Abstract
Background
Coronavirus disease-2019 (COVID-19) is caused by severe acute respiratory-syndrome coronavirus-2 that interfaces with the renin-angiotensin-aldosterone system (RAAS) through angiotensin-converting enzyme 2. This interaction has been proposed as a potential risk factor in patients treated with RAAS inhibitors.
Objectives
This study analyzed whether RAAS inhibitors modify the risk for COVID-19.
Methods
The RASTAVI (Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation) trial is an ongoing randomized clinical trial randomly allocating subjects to ramipril or control groups after successful transcatheter aortic valve replacement at 14 centers in Spain. A non-pre-specified interim analysis was performed to evaluate ramipril’s impact on COVID-19 risk in this vulnerable population.
Results
As of April 1, 2020, 102 patients (50 in the ramipril group and 52 in the control group) were included in the trial. Mean age was 82.3 ± 6.1 years, 56.9% of the participants were male. Median time of ramipril treatment was 6 months (interquartile range: 2.9 to 11.4 months). Eleven patients (10.8%) have been diagnosed with COVID-19 (6 in control group and 5 receiving ramipril; hazard ratio: 1.150; 95% confidence interval: 0.351 to 3.768). The risk of COVID-19 was increased in older patients (p = 0.019) and those with atrial fibrillation (p = 0.066), lower hematocrit (p = 0.084), and more comorbidities according to Society of Thoracic Surgeons score (p = 0.065). Admission and oxygen supply was required in 4.9% of patients (2 in the ramipril group and 3 in the control group), and 4 of them died (2 in each randomized group). A higher body mass index was the only factor increasing the mortality rate (p = 0.039).
Conclusions
In a high-risk population of older patients with cardiovascular disease, randomization to ramipril had no impact on the incidence or severity of COVID-19. This analysis supports the maintenance of RAAS inhibitor treatment during the COVID-19 crisis. (Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation [RASTAVI]; NCT03201185)
Key Words: COVID-19, renin-angiotensin, SARS-CoV-2, transcatheter aortic valve replacement
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; CI, confidence interval; COVID-19, coronavirus disease-2019; IQR, interquartile range; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory-syndrome coronavirus-2
Central Illustration
The coronavirus disease-2019 (COVID-19) outbreak is caused by a new coronavirus (1, 2, 3, 4), the severe acute respiratory-syndrome coronavirus-2 (SARS-CoV-2) (5, 6, 7, 8, 9). According to several investigators, the virus internalizes the cell by binding its trimeric spike protein to the human receptor angiotensin-converting enzyme II (ACE2) (10, 11, 12). Renin-angiotensin-aldosterone system (RAAS) inhibition up-regulates ACE2, which casts doubts on whether the administration of these drugs could predispose a patient to the disease or worsen the course of the disease (13, 14, 15). With this background, some investigators advise the discontinuation of RAAS blockers either with prophylactic purposes or, in infected patients, to avoid the evolution to SARS (16). On the other hand, high ACE2 levels have been shown to protect the lung in SARS (17), and a small series suggests an absence of a deleterious effect in hypertensive patients taking RAAS inhibitors (18) with a decrease in the peak viral load and the inflammatory markers in patients under these medications. Moreover, the well-known cardiorenal effects of RAAS blockade in cardiovascular disease should not be dismissed, particularly in COVID-19 patients, in whom cardiovascular comorbidities favor a severe course of the disease.
The ongoing RASTAVI (Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation) clinical trial is a randomized 1:1 open-label study involving 14 Spanish centers. Currently, Spain presents one of the highest rates of confirmed COVID-19 cases and of deaths per million in the world. The RASTAVI trial is investigating the effect of adding ramipril to the standard care in patients successfully treated with percutaneous aortic valve in terms of ventricular remodeling as assessed by cardiac magnetic resonance and in the major clinical outcomes (19,20).
By May 5, 2020, 3,525,116 people all over the world have been infected by SARS-CoV-2 (21), whereas thousands of patients worldwide are taking RAAS inhibitors daily. In this scenario, we describe for the first time the impact of the COVID-19 pandemic in high-risk patients from the RASTAVI trial who were randomly assigned to receive ramipril or standard care.
