Abstract
Meroterpenoids are natural products of hybrid biosynthetic origins – derived from both terpenoid and polyketide pathways – with a wealth of biological activities. Given their therapeutic potential, a general strategy to access these natural products in a concise and divergent fashion is highly desirable. Here, we report a modular synthesis of a suite of oxidized meroterpenoids using a hybrid synthetic strategy that is designed to harness the power of both biocatalytic and radical-based retrosynthetic logic. This strategy enables direct introduction of key hydroxyl groups and rapid construction of key bonds and stereocenters, facilitating the development of a concise route (7–12 steps from commercial materials) to eight oxidized meroterpenoids from two common molecular scaffolds. This work lays the foundation for rapid access to a wide range of oxidized meroterpenoids through the use of similar hybrid strategy that combines two synthetic approaches.
Multistep chemical synthesis relies almost exclusively on the use of retrosynthetic analysis.1 Using this conceptual framework, a target molecule is disconnected through a series of reverse reactions to arrive finally at greatly simplified or commercial starting materials. As each disconnection needs to be sensible in the forward synthetic direction, the choice of bond(s) to disconnect is highly dependent on the contemporary synthetic transformations available at the practitioner’s disposal. Throughout the history of organic chemistry, polar disconnections, due to their perceived robustness, permeate much of the discourse in the field. More recently, the emergence of new technologies in chemical synthesis has led to the formulation of alternative retrosynthetic strategies. Biocatalytic retrosynthesis2 has flourished into a highly powerful principle for multistep syntheses due to the unparalleled selectivity of enzymatic transformations and the ever-growing tools of protein engineering and directed evolution.3 Similarly, radical-based retrosynthetic disconnections4,5 have become increasingly popular, owing to the unique chemoselectivity and chemofidelity6 of radicals. Despite their respective advantages, these emerging strategies have largely developed as independent entities with minimal crosstalk. Here, we describe the development of a powerful strategy to streamline access to meroterpenoid natural products (eight total) by combining the unparalleled site-selectivity of enzymatic hydroxylation with the unique reactivity profile of radical-based transformations. The efficiency of our route highlights the benefits of employing such a hybrid synthetic strategy in the synthesis of complex molecules.
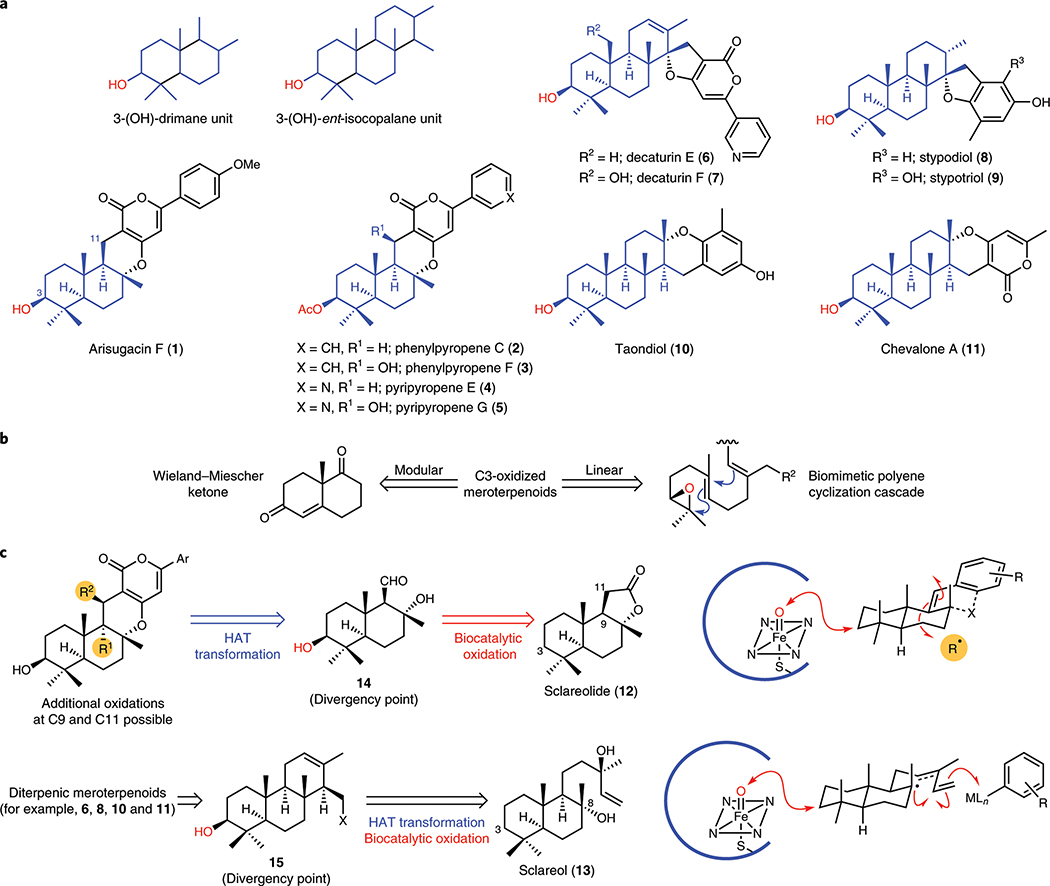
Commonly isolated from fungi, meroterpenoids are a large family of hybrid terpene natural products possessing a wide range of structural diversity (Fig. 1A).7 The α-pyrone meroterpenoids (e.g., 1–5) constitute a prominent subset of this family and possess a common C3-oxidised drimane unit that is attached to various polyketide-derived pyrone fragments at C11.8 Members of this family exhibit a broad range of bioactivity ranging from anti-cholinesterase activity to acyl-CoA/cholesterol acyltransferase (ACAT) inhibition. While not as common, meroterpenoids bearing a diterpene unit have also been found in nature.9 Members of this subset (e.g., 6–11) typically share a common C3-oxidised ent-isocopalane fragment that occurs in combination with various aromatics and have been known to exhibit anti-mycobacterial, insecticidal, and cytotoxic properties. Notably, the C3-oxidised ent-isocopalane fragment is also highly prevalent in many higher-order terpenes. Despite their structural diversity, most C3-oxidised meroterpenoids share a common biosynthetic logic10: they arise in nature from the union of polyisoprene pyrophosphate with a polyketide-derived aromatic unit, followed by enantioselective epoxidation and polyene cyclisation cascade.
