Abstract
Background
Coronavirus disease-2019 (COVID-19), a respiratory disease has been associated with ischemic complications, coagulation disorders, and an endotheliitis.
Objectives
To explore endothelial damage and activation-related biomarkers in COVID-19 patients with criteria of hospitalization for referral to intensive care unit (ICU) and/or respiratory worsening.
Methods
Analysis of endothelial and angiogenic soluble markers in plasma from patients at admission.
Results
Study enrolled 40 consecutive COVID-19 patients admitted to emergency department that fulfilled criteria for hospitalization. Half of them were admitted in conventional wards without any ICU transfer during hospitalization; whereas the 20 others were directly transferred to ICU. Patients transferred in ICU were more likely to have lymphopenia, decreased SpO2 and increased D-dimer, CRP and creatinine levels. In those patients, soluble E-selectin and angiopoietin-2 were significantly increased (p value at 0.009 and 0.003, respectively). Increase in SELE gene expression (gene coding for E-selectin protein) was confirmed in an independent cohort of 32 patients using a whole blood gene expression profile analysis. In plasma, we found a strong association between angiopoetin-2 and CRP, creatinine and D-dimers (with p value at 0.001, 0.001 and 0.003, respectively). ROC curve analysis identified an Angiopoietin-2 cut-off of 5000 pg/mL as the best predictor for ICU outcome (Se = 80.1%, Sp = 70%, PPV = 72.7%, NPV = 77%), further confirmed in multivariate analysis after adjustment for creatinine, CRP or D-dimers.
Conclusion
Angiopoietin-2 is a relevant predictive factor for ICU direct admission in COVID-19 patients. This result showing an endothelial activation reinforces the hypothesis of a COVID-19-associated microvascular dysfunction.
Keywords: COVID-19, Angiogenesis, Endothelial, Biomarker, E-selectin, Angiopoietin-2
Introduction
SARS-CoV-2 infection can be paucisymptomatic or lead to the coronavirus disease-2019 (COVID-19), which has a very large pattern of disease severity, in particular in patients with cardiovascular comorbidities [1]. COVID-19 infection is associated with a coagulopathy characterized by an increase in procoagulant factors such as fibrinogen, together with a strong elevation of D-dimers that have been associated with a higher mortality [1, 2]. Increased incidence of pulmonary embolism has been observed in several reports [3] and microvascular thrombosis in the lungs has been noted in autopsies series and in COVID-19 acute respiratory distress syndrome (ARDS) [4–6]. This microvascular thrombosis is observed while an inflammatory storm including IL-6 occurs in COVID-19 patients leading to the activation and recruitment of leukocytes. Activated leukocytes are able to damage capillary endothelium and to disrupt the thrombo-protective state of endothelial cells, probably participating to microvascular thrombosis. SARS-CoV-2 virus has been shown to infect blood vessels and induce vascular damage in vitro and in vivo [7–9]. SARS-CoV-2 can infect cells via the angiotensin-converting enzyme 2 (ACE2) receptor which is ubiquitous but largely expressed in endothelial cells [10]. In this report, more than the presence of virus in endothelial cells, authors describe both inflammation and endothelial cell death. This endotheliitis could be at the origin of impaired microcirculatory function affecting particularly the lungs and kidneys [8, 11].
Thrombo-inflammation has been described in arterial and/or venous thrombosis but also in cancer associated thrombosis with the complex interplay between blood coagulation and inflammation [12]. Markers of activation of endothelium such as soluble endoglin, soluble E-selectin (sE-selectin) or angiopoietin-2 have been involved in hemostasis, thrombo-inflammatory events or sepsis [13–15]. Indeed, endothelial activation has been found as a major determinant that mediates cerebral ischemia/reperfusion injury by promoting thrombo-inflammation [16].
Therefore, the aim of our study was to identify, in COVID-19 patients with criteria of hospitalization, soluble circulating endothelial and/or angiogenic markers at admission that could predict the need for hospitalization in intensive care unit (ICU) – i.e., respiratory failure and requiring mechanical ventilation – and anticipate subsequent disease worsening.
Patients and methods
Study design and population
This study was an observational cohort study conducted in Georges Pompidou European hospital in Paris (France). Consecutive patients with suspected SARS-CoV-2 infection were prospectively included. Inclusion criteria were an age over 18 years old, an infectious syndrome, a suspected COVID-19 with hospitalization criteria either in conventional wards or directly to ICU. All COVID-19-suspected patients were tested for SARS-CoV-2 infection by nasopharyngeal swabs and screened for hospitalization criteria based on local guidelines. For each included patient, clinical evaluation, chest computed tomography (CT) scan, and biological evaluation were performed. Baseline characteristics (demography, usual treatments, clinical manifestations, cardiovascular risk factors, and body mass index), biological data were retrieved from the computer-based patient records using a standardized data collection. For confirmation cohort of E-selectin expression, we used data from 32 patients with confirmed SARS-CoV-2 infection and various disease severity from Cochin Hospital in which a whole blood gene expression profile analysis was made using the Nanostring nCounter immunology panel. Healthy controls were asymptomatic adults, matched with cases on age with a negative SARS-CoV-2 RT-PCR testing at time of inclusion.
Both studies conformed to the principles outlined in the Declaration of Helsinki, and received approval by the appropriate Institutional Review Board (CPP2020-04-048/2020-A01048-31 / 20.04.21.49318).