Methods
Study population
The RASTAVI study is a national, multicenter, open-label, and randomized 1:1 trial aiming to determine the effect of ramipril on cardiac events, functional capacity, and cardiac remodeling on patients with aortic stenosis successfully treated with transcatheter aortic valve replacement. The first patient was included on March 26, 2018, and the study will continue recruiting until the end of 2021. Patients are randomized, after signing informed consent, between 1 and 5 days after transcatheter aortic valve replacement procedure to receive either standard care or an initial dose of ramipril (2.5 mg daily). Titration of the ramipril is performed at each monitored visit with the goal of a full dose (10 mg daily) if tolerated. Patients included in the control group if their blood pressure is beyond recommended parameters (140/90 mm Hg) receive any medication to control it, except for RAAS inhibitors (19).
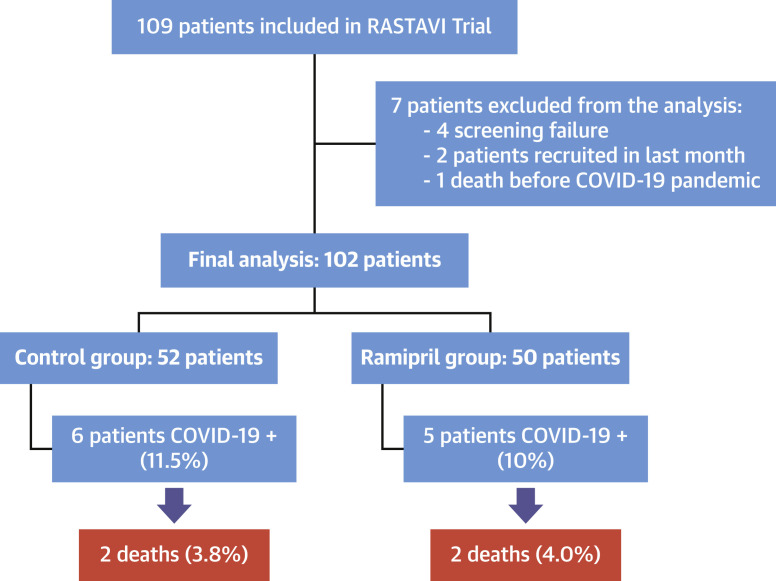
As of April 1, 2020, 109 patients have been included in the study (Figure 1 ). The aim of this non-pre-specified interim analysis is to investigate how SARS-CoV-2 infection has affected this high-risk population and to get further insight on the effect of RAAS inhibitors on the susceptibility to the disease. For this purpose, updated follow-up via phone calls and consulting electronic clinical reports was obtained for all patients. This follow-up was monitored and performed with the approval of the ethics committee.
Figure 1.
Patient Flowchart
Schematic flowchart of the patients included in the RASTAVI (Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation) trial and the interim analysis showing their rate of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection and the mortality. COVID-19 = coronavirus disease-2019.
COVID-19 diagnosis
All patients from the study were contacted by phone and a questionnaire was performed to determine the presence of symptoms (fever, cough, dyspnea, syncope, myalgia, or others) in the last 5 months, or contact with confirmed cases of the disease. Also, any admission in the last 5 months was recorded as per protocol, and the clinical reports as well as any laboratory exams were gathered. Confirmation of SARS-CoV-2 infection was performed through real-time reverse transcription polymerase chain reaction of nasopharyngeal sample, and the test was performed as clinically indicated.
Statistical analysis
Categorical variables are reported as number (percent) and continuous variables as mean ± SD or median (interquartile range [IQR]), depending on variable distribution. Comparisons between those who were and those who were not developing COVID-19 were performed to determine pre-disposing factors.
A Cox regression analysis was performed for investigating the effect of ramipril administration on the time symptoms of COVID-19 developed in the study patients. Ramipril was the only factor included in the analysis to avoid overfitting. Also, factors associated with mortality were estimated for patients with SARS-CoV-2 infection comparing the characteristics of those who died and those who survived the disease. Pearson chi-square test and Fisher exact test were performed in comparisons between groups with qualitative variables, and Student's t-test or Mann-Whitney U test were performed for continuous variables. All tests were 2-sided at the 0.05 significance level. Statistical analysis was performed with IBM SPSS Statistics version 25 (IBM, Armonk, New York).
