Abstract
BACKGROUND:
We sought to determine whether insulin-sensitizing therapy (thiazolidinediones or metformin) decreased the risk of developing atrial fibrillation compared with insulin-providing therapy (insulin, sulfonylurea, or a meglitinide). Thiazolidinediones are insulin sensitizers that also decrease the inflammatory response. Because inflammation is a risk factor for atrial fibrillation, we hypothesized that treating diabetes with thiazolidinediones might decrease the risk of developing atrial fibrillation.
METHODS:
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial enrolled patients with type 2 diabetes and documented coronary artery disease. All patients were randomized to insulin-sensitizing therapy or insulin-providing therapy.
RESULTS:
A total of 2319 patients entered the study, with 1160 assigned to the insulin-sensitization strategy and 1159 assigned to the insulin-provision strategy. Over a median follow-up of 4.2 years, 90 patients (3.9%) developed new-onset atrial fibrillation. In the intention-to-treat analysis, the incidence of atrial fibrillation was 8.7 per 1000 person-years in patients assigned to insulin sensitization compared with 9.5 in patients assigned to insulin provision with a hazard ratio (HR) of 0.91 (95% confidence interval [CI], 0.60–1.38, P = .66). In a time-varying exposure analysis, the incidence rate per 1000 person-years was 7.2 while exposed to thiazolidinediones and 9.7 while not exposed to thiazolidinediones with an adjusted HR of 0.80 (95% CI, 0.33–1.94, P = .62). In a subset of patients matched on propensity to receive a thiazolidinediones, the HR was 0.75 (95% CI, 0.43–1.30, P = .30).
CONCLUSIONS:
We did not find a significant reduction of atrial fibrillation incidence with use of thiazolidinediones.
Keywords: Atrial fibrillation, Diabetes mellitus, Insulin providers, Insulin sensitizers, Thiazolidinediones
INTRODUCTION
Patients with type 2 diabetes mellitus (diabetes) are at increased risk of atrial fibrillation, and patients with the combination of atrial fibrillation and diabetes are at particularly increased risk of stroke.1–4 The potential mechanisms underlying the predisposition for atrial fibrillation in patients with diabetes are not fully understood, but microvascular coronary disease accompanied by elevated C-reactive protein, inflammation, and oxidative stress on the endothelium, all present in both diabetes and atrial fibrillation, could play a role.5–12 In addition, increased left atrial size is known to be associated with increased risk of atrial fibrillation, and diabetes is known to induce an increase in left atrium size independent of hypertension and diastolic function.13–15 Finally, diabetes causes neural remodeling in the atrium, characterized by both parasympathetic and sympathetic denervation, which in itself could engender atrial fibrillation.16 Interventions that alter these processes might reduce the risk of atrial fibrillation in patients with diabetes.
Thiazolidinediones are insulin sensitizers that belong to the class of peroxisome proliferator-activated receptor gamma activators. Binding to this transcription factor alters glucose and lipid homeostasis, increases sensitivity to insulin, decreases the inflammatory response, and reduces levels of resistin.17–20 Elevated levels of serum resistin have been shown to be significantly associated with higher rates of incident atrial fibrillation.20 Thiazolidinediones reduce glucose levels in patients with diabetes, but they have also been associated with worsening heart failure, which prompted the US Food and Drug Administration to apply a black box warning on rosiglitazone and pioglitazone, the only thiazolidinediones still available in the United States after withdrawal of troglitazone.21 International guidelines recommend thiazolidinediones as a second-line treatment option for diabetes.22
It has been hypothesized that the use of thiazolidinediones could prevent atrial fibrillation through their anti-inflammatory effects and other mechanisms,23–26 but empirical evidence for this potential effect is scant. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial provides a unique opportunity to test whether treatment with thiazolidinediones decreases the risk of developing atrial fibrillation, because it randomized patients to a strategy of insulin sensitization, which included the use of thiazolidinediones, or a strategy of insulin provision to treat diabetes.
METHODS
The BARI 2D trial design has been described in detail.27 Briefly, BARI 2D enrolled patients with type 2 diabetes mellitus who had documented coronary artery disease of sufficient severity to warrant consideration of revascularization. Patients were excluded if they had heart failure symptoms equal to New York Heart Association class III or IV, an indication for immediate coronary revascularization, a creatinine level >2.0 mg/dL (177 μmol/L), a glycohemoglobin (HbA1C) level >13.0%, hepatic dysfunction, coronary artery bypass graft surgery within 12 months, or prior percutaneous coronary intervention. The institutional review boards at the University of Pittsburgh and at each participating site approved the protocol, and all patients provided written informed consent.
