Abstract
Recent advances in next generation sequencing have enabled panel gene testing, or simultaneous testing for mutations in multiple genes for a clinical condition. With more extensive and widespread genetic testing, there will be increased detection of genes with moderate penetrance without established clinical guidelines and of variants of uncertain significance (VUS), or genetic variants unknown to either be disease-causing or benign. This study surveyed 232 patients who underwent genetic counseling for hereditary breast and ovarian cancer to examine the impact of panel gene testing on psychological outcomes, patient understanding, and utilization of genetic information. The survey used standardized instruments including the Impact of Event Scale (IES), Multidimensional Impact of Cancer Risk Assessment (MICRA), Satisfaction with Decision Instrument (SWD), Ambiguity Tolerance Scale (AT-20), genetics knowledge, and utilization of genetic test results. Study results suggested that unaffected individuals with a family history of breast or ovarian cancer who received positive results were most significantly impacted by intrusive thoughts, avoidance, and distress. However, scores were also modestly elevated among unaffected patients with a family history of breast and ovarian cancer who received VUS, highlighting the impact of ambiguous results that are frequent among patients undergoing genetic testing with large panels of genes. Potential risk factors for increased genetic testing-specific distress in this study included younger age, black or African American race, Hispanic origin, lower education level, and lower genetic knowledge and highlight the need for developing strategies to provide effective counseling and education to these communities, particularly when genetic testing utilizes gene panels that more commonly return VUS. More detailed pre-test education and counseling may help patients appreciate the probability of various types of test results and how results would be used clinically, and allow them to make more informed decisions about the type of genetic testing to select.
Keywords: genetic counseling, genetic testing for cancer susceptibility, breast cancer, panel gene testing, MICRA, cancer genetics, hereditary breast and ovarian cancer
Introduction
Genetic testing for hereditary cancer risk assessment has become standard for breast and ovarian cancers. Five to ten percent of all breast cancers are hereditary and due to monogenic, highly penetrant genes, and 30% occur in women with a family history of breast cancer and may be due to genes and genetic variants of moderate risk (Lynch, Silva, Snyder, & Lynch, 2008). Historically, genetic testing for individuals with a personal or strong family history of breast cancer focused on germline mutations in BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) genes, as these are the most common monogenetic contributors to breast cancer. However, many other genes beyond BRCA1 or BRCA2 have been demonstrated to cause heritable breast cancers (Robson & Offit, 2007).
Protein-truncating mutations in other DNA repair genes, including PALB2 (OMIM 610355), CHEK2 (OMIM 604373), ATM (OMIM 607585), and NBN (OMIM 602667), have been associated with an increased risk of hereditary breast cancer (Easton et al., 2015; Hearle et al., 2006; Olivier et al., 2003; Pharoah, Guilford, Caldas, & Consortiu, 2001; Tan et al., 2012). Mutations in TP53 (OMIM 191170), PTEN (OMIM 601728) and CDH1 (OMIM 192090) are known to cause cancer syndromes that confer an increased risk of breast cancer (Bubien et al., 2013; Hwang, Lozano, Amos, & Strong, 2003; Liaw et al., 1997; Madanikia, Bergner, Ye, & Blakeley, 2012; Pharoah et al., 2001; Seminog & Goldacre, 2015; Tan et al., 2012; Walsh et al., 2010). Patients with strong family histories of breast cancer but no mutation in BRCA1/2 may have mutations in one or more of these other genes.
Recent advances in next generation sequencing (NGS), have enabled sequencing of multiple genes simultaneously, with increased speed and cost effectiveness and improved clinical sensitivity (Ku, Cooper, Iacopetta, & Roukos, 2013; LaDuca et al., 2014; McCarthy, McLeod, & Ginsburg, 2013). Cost savings with NGS has made genetic testing for hereditary breast cancer more widely available (Walsh et al., 2010). This has enabled panel gene testing, or simultaneous testing for mutations in multiple genes causing a single clinical condition (Ku et al., 2013; Walsh et al., 2010).
Though panel gene testing increases the sensitivity of genetic testing for breast cancer, thereby improving options for detection and treatment of hereditary breast cancer, it has several important limitations. While highly penetrant genes such as BRCA1 and BRCA2 have well-defined risk profiles and guidelines for clinical management of mutation carriers, mutations in other genes are not yet as well understood and the risk profiles are not yet defined or available to guide clinical management (Easton et al., 2015; Rainville & Rana, 2014; Walsh et al., 2010). Lifetime risks of breast cancer with BRCA1 and BRCA2 are well defined and high, estimated to be 50–70% (Antoniou et al., 2003; Chen & Parmigiani, 2007; Mavaddat et al., 2013). For women found to have disease-causing mutations in BRCA1 or BRCA2, there are enhanced screening options for early detection including mammography, magnetic resonance imaging (MRI), and ultrasonography, as well as options for risk reduction, including chemoprevention with tamoxifen or raloxifene, risk-reducing salpingo-oopherectomy, and risk-reducing mastectomy (Robson & Offit, 2007). However, with more widespread panel genetic testing, more women are being identified as carrying pathogenic variants in genes with moderate penetrance for which the management is less clear (LaDuca et al., 2014; Rainville & Rana, 2014). As more genes are tested in panels, more women are identified to have variants of uncertain significance (VUS), genetic variants that are unknown to either be benign or associated with disease. The frequency of returning VUS in panel gene testing for cancer has been 21.9 to 33.3% (Frey et al., 2015; Lincoln et al., 2015).
Prior to the availability of panel gene tests for hereditary cancer, previous studies have examined the psychological effects on patients of genetic testing for breast and ovarian cancer, with conflicting results. A meta-analysis of these studies looking specifically at anxiety and cancer-specific distress found that there was an increase in cancer-specific distress in the short-term, with return to baseline over time (Hamilton, Lobel, & Moyer, 2009). While most studies have found an increase in psychological distress, anxiety, and depression in the first few months after disclosure of results with adaptation beyond one year, other longer-term studies have shown persistent distress in mutation carriers (Bjornslett, Dahl, Sorebo, & Dorum, 2015; Crotser & Boehmke, 2009; Douglas, Hamilton, & Grubs, 2009; Heshka, Palleschi, Howley, Wilson, & Wells, 2008; Hirschberg, Chan-Smutko, & Pirl, 2015; Ringwald et al., 2016). A study looking at outcomes in patients with VUS results showed that they had the poorest ability to recall and report the clinical significance of their test results, and that their cancer worry was lower than mutation carriers and equivalent to those with negative test results (Hirschberg et al., 2015). Another study, however, showed that women who received BRCA1/2 VUS results had increased frustration, anxiety, and depression post-disclosure as compared with women who had no mutation identified (O’Neill et al., 2009).
The psychological impact of cancer genetic testing has not been well-studied in individuals undergoing panel gene testing. Understanding the impact of panel gene testing is important given the increased amount of information and uncertainty introduced by panel gene testing. The large number of choices of gene panels that patients now have for hereditary cancer testing allows the ability to tailor the genetic testing to the preferences of the patient, yet increases the complexity of pre-test education and counseling. In addition, due to the number of genes included in the panels, and therefore the higher probability for a patient to receive one or more VUS, and the relatively limited amount of information about penetrance and cancer spectrum associated with several of the recently described clinical conditions associated with the new hereditary cancer genes, there is a greater opportunity to receive medically impactful information and also uncertain information from panel gene testing that could increase the psychological burden of testing. In the first study of the psychological impact of panel gene testing for hereditary breast cancer, no short-term increase in anxiety, depression, uncertainty, or cancer worry following genetic testing was observed; however, a large subset of patients in this study declined multiplex testing due uncertainty or distress (Bradbury et al., 2016). Additional studies may help to identify how patient preference and understanding can guide genetic testing and counseling (Kurian et al., 2014).
This study aims to describe patient understanding, psychological outcomes, and utilization of genetic information among patients with a personal or family history of breast or ovarian cancer who were offered panel gene testing for hereditary breast and ovarian cancer according to the results received: pathogenic/likely pathogenic variants, VUS or normal results. These results may inform genetic testing and counseling for future patients undergoing panel gene testing.
Methods
Participants
The study population included all patients referred to the Columbia University Cancer Genetics Clinic for counseling for hereditary breast and ovarian cancer between June 2013 and May 2015. Non-English speaking patients, patients without a personal or family history of breast or ovarian cancer and patients who did not undergo genetic testing at the time of consultation were excluded from the study. Consent was obtained from all participants, and the study was approved by the Institutional Review Board at Columbia University.