Figure 1. Combining chemoenzymatic and radical-based retrosynthetic logic for collective synthetic access to oxidised meroterpenoids.
a. C3-oxidised meroterpenoids share a common 3-(OH)-drimane or 3-(OH)-ent-isocopalane unit. These ubiquitous decalin motifs are found in over 100 terpenoids. b. Prior synthetic strategies employed to access C3-oxidised meroterpenoids typically rely on inefficient manipulation of Wieland–Miescher ketone or a biomimetic polyene cyclisation cascade. These 2 electron-based strategies are tactically indirect, involve inefficient functional group manipulations, and result in sub-optimal chemo- and diastereoselectivity. c. In our synthesis design, the α-pyrone meroterpenoids were envisioned to arise from a range of radical-based transformations to install the requisite functional groups at C9 and C11. Intermediate 14 would be prepared via biocatalytic oxidation at C3 of sclareolide (12). Conversely, the diterpenic meroterpenoids could be accessed from a common intermediate 15, which would be synthesised from sclareol (13) via biocatalytic oxidation at C3 and radical-based transformation(s) to forge the C ring, including radical-based cross coupling. Overall, the unique reactivity profile of radicals (chemoselectivity and chemofidelity), and site-selectivity of enzymatic oxidation will allow a more direct access to a wide range of meroterpenoids. Key disconnections for pyrone meroterpenoids (above) and diterpenic meroterpenoids (below) are shown on the right. HAT, hydrogen atom transfer.
Two general synthetic approaches have been developed to stereoselectively access C3-oxidised meroterpenoids. The first aims to recapitulate the biosynthetic logic through the use of enantioselective dihydroxylation11 on a polyisoprenyl unit (e.g., farnesyl or geranylgeranyl), followed by epoxide formation, and Lewis acid-catalysed polyene cyclisation. This approach is linear in nature, and is often beset by slow rates of dihydroxylation, variable levels of regio- and enantioselectivity, and varying degrees of success in the biomimetic cyclisation step. For example, asymmetric dihydroxylation of farnesol derivatives is typically complicated by unwanted reaction at the internal double bond,12 and polyene cyclisation towards taondiol was reported to afford only 2% yield of the desired product.13 The dearth of efficient methods for introducing additional oxygenation on the polyisoprenyl unit further hinders the synthetic versatility of this approach for accessing more oxidised meroterpenoids. An alternative strategy commencing from Wieland–Miescher ketone has also been developed. However, this approach is strategically inefficient as it requires multiple tailoring steps on the decalin skeleton and nontrivial C–C bond formation steps for subsequent ring construction.14 For example, the use of organolithium addition to link the terpene and arene fragments of stypodiol resulted in a diastereomeric mixture of products,15 and the use of Robinson annulation to form the tricyclic core of the decaturins was plagued by unexpected racemization.16 These shortcomings provide a strong incentive for the development of an alternative synthetic strategy.
We envisioned a synthetic approach that is based on a site-selective oxidation of a simple drimane or isocopalane-like framework (Fig. 1B). Such unit is readily available in the form of sclareolide (12) and sclareol (13), common feedstocks in the perfume industry that can be accrued in kilogram quantities. With respect to α-pyrone meroterpenoids, a C3-selective hydroxylation of sclareolide will furnish the minimally-oxidised A/B ring of 1–5. In turn, the lactone moiety could be readily manipulated to provide advanced intermediate 14 that would allow maximal divergency to access a wide range of α-pyrone meroterpenoids. A similar strategy for the synthesis of quinone sesquiterpenoids has previously been conceived through the intermediacy of boronosclareolide.17 However, this approach suffers from the lack of ability to introduce additional oxygenations at C3, C9, and C11. To address these limitations, we envisioned the conversion of the aldehyde moiety of 14, to the corresponding olefin, which would offer versatile installation18 of various functionalities at C9 and C11. While this transform would lead to a retron containing multiple olefins, we reasoned that the abundance of options for olefin functionalization would allow for the identification of a suitable method with the desired chemoselectivity.