Patients in the BARI 2D trial were randomized in a 2-by-2 factorial design to (1) an insulin-sensitizing therapy or an insulin-provision strategy; and (2) to prompt coronary revascularization with optimal medical therapy or optimal medical therapy alone. Insulin-sensitizing therapy was defined as treatment with a thiazolidinedione or metformin, and insulin-provision therapy was defined as treatment with insulin, sulfonylurea, or a meglitinide. If the HbA1C level could not be maintained at less than 8.0% using the protocol-assigned drugs, patients assigned to insulin-sensitizing therapy were allowed use of an insulin-providing drug and vice versa. A total of 2368 participants were enrolled at 49 international clinical sites between January 1, 2001, and March 31, 2005. Treatment continued until the 6-year visit or December 1, 2008.
Most patients were already on treatment with a glucose-lowering drug at entry into BARI 2D, with 240 patients receiving rosiglitazone and 204 patients receiving pioglitazone at baseline. After random assignment to the insulin-sensitization versus insulin-provision groups, medical therapy was adjusted by the site diabetes specialist in accordance with the study protocol with the goal of achieving a target HbA1C level of <7.0%. Patients assigned to the insulin-provision strategy who were taking a thiazolidinedione at study entry (19.4%) were generally tapered off the thiazolidinedione over the first 6 months. Current medication use and discontinuation for thiazolidinediones, and for rosiglitazone and pioglitazone in particular, were recorded at trial entry and at each follow-up visit. These data were used to determine the on-treatment thiazolidinediones intervals and coincident events.
Outcomes
The primary outcome of interest of this investigation was the new onset of atrial fibrillation after randomization. Because atrial fibrillation was not a predefined study end point, we used data from electrocardiograms (ECGs) systematically recorded according to the trial protocol at baseline, 3 months, and each annual follow-up visit to identify atrial fibrillation. The protocol also required ECGs to be collected before and after revascularization procedures, and during evaluation for suspected myocardial infarction. The BARI 2D ECG Core Laboratory at the University of St Louis determined the rhythm for each submitted ECG and specifically noted the presence of atrial fibrillation. Atrial fibrillation types were classified by the timing of the electrocardiography recording as “baseline atrial fibrillation,” “postoperative atrial fibrillation,” or “new-onset atrial fibrillation.” All patients with baseline atrial fibrillation were excluded from the study cohort.
In a sub-study of the Economic outcomes of the BARI 2D trial, 2005 patients were contacted every 3 months by the BARI 2D Economics Core Laboratory at Stanford University to collect data on health care use and costs.28 For patients enrolled in the BARI 2D Economics sub-study, International Classification of Diseases 9th or 10th Revision codes with atrial fibrillation were available from collected for hospitalizations from some of the included sites. A hospitalization was categorized as an atrial fibrillation hospitalization if the primary or a secondary International Classification of Diseases 9th Revision code was 427.3 or if the primary International Classification of Diseases 10th Revision code was 148. The only hospital providing International Classification of Diseases 10th Revision codes did not provide secondary codes. Analyses using these data were censored at the end of follow-up in the economics sub-study, which was earlier than that of the main BARI 2D trial.
Statistical Analyses
We conducted 3 types of analyses: (1) an intention-to-treat-analysis, based on random assignment to the insulin-sensitization versus insulin-provision strategies; (2) a treatment received analysis, using time-updated information on thiazolidinedione status (prescription from the prior study visit); and (3) a propensity score-matched analysis based on 1:1 matching using a propensity for thiazolidinedione use. Subgroup analyses including only the economics sub-study patients were conducted using both an intention-to-treat approach and a propensity score-matched analysis.
Patient characteristics were compared according to insulin-sensitization or insulin-provision assignment status and by early thiazolidinedione use (yes or no). Early thiazolidinedione use was defined as maintenance of baseline thiazolidinedione use at 6 months or any new initiation of thiazolidinediones use within the first 6 months, as previously described.29 Categoric data were presented as counts with percentages, and statistical differences were tested using chi-square tests and Fisher exact tests where appropriate. Continuous variables were presented as mean with standard deviations for normally distributed data and as medians with interquartile range for non-normally distributed data. Statistical differences were tested using Student t test and Wilcoxon rank-sum test where appropriate.
Crude incidence rates of new-onset atrial fibrillation were calculated per 1000 person-years by assigned treatment and by thiazolidinedione status. The relative risk for new-onset atrial fibrillation were estimated using hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox proportional regression analyses. A P value <.05 was considered as statistically significant.
A Cox proportional hazards regression model with time-varying exposures was used to assess the association of current thiazolidinedione use with atrial fibrillation. Risk for the detection of atrial fibrillation at a visit was modeled as a function of whether or not a patient used thiazolidinedione between the previous and current visits. The time-varying analysis was adjusted for sex, age, US patient status, duration of diabetes, history of hypercholesterolemia, hypertension, myocardial infarction, heart failure, stroke or transient ischemic attack, prior coronary revascularization, sitting 60-second pulse, body mass index, level of activity, cigarette smoking category, low-density lipoprotein cholesterol, HbA1c, statin, angiotensin-converting enzyme inhibitor, and angiotensin II inhibitors.