Survey
A 56-question survey was designed by a certified genetic counselor and clinical geneticist using a combination of novel questions and standardized instruments (Supplemental Materials). The study questionnaire included novel questions about genetic knowledge, experience with genetic testing, and understanding of genetic test results. It also used standardized instruments including the Impact of Event Scale (IES), Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire, Satisfaction with Decision Instrument (SWD), and Revised Scale for Ambiguity Tolerance (AT-20) (Cella et al., 2002; Holmes-Rovner et al., 1996; A. P. MacDonald, 1970; Weiss & Marmar, 1997). The IES is an instrument used to assess the degree of distress experienced after a major life event, based upon the diagnostic criteria for post-traumatic stress disorder. It includes subscales for intrusive thoughts, avoidant behavior, and symptoms of hyperarousal (Weiss & Marmar, 1997). The MICRA is a questionnaire that was designed specifically to assess the psychological impact after genetic testing for hereditary cancer syndromes. The original study was conducted in patients undergoing genetic testing for BRCA1/2 mutations. The MICRA includes three sub-scales that assess feelings of distress, uncertainty, and positivity after receiving genetic test results (Cella et al., 2002). Utilization of test results was assessed by a set of novel yes-or-no questions asking whether genetic test results played a role in specific screening and treatment decisions. Perceived discrimination was assessed by a novel set of questions asking if individuals had worried about or experienced discrimination following genetic testing in obtaining insurance, in the workplace, or in interpersonal relationships. Our complete survey was piloted with ten individuals to identify and clarify ambiguous questions prior to implementation.
Procedures
A total of 466 patients were seen in the Columbia Cancer Genetics program for counseling for hereditary breast or ovarian cancer. Of those, two (0.4%) were excluded because they were deceased, 10 (2.1%) were non-English speaking, 31 (6.7%) did not have current contact information, six (1.3%) did not have a personal or family history of breast or ovarian cancer, and 50 (10.7%) did not undergo genetic testing at the time of consultation. An invitation letter was mailed to the remaining 367 eligible participants. Following distribution of invitation letters, participants were contacted by phone to request their participation in the study with a maximum of five phone attempts. In total, 283 patients (77.1%) agreed to participate. Questionnaires were distributed by email, mail, or phone, according to patient preference, between June and December 2015. Electronic surveys were distributed using Qualtrics via individualized email links, and all survey data were maintained in Qualtrics. Of the 283 patients who initially agreed to participate, 51 later declined participation. In total, 232 (63.2% of eligible 367) patients completed and returned the questionnaire (Figure 1).
Figure 1.
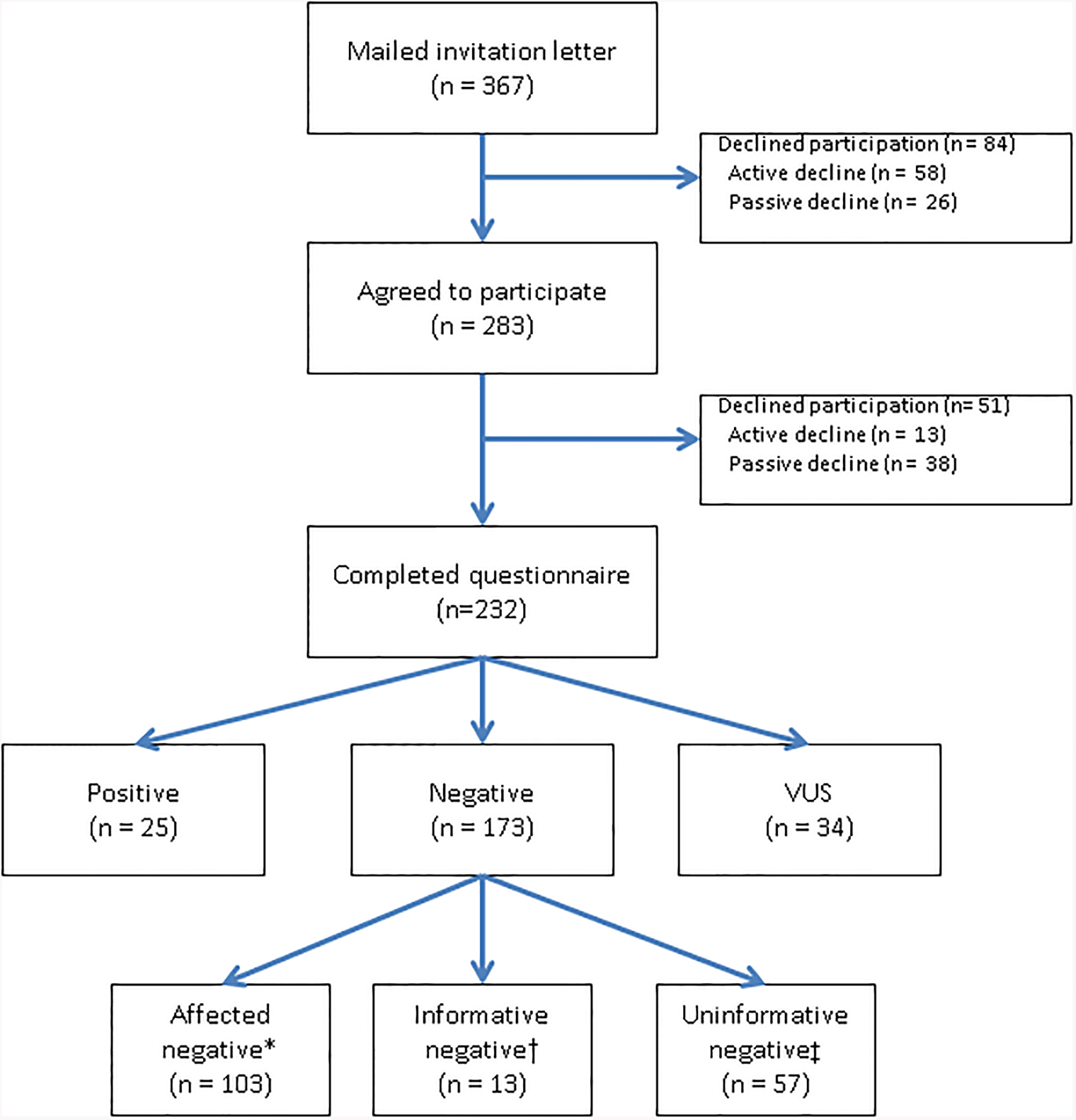
Study Flow Chart
*Affected negative: negative results in patient with personal history of breast or ovarian cancer
†Informative negative: negative for known familial mutation
‡Uninformative negative: negative test result in a patient with no known familial mutation and no personal history of breast or ovarian cancer
Study Groups
Participants were divided into six groups based on personal history of breast or ovarian cancer and genetic test results using the classification system recommended by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines (Richards et al., 2015). Outcomes were analyzed based on these groups as follows:
CA/mutation +:
11 patients (5%) with personal history of breast or ovarian cancer and positive (pathogenic/likely pathogenic) genetic test results
CA/VUS:
14 patients (6%) with personal history of breast or ovarian cancer and VUS
CA/mutation −:
104 patients (45%) with personal history of breast or ovarian cancer and negative (normal) genetic test results
No CA/mutation +:
14 unaffected patients (6%) with a family history of breast or ovarian cancer (no personal breast or ovarian cancer history) and positive (pathogenic/likely pathogenic) genetic test results
No CA/VUS:
20 unaffected patients (9%) with a family history of breast or ovarian cancer and VUS
No CA/mutation −:
69 unaffected patients (30%) with a family history of breast or ovarian cancer and negative genetic test results.
If a participant had a pathogenic/likely pathogenic variant in one gene and a VUS in a second gene, they were categorized as having a positive result. Genes in which pathogenic mutations or VUS were detected were classified based upon their risk profile. Genes classified as high risk included APC, BMPR1A, BRCA1/2, CDH1, CDKN2A, EPCAM, MLH1, MSH2, MSH6, MUTYH, PMS2, PTEN, SMAD4, STK11, TP53, and VHL. Genes classified as moderate risk included ATM, CHEK2, NF1, and PALB2. Newer genes with less clear risk profiles included AXIN2, BARD1, BRIP1, CDK1, FANCC, NBN, POLD1, POLE, RAD50, RAD51C, RAD51D, SCG5/GREM1, and XRCC2 (Richards et al., 2015).
