our study, coexpression of SSP IL-15 and IL-15Rα in mice resulted in high plasma levels of bioactive IL-15 and mobilization and/or expansion of NK and T cells in nonlymphoid organs, such as lung. We also found low, but detectable plasma levels of SSP IL-15 after in vivo delivery even in the absence of IL-15Rα (Fig. 5, ∆). This is the only report of monomeric SSP IL-15 able to be secreted. This discrepancy is explained by the fact that, after efficient hydrodynamic delivery, the SSP IL-15 cDNA is expressed in many cells, including cells expressing the endogenous IL-15Rα. Therefore, SSP IL-15 has the opportunity to meet IL-15Rα in some cells after hydrodynamic delivery into normal mice and be secreted as a complex. In this case, SSP IL-15 is proposed to compete with mouse IL-15 and to replace endogenous mouse IL-15/IL-15Rα complexes, rather than increasing them. In support of this hypothesis, the low SSP IL-15 levels detected in the plasma of mice injected with only SSP IL-15 DNA do not show any bioactivity over the mice injected with control vectors (Fig. 6, A and B).
Therefore, SSP IL-15 is secreted and bioactive when produced as a heterodimer with IL-15Rα in the same cell in vivo and can exert the same function as LSP IL-15. Once IL-15/IL-15Rα is secreted in the serum, no qualitative differences were found between the LSP and SSP IL-15-dependent actions; IL-15 effects correlate only with IL-15 plasma level, regardless of the isoform of IL-15 produced.
The protein levels produced by our expression vectors are high for both the single chain and the heterodimer IL-15. This is the result of careful optimization of multiple steps in gene expression, including efficient promoter, mRNA stabilization, and efficient export. The nature of the signal peptide also affects posttranslational steps, resulting in altered IL-15 stability. Replacing the natural signal peptides of IL-15 with that of tissue plasminogen activator, IgE, or GM-CSF led to significant increase in extracellular IL-15 levels both in the absence (2, 5, 14) and presence of IL-15Rα (25).
A role of SSP IL-15 on IL-15 regulation has been proposed both because of its unexpected nuclear/cytoplasmic localization and because of direct experimental evidence (7, 12, 39, 40). Nishimura et al. (12, 39) proposed that SSP IL-15 play a role on the negative feedback of endogenous production of IL-15, controlling the transcriptional activation of IL-15 gene. Transgenic mice expressing SSP IL-15 were unable to produce high levels of bioactive IL-15 and were more susceptible to Salmonella infection compared with wild-type mice. Thus, in vivo, increased SSP IL-15 expression, such as in the reported transgenic mice, is overall inhibitory. We also found that SSP IL-15 expression is antagonistic to LSP, in the absence of any transcriptional regulation. Our expression vectors use the CMV promoter and override all transcriptional control mechanisms; therefore, our results cannot formally address the possibility of transcriptional regulation by SSP IL-15. Yet, coexpression of SSP and LSP IL-15 and IL-15Rα in the same cells inhibited plasma levels and bioactivity of IL-15, compared with LSP IL-15 coexpressed with IL-15Rα (Fig. 7). These results suggest an additional explanation for the results of Nishimura et al. (39), namely, that transgenic mice with constitutive SSP IL-15 expression may inhibit IL-15 expression due to posttranslational regulatory mechanisms. We showed that when both LSP IL-15 and SSP IL-15 are produced in the same cell, they compete for the binding to IL-15Rα, resulting in lower levels of bioactive IL-15. Because both isoforms of IL-15 may be produced in the same cell by alternative splicing (6, 8, 10, 11), this would provide for an additional level of regulation in vivo. Therefore, coexpressed SSP IL-15 acts as competitive inhibitor of LSP IL-15 and may regulate the bioavailability of LSP IL-15 at the level of intracellular trafficking through engagement of the IL-15Rα.