Routine blood examinations
All samples were collected on EDTA, sodium heparin and 0.129 M trisodium citrate tubes (9NC BD Vacutainer, Plymouth, UK). Routine lab tests were complete blood count, creatinine, C-reactive protein (CRP) and high sensitivity cardiac troponin (Hs-cTnI, Beckman) on a DXI analyzer [17, 18]. Platelet poor plasma (PPP) was obtained after centrifugation twice at 2500 g for 15 min. Coagulation tests were PT ratio, fibrinogen and soluble fibrin monomer level (STA®-Liatest FM; Diagnostica Stago) explored on a STA-R® Max coagulometer (Stago) as previously described [19]. D-dimer concentrations were determined using the Vidas D-Dimer assay (BioMérieux) according to the manufacturer’s instructions. PPP was frozen after a second centrifugation at 2500 g for 15 min and stored at − 80 °C until analysis of vascular markers. Soluble E-selectin, endoglin, VEGF-A, PlGF, basic-FGF, VEGFR-2, angiopoietin-1 and -2, c-Kit and leptin concentrations were quantified in PPP with a Human Magnetic Luminex Assay from R&D systems (Lille, France). Data were assessed with the Bio-Plex 200 using the Bio-Plex Manager 5.0 software (Bio-Rad, Marnes-la-Coquette, France).
Gene expression analysis
As previously described [20], we analyzed 100 ng (5 μl) of total RNA from each sample using the Nanostring Human Immunology kit v2 according to the manufacturer’s instructions. The housekeeping genes were selected from the 15 candidate control genes provided by Nanostring, following the geNorm method. Briefly, after selection of genes with all values above the background level, for each two genes j ≠ k, pairwise variation coefficient Vjk is defined as
where aij is the number of counts for the gene j in the sample i. The gene stability measure Mj for control gene j is the arithmetic mean of all pairwise variations Vjk for k ≠ j. Mj evaluates the degree of correlation of gene j to other control genes (the smaller Mj is, the more correlated gene j is to other control genes). Genes were ranked by increasing M, and to determine a threshold, the normalization factors NFn was computed for all n (defined as the geometric mean of the housekeeping gene counts) of each sample when considering the n genes with lowest M as a housekeeping gene set. Correlations between consecutive normalization factors increased then decreased when adding the 6th gene with lowest M. This threshold was confirmed by studying the pairwise variation between consecutive NFns. The final housekeeping gene set consisted of the following 5 genes: TUBB, GUSB, SDHA, TBP, ABCF1. Normalization was performed as follows: the scaling factor for a sample was defined as the ratio of the average across all geometric means and the geometric mean of the sample. For each sample, all gene counts were multiplied by the corresponding scaling factor.
Respiratory mechanics and gas exchange
To evaluate the association between the level of angiopoietin-2 as a circulating endothelial and/or angiogenic markers and the pathophysiological process leading to severe respiratory failure (i.e., with the need of invasive mechanical ventilation), we analyzed respiratory measurements among 17 COVID-19 patients that fulfilling Berlin criteria for moderate or severe ARDS. Corresponding data were obtained while patients were ventilated using a high positive end-expiratory pressure (PEEP) strategy, as part of our respiratory bundle in such patients [10]. We present measurements obtained early during the invasive mechanical ventilation (IMV) course in deeply sedated and paralyzed patients. Importantly, no patient suffered from pulmonary embolism at the time of measurements. All patients were ventilated using the CareScape R860 ventilator (GE Healthcare, USA) allowing the following measurements:
Respiratory mechanics: plateau pressure (Pplateau), total PEEP (PEEPtot), driving pressure (DP), respiratory system compliance (Crs), end-expiratory lung volume (EELV) as determined by the nitrogen washing-washout method.
Gas exchanges: PaO2/FiO2 ratio
Statistical analysis
Continuous data were expressed as median [interquartile range: (IQR)] and categorical data as proportion. Patients were compared according to level of care (hospitalization in conventional medical unit or hospitalization directly in ICU). In this univariate analysis, we determined the differences in median using the unpaired t-test (Mann–Whitney U test) for continuous variable and differences in proportions were assessed with the Chi-square test or Fischer exact test if necessary.
We generated receiver operating characteristics (ROC) curves with a regression logistic model that included the angiopoietin-2 to assess the predictability of hospitalization in ICU. We calculated the area under the curve (AUC) and to obtain the optimal cut-off points we used the Youden index method [21]. We used logistic regression to assess the association between the level of angiopoeitin-2 (as a categorical variable dichotomized according to the cutoff of 5000 pg/mL) and the hospitalization in ICU. We performed 4 different logistic models that included Angiopoeitin-2: (i) unadjusted (the angiopoeitin-2 only); (ii) adjusted on the plasma creatinine level; (iii) adjusted on the D-dimers level; and (iv) adjusted on the CRP level. Plasma creatinine, D-dimers and CRP were included in the model as a categorical variable dichotomized according the median. We performed the correlation between routine biomarkers (plasma creatinine, D-dimers and CRP), vascular biomarkers (angiopoietin-2 and sE-selectin) and respiratory measurements (Pplateau, PEEPtot, DP, Crs, EELV). Each correlation was assessed using the Kendall coefficient correlation test.