Results
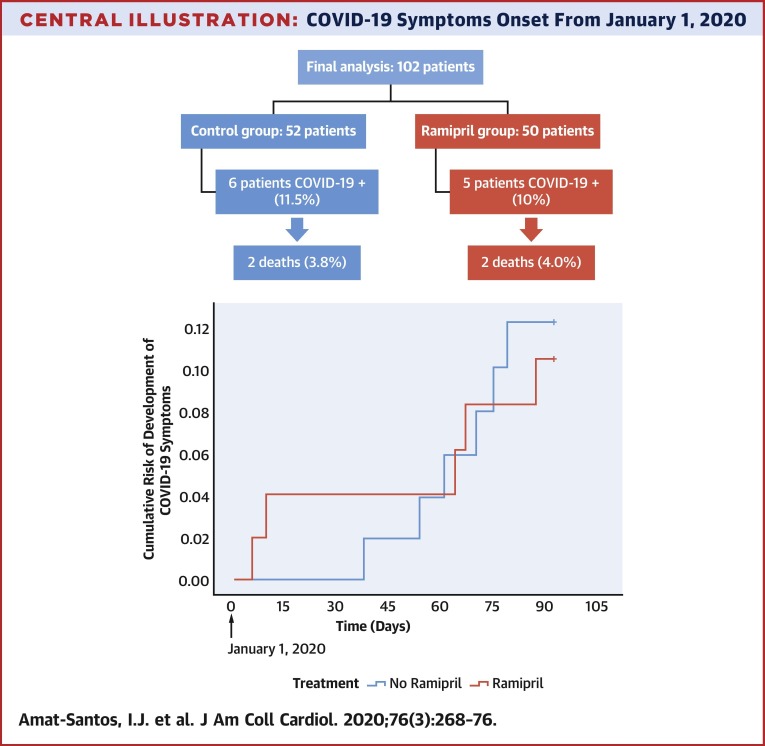
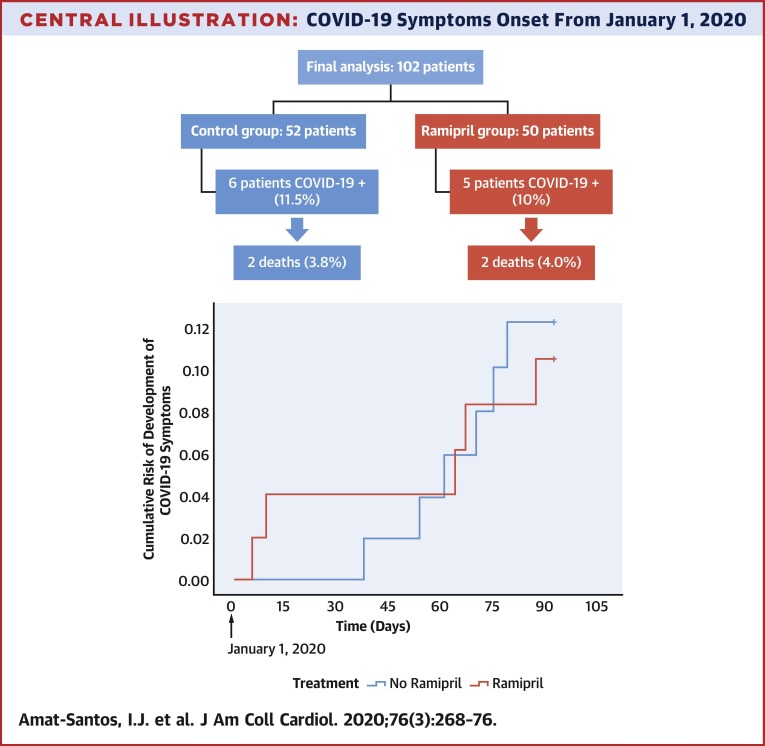
After careful assessment of the patients randomized in the study (Central Illustration ), a total of 102 patients (50 in the ramipril group and 52 in the control group) were included in this interim analysis. Of them, 11 patients (10.8%), presenting with clinical symptoms compatible with COVID-19, underwent SARS-CoV-2 reverse transcription polymerase chain reaction of nasopharyngeal sample with positive result, 5 of them from the ramipril group and 6 from the control group (p = 0.802). No other patients developed symptoms, but 7 of them had the SARS-CoV-2 test performed due to risk contacts and presented negative results.
Central Illustration.
COVID-19 Symptoms Onset From January 1, 2020
Development of symptoms from January 1, 2020, according to the administration of ramipril or standard care. COVID-19 = coronavirus disease-2019.