Data Analysis
Demographic data were compared using t-tests for continuous variables and chi-square tests for categorical variables. Non-parametric analysis of variance with Kruskal-Wallis testing was used to analyze the IES, MICRA, and SWD scores by the six study groups, due to the non-normal distribution of the outcome measures. Dunn’s test for pairwise comparison of groups was performed when Kruskal-Wallis tests were significant (Dunn, 1964). Median regression was used to analyze the association between age, race, education, time from genetic testing to questionnaire completion, ambiguity tolerance, and genetic knowledge with the IES, MICRA, and SWD scores. Adjusted analyses were performed using median regression. Only variables where we observed a statistically significant relationship with the IES, MICRA, or SWD scores were included in the model for that outcome. All statistical analyses were performed using STATA 14.1. P values <0.05 were considered statistically significant. No adjustments were made for missing data. All analyses were performed with all participants who responded to a given question. Of the 232 participants, 218 (94.0%) responded to all of the IES, MICRA, and SWD questions.
Results
Participant Characteristics
Of the 232 participants, mean age was 48.7 years (range 21–88), 224 (96.6%) were female, and 155 (66.8%) were of European ancestry. In total, 129 (55.6%) participants had a personal history of breast or ovarian cancer and 103 (44.4%) had no personal history of breast or ovarian cancer and had a family history of breast or ovarian cancer. Of the 135 patients who declined participation, mean age was 48.8 years (range 15–83), 131 (97.0%) were female, and 72 (53.3%) had a personal history of breast or ovarian cancer, similar to the participants in the study. Patients with a personal history of breast or ovarian cancer were significantly older than unaffected patients with a family history of cancer, with mean ages of 54.3 years (range 24–88) and 41.6 years (range 21–72), respectively. There was a statistically significant difference in the type of genetic testing performed and in the genetic test results between the two groups, with unaffected patients more frequently receiving targeted testing for a known familial mutation or multisite 3 testing for the Ashkenazi Jewish mutations in BRCA1/2. All other demographic data were similar between the two groups (Table 1). Among the 25 individuals who received positive test results, 24 (96%) had mutations in high-risk genes and one (4%) had a mutation in a moderate risk gene (Table 2). Thirty-four patients had VUS results, with a total of 40 VUS detected among these patients. Of the VUS results, 25 (62.5%) were in high-risk genes, 12 (30%) were in moderate risk genes, and 3 (7.5%) were in newer genes with undefined risk (Table 2) (Richards et al., 2015). On average, patients completed the questionnaire just over 12 months following genetic testing.
Table 1.
Patient Characteristics
| Personal history of breast or ovarian cancer n = 129 (%) | Unaffected with family history of breast or ovarian cancer n = 103 (%) | p value* | |
|---|---|---|---|
| Age at questionnaire, years | |||
| Mean (sd) | 54.3 (12.6) | 41.6 (13.0) | <0.001 |
| Range | 24–88 | 21–72 | |
| Age at breast cancer diagnosis | |||
| Mean (sd) | 49.6 (11.4) | n/a | |
| Range | 22–77 | ||
| Time from genetic testing to questionnaire, months | |||
| Mean (sd) | 13.3 (6.7) | 12.5 (6.3) | 0.33 |
| Range | 3–26 | 3–27 | |
| Gender | |||
| Female | 128 (99.2) | 96 (93.2) | 0.02† |
| Male | 1 (0.8) | 7 (6.8) | |
| Hispanic | |||
| Y | 23 (17.8) | 24 (23.3) | 0.30‡ |
| Race | |||
| Caucasian, non-Hispanic | 76 (58.9) | 62 (60.2) | 0.62‡ |
| Caucasian, Hispanic | 19 (14.7) | 19 (18.5) | |
| Black or African American | 14 (10.9) | 9 (8.7) | |
| Asian or Pacific Islander | 11 (8.5) | 4 (3.9) | |
| Other (includes American Indian or Alaska Native, black Hispanic, multiracial) | 9 (7.0) | 7 (6.8) | |
| No response | 0 (0) | 2 (1.9) | |
| Educational level | |||
| College graduate or higher | 87 (67.4) | 82 (79.6) | 0.053 |
| Some college or less | 42 (32.6) | 21 (20.4) | |
| Type of Genetic Testing | |||
| Single familial gene mutation | 1 (0.8) | 15 (14.6) | <0.001 |
| BRCA1 and BRCA2 | 25 (19.4) | 9 (8.7) | |
| Multisite 3 | 8 (6.2) | 14 (13.6) | |
| Small panel (5–6 genes) | 66 (51.2) | 38 (36.9) | |
| Medium panel (17–18 genes) | 11 (8.5) | 8 (7.8) | |
| Large panel (25+ genes) | 18 (14.0) | 19 (18.5) | |
| Genetic Test Results | |||
| Positive | 11 (8.5) | 14 (13.5) | 0.06 |
| Negative | 104 (80.6) | 69 (66.9) | |
| Variant of uncertain significance (VUS) | 14 (10.9) | 20 (19.4) |
Compared using t-tests for continuous variables and chi2 test for categorical variables
Fisher’s exact
“No response” omitted for chi2 test
Table 2.
Classification of pathogenic/likely pathogenic variants and VUS results by risk profile
| Pathogenic n=25 (%) | VUS n=40 (%)* | ||
|---|---|---|---|
| High risk | APC | 1 (4) | |
| BRCA1 | 9 (36) | 3 (7.5) | |
| BRCA2 | 10 (40) | 6 (15) | |
| CDH1 | 2 (5) | ||
| CDKN2A | 1 (2.5) | ||
| MLH1 | 1 (2.5) | ||
| MSH2 | 1 (2.5) | ||
| MSH6 | 3 (7.5) | ||
| MUTYH | 3 (12) | 1 (2.5) | |
| PMS2 | 3 (7.5) | ||
| PTEN | 3 (7.5) | ||
| STK11 | 1 (2.5) | ||
| TP53 | 1 (4) | ||
| Moderate risk | ATM | 8 (20) | |
| CHEK2 | 1 (2.5) | ||
| NF1 | 1 (2.5) | ||
| PALB2 | 1 (4) | 2 (5) | |
| Newer genes | RAD50 | 2 (5) | |
| RAD51 | 1 (2.5) |
Thirty-four study participants had VUS results, however five participants had more than one VUS detected.
Patient Understanding and Interpretation of Results
Of the 14 patients in the CA/VUS group, four (28.6%) reported that they had received a result of VUS and nine (64.3%) reported that they had received a negative test result. Of the patients in the No CA/mutation + group, 12 (80.0%) interpreted positive results to mean an increased risk of breast cancer and none interpreted positive results to mean that they were guaranteed to develop breast cancer. Of the patients in the No CA/mutation - group, 46 (67.6%) interpreted negative results to mean that they were less likely to develop breast cancer compared to the risk calculated prior to genetic testing, 13 (19.1%) felt these results did not clarify their breast cancer risk, and three (4.4%) interpreted these results to mean they had no risk of breast cancer.
Psychological Outcomes
Median Regression Analyses for Participant Characteristics
Age
There was a small but statistically significant decrease in median MICRA positive experience and total scores with increasing age. There was a small but statistically significant increase in median SWD scores with increasing age (Supplement Table 1Sa).
Race
Median IES total, intrusion, avoidance, and hyperarousal scores and MICRA distress subscale scores were significantly higher (worse) in African Americans as compared with Caucasians. SWD scores were significantly lower (worse) among Hispanics and Asians as compared with Caucasians. There was no significant difference in the median scores for the other MICRA or IES scales between different races (Supplement Table 1Sb).
Education
Median SWD scores were significantly lower among individuals with lower education level. There was no significant difference in any of the other outcome measures by education level (Supplement Table 1Sc).
Time from genetic testing to questionnaire completion
There was a small statistically significant positive relationship between median MICRA total scores and the time from genetic testing to questionnaire completion, and there was a statistically significant negative relationship between median SWD scores and time to questionnaire completion (Supplement Table 1Sd).
Ambiguity Tolerance
By median quantile regression, there was no significant relationship between AT-20 scores and the MICRA, IES, or SWD scores (Supplement Table 1Se).
Genetics Knowledge
Greater knowledge about genetics was significantly associated with lower IES avoidance scores and increased SWD scores (Supplement Table 1Sf).
Multidimensional Impact of Cancer Risk Assessment (MICRA)
There was a statistically significant difference between MICRA total, distress, and positive experience scores between groups (Table 3). For the MICRA total score and distress subscale, scores were highest in the No CA/mutation + group. MICRA total scores were also increased in the No CA/VUS and CA/mutation + groups (Table 3; Figure 2a). MICRA distress scores were also high in the CA/VUS group when compared to mutation - groups. MICRA positive scores were similar among the CA/mutation +, No CA/mutation + and No CA/VUS groups (Table 3, Figure 2c). There was no difference between groups for the MICRA uncertainty subscale (Table 2, Figure 2d).