Similarly, a C3-selective hydroxylation of sclareol will generate a synthetic intermediate that not only possesses the correct oxidation state at C3, but also the appropriate functional handles for the construction of the remaining rings of 6–11. In the forward sense, a C–C bond formation event would afford advanced intermediate 15, which acts as a divergency point to access a wide range of diterpenic meroterpenoids. It is worth noting that related tricyclic motifs have previously been constructed from 13. However, work in this area has been dominated by two electron-based transformations. For example, the only total synthesis of makassaric acid proceeded from 13 and enlisted the use of organolithium addition to an aldehyde, followed by Barton deoxygenation.19 Adaptation of such transformation onto routes that feature 3-hydroxysclareol would necessitate undesired protecting group manipulations and functional group interconversions. In contrast, we believe that radical-based transformations, with their unique reactivity profile, would allow us to bypass such concession steps.
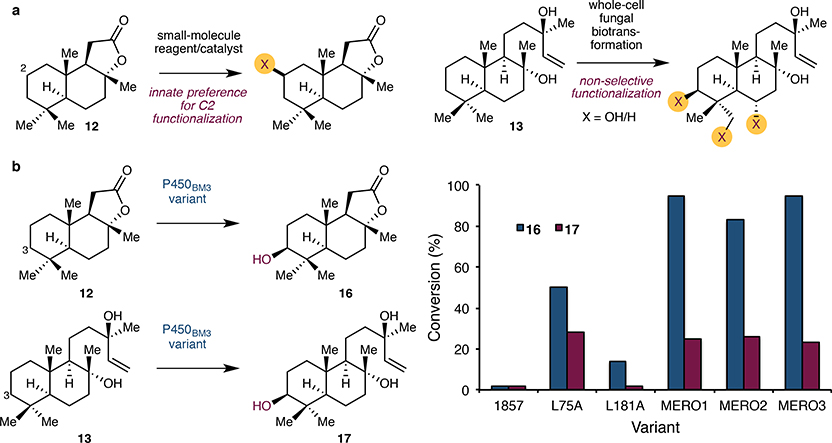
From a medicinal chemistry perspective, this overall strategy provides an ideal synthetic blueprint to target a wide spectrum of meroterpenoids from just two common intermediates. Nevertheless, the realization of this strategy is not without its challenges. Numerous chemical methods have been reported for selective C–H functionalisation of 12.20,21,22,23 However, they rely on the innate reactivity of the carbocyclic scaffold and result predominantly in C2 modification. While whole-cell fungal biotransformation of 12 has been reported to give C3-oxidised product, it is typically accompanied by over-oxidation to the ketone product and oxidation at other positions.24 At the outset of our work, C3-selective enzymatic hydroxylation of 12 has been reported to take place with a P450BM3 variant25 II-H8 (15 mutations from wild type), or CYP101B1, a P450 monooxygenase from N. aromaticivorans DSM12444.26 However, these bioconversions have only been carried out on up to 50 mg scale under high dilution conditions (1 mM substrate concentration with ca. 1000 total turnover number (TTN)), and their scalability remains to be addressed. Additionally, oxidation of sclareol has only been attempted with whole-cell fungal biotransformation and was reported to result in non-selective oxidation.24,27
Results and discussion
We began by conducting a brief survey of several P450BM3 variants harbouring similar mutations to II-H8 for the hydroxylation of sclareolide and sclareol (Fig. 2). With an eye towards practical and inexpensive hydroxylation method, small-scale screening of these variants was performed with lysate of E. coli cells expressing both the P450BM3 variant and a thermostabilised phosphate dehydrogenase (Opt13) for NADPH recycling.28 Here, we chose to employ pET22b(+)- and pRSF-based vectors for overexpression of the P450BM3 variant and Opt13 respectively, as they possess compatible origins of replication and allow the desired genes to be expressed under the control of strong T7lac promoter. Alanine scanning29 on variant 1857 (four amino acid substitutions from II-H8) revealed several mutants with varying levels of hydroxylation activity on 12. Gratifyingly, variant 1857 V328A (BM3 MERO1) afforded >95% conversion to 3-(OH)-sclareolide (corresponding to ca. 5000 TTN), with no detectable presence of the undesired C2-hydroxylated product. Mutations T235A and R471A, previously reported to enhance the organic solvent tolerance of P450BM3,30 were introduced in an attempt to perform the biocatalytic oxidation at higher substrate concentration and amount of organic cosolvent. Unfortunately, this variant (BM3 MERO2) proved to be inferior to BM3 MERO1 for the hydroxylation of 12. Reversion of thermostabilising mutations C47R and I94K29 resulted in variant BM3 MERO3, which showed comparable conversion to BM3 MERO1 on both small-scale and preparative-scale reactions, suggesting that the thermostabilising mutations are not necessary for achieving high hydroxylation activity on 12. The aforementioned variants were also tested for their ability to hydroxylate sclareol, revealing several variants with modest hydroxylation activity (ca. 30% conversion). Importantly, all variants examined proved selective for C3 hydroxylation and provided initial validation for our hybrid strategy in the synthesis of diterpenic meroterpenoids.