For whole blood gene expression analysis of 32 patients and 13 healthy donors from Cochin hospital gene, data were imported into nSolver analysis software (version 2.5) for quality checking, then exported as a table, and all subsequent analyses were performed using R version 4.3, using ggplot2 for plots. Background level was computed as mean + 2 standard deviation of the negative control probes, for all samples. We then compared the level of E-selectin normalized RNA count between the 3 groups (control, patients hospitalized in medical conventional wards and patients hospitalized in ICU) using the Kruskal–Wallis test followed by Dunn’s post-test for multiple group comparisons with median reported; *p < 0.05; **p < 0.01; ***p < 0.001.
All analyses were 2-sided and a p value of p < 0.05 was considered statistically significant. Statistical analysis was performed using R studio software (R Development Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
sE-selectin and angiopoietin-2 at entrance in hospital are discriminant biomarkers for direct ICU admission
The study cohort included 40 consecutive patients who presented at the emergency department and were diagnosed positive for COVID-19. They were divided into two groups: patients hospitalized in medical conventional wards (n = 20) and patients directly admitted to the ICU (n = 20). The two populations were strictly comparable in terms of age and time from onset of first symptoms (Table 1). However, COVID-19 patients directly admitted to the ICU had a significant lower SpO2 (p = 0.003) and higher respiratory rate-breath per minutes (p = 0.003). COVID-19 patients admitted in ICU had a more pronounced lymphopenia (p = 0.038, Table 2). Regarding hemostasis, COVID-19 patients admitted in ICU had higher D-dimer levels at entrance (p = 0.008). No difference in PT ratio was observed between groups (Table 2). Fibrin monomers being negative and fibrinogen level elevated, results were not in favor of a COVID-19 associated disseminated intravascular coagulation at admission. Also, COVID-19 patients admitted in ICU had significantly higher CRP level (p = 0.002) and serum creatinine levels (p = 0.001).
Table 1.
Demographic and clinical characteristics of COVID-19 patients at admission according to level of care (medical ward or ICU)
| Medicine patients (n = 20) | ICU patients | p value | |
|---|---|---|---|
| (n = 20) | |||
| Male sex—n (%) | 11 (55) | 17 (85) | 0.084 |
| Age—years*, median [IQR] | 53 [37, 65.4] | 59.5 [54.25, 70.5] | 0.11 |
| BMI—kg/m2* | 26.6 [24.4, 27.6] | 29.1 [23.9, 31.6] | 0.487 |
| Time from illness onset to hospital admission—days* | 5.5 [3, 7] | 6.5 [4, 8.3] | 0.215 |
| CV risk factors, n (%) | |||
| Hypertension | 6 (30) | 10 (50) | 0.333 |
| Dyslipidemia | 3 (15) | 7 (35) | 0.273 |
| Diabetes | 2 (10) | 6 (30) | 0.127 |
| Sedentarity | 0 (0) | 2 (10) | 0.198 |
| Chronic kidney disease | 1 (5) | 2 (10) | 0.99 |
| Medical history, n (%) | |||
| Cancer | 1 (5) | 1 (5) | 0.99 |
| Coronary heart disease | 0 (0) | 2 (10) | NA |
| Stroke | 2 (10) | 1 (5) | 0.99 |
| Treatments, n (%) | |||
| Statins | 3 (15) | 3 (15) | 0.193 |
| Oral antidiabetic agents | 1 (5) | 5 (25) | 0.184 |
| Insulin | 1 (5) | 2 (10) | 0.99 |
| β-blocker | 1 (5) | 2 (10) | 0.99 |
| Calcium channel blockers | 1 (5) | 4 (20) | 0.339 |
| ACEi or ARBs | 3 (15) | 8 (40) | 0.157 |
| ARBs | 2 (10) | 5 (25) | 0.207 |
| Diuretics | 1 (5) | 3 (15) | 0.598 |
| Clinical features, n (%) | |||
| Fever | 20 (100) | 19 (95) | 0.99 |
| Headache | 7 (35) | 12 (60) | 0.205 |
| Cough | 18 (90) | 16 (80) | 0.658 |
| Productive cough | 3 (15) | 2 (10) | 0.99 |
| Dyspnea | 9 (45) | 18 (90) | 0.007 |
| Myalgia | 7 (35) | 8 (40) | 0.99 |
| Diarrhea | 4 (20) | 3 (15) | 0.99 |
| Pneumonia | 16 (80) | 19 (95) | 0.339 |
| SpO2—%a | 95 [91.8, 97] | 90 [87, 92.3] | 0.003 |
| Respiratory rate—Breathes per min* | 18 [16, 20] | 22 [21, 38] | 0.003 |
| Pulse—Beats per min* | 92 [81.5, 112.5] | 95 [83.5, 106] | 0.953 |
ICU intensive care unit, BMI body mass index, CV cardiovascular, ACEi angiotensin conversion enzyme inhibitor, ARB-2 antagonist of angiotensin 2 receptor blocker, SpO2 pulse oximetric saturation, ARDS acute respiratory distress syndrome, IQR interquartile range, CRP C-reactive protein
*Variable expressed as median [IQR]
Table 2.