Propensity scores were constructed using a logistic regression model developed to predict which patients assigned to the insulin-sensitization strategy would receive a thiazolidinedione within 6 months compared with patients assigned to the insulin-sensitization group who did not receive a thiazolidinedione within 6 months. The variates included in the propensity score were sex, age, country (United States or other), duration of diabetes, history of hypercholesterolemia, hypertension, myocardial infarction, heart failure, stroke or transient ischemic attack, prior coronary revascularization, sitting 60-second pulse, body mass index, level of activity, cigarette smoking category, low-density lipoprotein cholesterol, HbA1c, statin, angiotensin-converting enzyme inhibitor, and angiotensin II inhibitors. Patients with missing values in these variables were not included in the matched analysis. Patients who received a thiazolidinedione in the insulin-sensitization group were then matched 1:1, without replacement, based on the propensity score with patients randomly assigned to the insulin-provision strategy who did not receive a thiazolidinedione within 6 months, using a greedy matching algorithm, with a caliper distance of 0.02. The matched analysis was performed to compare patients receiving thiazolidinedione from the insulin-sensitization group with patients from the insulin-provision group, who would have been likely to receive thiazolidinedione if they had been randomized to the insulin-sensitization group instead of insulin-provision group.
In the subgroup analysis of 2005 patients from the economics sub-study, risk of atrial fibrillation was investigated as both an intention-to-treat analysis and a propensity score–matched analysis using a similar approach as in the main analyses.
RESULTS
Cohort Summary
Of the 2368 patients enrolled in the BARI 2D study, 31 had atrial fibrillation at study baseline and 18 had no follow-up time, yielding a final study population of 2319 patients, 1160 of whom were assigned to the insulin-sensitization strategy and 1159 of whom were assigned to insulin-provision strategy (Figure 1). The 2 groups had similar baseline demographics, medical history, and laboratory values (Table 1), as expected by randomization.
Figure 1.
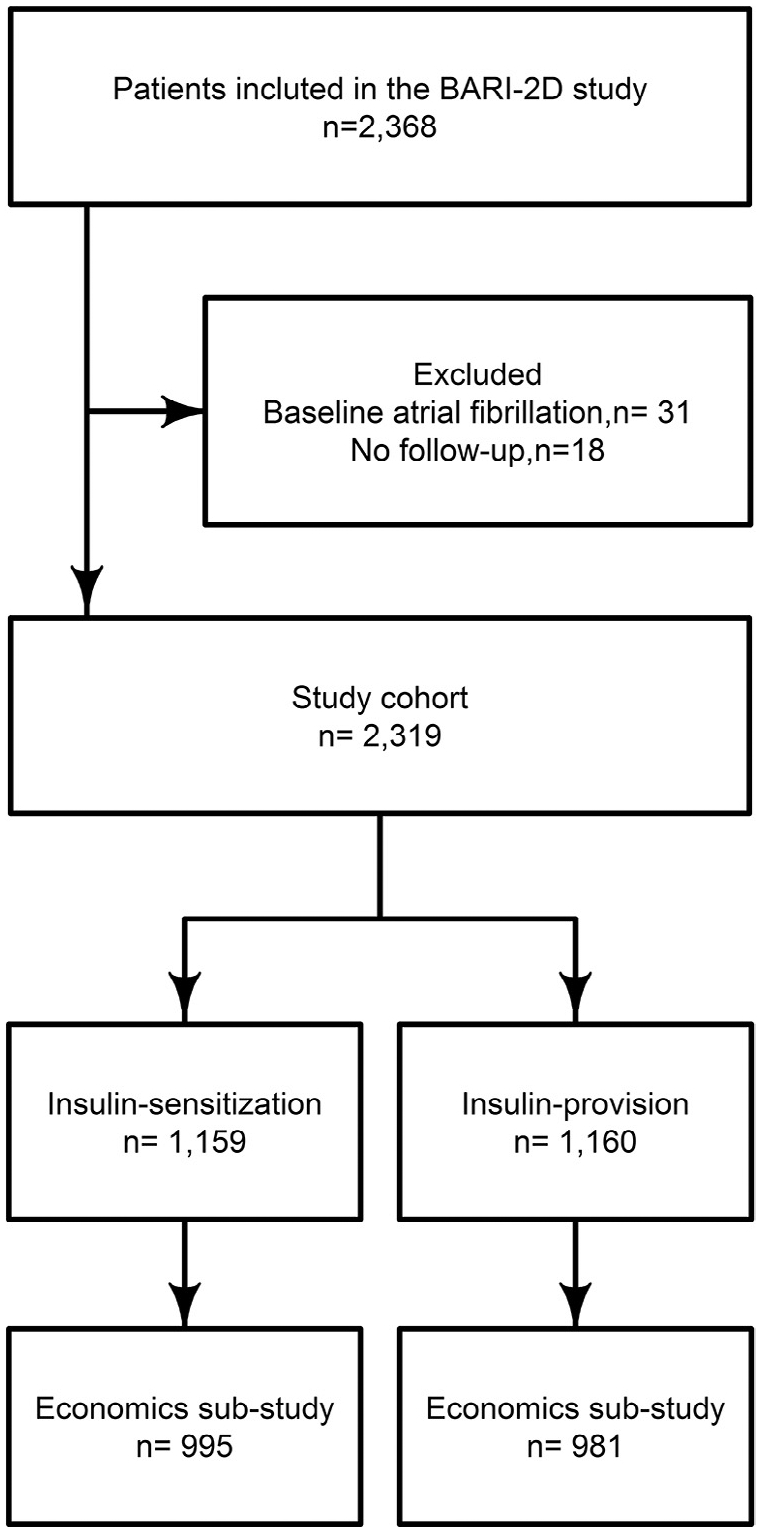
Flowchart of intention-to-treat analysis. BARI-2D = Bypass Angioplasty Revascularization Investigation 2 Diabetes.