The apparent stability of SSP IL-15/IL-15Rα complex both in vitro and in vivo is lower compared with LSP IL-15/IL-15Rα complex, as revealed by direct comparisons (Fig. 2, A and B; Fig. 4, A and B; and Fig. 5). This results in lower production of secreted bioactive IL-15. Because SSP and LSP IL-15 have identical amino acid sequence after signal peptide processing, the difference in stability measured experimentally is the result of the different processing and trafficking of the two molecules. In addition, alternative splicing generating different functional IL-15Rα variants has been described (41, 42). One possibility is that SSP IL-15 might preferentially be coexpressed and/or interact with particular IL-15Rα isoforms, resulting in a complex with different biochemical features. Thus, alternative splicing may provide the cell with the ability to produce different levels of bioactive IL-15/IL-15Rα. It is interesting that SSP IL-15 mRNA is expressed at high levels in the thymus and in thymus cell lines (6, 8, 10, 11). An interesting hypothesis is that SSP IL-15 is important for intrathymic effects on lymphocytes. Perhaps thymocyte regulation requires either low levels or short-acting IL-15. SSP IL-15/IL-15Rα complexes can achieve this objective by lower production and by competition with LSP IL-15/IL-15Rα complex formation. Expression of both SSP and LSP forms of IL-15 appears to be conserved in many mammals (Fig. 1), suggesting that SSP may be important for providing a form of IL-15 with lower activity. Elucidation of IL-15 function is essential to harness the power of this cytokine for promising immunotherapy applications. Our results assign a function to the previously puzzling variant SSP IL-15, and suggest several testable hypotheses for further work. It will be of interest to determine which cells express SSP and LSP IL-15 and at what ratios, and how they regulate in vivo levels and activity of IL-15.
Acknowledgments
We thank A. Valentin for valuable discussions and assistance; K. Nandy and S. Lockett for help with confocal microscopy; J. Bear and J. Smith for technical assistance; and T. Jones for editorial assistance.
This work was supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations used in this paper:
- LSP
long signal peptide
- HA
hemagglutinin
- SSP
short signal peptide
- BGH
bovine growth hormone
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bamford RN, Battiata AP, Burton JD, Sharma H, and Waldmann TA 1996. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA 93: 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford RN, DeFilippis AP, Azimi N, Kurys G, and Waldmann TA 1998. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J. Immunol 160: 4418–4426. [PubMed] [Google Scholar]
- 3.Duitman EH, Orinska Z, Bulanova E, Paus R, and Bulfone-Paus S 2008. How a cytokine is chaperoned through the secretory pathway by complexing with its own receptor: lessons from interleukin-15 (IL-15)/IL-15 receptor α. Mol. Cell. Biol 28: 4851–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurys G, Tagaya Y, Bamford R, Hanover JA, and Waldmann TA 2000. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J. Biol. Chem 275: 30653–30659. [DOI] [PubMed] [Google Scholar]
- 5.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y, et al. 2005. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol 175: 112–123. [DOI] [PubMed] [Google Scholar]
- 6.Onu A, Pohl T, Krause H, and Bulfone-Paus S 1997. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J. Immunol 158: 255–262. [PubMed] [Google Scholar]
- 7.Tagaya Y, Bamford RN, DeFilippis AP, and Waldmann TA 1996. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4: 329–336. [DOI] [PubMed] [Google Scholar]
- 8.Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, and Waldmann TA 1997. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc. Natl. Acad. Sci. USA 94: 14444–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meazza R, Gaggero A, Neglia F, Basso S, Sforzini S, Pereno R, Azzarone B, and Ferrini S 1997. Expression of two interleukin-15 mRNA isoforms in human tumors does not correlate with secretion: role of different signal peptides. Eur. J. Immunol 27: 1049–1054. [DOI] [PubMed] [Google Scholar]
- 10.Meazza R, Verdiani S, Biassoni R, Coppolecchia M, Gaggero A, Orengo AM, Colombo MP, Azzarone B, and Ferrini S 1996. Identification of a novel interleukin-15 (IL-15) transcript isoform generated by alternative splicing in human small cell lung cancer cell lines. Oncogene 12: 2187–2192. [PubMed] [Google Scholar]
- 11.Nishimura H, Washizu J, Nakamura N, Enomoto A, and Yoshikai Y 1998. Translational efficiency is up-regulated by alternative exon in murine IL-15 mRNA. J. Immunol 160: 936–942. [PubMed] [Google Scholar]
- 12.Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, and Yoshikai Y 2005. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J 19: 19–28. [DOI] [PubMed] [Google Scholar]
- 13.Gaggero A, Azzarone B, Andrei C, Mishal Z, Meazza R, Zappia E, Rubartelli A, and Ferrini S 1999. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur. J. Immunol 29: 1265–1274. [DOI] [PubMed] [Google Scholar]
- 14.Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, Zhang GM, Sidhu MK, Eldridge JH, Weiner DB, et al. 2007. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol 26: 827–840. [DOI] [PubMed] [Google Scholar]