Biological characteristics of COVID-19 patients at admission according to level of care (medical ward or ICU direct admission)
| Medicine patients (n = 20) | ICU patients (n = 20) | p value | |
|---|---|---|---|
| Biological parameters, median [IQR] | |||
| White blood cells—× 109 per L | 6.15 [4.30, 7.45] | 7.20 [4.75, 11.25] | 0.267 |
| Hemoglobin—g/L | 143 [127.8, 152.5] | 128 [120.5, 139] | 0.066 |
| Platelet count—× 109 per L | 205.5 [151.3, 238] | 165 [135, 217] | 0.361 |
| Polynuclear neutrophils—× 109 per L | 4.43 [3.10, 5.53] | 4.38 [3.58, 10.48] | 0.187 |
| Lymphocytes—× 109 per L | 0.95 [0.80, 1.35] | 0.68 [0.46, 1.10] | 0.038 |
| Monocytes—× 109 per L | 0.38 [0.29, 0.55] | 0.33 [0.19, 0.62] | 0.391 |
| CRP—mg/L | 76.8 [31.9, 101.2] | 146 [101.3, 204.8] | 0.002 |
| Plasma creatinine—µmol/L | 67 [61.3, 83.5] | 115 [76.5, 215.5] | 0.001 |
| PT ratio | 0.96 [0.94, 0.10] | 0.88 [0.81, 12] | 0.132 |
| Fibrinogen—g/L | 5.3 [4.6, 5.9] | 6.3 [5.4, 6.7] | 0.019 |
| D-dimers ≥ 1000 ng/mL—n (%) | 6 (30) | 12 (60) | 0.069 |
| D-dimers—ng/mL | 732 [512, 1063] | 1128 [885, 2151] | 0.008 |
| Fibrin monomers—µg/mL | < 7 [< 7, < 7] | < 7 [< 7, < 7] | 0.270 |
| Angiogenic parameters (pg/mL), median [IQR] | |||
| Angiopoietin-1 | 4139.4 [3042.2, 8755.5] | 5083.2 [2928.5, 7349.9] | 0.935 |
| Soluble endoglin | 1958.6 [1357.5, 2421.6] | 1934.9 [1568.8, 2255.1] | 0.925 |
| Leptin | 8531.4 [5059.2, 19,938.5] | 11,400.7 [5724.1, 25,004.7] | 0.552 |
| Soluble E-selectin | 37,930.5 [22227.1, 45,844.3] | 52,937.6 [35649, 71964] | 0.009 |
| VEGF-A | 23.6 [17.5, 34] | 24.5 [16.9, 34.5] | 0.839 |
| Angiopoietin-2 | 4385.7 [3185.9, 5667.1] | 6574.1 [5354.4, 11,375.9] | 0.003 |
| c-Kit | 12,809.7 [10535.3, 22,153.7] | 10,176.4 [6353.1, 22,024.2] | 0.218 |
| FGFb | 48.9 [34.2, 58.9] | 45 [34.8, 63.7] | 0.935 |
| PlGF | 42 [36.4, 47.6] | 43.7 [35.2, 53.4] | 0.482 |
| sVEGFR-2 | 15,153.2 [11619.2, 16,659.6] | 13,097.5 [10482.7, 17926] | 0.646 |
ICU intensive care unit
*Variable expressed as median [IQR]
We then analyzed plasma levels of vascular cytokines. In the ICU group, sE-selectin and angiopoietin-2 levels were significantly increased (p value at 0.009 and 0.003, respectively) (Table 1), while other biomarkers were comparable between groups (Table 1). Interestingly, sE-selectin and angiopoietin-2 levels were correlated (r = 0.25, p = 0.004) (Fig. 1).
Fig. 1.
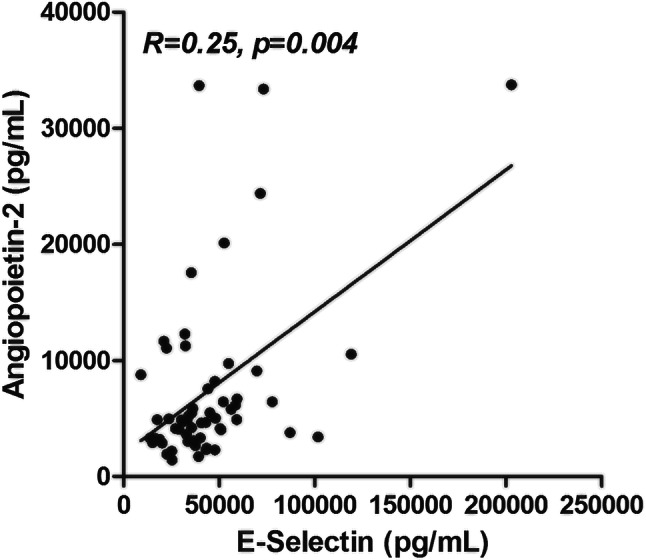
Correlations between plasma angiopoietin-2 and sE-selectin levels in COVID-19 patients. R for Kendall rank correlation coefficient
To confirm at gene level the increase of circulating endothelial biomarkers, we analyzed an independent COVID-19 cohort of 32 patients with various disease severity (21 patients in Medicine and 11 patients in ICU), for which whole blood gene expression analysis was performed [20]. Critically ill ICU COVID-19 patients had a significant increased expression of E-selectin with a grade-dependent manner (Fig. 2, p = 0.007).
Fig. 2.