Figure 2. Optimisation of P450BM3 variants for practical and selective C3 hydroxylation of 12 and 13.
a. Chemical methods reported for C–H functionalisation of sclareolide (12) have an innate preference for C2 functionalisation20–23 while whole-cell fungal biotransformation of sclareol (13) has been reported to form regioisomeric mixture of hydroxylated products. b. Alanine scanning on variant 1857 led to the identification of several variants with improved hydroxylation activities on 12 and 13. Among the variants tested, variants BM3 MERO1 and BM3 MERO3 were identified as the optimal variants for the hydroxylation of 12 and 13. The identities of the latter variants are as follows: BM3 MERO1 = 1857 V328A, BM3 MERO2 = 1857 T235A V328A R471A, BM3 MERO3 = 1857 C47R I94K V328A. Reaction conditions for terpene hydroxylation: 12 or 13 (5.0 mM), NADP+ (1.0 mM), NaHPO3 (100 mM), clarified lysate of E. coli BL21(DE3) expressing the appropriate P450BM3 variant and Opt13 (suspension in 50 mM pH 8.0 kPi, pre-lysis OD600 = 15), 20 h at 20 ºC.
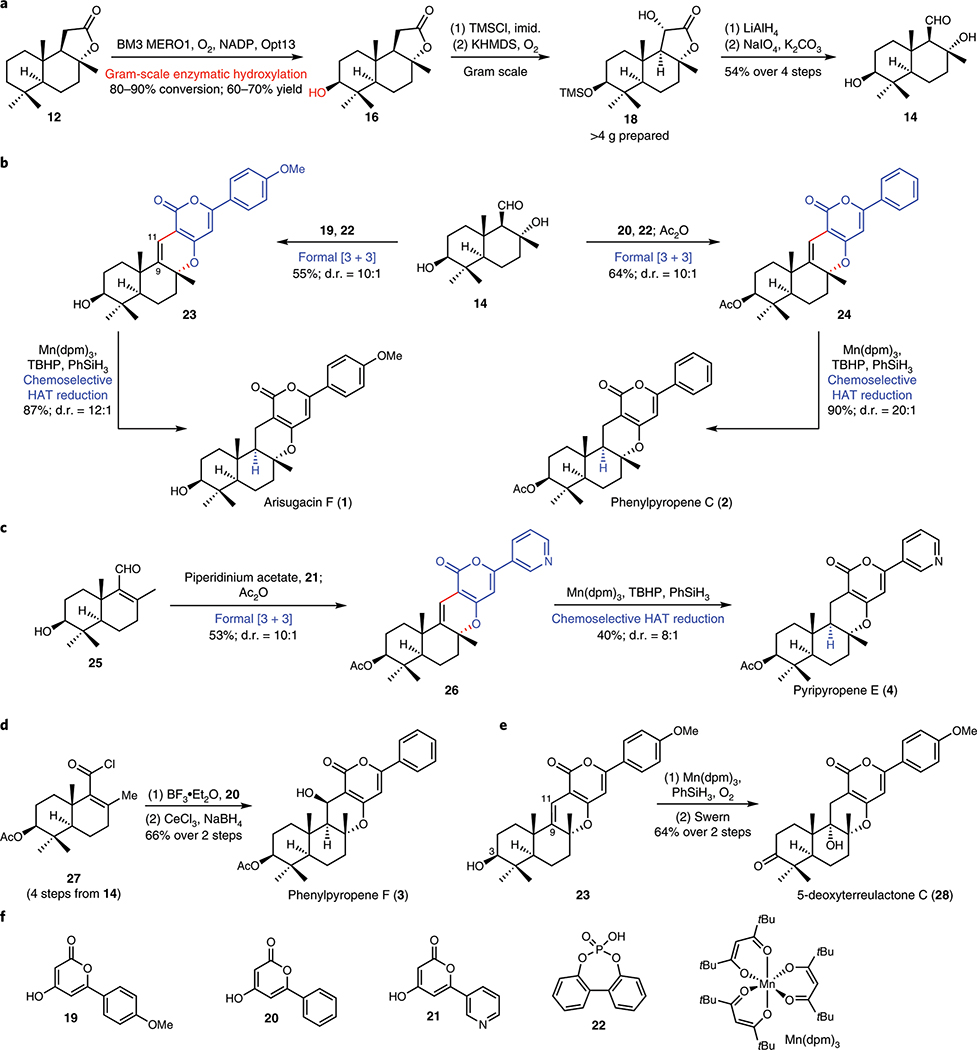
With optimized P450BM3 variants for sclareolide hydroxylation in hand, we sought to establish a robust route to prepare a suitable precursor for fragment coupling with the pyrone unit (Fig. 3A). Enzymatic hydroxylation of sclareolide could be routinely conducted on gram scale with BM3 MERO1 with excellent conversion and isolated yield. The supplementation of Opt13 allowed for substoichiometric use of NADPH, lowering the overall cost of conducting large-scale reactions. As a testament to the scalability of the biotransformation, lactone 18 has been prepared in > 4g quantity to date. Intermediate 14 could be quickly accessed through tailoring of the C-ring lactone in 2 steps from 18, setting the stage for the key coupling with pyrones 19–21. In contrast, preparation of 14 from Wieland-Miescher ketone is projected to require at least 8 steps, highlighting the tactical directness of our strategy. A brief survey of Brønsted and Lewis acids yielded phosphoric acid 2231 as an ideal catalyst for in situ alcohol dehydration and formal [3 + 3] union. Under these conditions, 23 and 24 were obtained in 55% and 64% yields, respectively. Coupling with pyrone 21 necessitated the use of enal 25 as a substrate, which readily underwent a formal [3 + 3] with 21 in the presence of piperidinium acetate.32
Figure 3. Modular chemoenzymatic synthesis of α-pyrone meroterpenoids.