Table 1.
Baseline Characteristics by Random Treatment Assignment
| Insulin Sensitization | Insulin Provision | P Value | |
|---|---|---|---|
| n | 1159 | 1160 | |
| Demographics | |||
| Female, n (%) | 349 (30.1) | 346 (29.8) | .92 |
| Age (mean ± SD) | 61.8 (9.1) | 61.9 (8.6) | .82 |
| Non-US patient, n (%) | 435 (37.5) | 427 (36.8) | .75 |
| Medical Insurance (%) | .87 | ||
| Medicare | 301 (26.1) | 293 (25.3) | |
| Other public | 504 (43.6) | 503 (43.5) | |
| Private | 298 (25.8) | 313 (27.1) | |
| None/self-pay | 52 (4.5) | 47 (4.1) | |
| Medical history | |||
| Duration of diabetes in years (median [IQR]) | 8.3 [3.8–14.8] | 9.1 [3.7–15.7] | .15 |
| History of CHF, n (%) | 72 (6.3) | 72 (6.2) | 1.00 |
| History of hypercholesterolemia, n (%) | 924 (80.8) | 950 (83.0) | .19 |
| History of stroke/TIA, n (%) | 114 (9.9) | 111 (9.6) | .85 |
| History of hypertension, n (%) | 929 (81.1) | 960 (83.8) | .09 |
| History of hypoglycemia, n (%) | 229 (20.0) | 270 (23.6) | .04 |
| History of MI, n (%) | 374 (32.8) | 361 (31.6) | .55 |
| Prior coronary revascularization, n (%) | 265 (22.9) | 279 (24.1) | .53 |
| Physical exam | |||
| Serum creatinine mg/dL (mean (±SD)) | 1.0 (0.3) | 1.1 (0.3) | .47 |
| Sitting diastolic BP, mm Hg (mean (±SD)) | 74.6 (11.4) | 74.6 (11.1) | .98 |
| Sitting systolic blood pressure, mm Hg (mean (±SD)) | 131.7 (19.8) | 131.9 (20.3) | .88 |
| ABI <0.90, n (%) | 213 (20.5) | 226 (21.5) | .63 |
| Sitting 60-sec pulse (mean (±SD)) | 68.5 (10.7) | 67.9 (10.5) | .12 |
| BMI, kg/m2 (median [IQR]) | 30.9 [27.6–34.7] | 30.9 [27.6–34.8] | .94 |
| Lifestyle | |||
| Level of activity, n (%) | .55 | ||
| Sedentary | 258 (22.4) | 251 (21.8) | |
| Mild | 488 (42.3) | 468 (40.7) | |
| Moderate/strenuous | 407 (35.3) | 431 (37.5) | |
| Cigarette Smoking Category, n (%) | .12 | ||
| Never smoked | 403 (34.8) | 363 (31.4) | |
| Former smoker | 607 (52.4) | 654 (56.6) | |
| Current smoker | 148 (12.8) | 138 (11.9) | |
| Core Lab Measures from the Biochemistry Laboratories | |||
| HDL, mg/dL (mean (±SD)) | 37.9 (10.0) | 38.5 (10.6) | .22 |
| LDL mg/dL (mean (±SD)) | 95.6 (33.9) | 96.92 (33.0) | .35 |
| Total cholesterol, mg/dL (median [IQR]) | 163 [140–190] | 167 [144–193] | .08 |
| Triglycerides cholesterol, mg/dL (median [IQR]) | 147 [101–221] | 149 [108–219] | .34 |
| HbA1c % (median [IQR]) | 7.3 [6.4–8.6] | 7.3 [6.5–8.5] | .51 |
| Core lab measures from the ECG Core Laboratory | |||
| Presence of abnormal Q-waves, n (%) | 227 (20.2) | 195 (17.3) | .10 |
| Abnormal ST depression, n (%) | 179 (17.0) | 180 (17.1) | .98 |
| Abnormal T-waves, n (%) | 448 (42.5) | 446 (42.4) | 1.00 |
| Abnormal LVEF, n (%) | 203 (18.1) | 182 (16.2) | .26 |
| Medications | |||
| Insulin, n (%) | 316 (27.3) | 329 (28.4) | .58 |
| Biguanide, n (%) | 634 (54.8) | 620 (53.5) | .56 |
| Thiazolidinedione, n (%) | 224 (19.4) | 213 (18.4) | .58 |
| Sulfonylurea, n (%) | 625 (54.0) | 611 (52.7) | .56 |
| Statin, n (%) | 866 (74.9) | 861 (74.4) | .82 |
| Beta-blocker, n (%) | 860 (74.4) | 825 (71.3) | .10 |
| Calcium channel blockers, n (%) | 346 (29.9) | 383 (33.1) | .11 |
| Nonsublingual nitrate, n (%) | 369 (32.0) | 357 (30.8) | .58 |
| ACE inhibitor, n (%) | 738 (63.8) | 755 (65.3) | .49 |
| Angiotensin receptor blocker, n (%) | 172 (14.9) | 163 (14.1) | .63 |
| Aspirin, n (%) | 1022 (88.6) | 1016 (87.9) | .62 |
| Antiplatelet therapy (nonaspirin), n (%) | 232 (20.1) | 219 (18.9) | .52 |
| Diuretic, n (%) | 440 (38.0) | 450 (38.9) | .70 |
ABI = ankle-brachial index; ACE = angiotensin-converting enzyme; BMI = body mass index; CHF = congestive heart failure; ECG = electrocardiogram; HbA1c = glycohemoglobin; HDL = high-density lipoprotein; IQR = interquartile range; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; MI = myocardial infarction; SD = standard deviation; TIA = transient ischemic attack.