Table 3.
Comparison of Impact of Event Scale, Multidimensional Impact of Cancer Risk Assessment, and Satisfaction with Decision Instrument Scores between Sub-Groups
| CA/mutation + (n = 11) | CA/VUS (n = 14) | CA/mutation − (n = 104) | No CA/mutation + (n = 14) | No CA/VUS (n = 20) | No CA/mutation − (n = 69) | Non-parametric group comparison (p) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (sd) Median (IQR) | n | Mean (sd) Median (IQR) | n | Mean (sd) Median (IQR) | n | Mean (sd) Median (IQR) | n | Mean (sd) Median (IQR) | n | Mean (sd) Median (IQR) | ||
| IES, total | 11 | 6.8 (12) 2 (0, 6) | 13 | 7.6 (9.9) 3 (2, 10) | 100 | 8.0 (15) 1 (0, 11) | 14 | 18.1 (12) 17 (8, 24) | 18 | 8.8 (11) 3.5 (1, 14) | 66 | 6.7 (11) 1 (0, 11) | 0.01 |
| IES, intrusion | 11 | .2 (.4) 0 (0, 0.1) | 13 | .3 (.4) 0.1 (0, 0.5) | 99 | .3 (.7) 0 (0, 0.4) | 14 | 1 (.8) 0.7 (0.5, 1.6) | 18 | .4 (.5) 0.3 (0, 0.5) | 66 | .3 (.5) 0 (0, 0.5) | 0.0009 |
| IES, avoidance | 11 | .4 (.7) 0 (0, 0.8) | 13 | .5 (.7) 0.3 (0.1, 0.5) | 100 | .5 (.9) 0 (0, 0.6) | 14 | 1 (.6) 1.0 (0.5, 1.5) | 18 | .5 (.6) 0.3 (0, 1.1) | 66 | .4 (.7) 0 (0, 0.5) | 0.01 |
| IES, hyperarousal | 11 | .3 (.6) 0 (0, 0.2) | 13 | .2 (.3) 0 (0, 0.2) | 100 | .3 (.7) 0 (0, 0.2) | 14 | .5 (.7) 0.2 (0.2, 0.7) | 18 | .2 (.4) 0 (0, 0.2) | 66 | .2 (.4) 0 (0, 0.2) | 0.07 |
| MICRA, total | 11 | 19.4 (12.2) 19 (10, 26) | 13 | 12.4 (8.6) 14 (3, 18) | 100 | 11.6 (9.4) 10 (3, 18) | 13 | 29.6 (14.0) 25 (22, 42) | 17 | 19.0 (10.8) 18 (11, 22) | 67 | 12.4 (8.6) 12 (5, 20) | 0.0001 |
| MICRA, distress | 11 | 4.5 (6.7) 0 (0, 9) | 12 | 3.3 (3.7) 3 (0, 6) | 99 | 1.4 (3.1) 0 (0, 1) | 13 | 10.9 (5.7) 9 (8, 13) | 17 | 3.3 (5.8) 0 (0, 1) | 69 | 1.5 (3.1) 0 (0, 3) | 0.0001 |
| MICRA, uncertainty | 11 | 3.9 (4.2) 2 (0, 8) | 13 | 4.8 (5.3) 4 (0, 6) | 99 | 4.2 (5.2) 3 (0, 6) | 13 | 9.6 (7.7) 6 (3, 15) | 17 | 6.0 (7.3) 3 (2, 7) | 67 | 4.3 (5.3) 3 (0, 7) | 0.13 |
| MICRA, positive experience | 11 | 10.9 (5.5) 10 (7, 16) | 13 | 4.7 (3.5) 4 (2, 6) | 100 | 6.1 (6.7) 4 (0, 10) | 13 | 9.1 (4.6) 10 (6, 11) | 17 | 9.7 (7.1) 10 (3, 16) | 67 | 6.6 (7.3) 4 (0, 12) | 0.03 |
| SWD | 11 | 20.5 (6.2) 22 (20, 25) | 13 | 21.5 (5.6) 25 (20, 25) | 100 | 20.9 (5.9) 23 (20, 25) | 14 | 21.7 (3.3) 21 (20, 25) | 18 | 23.1 (2.2) 25 (21, 25) | 66 | 22.2 (4.2) 25 (20, 25) | 0.80 |
IES: Impact of Event Scale; MICRA: Multidimensional Impact of Cancer Risk Assessment; SWD: Satisfaction with Decision Instrument
Figure 2.
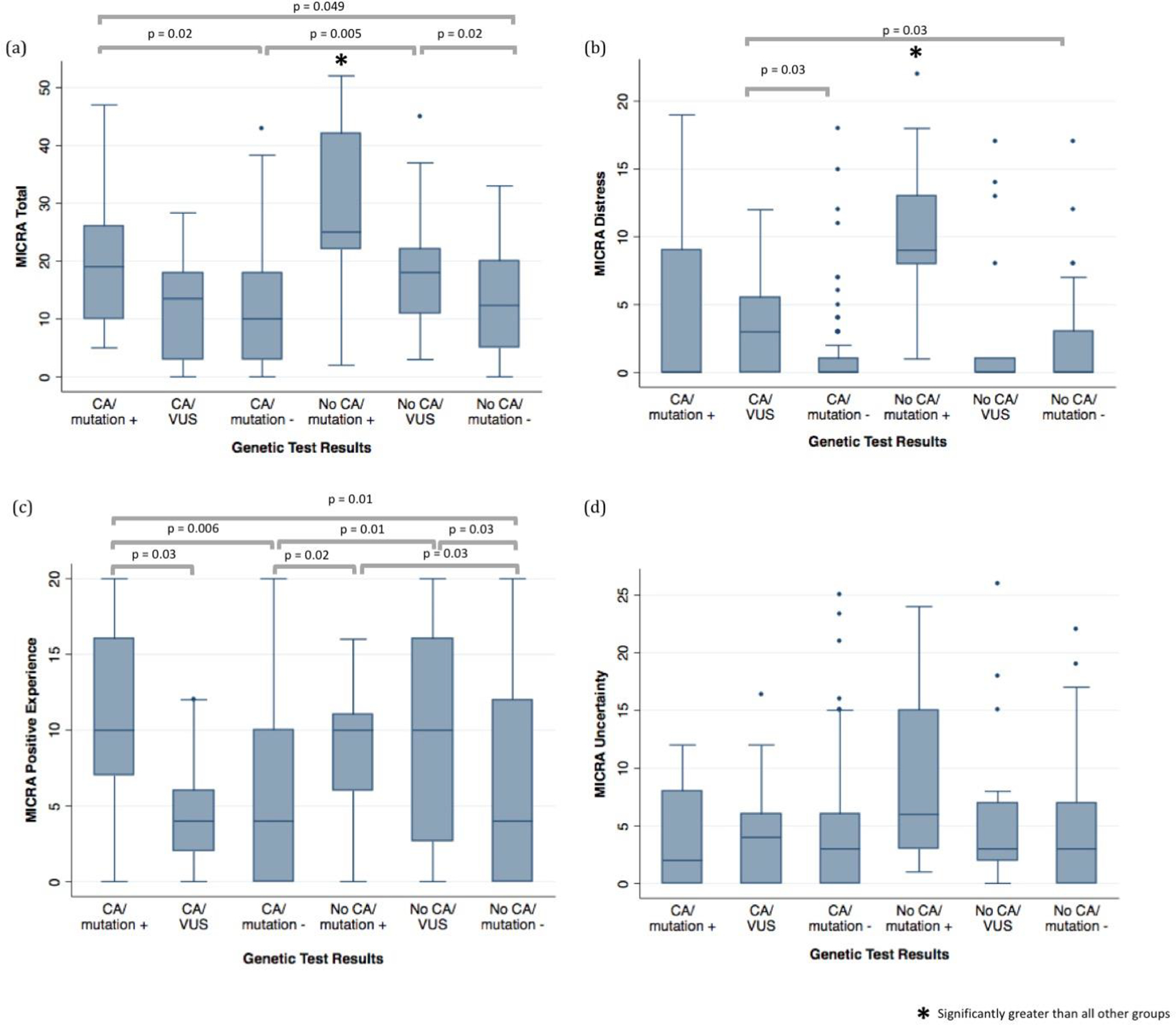
Multidimensional Impact of Cancer Risk Assessment (MICRA) total, distress, positive experience, and uncertainty scores by genetic test results compared using Kruskal-Wallis test and Dunn’s test for pairwise comparison of groups if applicable
When adjusted for age, race, education, time to questionnaire completion, and genetic knowledge, the MICRA total, distress, and positive experience scores were significantly different between the study groups (Table 4). In subsequent pairwise comparisons between each of the study groups for these MICRA scores, several significant differences were observed. MICRA total scores were greater in the No CA/mutation + group than in either of the mutation - groups or in the CA/VUS group (Supplement Figure 1Sa). MICRA distress scores were greater in the No CA/mutation + group than in any of the other groups, and the CA/VUS group was observed to have higher MICRA distress scores than all groups other than the No CA/mutation + group (Supplement Figure 1Sb). The No CA/VUS and the CA/mutation + groups had worse positive experience scores than any patients with negative genetic test results (Supplement Figure 1Sc).