Baseline characteristics
Main baseline characteristics from the 102 patients according to diagnosis of COVID-19 are summarized in Table 1 . Mean age was 82.3 ± 6.1 years and 56.9% were male. In those randomized to the drug, median time under treatment with ramipril was 6 months (IQR: 2.9 to 11.4 months), with all the included cases receiving the therapy at least for 1 month. Time from January 1, 2020, to onset of COVID-19 symptoms is presented in the Central Illustration. The prior administration of ramipril presented a hazard ratio of 1.150 (95% confidence interval [CI]: 0.351 to 3.768) for the development of COVID-19. Patients developing COVID-19 were significantly older (median: 86 years [IQR: 84 to 88 years] vs. 83 years [IQR: 78 to 86 years]; p = 0.019) and presented a trend to higher rate of prior atrial fibrillation and anemia. No significant differences existed regarding the rate of main cardiovascular risk factors between patients experiencing COVID-19 and those free of the infection, including hypertension, diabetes mellitus, and dyslipidemia. Globally, there were no differences in major comorbidities including coronary artery disease, moderate or severe chronic obstructive pulmonary disease, and chronic kidney disease. However, there was a trend toward worse baseline risk according to the Society of Thoracic Surgeons score (median: 3.90 [IQR: 2.64 to 6.60] vs. 3.06 [IQR: 1.82 to 4.02]; p = 0.065).
Table 1.
Baseline Characteristics of the RASTAVI Study Population According to COVID-19 Diagnosis
| COVID-19–Positive (n = 11) | COVID-19–Negative (n = 91) | p Value | |
|---|---|---|---|
| Age, yrs | 86.0 (84.0–88.0) | 83.0 (78.0–86.0) | 0.019 |
| Body mass index, kg/m2 | 26.3 (24.9–28.7) | 27.1 (24.6–30.5) | 0.580 |
| Female | 5 (45.5) | 53 (52.8) | 0.524 |
| Hypertension | 6 (54.5) | 49 (53.8) | 0.965 |
| Diabetes | 2 (18.2) | 19 (20.9) | 0.834 |
| Dyslipidemia | 6 (54.5) | 60 (65.9) | 0.512 |
| Prior atrial fibrillation | 6 (54.5) | 22 (24.2) | 0.066 |
| Coronary artery disease | 2 (18.2) | 24 (26.4) | 0.724 |
| Prior myocardial infarction | 0 (0.0) | 6 (6.6) | 0.635 |
| Prior PCI | 2 (18.2) | 18 (19.8) | 0.999 |
| CKD, eGFR <60 ml/min | 4 (36.4) | 29 (31.9) | 0.744 |
| Moderate or severe COPD | 1 (9.1) | 5 (5.5) | 0.663 |
| Peripheral vascular disease | 2 (18.2) | 9 (9.9) | 0.338 |
| Prior stroke/TIA | 1 (9.1) | 12 (13.2) | 0.999 |
| Prior blood test parameters | |||
| Hematocrit, % | 31 (28.6–33.4) | 33.1 (31–36.6) | 0.084 |
| Creatinine, mg/dl | 0.90 (0.80–1.15) | 0.80 (0.70–1.10) | 0.470 |
| NT-proBNP, pg/ml | 1,284 (918–1,894) | 1,140 (522–2,724) | 0.719 |
| Prior treatment | |||
| Oral anticoagulation | 6 (54.5) | 28 (31.1) | 0.175 |
| Statins | 6 (54.4) | 61 (67.8) | 0.501 |
| Oral hypoglycemic drug | 1 (9.1) | 15 (16.7) | 0.999 |
| Barthel index | 92.5 (75.0–100.0) | 95.0 (90.0–100.0) | 0.584 |
| NYHA functional class ≥II | 11 (100.0) | 78 (85.7) | 0.351 |
| EuroSCORE II, % | 5.02 (3.90–5.95) | 3.89 (3.20–5.26) | 0.112 |
| STS-PROM, % | 3.90 (2.64–6.60) | 3.06 (1.82–4.02) | 0.065 |
| Echocardiographic findings | |||
| LVEF—Simpson’s method, % | 60.0 (50.0–65.0) | 61.5 (56.0–66.0) | 0.472 |
| Residual aortic regurgitation ≥3 | 0 (0.0) | 4 (4.4) | 0.478 |
| Residual peak aortic gradient, mm Hg | 18.0 (10.5–21.0) | 7.0 (4.5–9.0) | 0.276 |
| Aortic velocity-time integral | 17.0 (16.0–19.0) | 22.0 (19.5–28.5) | 0.071 |
| Septal width, mm | 13.0 (11.5–15.5) | 13.0 (12.0–15.0) | 0.947 |
Values are median (interquartile range) or n (%).
CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease-2019; eGFR = estimated glomerular filtration rate; EuroSCORE = European System for Cardiac Operative Risk Evaluation; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; RASTAVI = Renin-Angiotensin System Blockade Benefits in Clinical Evolution and Ventricular Remodeling After Transcatheter Aortic Valve Implantation; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TIA = transient ischemic attack.
Main features of patients with COVID-19
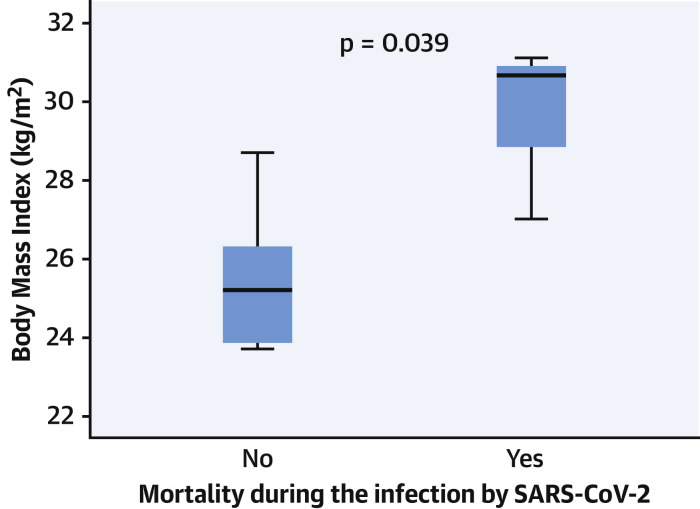
Among patients with COVID-19 (n = 11), fever was the most frequent symptom (63.5%), followed by cough (54.5%), dyspnea (27.3%), and myalgia (9.1%). Five patients presented with severe respiratory disease that required admission to a health care institution and specific treatment (lopinavir and ritonavir, hydroxychloroquine, azithromycin, and corticoids) as opposed to only symptomatic treatment in those who did not require hospitalization. Four of these 5 hospitalized patients (36.4% of the COVID-19 cases) died as a consequence of bilateral pneumonia with severe acute respiratory distress. The specific characteristics of each of the 4 patients who died are described in detail in Table 2 . No differences were found according to the drug randomization, with 2 of the patients undergoing RAAS-inhibitors treatment and 2 undergoing standard care. All of them presented with persistent heart failure despite successful treatment of their valvular disease. The only factor related to higher mortality in patients diagnosed with COVID-19 was a greater median body mass index (30.7 [IQR: 28.8 to 30.9] in those who died vs. 25.2 [IQR: 23.9 to 26.3] in those who did not; p = 0.039) (Figure 2 ).
Table 2.