Intention-to-Treat Analysis
A total of 90 patients (3.9%) developed new-onset atrial fibrillation based on ECG Core Laboratory classification over a median follow-up of 4.2 years (interquartile range, 3.9–5.3 years), 43 patients assigned to the insulin-sensitization strategy and 47 assigned to the insulin-provision strategy. Post-operative atrial fibrillation after a revascularization procedure developed in an additional 27 patients (15 in the insulin-sensitization strategy group and 12 in the insulin-provision strategy group), which was not classified as new-onset atrial fibrillation in this study. The incidence rate of new-onset atrial fibrillation was 8.7 per 1000 person-years in patients assigned insulin sensitization, compared with 9.5 in patients assigned to insulin provision (Figure 2). In the intention-to-treat analysis, the relative risk of atrial fibrillation was 9% lower in the insulin-sensitization group, with an HR of 0.91 (95% CI, 0.60–1.38, P = .59).
Figure 2.
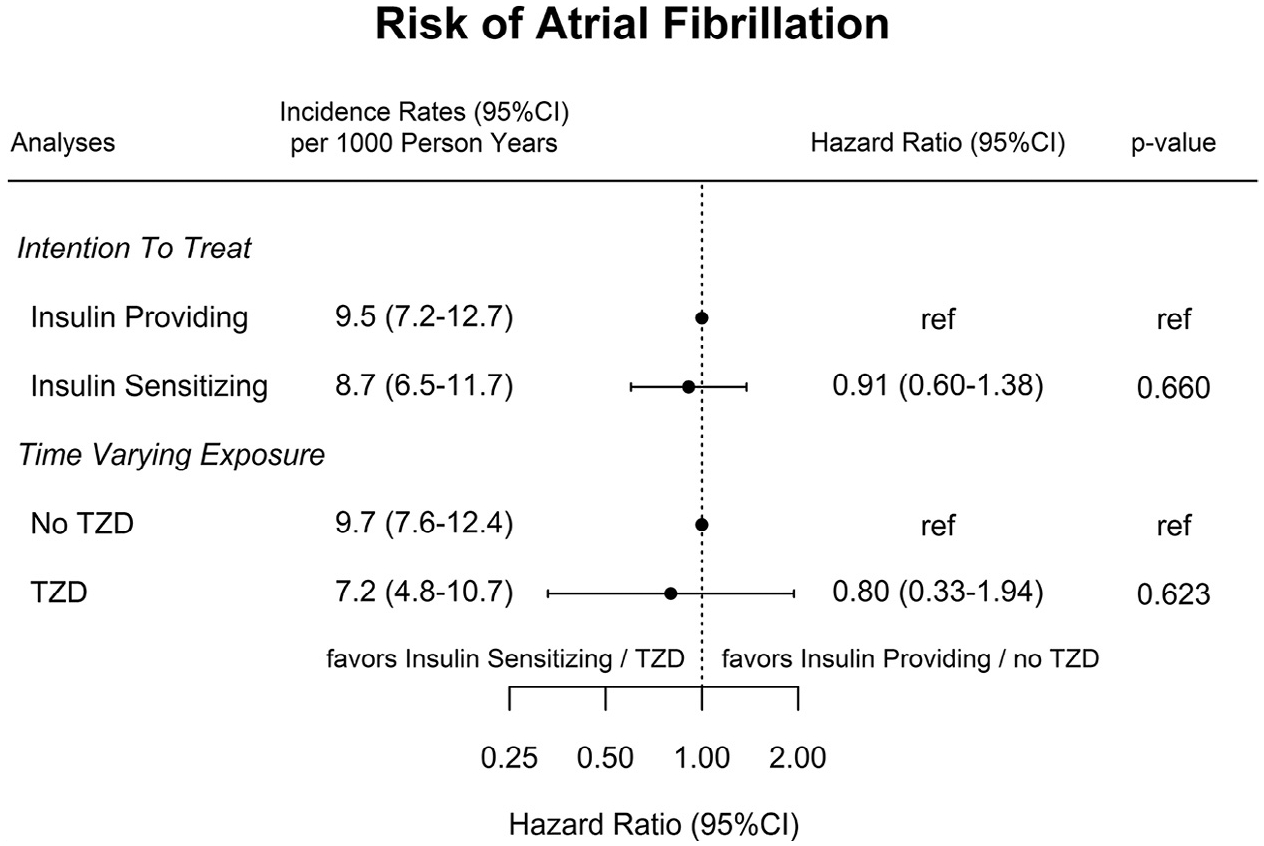
Incidence rates and HRs of atrial fibrillation. The time-varying exposure analysis was adjusted for US patients’ status, duration of diabetes, history of hypercholesterolemia, hypertension, myocardial infarction, heart failure, stroke or transient ischemic attack, prior coronary revascularization, sitting 60-second pulse, body mass index, level of activity, cigarette smoking category, low-density lipoprotein cholesterol, HbA1c, statin, angiotensin-converting enzyme inhibitor, and angiotensin II inhibitors. CI = confidence interval; TZD = thiazolidinedione.