Table 4.
Multiple median regression models for outcomes adjusted for age, race, education, time to questionnaire completion, and genetics knowledge
| CA/mutation + (n = 11) | CA/VUS (n = 14) | CA/mutation − (n = 104) | No CA/mutation + (n = 14) | No CA/VUS (n = 20) | No CA/mutation − (n = 69) | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Predicted median (SE) | n | Predicted median (SE) | n | Predicted median (SE) | n | Predicted median (SE) | n | Predicted median (SE) | n | Predicted median (SE) | ||
| IES, total | 11 | 2.4 (3.3) | 13 | 3.4 (3.0) | 100 | 1.4 (1.1) | 14 | 17.4 (2.9) | 18 | 4.4 (2.6) | 66 | 1.4 (1.3) | <0.001 |
| IES, intrusion | 11 | 0.03 (0.1) | 13 | 0.2 (0.1) | 98 | 0.03 (0.03) | 14 | 0.7 (0.09) | 17 | 0.3 (0.08) | 65 | 0.03 (0.04) | <0.001 |
| IES, avoidance | 11 | 0.09 (0.25) | 13 | 0.3 (0.2) | 96 | 0.09 (0.08) | 14 | 1.0 (0.2) | 17 | 0.3 (0.2) | 65 | 0.09 (0.1) | 0.012 |
| IES, hyperarousal | 11 | 0.02 (0.05) | 13 | 0.02 (0.04) | 100 | 0.02 (0.02) | 14 | 0.18 (0.04) | 18 | 0.02 (0.04) | 66 | 0.02 (0.02) | 0.014 |
| MICRA, total | 11 | 18.6 (4.2) | 13 | 12.2 (3.8) | 100 | 11.4 (1.5) | 13 | 25.2 (3.9) | 17 | 18.6 (3.4) | 67 | 11.2 (1.7) | 0.01 |
| MICRA, distress | 11 | 0 (0.79) | 12 | 3 (0.8) | 97 | 0 (0.3) | 12 | 9 (0.7) | 16 | 0 (0.6) | 66 | 0 (0.3) | <0.001 |
| MICRA, uncertainty | 11 | 2 (2.1) | 13 | 4 (1.9) | 99 | 3 (0.70) | 13 | 6 (1.9) | 17 | 3 (1.7) | 66 | 3 (0.84) | 0.73 |
| MICRA, positive experience | 11 | 10 (2.7) | 13 | 4.1 (2.4) | 100 | 3.7 (0.95) | 13 | 9.3 (2.5) | 17 | 9.7 (2.2) | 67 | 3.6 (1.1) | 0.01 |
| SWD | 11 | 22.0 (1.5) | 13 | 23.0 (1.4) | 98 | 23.1 (0.5) | 13 | 22.5 (1.4) | 18 | 23.6 (1.2) | 66 | 23.4 (0.6) | 0.95 |
IES: Impact of Event Scale; MICRA: Multidimensional Impact of Cancer Risk Assessment; SWD: Satisfaction with Decision Instrument
Impact of Event Scale
Median IES total, intrusion, and avoidance scores were significantly different among the six study groups. Using Dunn’s test for pairwise comparisons of groups, we observed that IES total, intrusion and avoidance scores were significantly higher the No CA/mutation + group than any of the other groups (Table 3, Figure 3a, 3b, 3c). No statistically significant differences between groups were observed with any of the other pairwise comparisons (Figure 3).
Figure 3.
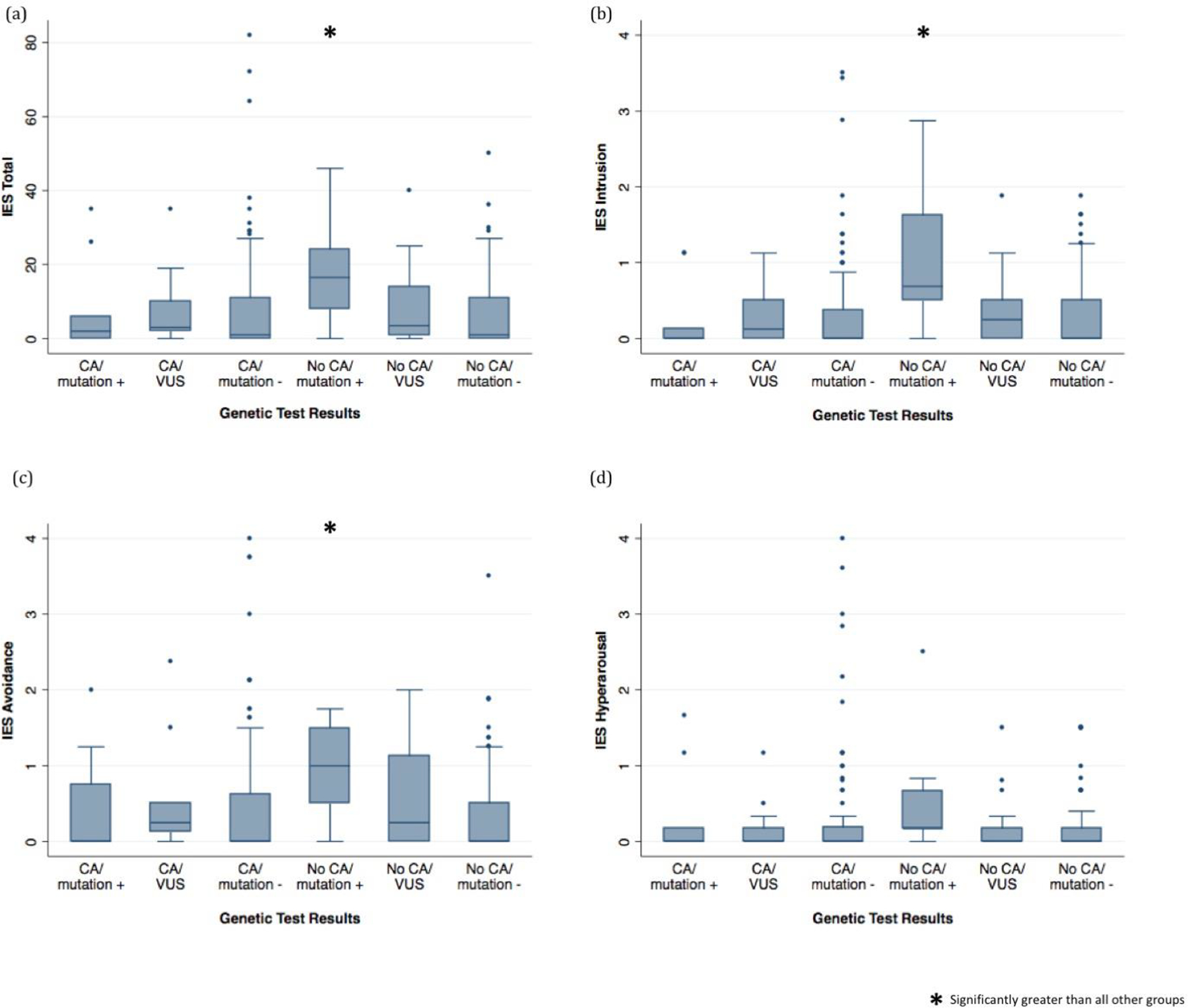
Impact of Event Scale (IES) total, intrusion, avoidance, and hyperarousal scores by genetic test results compared using Kruskal-Wallis test and Dunn’s test for pairwise comparison of groups test if applicable
When adjusted for age, race, education, time to questionnaire completion, and genetics knowledge in median regression models, overall between-group differences for IES remained significant, and the difference between sub-groups on the IES hyperarousal sub-scale was significant (Table 4, Supplement Figure 2S). Using pairwise comparisons in the adjusted model, we observed that median IES total, avoidance, intrusion, and hyperarousal scores were significantly higher in the No CA/mutation+ group than in any of the other groups (Supplement Figure 2S). IES intrusion scores were also significantly higher in the No CA/VUS group than any patients with negative results (Supplement Figure 2Sb).