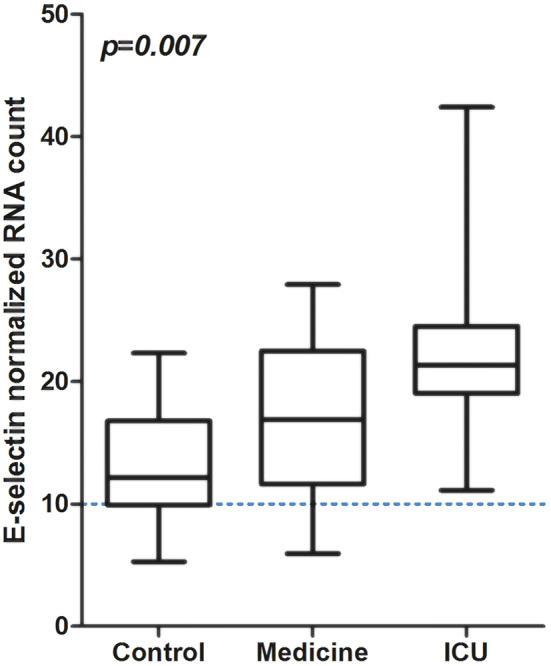
E-selectin gene expression profile according to admission of patients in medical ward or ICU versus controls. ICU for intensive care unit; RNA for Ribonucleic Acid; Difference between groups evaluated with Kruskal–Wallis test
Angiopoietin-2 level at admission is the most relevant factor to predict transfer to ICU
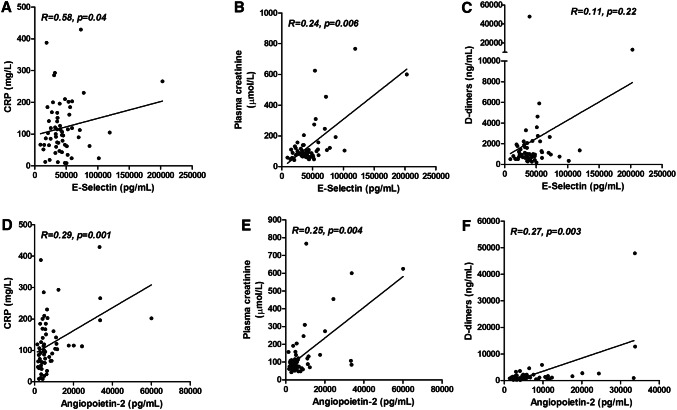
In the thrombo-inflammation context of COVID-19, and since D-dimers, CRP and creatinine at admission were associated to transfer to ICU; we analyzed the association of sE-selectin and angiopoietin-2 with these three markers. While a significant association was found between sE-selectin and both CRP and creatinine (respectively with p value at 0.04 and 0.006), no association existed with D-dimers (p value at 0.22) (Fig. 3a, b, c). In contrast, angiopoietin-2 levels were significantly associated with CRP, creatinine and D-dimers (respectively with p value at 0.001, 0.001 and 0.003) (Fig. 3d, e, f).
Fig. 3.
Correlations between E-selectin and angiopoietin-2 and biological parameters of thrombo-inflammation. a–c correlations between sE-selectin and CRP, plasma creatinine and D-dimers. d–f: correlations between angiopoietin-2 and CRP, plasma creatinine and D-dimers. R for Kendall rank correlation coefficient
ROC curve analysis was performed to define the optimal cut-off of angiopoietin-2 level to predict admission to ICU (Fig. 4). We identified angiopoietin-2 above 5000 pg/mL as potential inclusion criteria for ICU entrance in COVID-19 patients (AUC 77.2, 95% CI 62.4–91.9). Using this cut-off, ROC curve yielded a sensitivity of 80.1% (95% CI 55–93), a specificity of 70% (95% CI 45–87), a positive predictive value of 72.7% (95% CI 49–88) and a negative predictive value of 77.7% (95% CI 51%–92%) (Fig. 4). Table 3 confirms the link between angiopoietin-2 cut-off at 5000 pg/mL and ICU outcome using a logistic regression model (OR 9.33, 95% CI 2.35–44.91, p = 0.003). Strikingly, when adjusted to D-dimers, CRP or creatinine, the adjusted OR remained significant (p value at 0.009, 0.01, and 0.04, respectively).
Fig. 4.
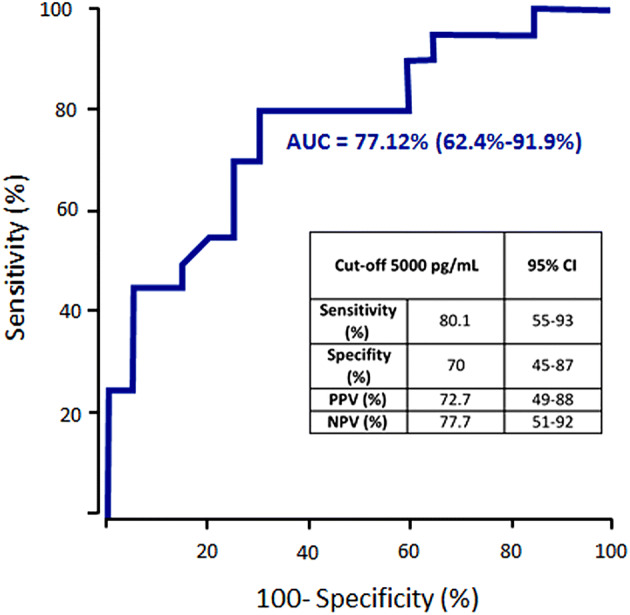
ROC curve for angiopoietin-2 cut-off in direct ICU admission. Angiopoietin-2 level above 5000 pg/mL was identified using Youden index method as a potential criteria for COVID-19 transfer in ICU (AUC 77.12, 95% CI 62.4–91.9). AUC for area under the curve; CI for confidence interval; PPV for positive predictive value; NPV for negative predictive value. R for Kendall rank correlation coefficient; CRP for C-reactive protein
Table 3.