Thiazolidinedione Time-Varying Exposure
Patients in BARI 2D were exposed to a thiazolidinedione for 3418 years (34%) of the 10,006 person-years of follow-up.New-onset atrial fibrillation developed in 29 patients while exposed to thiazolidinedione, an incidence rate of 7.2 (95% CI, 4.8–10.7) per 1000 person-years, and in an additional 61 patients while not exposed to a thiazolidinedione, equivalent to an incidence rate of 9.7 (95% CI, 7.6–12.4). The unadjusted HR was 0.73 (95% CI, 0.46–1.18, P = .20), and the adjusted HR was 0.80 (95% CI, 0.33–1.94, P = .62) (Figure 2).
Propensity Score-Matched Analyses
The propensity score developed with a logistic regression model to identify the thiazolidinedione users in the insulin-sensitization group had good predictive properties, with a C-statistic of 0.78. In the matched analysis, 663 patients entered each group with a 98.8% propensity score match between the 2 groups. A nonstatistically significant decreased risk of atrial fibrillation was found in the thiazolidinedione group (22 patients) compared with the nonthiazolidinedione group (27 patients), with an HR of 0.75 (95% CI, 0.43–1.30, P = .30) (Table 2).
Table 2.
Matched Analysis and Cost-Effectiveness Study
| BARI 2D Cohort Propensity Score–Matched Analysis* |
HR (95% CI) | P Value |
|---|---|---|
| Thiazolidinedione users in the insulin sensitization group compared with nonthiazolidinedione users in the insulin provision group as reference* | 0.75 (0.43–1.30) | .30 |
| Economics sub-study | HR (95% CI) | P Value |
| Insulin sensitization vs insulin provision intention to treat | 0.89 (0.62–1.28) | .54 |
| Thiazolidinedione users in the insulin sensitization group compared with nonthiazolidinedione users in the insulin providers group as reference matched analysis* | 0.79 (0.49–1.26) | .32 |
BARI 2D = Bypass Angioplasty Revascularization Investigation 2 Diabetes; CI = confidence interval; HR = hazard ratio.
Matched by propensity score based on country (United States or other), duration of diabetes, history of hypercholesterolemia, hypertension, myocardial infarction, heart failure, stroke or transient ischemic attack, prior coronary revascularization, sitting 60-second pulse, body mass index, level of activity, cigarette smoking category, low-density lipoprotein cholesterol, HbA1c, statin, angiotensin-converting enzyme inhibitor, and angiotensin II inhibitors.
Subgroup Analysis from the economics Sub-Study
After excluding 29 patients with baseline atrial fibrillation, 1976 patients entered the economic sub-study, 995 (50.4%) assigned to the insulin sensitization strategy and 981 (49.6%) assigned to the insulin provision strategy. Of the 118 cases of new-onset atrial fibrillation, 42 (36%) came from hospital discharge diagnosis, 57 (48%) came from ECG, and 19 (16%) came from both discharge diagnosis and ECG. In the intention-to-treat analysis, assignment to the insulin-sensitization strategy decreased risk of atrial fibrillation with an HR of 0.89 (95% CI, 0.62–1.28, P = .54) compared with the insulin-provision strategy, and in the matched analysis thiazolidinedione decreased risk of atrial fibrillation with an HR of 0.79 (95% CI, 0.49–1.26, P = .32) compared with matched patients who did not receive a thiazolidinedione; thus, none of the results were significant (Table 2).