a. Gram-scale biocatalytic hydroxylation of sclareolide (12), followed by oxidative degradation of its lactone moiety, enabled rapid access to key aldehyde 14. b. Compound 14 was converted to arisugacin F (1) and phenylpyropene C (2) via [3 + 3] coupling to append the appropriate pyrone units and HAT hydrogenation to selectively reduce the C9–C11 olefin. c. Compound 14 was dehydrated to enal 25, which in turn could be converted to pyripyropene E (4) via a similar [3 + 3] coupling/HAT hydrogenation sequence. 1, 2 and 4 are obtained in 7–8 steps from 12; biocatalytic installation of C3–OH and the use of radical-based logic for reduction at C9 are important transformations in their syntheses. d. Acid chloride 27, available from 14 in 4 steps, was converted to phenylpyropene F (3) via [3 + 3] coupling, followed by Luche reduction of the C11 ketone. e. A chemoselective Mukaiyama hydration of the C9–C11 olefin of 23 completed the synthesis of 5-deoxyterreulactone C (28). f. Chemical structures of reagents used in the transformations shown in this figure. NADP, nicotinamide adenine dinucleotide phosphate; TMSCl, trimethylsilyl chloride; imid., imidazole; KHMDS, potassium bis(trimethylsilyl)amide; dpm, 2,2,6,6-tetramethyl-3,5-heptanedionato; TBHP, tert-butyl hydrogen peroxide.
With the carbon framework fully installed, attention turned to the reduction of the C9–C11 alkene. A gamut of hydrogenation conditions in the presence of Rh, Pt, and Pd catalysts was surveyed, only to yield reduction and/or over-reduction of the pyrone motif. We reasoned that hydrogen atom transfer-based (HAT-based) hydrogenation would provide a viable solution to this problem.33,34 The steric bulk of the catalyst would suppress any undesired reactivity with the tetrasubstituted alkene, and between the two trisubstituted alkenes, reduction of Δ9,11 should be preferred due to the greater stability of the incipient radical at C9. Furthermore, HAT-based reduction is known to deliver the thermodynamic hydrogenation product, providing trans-decalin selectively from Δ9,10-octalin. Indeed, subjecting 23, 24, and 26 to Shenvi’s33 reduction conditions (Mn(dpm)3, tert-butyl hydrogen peroxide (TBHP), and PhSiH3) provided arisugacin F (1), phenylpyropene C (2), and pyripyropene E (4) in moderate to high yields and excellent diastereoselectivities (Fig. 3B, 3C).
The modularity of the designed sequence was further underscored by the ability to obtain phenylpyropene F (3), a more oxidised member of the α-pyrone meroterpenoid family, in a concise manner (Fig. 3D). Here, aldehyde 14 was converted to acid chloride 27, which was then subjected to a Friedel-Crafts acylation with pyrone 20, followed by a cyclisation/reduction sequence to complete the synthesis of 3 in 11 steps. The C9–C11 alkene proved to be a versatile functional handle, as Mukaiyama hydration35 on this moiety proceeded in a highly selective fashion (>20:1 dr) to generate the corresponding 3° alcohol at C9 (Fig. 3E). In contrast, previous approach to generate this alcohol required the intermediacy of the corresponding epoxide, which was then subjected to nucleophilic opening and alcohol reduction. In combination with C3 alcohol oxidation, this approach enabled rapid access to 5-deoxyterreulactone C (28) from 23 in 64% yield over 2 steps.