Logistic regression model evaluating angiopoietin-2 cut-off at > 5000 pg/mL for ICU outcome in COVID-19 patients
| Logistic regression model | OR univariable (95% CI) | OR multivariable (95% CI) | |
|---|---|---|---|
| 1 | Angiopoietin-2 > 5000 pg/mL | 9.33 (2.35–44.91, p = 0.003) | – |
| 2 | Adjusted on creatinine | 5.18 (1.08–29.29, p = 0.04) | |
| 3 | Adjusted on CRP | 6.96 (1.57–36.13, p = 0.01) | |
| 4 | Adjusted on D-dimers | 7.34 (1.77–36.20, p = 0.009) |
We performed 4 separated logistic models: (1) unadjusted (Angiopoietin-2 level only); (2) adjusted on the plasma creatinine level; (3) adjusted on the D-dimer level; and (4) adjusted on the CRP level
Plasma creatinine, D-dimers and CRP were included in the model as a categorical variables dichotomized according the median: OR for Odds Ratio; CRP for C-reactive protein
High level of angiopoietin-2 at hospital entrance is correlated to decreased respiratory system compliance
To confirm the relevance of angiopoietin-2 levels with the clinical course and disease severity of COVID-19 patients, we explored the relationship between angiopoietin-2 levels at admission and respiratory mechanic and gas exchange values in 17 consecutive patients admitted to the ICU and requiring IMV. Strikingly, a significant negative correlation was observed between angiopoietin-2 level and Crs (mL/cmH2O, r = − 0.46, p = 0.01), while no significant correlations were found with other parameters (Table 4).
Table 4.
Correlation between angiopoietin-2 level and respiratory mechanic and gas exchange measurements in 17 COVID-19 ARDS patients
| Correlation coefficient with angiopoietin-2 level | p value | ||
|---|---|---|---|
| Respiratory mechanics, median [IQR] | |||
| Pplateau (cmH2O) | 27 [25, 28] | 0.26 | 0.16 |
| PEEPtot (cmH2O) | 16 [16, 18] | 0.01 | 0.99 |
| DP (cmH2O) | 10 [9, 12] | 0.27 | 0.15 |
| Crs (mL/cmH2O) | 39 [33, 42.50] | − 0.46 | 0.01 |
| EELV (mL) | 2101 [1790, 2447] | − 0.27 | 0.14 |
| Gas exchanges, median [IQR] | |||
| PaO2/FiO2 | 185 [168, 284] | − 0.05 | 0.77 |
| Angiopoietin-2 pg/mL (median [IQR]) | 9743.54 [5787.62, 12,289.77] | – | – |
The correlations between variables were assessed using the Kendall rank correlation test
Discussion
In this prospective study, we hypothesized that SARS-CoV-2 infection could induce endothelial dysfunction at the origin or associated to microvascular pulmonary damage. We provided evidence that levels of soluble endothelial markers of activation, i.e., sE-selectin and angiopoietin-2 were increased in critically ill COVID-19 patients. Angiopoietin-2 was the best biomarker to predict transfer to the ICU and was associated with poor lung compliance in COVID-19 patients.
Endothelial dysfunction and/or impaired angiogenesis may contribute to microvascular dysfunction in all organs. Markers of endothelial dysfunction and angiogenesis were shown to be predictors of disease severity in heart failure [22], in renal failure [23] and after cardiac surgery [24]. Circulating biomarkers of endothelial activation and/or injury can be both the cause and/or the consequence of microvascular dysfunction. Integrity of endothelial cells allows providing an antithrombotic environment that is reversed upon the burst of inflammation related to IL-6 in COVID-19 patients. Therefore, this endothelial thrombo-protective barrier is disrupted, probably leading to the coagulopathy largely described in COVID-19 patients, along with a highly increased level of D-dimers related to in-hospital mortality [1, 2]. The thrombo-inflammation and impaired immune response associated with endothelial dysfunction can be reflected by circulating markers in pre-eclampsia or in cancer associated thrombosis. Indeed, for example in pre-eclampsia, the soluble endoglin or placental growth factor are now well recognized biomarkers of renal or endothelial damage, but also of oxidative stress secondary to the pathophysiological changes that precede the clinical onset of pre-eclampsia [25].