DISCUSSION
Atrial fibrillation is a common, serious clinical problem among patients with diabetes and coronary disease. This analysis of the BARI 2D randomized trial suggests that the incidence of atrial fibrillation might be 20% to 25% lower in patients treated with an insulin-sensitizing thiazolidinedione, but the confidence limits on these observations were very wide, and the trial lacked the statistical power to establish or exclude a clinically important effect of thiazolidinediones on atrial fibrillation, because of the relatively low incidence of documented atrial fibrillation (<1% per year).
The potential of thiazolidinediones to lower the risk of developing atrial fibrillation has been suggested by a few earlier studies. In a Danish nationwide cohort study, a decreased risk of atrial fibrillation with thiazolidinedione of 24% was found in 108,624 patients with diabetes.30 This was similar to the study by Chao et al,25 who reported a 30% lower risk of developing atrial fibrillation with the use of thiazolidinediones in a nationwide, observational study of 12,065 patients with diabetes (HR, 0.69; 95% CI, 0.49–0.91; P = .03). The PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) randomized controlled trial comparing pioglitazone with placebo for the treatment of diabetes was not designed to test differences in atrial fibrillation,31 but noted that it developed in 42 patients (1.6%) assigned to proglitazone compared with 51 patients (1.9%) assigned to placebo (rate ratio, 0.82, P = .37).31 The difference between the 2 register-based studies finding a reduced risk of atrial fibrillation with thiazolidinedione and the PROactive study and the BARI 2D study is both the design of the studies, register versus randomized controlled trial, and the size of the cohorts. Atrial fibrillation was also a predefined end point in the 2 register-based studies whereas in this study and in the PROactive trial atrial fibrillation was not a predefined endpoint but identified in post hoc analyses.
The effect of thiazolidinedione on the risk of developing atrial fibrillation has also been investigated in patients after cardiac procedures.23,24 A study of 188 patients with diabetes who underwent cardiothoracic surgery, 40 of whom were treated with a thiazolidinedione, reported a lower risk of developing postoperative atrial fibrillation, with an adjusted odds ratio of 0.80 (95% CI, 0.32–1.99, P = .63). The risk of recurrent atrial tachycardia after catheter ablation was investigated in diabetic patients who were randomized to pioglitazone versus no treatment: Pioglitazone decreased the risk of atrial tachycardia recurrence, with an odds ratio of 0.32 (95% CI, 0.12–0.86, P = .02).
A decreased thiazolidinedione use was seen after the black-box warnings restrictions, although these restrictions subsequently were lifted by the Food and Drug Administration, because there was no convincing evidence from the RECORD trial that myocardial infarction risk was higher, thiazolidinediones never regained their popularity. However, because of the possible beneficial effects, such as lower atrial fibrillation rates, there may be reasons for more widespread use.
LIMITATIONS
The BARI 2D was not designed to investigate the risk of atrial fibrillation and therefore did not monitor patients systematically for its development, such as with long-term ECG monitors. This could explain to some extent the low number of patients with atrial fibrillation at baseline and the low number of cases of new-onset atrial fibrillation found in the study. A little more than half of the patients assigned to the insulin sensitization strategy received a thiazolidinedione, and most of the remaining patients received metformin. Consequently, the intention-to-treat comparison between randomized diabetes management strategies includes many patients in both arms who did not receive a thiazolidinedione, which diluted this study’s ability to document an effect of thiazolidinediones on atrial fibrillation. We attempted to overcome this limitation by developing a propensity score model to identify which patients assigned to the insulin-sensitization strategy were prescribed a thiazolidinedione instead of metformin or other medications. In this subset, there was a stronger signal of an effect of the randomized assignment on atrial fibrillation (HR, 0.75 in the matched patients vs 0.91 in the entire population), but the confidence limits were again wide because relatively few outcome events were documented.
CONCLUSIONS
Our findings suggest no significant reduction of atrial fibrillation incidence with the use of thiazolidinediones. Larger studies with closer ECG monitoring are warranted among patients at increased risk of atrial fibrillation to provide a more conclusive evaluation of whether thiazolidinediones might reduce the risk of atrial fibrillation.
CLINICAL SIGNIFICANCE.
A total of 2319 patients with coronary disease were randomized to insulin-sensitization therapy (thiazolidinediones or metformin) or insulin-provision therapy (insulin, sulfonylurea, or a meglitinide).
The incidence rate of new-onset atrial fibrillation was 8.7 per 1000 person-years in patients assigned to insulin sensitization compared with 9.5 in patients assigned to insulin provision.
This gave a 20% to 25% lower risk of atrial fibrillation in patients treated with an insulin-sensitizing thiazolidinedione compared with insulin-provision therapy. This finding was not significant.