Main Characteristics of the Patients Presenting With COVID-19 That Died According to Prior Randomization to Ramipril or Standard of Care
| Group |
||||
|---|---|---|---|---|
| Randomized to Ramipril |
Randomized to Control |
|||
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | |
| Baseline characteristics | ||||
| Sex | Male | Female | Female | Male |
| Age, yrs | 86 | 83 | 88 | 89 |
| Body mass index, kg/m2 | 27.01 | 30.67 | 29.2 | 31.12 |
| Hypertension | No | Yes | No | Yes |
| Diabetes mellitus | No | No | No | No |
| Dyslipidemia | No | Yes | No | Yes |
| Smoker | No | No | No | No |
| Coronary artery disease | Yes | No | No | No |
| Chronic pulmonary disease | No | No | No | No |
| Persistent heart failure | Yes | Yes | Yes | Yes |
| NYHA functional class | III | II | II | II |
| CKD, eGFR <60 ml/min | Yes | Yes | No | Yes |
| Moderate or severe COPD | No | No | No | No |
| Atrial fibrillation | Yes | No | Yes | Yes |
| Anticoagulation | Dabigatran | No | Apixaban | Edoxaban |
| Peripheral artery disease | No | No | Yes | Yes |
| Date of TAVR procedure | April 24, 2018 | May 17, 2019 | September 21, 2018 | September 11, 2019 |
| Implanted TAVR device∗ | Evolut | Evolut | Allegra | Evolut |
| Residual aortic regurgitation | I | 0 | I | 0 |
| EuroSCORE II, % | 4.55 | 3.08 | 9.32 | 6.63 |
| STS-PROM, % | 3.95 | 3.84 | 12.77 | 6.60 |
| COVID-19 features | ||||
| Fever | Yes | Yes | No | Yes |
| Cough | No | Yes | No | Yes |
| Dyspnea | Yes | Yes | Yes | Yes |
| Specific COVID-19 treatment | L/R+HC+A+C | L/R+HC+A+C | L/R+HC+A+C | L/R+HC+A+C+T |
| Days from diagnosis to death | 15 | 17 | 12 | 21 |
| ICU admission | No | No | No | No |
| Noninvasive mechanical ventilation | No | No | No | Yes |
| Invasive mechanical ventilation | No | No | No | No |
A= azithromycin; C = corticoids; HC = hydroxychloroquine; ICU = intensive care unit; L/R = lopinavir and ritonavir; T = tocilizumab; TAVR = transcatheter aortic valve implantation; other abbreviations as in Table 1.
Evolut (Medtronic, Minneapolis, Minnesota); Allegra (New Valve Technology, Muri, Switzerland).
Figure 2.
Impact of BMI on Mortality
Body mass index (BMI) in patients with coronavirus disease (COVID-19) according to mortality. The box shows the interquartile range and the T-bars represent the highest and lowest values (the range). The horizontal line in the box is the median. SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
Discussion
In the beginning of the COVID-19 pandemic, certain publications called for the discontinuation of RAAS inhibitors, both to potentially—we must not miss the hypothetical nuance—prevent the disease and to improve the prognosis in patients who were already diagnosed with or who were suspected of having COVID-19 (16,22, 23, 24, 25). This call for caution has prevailed in the medical community and the media despite the rise of discordant voices defending alternative hypothesis (26) and has led the American College of Cardiology to issue a request for urgent research to clarify this aspect (27). Hypertension has been described as one of the most common coexisting conditions in patients admitted in hospital due to COVID-19 (28), probably as a result of its higher prevalence in older patients. Often, this cardiovascular condition is treated with RAAS inhibitors, which explains the great clinical relevance of clarifying the potential role of these drugs in COVID-19 pandemic.
The main findings of our study suggest that: 1) age, but not hypertension, seems to be the only factor significantly associated with risk for developing COVID-19 in this comorbid population; 2) >10% of the patients developed COVID-19 and one-third of them died, but the use of RAAS inhibitors was associated with neither risk for the clinically symptomatic infection nor poorer outcomes in the course of the disease; and 3) patients who died due to the infection presented higher body mass index as a unique and characteristic feature. Obesity is a frequent condition among patients who are hospitalized with COVID-19 (29) and is present in up to 42% of those admitted to the hospital (30), but its influence in the prognosis has not been well described yet. However, experiences with other viruses, such as H1N1 influenza, suggest that patients with obesity (31), even of young age, may evolve toward severe alveolitis with respiratory failure and death (32). Therefore, this is not a minor finding and warrants future analysis and research.
Outbreak of COVID-19
As of late 2019, 6 coronavirus species have been described to cause human disease. Four of them—229E, OC43, NL63, and HKU1—are responsible for light or mild respiratory disease (1). The 2 other species can cause severe disease and were the causal agents of the 2002 SARS-CoV (2,3) and the 2012 Middle East respiratory syndrome coronavirus (4) outbreaks. In early December 2019 in Wuhan, China, the first cases of pneumonia due to an unidentified microbial agent were described (5,6). Reverse transcription polymerase chain reaction of lower respiratory tract samples allowed the sequencing of the genome of a novel ribonucleic acid virus with 79.6% of similarities with SARS-CoV, hence it was named SARS-CoV-2 (7,8). Person-to-person transmission was rapidly described (9).