Satisfaction with Decision
There was no significant difference in satisfaction with the decision to undergo genetic testing between groups in either the unadjusted or adjusted analysis (Tables 3 and 4).
Utilization of Test Results
The No CA/mutation + group most frequently made decisions based on genetic test results. Of the 14 patients in this group, 13 (92.9%) reported that their genetic test results impacted their decision to have more frequent or additional cancer screening, and one (7.1%) reported that her results impacted her decision to undergo prophylactic surgery. Positive genetic test results among patients with a personal history of breast or ovarian cancer affected the decision about the type of surgery to undergo in four (36.4%) patients. Patients with VUS both with and without a personal history of breast or ovarian cancer reported that they had additional or more frequent screening following genetic testing. Of the 20 patients in the No CA/VUS group, seven (35.0%) reported that their genetic test results would impact their decision to have additional screening. Of the 14 patients in the CA/VUS group, three (21.4%) reported that their genetic test results would impact their decision to have additional screening (Table 5).
Table 5. Utilization of Genetic Testing Information.
Table reflects percentages of patients who answered “yes” to “Did your genetic test results play a role in your decision to do any of the following?”
| Positive n = 11 (%) | VUS n = 14 (%) | Negative n = 104 (%) | ||
|---|---|---|---|---|
| Personal history of breast or ovarian cancer | Type of surgery to treat breast cancer (mastectomy vs. lumpectomy) | 4 (36.4) | 2 (14.3) | 17 (16.4) |
| Receive radiation | 2 (18.2) | 2 (14.3) | 13 (12.5) | |
| Receive chemotherapy | 1 (9.1) | 2 (14.3) | 13 (12.5) | |
| Receive another type of treatment | 2 (18.2) | 1 (7.1) | 9 (8.7) | |
| Change treatment | 1 (9.1) | 1 (7.1) | 2 (1.9) | |
| Stop treatment | 0 (0.0) | 1 (7.1) | 3 (2.9) | |
| Have prophylactic surgery | 2 (18.2) | 1 (7.1) | 11 (10.6) | |
| Receive a drug to prevent breast cancer | 1 (9.1) | 3 (21.4) | 16 (15.4) | |
| Have more frequent or additional cancer screening | 1 (9.1) | 3 (21.4) | 16 (15.4) | |
| Positive n = 14 (%) | VUS n = 20 (%) | Negative n = 69 (%) | ||
| No personal history of breast or ovarian cancer | Have prophylactic surgery | 1 (7.1) | 3 (15.0) | 7 (10.1) |
| Receive a drug to prevent breast cancer | 0 (0.0) | 1 (5.0) | 4 (5.8) | |
| Have more frequent or additional cancer screening | 13 (92.9) | 7 (35.0) | 11 (15.9) |
Perceived Discrimination
Concerns about genetic discrimination were generally low. More patients reported worrying about discrimination based on their genetic test results than had actually experienced discrimination. The most common concerns were about problems with life insurance and with someone accessing personal genetic information (Figure 4). While worry about discrimination was higher in unaffected patients with a family history of breast or ovarian cancer, a greater percentage of patients with a personal history of breast or ovarian cancer reported experiencing discrimination, possibly due to their cancer rather than their genetic results (Figure 4). Two unaffected patients with VUS results reported problems with accessing life insurance and/or long term care insurance.
Figure 4.
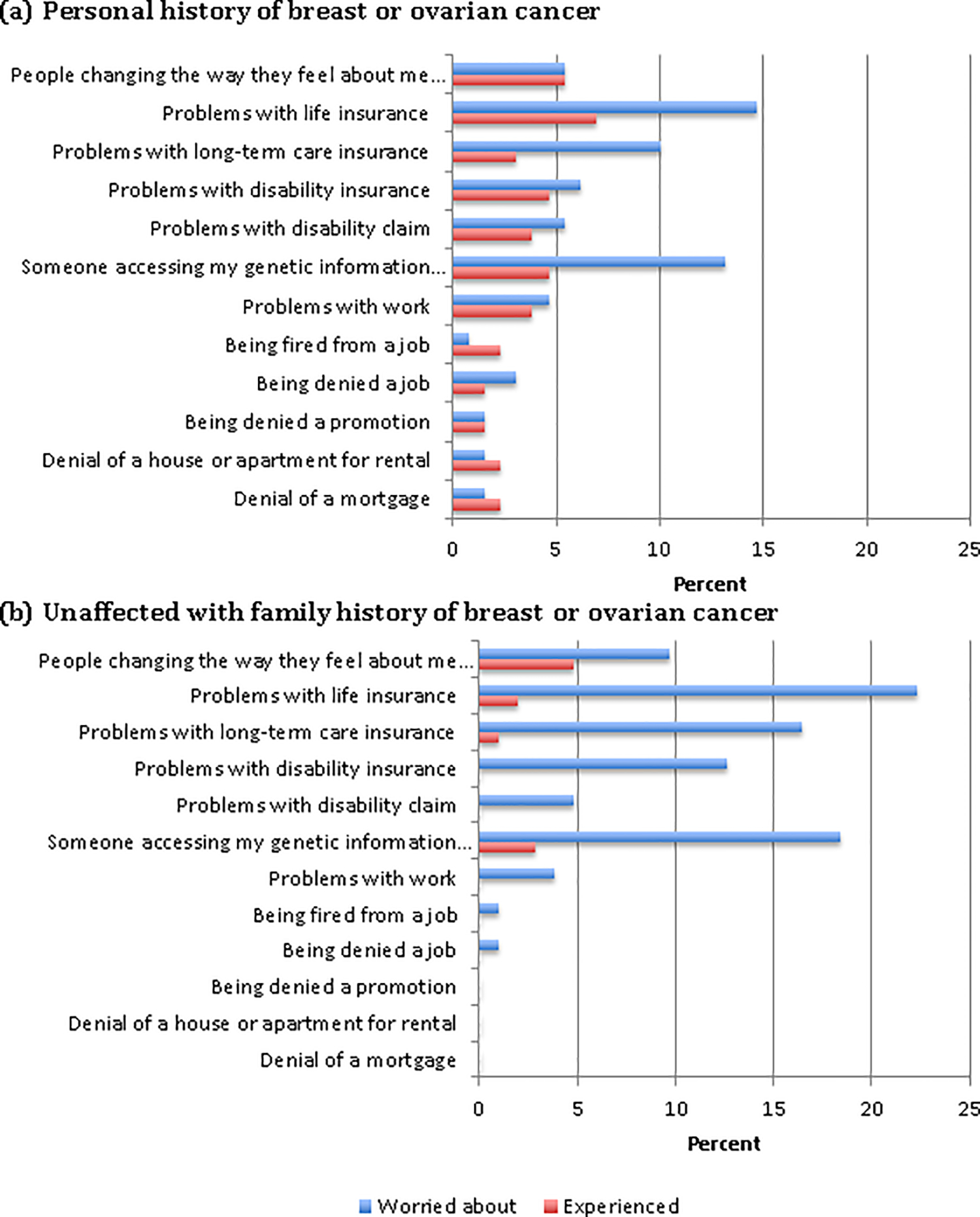
Perceived Discrimination Following Genetic Testing
Discussion
Among patients undergoing panel gene testing for hereditary breast and ovarian cancer, unaffected patients with a family history of breast or ovarian cancer who received positive results tended towards higher levels of post-testing distress with intermediate levels of some measures of distress among unaffected patients with VUS results. As expected, within this cohort, patients with positive results had the highest scores for intrusive thoughts, avoidance, and distress. These results were similar to the findings in a prior study looking at women undergoing testing for mutations in BRCA 1/2, in which a more positive impact was observed in women who had a personal history of breast cancer and positive results, as compared to those with no personal history of cancer (Low, Bower, Kwan, & Seldon, 2008). However, what was unique in our study was that scores were also increased to an intermediate level among unaffected patients with a family history of breast or ovarian cancer with VUS, relative to those with negative results. Patients with VUS results, both those with a personal history of breast or ovarian cancer and those unaffected with only a family history, frequently reported an intention to increase their cancer screening despite counseling that screening should be guided by personal history and family history alone. This study therefore highlights an important at-risk subset of patients: unaffected patients who receive VUS, a group that will continue to grow with the increasing availability and affordability of panel gene testing and increasing size of gene panels (Frey et al., 2015; Lincoln et al., 2015). Because ethnic minority patients currently have a higher frequency of VUS and may also have lower health literacy, there is a higher likelihood that panel gene tests will yield ambiguous results that are more difficult to understand in ethnic minority patients.