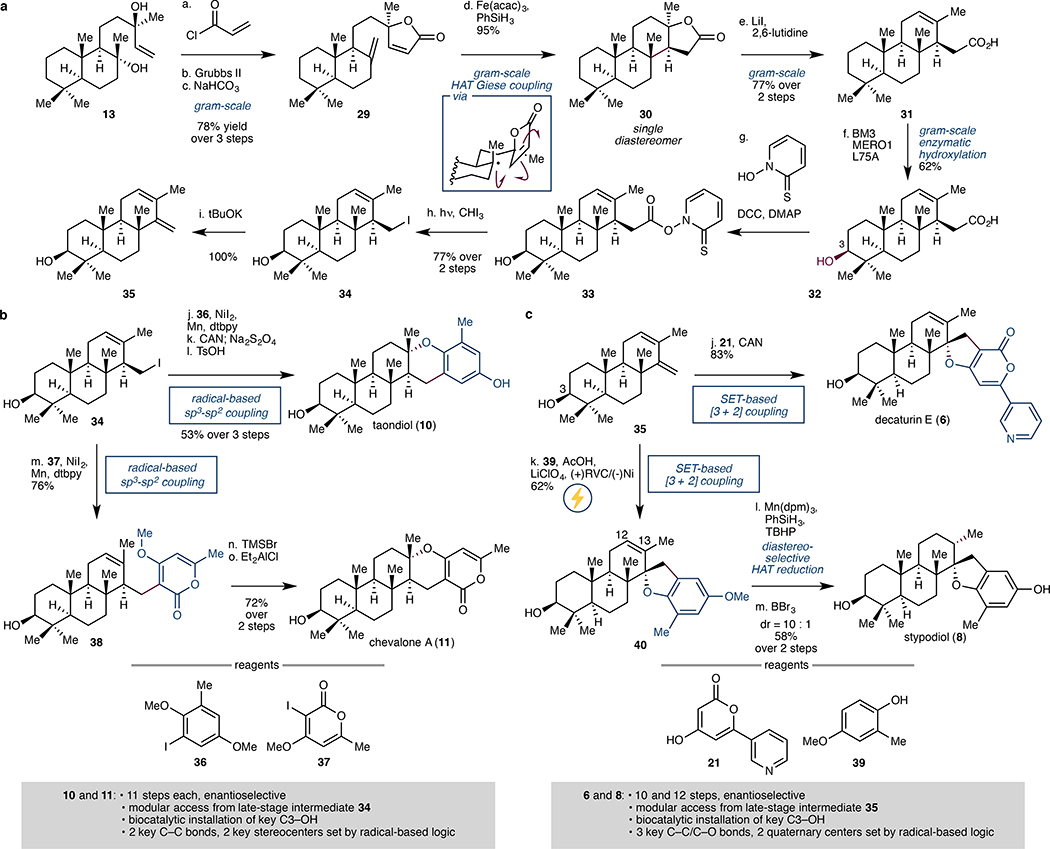
In parallel, route scouting for efficient access to diterpenic meroterpenoids was undertaken, affording a five-step synthesis of tricycle 31 as a single diastereomer (Fig. 4A). Key to this success is the use of HAT-based intramolecular Giese coupling36 to forge the C ring with complete diastereoselectivity and excellent yield. This outcome could be rationalised by invoking rapid pyramidalisation of the incipient 3° radical and cyclic stereocontrol imposed by the rigid trans-decalin and butyrolactone moieties. In contrast, previous approach to construct methyl ent-isocopalate from sclareol using polar 2-electron-based transformations resulted only in 82% de of the desired isomer.37 Intermediates 13, 29–31 were tested for enzymatic hydroxylation with various P450BM3 mutants. From this combinatorial experiment, the pairing of acid 31 and P450BM3 variant BM3 MERO1 yielded the most promising outcome (33% yield). Further alanine scanning on MERO1 identified variant MERO1 L75A as a superior biocatalyst, providing the desired C3-oxidized product 32 in 62% yield with complete regio- and diastereoselectivity. Gratifyingly, this key transformation could be routinely conducted on gram-scale without any appreciable decrease in conversion and isolated yield.
Figure 4. Modular chemoenzymatic synthesis of diterpenic meroterpenoids.
a. Building blocks 34 and 35 were synthesised from sclareol (13) in 8 and 9 steps, respectively. Key transformations in the sequence include the use of HAT-based intramolecular Giese coupling to form the C-ring with complete diastereoselectivity and the use of biocatalytic hydroxylation with an engineered P450BM3 to selectively install the C3 alcohol. b. Taondiol (10) and chevalone A (11) were synthesised in modular fashion from late-stage intermediate 34 via Ni-catalysed cross coupling with aryl iodides 36 and 37, respectively. Both 10 and 11 are accessed enantioselectively in 11 steps from 13; the full route involves biocatalytic installation of the key C3–OH and the formation of 2 key C–C bonds and 2 key stereocentres using radical-based logic. c. Decaturin E (6) and stypodiol (8) were synthesised in modular fashion from late-stage intermediate 35 via SET-based [3 + 2] coupling with pyrone 21 and phenol 39, respectively. Enantioselective formation of 6 and 8 occurs in 10 and 12 steps, respectively, from 13. Key transformations include the biocatalytic installation of C3–OH and the formation of 3 C–C/C–O bonds and 2 quaternary centres using radical-based logic. Acac, acetyl acetone; DCC, N,N’-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine; dtbpy, 4,4´-di-tert-butyl-2,2´-dipyridyl; CAN, ceric ammonium nitrate; SET, single electron transfer; TMSBr, trimethylsilyl bromide; RVC, reticulated vitreous carbon.