Interplay of SARS-CoV-2 and ACE2
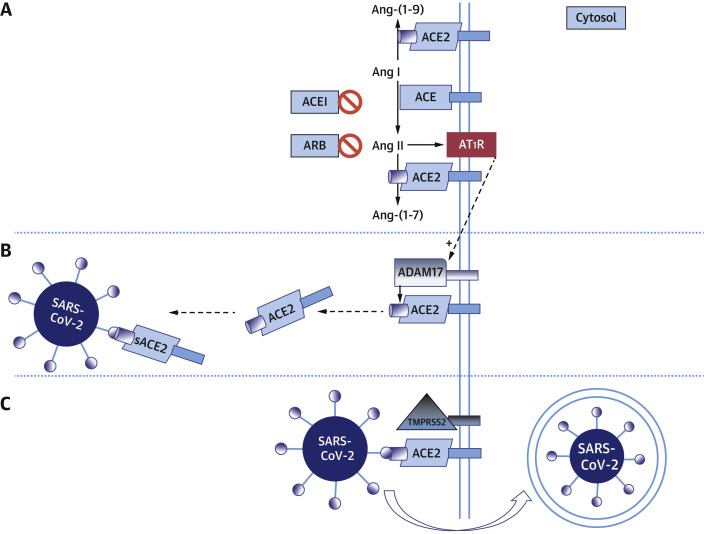
SARS-CoV-2 presents a spike protein to facilitate entry into target cells. It links to ACE2 and is internalized after priming by the transmembrane protease serine 2 (7,10, 11, 12) (Figure 3 ). Membrane-bound ACE2 is part of a 3-enzyme system to convert angiotensin II to angiotensin-(1-7), with opposing properties to angiotensin II to achieve balance in the human body (Figure 3A). ACE2 is considerably similar to ACE, which converts angiotensin I to angiotensin II (28,33), and this second, not ACE2, is the target of ACE inhibitors. ACE2 is 2% in a soluble form—which is not valid for SARS-CoV-2 binding—after cleavage by A disintegrin and metalloprotease 17. Angiotensin I upregulates A disintegrin and metalloprotease 17, thus increasing soluble ACE2 levels (13,14) (Figure 3B). Furthermore, A disintegrin and metalloprotease 17also mediates the release of membrane-bound precursors of proinflammatory cytokines (tumor necrosis factor-α, interferon-γ, and interleukin-4) into the circulation. These cytokines down-regulate ACE2 cell surface expression reducing the capability of SARS-CoV-2 to cause damage (15) (Figure 3C). The hypothesis suggesting that RAAS inhibitors might increase the susceptibility of patients who are taking them to COVID-19 and proposing the discontinuation of RAAS inhibitors has its basis in this pathophysiologic explanation (16). On the other hand, Meng et al. (18) found lower interleukin-6 and peak viral load in peripheral blood and increased CD3 and CD8 T cells in patients with hypertension treated with RAAS inhibitors. Despite this contradictory evidence, the most recent clinical data suggest that the up-regulation of inflammatory cytokines by angiotensin II, which is harmful to the outcomes of patients with COVID-19, can be decreased thanks to the reduced formation of angiotensin II caused by RAAS inhibitors, thus explaining the better clinical outcomes detected by leading-edge research groups (18,26,30).
Figure 3.
Interplay of RAAS and SARS-CoV-2
Hypothetic model of renin-angiotensin-aldosterone system (RAAS) activation and severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) cell entry. (A) Angiotensin-converting enzyme 2 (ACE2) converts angiotensin (Ang) I to Ang-(1-9) and Ang II to Ang-(1-7). When angiotensin-converting enzyme inhibitors (ACEIs) are present, they prevent the conversion of Ang I to Ang II. Angiotensin-receptor blockers (ARBs) act at angiotensin II type 1 receptor (AT1R). (B) When A disintegrin and metalloprotease 17 (ADAM17) binds ACE2, it results in the occurrence of soluble (s) ACE2, which can no longer mediate SARS-CoV-2 entry and which might even prevent such entry by keeping the virus in solution. (C) The SARS-CoV-2 spike links to ACE2 and is internalized after priming by the transmembrane protease serine 2 (TMPRSS2).
Clinical impact of management of RAAS inhibitors during COVID-19 pandemic
The recommendation of discontinuation of RAAS inhibitors was initially accepted by part of the medical community as a prophylactic measurement aiming to potentially reduce the risk of a severe respiratory disease. However, in this discussion an important issue was forgotten: loss-of-function experiments using ACE2 knockout mice have demonstrated increased susceptibility to myocardial infarction, hypertension and myocardial hypertrophy, microvascular complications, inflammation, fibrosis, diastolic and systolic dysfunction, and oxidative stress (19). Translated into humans, avoiding these drugs, where indicated, may increase major cardiovascular events causing more damage than the potential of increased susceptibility to SARS-CoV-2.