Increased genetic testing-specific distress among individuals with VUS results as compared with those with negative results has been observed in a prior study. O’Neill et al. (2009) described increased genetic testing-specific distress in individuals with a VUS result that persisted at one year following testing. However, in other studies, including in a meta-analysis of emotional distress after BRCA1/2 testing, an increase in distress with VUS results or inconclusive results as compared with negative test results was not observed (Culver et al., 2013; Hamilton et al., 2009; Richter et al., 2013; van Dijk et al., 2004). Our study is the first, however, to look at the impact of a VUS result on genetic testing-specific distress following panel gene testing for hereditary breast and ovarian cancer, as previous studies focused only on a VUS result following BRCA1/2 testing. Our study suggests increased distress with VUS results as well confusion regarding the interpretation of VUS, and our study identifies a need for more targeted genetic education and counseling about VUS. Our study also highlights the need to help optimize the number and types of genes tested in a patient based upon his/her individual likelihood of receiving a VUS result by ethnicity, as well as how that individual anticipates using the information.
Prior studies have identified patient characteristics as risk factors for distress following genetic testing, including pre-existing anxiety or depression, psychopharmacologic medication use, pretest anxiety, passive coping style, and increased risk perception, as well as family risk factors, including being the first family member to pursue testing, loss of a parent at a young age due to cancer, single status and having children (Hirschberg et al., 2015; D. J. MacDonald, Sarna, Weitzel, & Ferrell, 2010; Voorwinden & Jaspers, 2015). This study highlights demographic risk factors for increased genetic testing-specific distress not previously identified that have important implications in the era of panel gene testing. Black or African American race and Hispanic origin were associated with increased genetic testing specific distress, independent of test results in our study. African American race was associated specifically with increased distress, intrusion, and avoidance as compared with Caucasians. Hispanic and Asian race were associated with lower satisfaction with the decision to undergo genetic testing, as compared with Caucasians. This may be in part a reflection of cultural differences in the experience with and the perception of genetic testing. Regardless, this has important implications for genetic testing and counseling, because less is known about ethnic-specific genetic variants in minority populations, due to limited genetic data in these populations. VUS are therefore more frequent and more problematic in these minority populations, and counseling for minorities should consider this difference in potential genetic test results.
Lower education level, lower genetic knowledge scores, and younger age were all associated with poorer psychological outcomes and highlight the need for improved education and counseling for these subgroups. Lower genetic knowledge was associated with decreased satisfaction with the decision to undergo genetic testing. Younger age was associated with worse positive experience scores and increased total MICRA scores, indicating poor psychological adaptation to the test results. This may be due to the fact that younger individuals have had less experience with the potential for serious illness. Other studies have demonstrated an increase in distress scores with increasing intolerance of uncertainty, specifically after BRCA1/2 testing (O’Neill et al., 2006). However, our study showed no change in outcomes with increasing ambiguity tolerance.
Study Limitations
This study has several important limitations. The questionnaire was administered, on average, more than one year after genetic testing, and the time between disclosure of the genetic results and completion of the survey was variable. Some of the initial distress associated with genetic testing may have diminished over time, as described in previous studies (Hamilton et al., 2009). However, other studies have demonstrated a persistent psychological response in some patients up to one year after genetic testing (O’Neill et al., 2009). While our response rate was over 65%, this still leaves a subset that is not captured by our study. Though the patients who declined participation were demographically similar to those who enrolled in our study and our total sample was large for a single site study, some of the sub-groups in our analysis were small, with as few as four participants. This limits the conclusions that can be drawn, particularly with regard to race and ethnicity. This study was also comprised predominantly of Caucasian, English-speaking, well-educated individuals, and the results therefore may not be generalizable to a more diverse population. Our study suggests the need to study outcomes in under-represented minority patients to better understand the impact of VUS in these patients and to develop better strategies for pre-test education and counseling about VUS in these communities.
Practice Implications
This is the first study to highlight the impact of panel gene testing on individuals and the impact of VUS results. Reactions to panel gene testing were similar to reactions in prior studies examining patients undergoing testing for BRCA1/2 alone, which allays one of the primary concerns about panel gene testing. The MICRA scores in the group with VUS results were intermediate between those individuals who received positive test results and those who received negative test results. Overall MICRA scores in this study were similar to or lower than previously published MICRA scores after hereditary cancer genetic testing (Graves et al., 2012; Halbert et al., 2011; Hirschberg et al., 2015; Oberguggenberger et al., 2016). This study highlights potential risk factors for negative psychological outcomes following genetic testing, including young age, non-Caucasian ancestry, and lower education level. This suggests that pretest genetic counseling and education for panel gene testing in young, non-Caucasian, and/or less educated patients should clearly explain the probability of uncertain results and the implications when uncertain results are returned. Each patient who chooses to pursue genetic testing will need to choose the right size gene panel to optimize the chance of receiving results that will have clinical utility and minimizing the chance of receiving results that have disutility and increase anxiety. Although our study focused on patients undergoing cancer risk assessment, the results are likely also applicable to other clinical situations for which there are gene panels to identify future disease risk such as cardiology or neurology.
Research Recommendations
Further research is necessary to expand our understanding of the impact of VUS results, as these results continue to increase in frequency with increased uptake of panel gene testing. Additional studies of the psychological outcomes and understanding following panel gene testing for hereditary cancers and other heritable medical conditions in a more diverse population are needed.
Supplementary Material
HUMAN STUDIES AND INFORMED CONSENT STATEMENT.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and nation) and with the Helsinski Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
ACKNOWLEDGMENTS
We would like to acknowledge the patients and their families who generously contributed their time to participation in our study. We would like to acknowledge David Cella for permitting and assisting in the use of the MICRA questionnaire in this study. This work was supported in part by NIA Grant T35 AG 044303.
Footnotes
CONFLICT OF INTEREST
Heidi S. Lumish declares that she has no conflict of interest.
Hallie Steinfeld declares that she has no conflict of interest.
Carrie Koval declares that she has no conflict of interest.
Donna Russo declares that she has no conflict of interest.
Elana Levinson declares that she has no conflict of interest.
Julia Wynn declares that she has no conflict of interest.
Wendy K. Chung declares that she has no conflict of interest.
ANIMAL STUDIES
No animal studies were carried out by the authors for this article.