In COVID-19, the hypothesis of kidney microthrombosis was proposed since the elevation of serum creatinine was associated with higher levels of D-dimers (> 500 ng/mL) [26]. The SARS-CoV-2 receptor (ACE2) is strongly expressed in endothelial cells [10]. Infection of endothelial cells could therefore induce endothelial lesions triggering massive activation of coagulation and diffuse microthrombotic process impairing renal function and respiratory mechanics and gas exchanges. To explore this hypothesis, we quantified a panel of soluble markers exploring the endothelial dysfunction and angiogenesis and found increased levels of E-selectin and angiopoietin-2 in COVID-19 patients directly admitted to the ICU. These two markers are both increased in the context of inflammation and/or activation of endothelial cells. Indeed, E-selectin (CD62E) is a leukocyte adhesion molecule expressed on activated endothelial cells. Endothelial cells from normal skin and bone marrow [27] or in infantile hemangioma [28] constitutively express E-selectin, contrasting with endothelial cells from other tissues that do not constitutively express it. However, its expression is strongly upregulated by inflammatory cytokines [29]. Soluble form of E-selectin is released during inflammation and has been widely proposed as a biomarker of endothelial dysfunction, in particular in sepsis [30]. Interestingly, targeting E-selectin is currently evaluated for the treatment of venous thrombosis and associated inflammatory events [14]. Thus, increased circulating sE-selectin together with increased mRNA confirm the endothelial dysfunction during COVID-19 disease and its relationship with severity. mRNA E-Selectin expression was indeed quantified within a huge panel of genes detected with Nanostring, mainly focusing on "immunology genes" [20]. E-selectin and Angiopoietin-2 are both endothelial related molecules, respectively, present after endothelial activation on cell membrane for E-Selectin and in secreted molecule from the Weibel palade bodies for angiopoietin-2. However, as E-selectin was the only endothelial/angiogenic gene available in this panel, it was therefore used in the present work to confirm our protein data at the gene expression level. Importantly, results of mRNA E-selectin used in the present work allowed replicating the relevance of circulating endothelial proteins in an independent COVID-19 cohort and at the gene expression level. Recent published data on endotheliitis are in line with our results [8]. Our results are, to our knowledge, the first proof of increased circulating markers of endothelial lesion in COVID-19 infection associated with severity.
Angiopoietin-2 is an angiogenesis regulator stored in Weibel Palade bodies, the endothelial storage organelles that contain multiple proteins, including von Willebrand factor. It can be rapidly released by activated endothelium upon thrombin or inflammatory cytokines. Angiopoietin-2 is part of the angiopoietin/tie-2 pathway, a crucial system regulating endothelial homeostasis, angiogenesis [31] and proliferation [32]. Angiopoietin-2 participates to the responsiveness of endothelium to inflammatory, hyperpermeability, apoptosis and vasoreactive stimuli but also induces inflammation and vascular hyperpermeability [31]. Circulating angiopoietin-2 levels have been found increased in patients with sepsis or ARDS [31] and predictive of mortality in ARDS patients [33]. In the present work, angiopoietin-2 levels were significantly higher in patients admitted to the ICU by comparison to those admitted to conventional wards, confirming the importance of angiopoietin-2 pathway in endothelial inflammation. Angiopoietin-2 levels were associated to CRP and D-dimers, the latter reflecting the link between the Covid-19 coagulopathy and endothelial dysfunction. Since, angiopoietin-2 could reflect coagulopathy/inflammation and endothelial dysfunction; we explored its association with respiratory parameters in 17 IMV ICU COVID-19 patients. We found an association with Crs quantified early during the course of protective mechanical ventilation. As Crs could be a marker of the extent of the COVID-19 pulmonary insult, in link with alveolar damages, one can suspect than the pulmonary endothelial insult could be in parallel a co-marker of pulmonary disease severity. Thus, these results strongly support the role of diffuse microcirculatory dysfunction in critically ill COVID-19 patients, in addition to alveolar insult.
In conclusion, our data strongly support the relevance of levels of biomarker of endothelial dysfunction in COVID-19 patients at hospital admission, since vascular disorders and thrombosis seem to be a crucial point. Further prospective studies should evaluate the role of angiopoietin-2 to predict admittance in ICU but also to evaluate its prognostic value during the follow-up of patients and its relevance in respiratory and thrombotic related disorders. Such data could support the use of vascular protecting drugs or anticoagulation in the early phase of the disease to prevent respiratory and renal micro thrombotic manifestations during COVID-19 evolution.
Acknowledgements
We would like to acknowledge all nurses, technicians and physicians involved in the Vascular medicine, Internal medicine, Respiratory medicine, Intensive care, Clinical investigation center and Hematology departments of the George Pompidou European Hospital and Cochin Hospital for their help in taking care of patients and including them in the study. We thank AP-HP for promotion of the SARCODO Project. We thank the unit of clinical research URC HEGP CIC-EC1418 (Natacha Nohile, Pauline Jouany and Dr Juliette Djadi-Prat) and Helene Cart-Grandjean from AP-HP for their involvement in SARCODO project.
Author contributions
DMS, TM and JLD conceived and supervised the study. CG, GG, LK, NG, RC and BD analyzed the data. RC analyzed the data and supervised statistical analysis. All authors' interpreted data, drafted and revised the manuscript, and approved the final version.
Funding
This work has been funded with grants from French national agency for research ANR SARCODO and from "Fonds IMMUNOV, pour l’Innovation en Immunopathologie".