- 15.Burkett PR, Koka R, Chien M, Chai S, Boone DL, and Ma A 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med 200: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois S, Mariner J, Waldmann TA, and Tagaya Y 2002. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17: 537–547. [DOI] [PubMed] [Google Scholar]
- 17.Lodolce JP, Burkett PR, Boone DL, Chien M, and Ma A 2001. T cell-independent interleukin 15Rα signals are required for bystander proliferation. J. Exp. Med 194: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandau MM, Schluns KS, Lefrancois L, and Jameson SC 2004. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Rα by the same cells. J. Immunol 173: 6537–6541. [DOI] [PubMed] [Google Scholar]
- 19.Schluns KS, Klonowski KD, and Lefrancois L 2004. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood 103: 988–994. [DOI] [PubMed] [Google Scholar]
- 20.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, and Aguila HL 2004. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor α expression. Proc. Natl. Acad. Sci. USA 101: 5616–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, and Boone DL 2003. IL-15R α expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA 100: 4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, and Ma A 2003. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J. Exp. Med 197: 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giron-Michel J, Giuliani M, Fogli M, Brouty-Boye D, Ferrini S, Baychelier F, Eid P, Lebousse-Kerdiles C, Durali D, Biassoni R, et al. 2005. Membrane-bound and soluble IL-15/IL-15Rα complexes display differential signaling and functions on human hematopoietic progenitors. Blood 106: 2302–2310. [DOI] [PubMed] [Google Scholar]
- 24.Zambello R, Facco M, Trentin L, Sancetta R, Tassinari C, Perin A, Milani A, Pizzolo G, Rodeghiero F, Agostini C, et al. 1997. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood 89: 201–211. [PubMed] [Google Scholar]
- 25.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, and Pavlakis GN 2008. Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem 283: 4189–4199. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, and Sprent J 2006. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl. Acad. Sci. USA 103: 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoklasek TA, Schluns KS, and Lefrancois L 2006. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J. Immunol 177: 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois S, Patel HJ, Zhang M, Waldmann TA, and Muller JR 2008. Preassociation of IL-15 with IL-15Rα-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol 180: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 29.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, and Jacques Y 2006. Soluble interleukin-15 receptor α (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rβ/γ: hyperagonist IL-15 × IL-15Rα fusion proteins. J. Biol. Chem 281: 1612–1619. [DOI] [PubMed] [Google Scholar]
- 30.Mortier E, Woo T, Advincula R, Gozalo S, and Ma A 2008. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med 205: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, and Pavlakis GN 2005. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J. Virol 79: 8480–8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider R, Campbell M, Nasioulas G, Felber BK, and Pavlakis GN 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol 71: 4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauber R, Gaitanaris GA, and Pavlakis GN 1995. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology 213: 439–449. [DOI] [PubMed] [Google Scholar]
- 34.Zolotukhin AS, Tan W, Bear J, Smulevitch S, and Felber BK 2002. U2AF participates in the binding of TAP (NXF1) to mRNA. J. Biol. Chem 277: 3935–3942. [DOI] [PubMed] [Google Scholar]
- 35.Zolotukhin AS, Valentin A, Pavlakis GN, and Felber BK 1994. Continuous propagation of RRE and Rev RRE human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J. Virol 68: 7944–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Yamato E, and Miyazaki J 2001. Intravenous delivery of naked plasmid DNA for in vivo cytokine expression. Biochem. Biophys. Res. Commun 289: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Song Y, and Liu D 1999. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 38.Ortaldo JR, Winkler-Pickett RT, Bere EW Jr., Watanabe M, Murphy WJ, and Wiltrout RH 2005. In vivo hydrodynamic delivery of cDNA encoding IL-2: rapid, sustained redistribution, activation of mouse NK cells, and therapeutic potential in the absence of NKT cells. J. Immunol 175: 693–699. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, Matsuguchi T, Suzuki M, Ohashi PS, and Yoshikai Y 2000. Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo. J. Exp. Med 191: 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, et al. 2007. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med 13: 927–934. [DOI] [PubMed] [Google Scholar]
- 41.Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, Reiling N, and Bulfone-Paus S 2007. Soluble IL-15Rα is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem 282: 13167–13179. [DOI] [PubMed] [Google Scholar]
- 42.Bulanova E, Budagian V, Orinska Z, Krause H, Paus R, and Bulfone-Paus S 2003. Mast cells express novel functional IL-15 receptor α isoforms. J. Immunol 170: 5045–5055. [DOI] [PubMed] [Google Scholar]