At this stage, radical cross-coupling employing redox-active ester derivatives38 of 32 was attempted to access taondiol and chevalone A. However, in both cases, no desired product formation was observed. As a workaround, 32 was first converted to the corresponding iodide (34), which was then subjected to nickel-catalysed cross coupling using Weix’s procedure39 with 36 and 37 (Fig. 4B). Using this procedure, the desired coupling products could be obtained in 85% and 76% yields, respectively. Finally, acid-catalysed cyclisations forged the final dihydropyran rings and completed the total syntheses of 10 and 11. The versatility of this synthetic approach is further highlighted by the ability to use the same iodo intermediate 34 to access decaturin E (6) and stypodiol (8) in a modular and efficient manner (Fig. 4C). Treatment of 34 with tBuOK afforded the corresponding diene (35) and set the stage for the final fragment coupling steps. The synthesis of 6 was realised by the use of a single electron transfer-based (SET-based) formal [3 + 2] coupling of 35 and pyrone 21. Initial attempts to realise this transformation in the presence of ceric ammonium nitrate40 were accompanied by competing oxidation of the C3 alcohol to the ketone. We hypothesised that increasing the equivalents of pyrone 21 would allow preferential single electron oxidation of this fragment and suppress undesired alcohol oxidation. Indeed, increasing the equivalents of pyrone 21 to 5 equivalents led to formation of 6 in 83% yield with no observable over-oxidation side product. Attempts to effect this CAN-mediated coupling with phenol 39 led to exclusive oxidation of 39 to the corresponding quinone. Hypothesising that electrochemical methods41 would allow a more controlled electron delivery to the phenol fragment, we subjected 35 and 39 to constant potential electrolysis. Gratifyingly, this approach was able to provide the desired [3 + 2] coupling product 40 as a single diastereomer in 62% yield. At this stage, the C12–C13 olefin needed to be reduced to the thermodynamic product containing an equatorial methyl group at C13. Given its thermodynamic preference, we turned to Shenvi’s HAT hydrogenation, which provided the correct stereochemical disposition at C13 with 10:1 dr. Once again, these radical-based conditions were found to be crucial as other reduction conditions led predominantly to the formation of the wrong diastereomer. Routine phenol demethylation finally completed the total synthesis of 8. Stypodiol has been shown to undergo ready conversion to stypodione42 and stypotriol.43 Thus, our approach also constitutes a 13-step and 14-step formal synthesis of stypodione and stypotriol, respectively.
The collective synthesis of various meroterpenoids presented in this work proceeded in 7–12 steps, comprising some of the most concise routes to the target molecules to date. Our hybrid approach results not only in favourable step count, but also significant improvement in overall yields for most of the targets (up to five-fold improvement, see Supplementary Table 1 for comparison). This work also constitutes the first total syntheses of 2, 3, 6 and 11. The orthogonal site-selectivity44,45,46 of biocatalytic C–H oxidation methods provided the opportunity to develop a novel disconnection for terpene synthesis and its combination with radical-based transformations4,5 allowed us to achieve exquisite stereocontrol and chemoselectivity in all of the key bond forming steps. Such features represent a distinct departure from previous approaches to bioactive meroterpenoids and highlight the benefits of combining45,47,48 modern synthetic paradigms in complex molecule synthesis. Importantly, eight synthetic targets could be accessed divergently from just two key intermediates, suggesting that this route can be readily adapted for the preparation of further synthetic derivatives as well as other meroterpenoid natural products bearing similar decalin architecture.49,50 Work in this area is underway and will be reported in due course.
Data Availability
Full experimental details, information about enzyme variants, and Supplementary Tables 1–9 are available in the Supplementary Information. Any additional information is available from the corresponding author upon request.
Supplementary Material
Acknowledgements
This work is supported by the National Institutes of Health Grant GM128895. We thank Profs. Phil S. Baran and Keary M. Engle for discussions and assistance in manuscript preparation. We acknowledge Prof. Frances H. Arnold (California Institute of Technology) and Prof. Huimin Zhao for providing plasmids encoding for P450BM3 variant 1857 and phosphite dehydrogenase variant Opt13, respectively. We thank the Shen lab and the Roush lab for generous access to their instrumentations.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Corey EJ & Cheng X-M The Logic of Chemical Synthesis (Wiley, 1995). [Google Scholar]
- 2.Turner NJ & O’Reilly E Biocatalytic retrosynthesis. Nat. Chem. Biol. 9, 285–288 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Turner NJ Directed evolution drives the next generation of biocatalysts. Nat. Chem. Biol. 5, 567–573 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Hung K, Hu X & Maimone T Total synthesis of complex terpenoids employing radical cascade processes. Nat. Prod. Rep. 35, 174–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JM, Harwood SJ & Baran PS Radical retrosynthesis. Acc. Chem. Res. 51, 1807–1817 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green SA et al. The high chemofidelity of metal-catalyzed hydrogen atom transfer. Acc. Chem. Res. 51, 2628–2640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda Y & Abe I Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 33, 26–53 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sunazuka T & Ōmura S Total synthesis of α-pyrone meroterpenoids, novel bioactive microbial metabolites. Chem. Rev. 105, 4559–4580 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Macías FA, Carrera C & Galindo JCG Brevianes revisited. Chem. Rev. 114, 2717–2732 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Itoh T et al. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2, 858–864 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Corey EJ, Noe MC & Lin S A mechanistically designed bis-cinchona alkaloid ligand allows position- and enantioselective dihydroxylation of farnesol and other oligoprenyl derivatives at the terminal isopropylidene unit. Tetrahedron Lett. 36, 8741–8744 (1995). [Google Scholar]