REFERENCES
- Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. (2003). Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. American Journal of Human Genetics, 72(5), 1117–1130. doi: Doi 10.1086/375033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornslett M, Dahl AA, Sorebo O, & Dorum A (2015). Psychological distress related to BRCA testing in ovarian cancer patients. Fam Cancer, 14(4), 495–504. doi: 10.1007/s10689-015-9811-2 [DOI] [PubMed] [Google Scholar]
- Bradbury AR, Patrick-Miller LJ, Egleston BL, DiGiovanni L, Brower J, Harris D, et al. (2016). Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genet Med, 18(1), 25–33. doi: 10.1038/gim.2015.19 [DOI] [PubMed] [Google Scholar]
- Bubien V, Bonnet F, Brouste V, Hoppe S, Barouk-Simonet E, David A, et al. (2013). High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet, 50(4), 255–263. doi: 10.1136/jmedgenet-2012-101339 [DOI] [PubMed] [Google Scholar]
- Cella D, Hughes C, Peterman A, Chang CH, Peshkin BN, Schwartz MD, et al. (2002). A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychology, 21(6), 564–572. doi: 10.1037//0278-6133.21.6.564 [DOI] [PubMed] [Google Scholar]
- Chen S, & Parmigiani G (2007). Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol, 25(11), 1329–1333. doi: 10.1200/JCO.2006.09.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotser CB, & Boehmke M (2009). Survivorship considerations in adults with hereditary breast and ovarian cancer syndrome: state of the science. J Cancer Surviv, 3(1), 21–42. doi: 10.1007/s11764-008-0077-7 [DOI] [PubMed] [Google Scholar]
- Culver JO, Brinkerhoff CD, Clague J, Yang K, Singh KE, Sand SR, et al. (2013). Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clinical Genetics, 84(5), 464–472. doi: 10.1111/cge.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas HA, Hamilton RJ, & Grubs RE (2009). The effect of BRCA gene testing on family relationships: A thematic analysis of qualitative interviews. J Genet Couns, 18(5), 418–435. doi: 10.1007/s10897-009-9232-1 [DOI] [PubMed] [Google Scholar]
- Dunn OJ (1964). Multiple Comparisons Using Rank Sums. Technometrics, 6(3), 241–&. doi: Doi 10.2307/1266041 [DOI] [Google Scholar]
- Easton DF, Pharoah PDP, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. (2015). Gene-Panel Sequencing and the Prediction of Breast-Cancer Risk. New England Journal of Medicine, 372(23), 2243–2257. doi: 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MK, Kim SH, Bassett RY, Martineau J, Dalton E, Chern JY, et al. (2015). Rescreening for genetic mutations using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol Oncol, 139(2), 211–215. doi: 10.1016/j.ygyno.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Graves KD, Vegella P, Poggi EA, Peshkin BN, Tong A, Isaacs C, et al. (2012). Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice. Cancer Epidemiol Biomarkers Prev, 21(3), 445–455. doi: 10.1158/1055-9965.EPI-11-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CH, Stopfer JE, McDonald J, Weathers B, Collier A, Troxel AB, et al. (2011). Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women’s concerns? J Clin Oncol, 29(32), 4302–4306. doi: 10.1200/JCO.2010.33.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M, & Moyer A (2009). Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol, 28(4), 510–518. doi: 10.1037/a0014778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJP, et al. (2006). Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clinical Cancer Research, 12(10), 3209–3215. doi: 10.1158/1078-0432.CCR-06-0083 [DOI] [PubMed] [Google Scholar]
- Heshka JT, Palleschi C, Howley H, Wilson B, & Wells PS (2008). A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genetics in Medicine, 10(1), 19–32. doi: 10.1097/GIM.0b013e31815f524f [DOI] [PubMed] [Google Scholar]
- Hirschberg AM, Chan-Smutko G, & Pirl WF (2015). Psychiatric implications of cancer genetic testing. Cancer, 121(3), 341–360. doi: 10.1002/cncr.28879 [DOI] [PubMed] [Google Scholar]
- Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. (1996). Patient satisfaction with health care decisions: The satisfaction with decision scale. Medical Decision Making, 16(1), 58–64. doi: Doi [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Lozano G, Amos CI, & Strong LC (2003). Germline p53 mutations in a cohort with childhood sarcoma: sex differences in cancer risk. American Journal of Human Genetics, 72(4), 975–983. doi: 10.1086/374567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CS, Cooper DN, Iacopetta B, & Roukos DH (2013). Integrating next-generation sequencing into the diagnostic testing of inherited cancer predisposition. Clinical Genetics, 83(1), 2–6. doi: Doi 10.1111/Cge.12028 [DOI] [PubMed] [Google Scholar]
- Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, et al. (2014). Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol, 32(19), 2001–2009. doi: 10.1200/JCO.2013.53.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDuca H, Stuenkel AJ, Dolinsky JS, Keiles S, Tandy S, Pesaran T, et al. (2014). Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med, 16(11), 830–837. doi: 10.1038/gim.2014.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. (1997). Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet, 16(1), 64–67. doi: 10.1038/ng0597-64 [DOI] [PubMed] [Google Scholar]
- Lincoln SE, Kobayashi Y, Anderson MJ, Yang S, Desmond AJ, Mills MA, et al. (2015). A Systematic Comparison of Traditional and Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Genes in More Than 1000 Patients. J Mol Diagn, 17(5), 533–544. doi: 10.1016/j.jmoldx.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Low CA, Bower JE, Kwan L, & Seldon J (2008). Benefit finding in response to BRCA1/2 testing. Ann Behav Med, 35(1), 61–69. doi: 10.1007/s12160-007-9004-9 [DOI] [PubMed] [Google Scholar]
- Lynch HT, Silva E, Snyder C, & Lynch JF (2008). Hereditary breast cancer: part I. Diagnosing hereditary breast cancer syndromes. Breast J, 14(1), 3–13. doi: 10.1111/j.1524-4741.2007.00515.x [DOI] [PubMed] [Google Scholar]
- MacDonald AP (1970). Revised scale for ambiguity tolerance: Reliability and validity. Psychological Reports, 26(3), 791–798. [Google Scholar]
- MacDonald DJ, Sarna L, Weitzel JN, & Ferrell B (2010). Women’s perceptions of the personal and family impact of genetic cancer risk assessment: focus group findings. J Genet Couns, 19(2), 148–160. doi: 10.1007/s10897-009-9267-3 [DOI] [PubMed] [Google Scholar]
- Madanikia SA, Bergner A, Ye X, & Blakeley JO (2012). Increased risk of breast cancer in women with NF1. Am J Med Genet A, 158A(12), 3056–3060. doi: 10.1002/ajmg.a.35550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. (2013). Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst, 105(11), 812–822. doi: 10.1093/jnci/djt095 [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, McLeod HL, & Ginsburg GS (2013). Genomic Medicine: A Decade of Successes, Challenges, and Opportunities. Science Translational Medicine, 5(189). doi: 10.1126/scitranslmed.3005785 [DOI] [PubMed] [Google Scholar]
- O’Neill SC, Demarco T, Peshkin BN, Rogers S, Rispoli J, Brown K, et al. (2006). Tolerance for uncertainty and perceived risk among women receiving uninformative BRCA1/2 test results. American Journal of Medical Genetics Part C-Seminars in Medical Genetics, 142C(4), 251–259. doi: 10.1002/ajmg.c.30104 [DOI] [PubMed] [Google Scholar]
- O’Neill SC, Rini C, Goldsmith RE, Valdimarsdottir H, Cohen LH, & Schwartz MD (2009). Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psycho-Oncology, 18(10), 1088–1096. doi: 10.1002/pon.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberguggenberger A, Sztankay M, Morscher RJ, Sperner-Unterweger B, Weber I, Hubalek M, et al. (2016). Psychosocial outcomes and counselee satisfaction following genetic counseling for hereditary breast and ovarian cancer: A patient-reported outcome study. J Psychosom Res, 89, 39–45. doi: 10.1016/j.jpsychores.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Olivier M, Goldgar DE, Sodha N, Ohgaki H, Kleihues P, Hainaut P, et al. (2003). Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Research, 63(20), 6643–6650. [PubMed] [Google Scholar]
- Pharoah PDP, Guilford P, Caldas C, & Consortiu IGCL (2001). Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology, 121(6), 1348–1353. doi: 10.1053/gast.2001.29611 [DOI] [PubMed] [Google Scholar]
- Rainville IR, & Rana HQ (2014). Next-Generation Sequencing for Inherited Breast Cancer Risk: Counseling through the Complexity. Current Oncology Reports, 16(3). doi: 10.1007/S11912-013-0371-Z [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Haroun I, Graham TC, Eisen A, Kiss A, & Warner E (2013). Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Annals of Oncology, 24, 69–74. doi: 10.1093/annonc/mdt312 [DOI] [PubMed] [Google Scholar]
- Ringwald J, Wochnowski C, Bosse K, Giel KE, Schaffeler N, Zipfel S, et al. (2016). Psychological Distress, Anxiety, and Depression of Cancer-Affected BRCA1/2 Mutation Carriers: a Systematic Review. J Genet Couns, 25(5), 880–891. doi: 10.1007/s10897-016-9949-6 [DOI] [PubMed] [Google Scholar]
- Robson M, & Offit K (2007). Clinical practice. Management of an inherited predisposition to breast cancer. N Engl J Med, 357(2), 154–162. doi: 10.1056/NEJMcp071286 [DOI] [PubMed] [Google Scholar]
- Seminog OO, & Goldacre MJ (2015). Age-specific risk of breast cancer in women with neurofibromatosis type 1. Br J Cancer, 112(9), 1546–1548. doi: 10.1038/bjc.2015.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, & Eng C (2012). Lifetime cancer risks in individuals with germline PTEN mutations. Clinical Cancer Research, 18(2), 400–407. doi: 10.1158/1078-0432.CCR-11-2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk S, van Asperen CJ, Jacobi CE, Vink GR, Tibben A, Breuning MH, et al. (2004). Variants of uncertain clinical significance as a result of BRCA1/2 testing: impact of an ambiguous breast cancer risk message. Genet Test, 8(3), 235–239. doi: 10.1089/gte.2004.8.235 [DOI] [PubMed] [Google Scholar]
- Voorwinden JS, & Jaspers JP (2015). Prognostic Factors for Distress After Genetic Testing for Hereditary Cancer. J Genet Couns. Doi: 10.1007/s10897-015-9894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Lee MK, Casadei S, Thornton AM, Stray SM, Pennil C, et al. (2010). Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A, 107(28), 12629–12633. doi: 10.1073/pnas.1007983107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, & Marmar CR (1997). The Impact of Event Scale - Revised In Wilson JP & Keane TM (Eds.), Assessing psychological trauma and PTSD (pp. xiv, 577 p.). New York: Guilford Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