Compliance with ethical standards
Conflict of interest
All authors declare nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Li D, Wang X, Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 4.Fox S, Akmatbekov A, Harbert J, Li G, Brown G, Vander Heide R. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Liu X, Xu Y, Xu Z, Huang Y, Chen S, et al. Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19 associated ARDS. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0373LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qanadli SD, Beigelman-Aubry C, Rotzinger DC. Vascular changes detected with thoracic CT in coronavirus disease (COVID-19) might be significant determinants for accurate diagnosis and optimal patient management. AJR Am J Roentgenol. 2020;7:W1. doi: 10.2214/AJR.20.23185. [DOI] [PubMed] [Google Scholar]
- 10.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 11.Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessandro E, Becker C, Bergmeier W, Bode C, Bourne JH, Brown H, et al. Thrombo-inflammation in cardiovascular disease: an expert consensus document from the third Maastricht consensus conference on thrombosis. Thromb Haemost. 2020;120(4):538–564. doi: 10.1055/s-0040-1708035. [DOI] [PubMed] [Google Scholar]
- 13.Rossi E, Pericacho M, Bachelot-Loza C, Pidard D, Gaussem P, Poirault-Chassac S, et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell Mol Life Sci. 2018;75(7):1269–1284. doi: 10.1007/s00018-017-2694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culmer DL, Dunbar ML, Hawley AE, Sood S, Sigler RE, Henke PK, et al. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb Haemost. 2017;117(6):1171–1181. doi: 10.1160/TH16-04-0323. [DOI] [PubMed] [Google Scholar]
- 15.Statz S, Sabal G, Walborn A, Williams M, Hoppensteadt D, Mosier M, et al. Angiopoietin 2 levels in the risk stratification and mortality outcome prediction of sepsis-associated coagulopathy. Clin Appl Thromb Hemost. 2018;24(8):1223–1233. doi: 10.1177/1076029618786029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhanesha N, Prakash P, Doddapattar P, Khanna I, Pollpeter MJ, Nayak MK, et al. Endothelial cell-derived von willebrand factor is the major determinant that mediates von willebrand factor-dependent acute ischemic stroke by promoting postischemic thrombo-inflammation. Arterioscler Thromb Vasc Biol. 2016;36(9):1829–1837. doi: 10.1161/ATVBAHA.116.307660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58(11):1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 19.Latremouille C, Carpentier A, Leprince P, Roussel JC, Cholley B, Boissier E, et al. A bioprosthetic total artificial heart for end-stage heart failure: results from a pilot study. J Heart Lung Transpl. 2018;37(1):33–37. doi: 10.1016/j.healun.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Pere H et al (2020) Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. medRxiv. 10.1101/2020.04.19.20068015 [DOI] [PMC free article] [PubMed]
- 21.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med. 2013;4(2):627–635. [PMC free article] [PubMed] [Google Scholar]
- 22.Bank AJ, Lee PC, Kubo SH. Endothelial dysfunction in patients with heart failure: relationship to disease severity. J Card Fail. 2000;6(1):29–36. doi: 10.1016/S1071-9164(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CE, Hamm LL, Batuman G, Kumbala DR, Chen CS, Kallu SG, et al. The association of angiogenic factors and chronic kidney disease. BMC Nephrol. 2018;19(1):117. doi: 10.1186/s12882-018-0909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour SG, Zhang WR, Moledina DG, Coca SG, Jia Y, Thiessen-Philbrook H, et al. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am J Kidney Dis. 2019;74(1):36–46. doi: 10.1053/j.ajkd.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamshirsaz AA, Paidas M, Krikun G. Preeclampsia, hypoxia, thrombosis, and inflammation. J Pregnancy. 2012;2012:374047. doi: 10.1155/2012/374047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Luo R, Wang K, Zhang M, Wang M, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva M, Videira PA, Sackstein R. E-selectin ligands in the human mononuclear phagocyte system: implications for infection, inflammation, and immunotherapy. Front Immunol. 2018;8:1878. doi: 10.3389/fimmu.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smadja DM, Mulliken JB, Bischoff J. E-selectin mediates stem cell adhesion and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2012;181(6):2239–2247. doi: 10.1016/j.ajpath.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balta S. Endothelial dysfunction and inflammatory markers of vascular disease. Curr Vasc Pharmacol. 2020 doi: 10.2174/1570161118666200421142542. [DOI] [PubMed] [Google Scholar]
- 30.Cummings CJ, Sessler CN, Beall LD, Fisher BJ, Best AM, Fowler AA. 3rd. Soluble E-selectin levels in sepsis and critical illness. Correlation with infection and hemodynamic dysfunction. Am J Respir Crit Care Med. 1997;156(2 Pt 1):431–437. doi: 10.1164/ajrccm.156.2.9509017. [DOI] [PubMed] [Google Scholar]
- 31.Nicolini G, Forini F, Kusmic C, Iervasi G, Balzan S. Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci. 2019;15(239):117080. doi: 10.1016/j.lfs.2019.117080. [DOI] [PubMed] [Google Scholar]
- 32.Smadja DM, Laurendeau I, Avignon C, Vidaud M, Aiach M, Gaussem P. The angiopoietin pathway is modulated by PAR-1 activation on human endothelial progenitor cells. J Thromb Haemost. 2006;4(9):2051–2058. doi: 10.1111/j.1538-7836.2006.02101.x. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Yin R, Guo Q. Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies. Ther Adv Respir Dis. 2020;14:1753466620905274. doi: 10.1177/1753466620905274. [DOI] [PMC free article] [PubMed] [Google Scholar]