Funding:
The original Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) was funded by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804, R21HL121495). The original BARI 2D received support from the following companies: GlaxoSmithKline; Lantheus Medical Imaging, Inc (formerly Bristol-Myers Squibb Medical Imaging, Inc); Astellas Pharma US, Inc; Merck & Co., Inc; Abbott Laboratories, Inc; Pfizer, Inc; MediSense Products; Bayer Diagnostics; Becton, Dickinson and Company; cJ. R. Carlson Labs; Centocor, Inc; Eli Lilly and Company; LipoScience, Inc; Merck Sante; Novartis Pharmaceuticals Corporation; and Novo Nordisk, Inc. JLP has received research funding from Boehringer-Ingelheim and Bayer. MMB has received a research grant from Gilead Sciences Inc. BC has received consult fees from Lilly, Novo Nordisk, and Merck outside the submitted work.
Footnotes
Conflicts of Interest: None.
References
- 1.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108(1):56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez MV, Wang PJ, Larson JC, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women’s Health Initiative Observational Study. Heart. 2013;99(16):1173–1178. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 3.Pallisgaard JL, Lindhardt TB, Olesen JB, Hansen ML, Carlson N, Gislason GH. Management and prognosis of atrial fibrillation in the diabetic patient. Expert Rev Cardiovasc Ther. 2015;13(6):643–651. doi: 10.1586/14779072.2015.1043892. [DOI] [PubMed] [Google Scholar]
- 4.Pallisgaard JL, Schjerning AM, Lindhardt TB, et al. Risk of atrial fibrillation in diabetes mellitus: a nationwide cohort study. Eur J Prev Cardiol. 2016;23(6):621–627. doi: 10.1177/2047487315599892. [DOI] [PubMed] [Google Scholar]
- 5.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 6.Ahlehoff O, Gislason GH, Jørgensen CH, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33(16):2054–2064. doi: 10.1093/eurheartj/ehr285. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen SL, Lindhardsen J, Ahlehoff O, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. 2014;16(4):477–484. doi: 10.1093/europace/eut312. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26(20):2083–2092. doi: 10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 10.Picchi A, Capobianco S, Qiu T, et al. Coronary microvascular dysfunction in diabetes mellitus: a review. World J Cardiol. 2010;2(11):377–390. doi: 10.4330/wjc.v2.i11.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovasc Ther. 2014;32(5):233–241. doi: 10.1111/1755-5922.12089. [DOI] [PubMed] [Google Scholar]
- 12.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: pathophysiological mechanisms and potential upstream therapies. Int J Cardiol. 2015;184:617–622. doi: 10.1016/j.ijcard.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 13.Kadappu KK, Boyd A, Eshoo S, et al. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging. 2012;13(12):1016–1023. doi: 10.1093/ehjci/jes084. [DOI] [PubMed] [Google Scholar]
- 14.Henry WL, Morganroth J, Pearlman AS, et al. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976;53(2):273–279. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y-S, Hyun DW, Jung BC, et al. Left atrial volume index as a predictor for occurrence of atrial fibrillation after ablation of typical atrial flutter. J Cardiol. 2010;56(3):348–353. doi: 10.1016/j.jjcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Otake H, Suzuki H, Honda T, Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50(5):627–641. doi: 10.1536/ihj.50.627. [DOI] [PubMed] [Google Scholar]
- 17.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23(5):205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28(12):551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 20.Ermakov S, Azarbal F, Stefanick ML, et al. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102(17):1354–1362. doi: 10.1136/heartjnl-2015-308927. [DOI] [PubMed] [Google Scholar]
- 21.Drugs@FDA: FDA Approved Drug Products. Available at: https://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed October 19, 2015.
- 22.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Gu J, Liu X, Wang X, et al. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace. 2011;13(9):1256–1261. doi: 10.1093/europace/eur131. [DOI] [PubMed] [Google Scholar]
- 24.Anglade MW, Kluger J, White CM, Aberle J, Coleman CI. Thiazolidinedione use and post-operative atrial fibrillation: a US nested case-control study. Curr Med Res Opin. 2007;23(11):2849–2855. doi: 10.1185/030079907X242494. [DOI] [PubMed] [Google Scholar]
- 25.Chao T-F, Leu H-B, Huang C-C, et al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol. 2012;156(2):199–202. doi: 10.1016/j.ijcard.2011.08.081. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Korantzopoulos P, Li G, Li J. The potential role of thiazolidinediones in atrial fibrillation. Int J Cardiol. 2008;128(1):129–130. doi: 10.1016/j.ijcard.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 27.Brooks MM, Frye RL, Genuth S, et al. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am J Cardiol. 2006;97(12A):9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Hlatky MA, Boothroyd DB, Melsop KA, et al. Economic outcomes of treatment strategies for type 2 diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation. 2009;120(25):2550–2558. doi: 10.1161/CIRCULATIONAHA.109.912709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach RG, Brooks MM, Lombardero M, et al. Response to letter regarding article, “rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D). Trial”. Circulation. 2014;129(16):e460–e461. doi: 10.1161/CIRCULATIONAHA.113.008033. [DOI] [PubMed] [Google Scholar]
- 30.Pallisgaard JL, Lindhardt TB, Staerk L, et al. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. 2016; doi: 10.1093/ehjcvp/pvw036. [DOI] [PubMed] [Google Scholar]
- 31.Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]