Therefore, in view of the overwhelming evidence of mortality reduction in cardiovascular disease and the lack of impact found in our research, we believe that RAAS inhibitors should be maintained or even initiated in patients with new onset heart failure, hypertension, or myocardial infarction according to current guidelines as tolerated, irrespective of SARS-CoV-2 disease outbreak. The frequently updated information on the disease suggests that, not only has mortality due to COVID-19 has increased, but also other comorbidities are experiencing a rise in their mortality rates. Cardiovascular events are still the greatest cause of mortality in our society and inadequate handling of secondary prevention can have a catastrophic impact on the health status of the population (26,29,34). However, as pointed out by Vaduganathan et al. (26), the effects on ACE2 should not be assumed to be uniform across RAAS inhibitors or even in response to therapies within a given drug class. For this reason, we need to remark that the current research only provided accurate evidence for ramipril and not for other drugs of this group.
Study limitations
The main limitations of the present study include its retrospective nature and the limited number of cases with confirmed COVID-19 infection. Detection of immunization should be undertaken as soon as confinement allows testing all patients in the trial to verify the real incidence of COVID-19 in this population, otherwise the real incidence of the disease remains unknown. Reporting this information in the near future is warranted but, still, reporting the impact of ramipril on prognosis of symptomatic infection is clinically relevant. The relatively short length of the follow-up precludes us from drawing further conclusions regarding the long term, but a continuous monitoring of patients at risk from the RASTAVI study will be performed.
Conclusions
More than 10% of the high-risk patients in the RASTAVI population developed COVID-19 and one-third of them died. Older patients with higher body mass index presented higher risk of infection and mortality. However, the use of ramipril did not increase the risk of infection or impair the prognosis. Hence, the use of RAAS inhibitors during the COVID-19 pandemic seems safe.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: In a subset of patients with hypertension after transcatheter aortic valve replacement who were enrolled in a randomized trial, the ACE inhibitor ramipril did not increase the risk of COVID-19 infection or adversely affect its prognosis.
TRANSLATIONAL OUTLOOK: Studies of larger populations are needed to determine whether angiotensin II receptor blockers differ from ACE inhibitors with regard to the risk of infection after exposure to the SARS-CoV-2 or severity of COVID-19.
Footnotes
This project was supported by the Insituto de Salud Carlos III (grant PI17/02237). Dr. Amat-Santos has served as proctor for Boston Scientific. Dr. Nombela-Franco has served as a proctor for Abbott; and has received speaker honoraria from Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Florian Rader, MD, MSc, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Corman V.M., Lienau J., Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist (Berl) 2019;60:1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong N.S., Zheng B.J., Li Y.M. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C., Günther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zhao X., Li J. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 14.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 Mar 26 doi: 10.1161/CIRCULATIONAHA.120.047049. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Sommerstein R., Kochen M.M., Messerli F.H., Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9:e016509. doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng J., Xiao G., Zhang J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Gabella T., Catalá P., Muñoz-García A.J. Renin-angiotensin system inhibition following transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74:631–641. doi: 10.1016/j.jacc.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Amat-Santos I.J., Catalá P., Diez Del Hoyo F. Impact of renin-angiotensin system inhibitors on clinical outcomes and ventricular remodelling after transcatheter aortic valve implantation: rationale and design of the RASTAVI randomised multicentre study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Coronavirus Disease 2019: Situation Report–76. April 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200405-sitrep-76-covid-19.pdf?sfvrsn=6ecf0977_4 Available at:
- 22.Zhong J., Basu R., Guo D. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 23.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 25.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa041. taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt B., Kovacs R., Harrington B. HFSA/ACC/AHA Statement Addressed Concerns Re: Using RAAS Antagonists in COVID-19. ACC news story. March 17, 2020 doi: 10.1016/j.cardfail.2020.04.013. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 Available at: Accessed May 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 2020;28:1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 32.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases: a statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuster G.M., Pfister O., Burkard T. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020 Mar 24 doi: 10.1001/jama.2020.4812. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]