- 12.Smith AB III,, Kinso T, Sunazuka T & Ōmura S . Biomimetic total synthesis of the ACAT inhibitor (+)-pyripyropene E. Tetrahedron Lett. 37, 6461–6464 (1996). [Google Scholar]
- 13.Kumanireng AS, Kato T & Kitahara Y Cyclization of polyenes x. biogenetic type synthesis of dl-taodiol. Chem. Lett. 2, 1045–1047 (1973). [Google Scholar]
- 14.Nagamitsu T et al. Total synthesis of (+)-pyripyropene A, a potent, orally bioavailable inhibitor of Acyl-CoA:cholesterol acyltransferase. J. Org. Chem. 60, 8126–8127 (1995). [Google Scholar]
- 15.Abad A et al. An efficient stereoselective synthesis of stypodiol and epistypodiol. J. Org. Chem. 63, 5100–5106 (1998). [Google Scholar]
- 16.Takikawa H, Imamura Y & Sasaki M Synthesis and absolute configuration of brevione B, an allelochemical isolated from Penicillium sp.. Tetrahedron 62, 39–48 (2006). [Google Scholar]
- 17.Dixon DD, Lockner JW, Zhou Q & Baran PS Scalable, divergent synthesis of meroterpenoids via “borono-sclareolide”. J. Am. Chem. Soc. 134, 8432–8435 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Crossley SWM, Obradors C, Martinez RM & Shenvi RA Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basabe P et al. Synthesis of (+)-makassaric acid, a protein kinase MK2 inhibitor. Tetrahedron 66, 6008–6012 (2010). [Google Scholar]
- 20.Chen MS & White MC Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Quinn RK et al. Site-selective aliphatic C–H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc. 138, 696–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamata, et al. Scalable, electrochemical oxidation of unactivated C–H bonds. J. Am. Chem. Soc. 139, 7448–7451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiappini ND, Mack JBC & Du Bois J Intermolecular C(sp3)−H amination of complex molecules. Angew. Chem. Int. Ed. 57, 4956–4959 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Frija LMT, Frade RFM & Afonso CAM Isolation, chemical, and biotransformation routes of labdane-type diterpenes. Chem. Rev. 111, 4418–4452 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, El Damaty S & Fasan R P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J. Am. Chem. Soc. 133, 3242–3245 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Hall EA, Sarkar MR, Lee JHZ, Munday SD & Bell SG Improving the monooxygenase activity and the regio- and stereoselectivity of terpenoid hydroxylation using Ester directing groups. ACS Catal. 6, 6306–6317 (2016). [Google Scholar]
- 27.Aranda G et al. Microbial transformation of diterpenes: Hydroxylation of sclareol, manool and derivatives by Mucor plumbeus. Tetrahedron 47, 8339–8350 (1991). [Google Scholar]
- 28.McLachlan MJ, Johannes TW & Zhao H Further improvement of phosphite dehydrogenase thermostability by saturation mutagenesis. Biotechnol. Bioeng. 99, 268–274 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Lewis JC et al. Combinatorial alanine substitution enables rapid optimization of cytochrome P450BM3 for selective hydroxylation of large substrates. ChemBioChem 11, 2502–2505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong TS, Arnold FH & Schwaneberg U Laboratory evolution of cytochrome P450 BM-3 monooxygenase for oganic co-solvents. Biotechnol. Bioeng. 85, 351–358 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Hubert C et al. Brønsted acid-catalyzed synthesis of pyrans via a formal [3+3] cycloaddition. Adv. Synth. Catal. 350, 40–42 (2008). [Google Scholar]
- 32.Hsung RP, Kurdyumov AV & Sydorenko N A formal [3 + 3] cycloaddition approach to natural-product synthesis. Eur. J. Org. Chem. 1, 23–44 (2005). [Google Scholar]
- 33.Iwasaki K, Wan KK, Oppedisano A, Crossley SWM & Shenvi RA Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am. Chem. Soc. 136, 1300–1303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King SM, Ma X & Herzon SB A method for the selective hydrogenation of alkenyl halides to alkyl halides. J. Am. Chem. Soc. 136, 6884–6887 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Isayama S & Mukaiyama T A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 1071–1074 (1989). [Google Scholar]
- 36.Lo JC et al. Fe-catalyzed C–C bond construction from olefins via radicals. J. Am. Chem. Soc. 139, 2484–2503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua S-K, Wang J, Chen X-B, Xu Z-Y & Zeng B-B Scalable synthesis of methyl ent-isocopalate and its derivatives. Tetrahedron 67, 1142–1144 (2011). [Google Scholar]
- 38.Sandfort F, O’Neill MJ, Cornella J, Wimmer L & Baran PS Alkyl−(hetero)aryl bond formation via decarboxylative cross-coupling: a systematic analysis. Angew. Chem. Int. Ed. 56, 3319–3323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everson DA, Shrestha R & Weix DJ Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 132, 920–921 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Yokoe H et al. Enantiocontrolled total syntheses of breviones A, B, and C. J. Am. Chem. Soc. 133, 8854–8857 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Chiba K, Fukuda M, Kim S, Kitano Y & Tada M Dihydrobenzofuran synthesis by an anodic [3 + 2] cycloaddition of phenols and unactivated alkenes. J. Org. Chem. 64, 7654–7656 (1999). [Google Scholar]
- 42.Falck JR et al. Total synthesis of the spiro-o-benzoquinonefuran (−)-stypoldione. J. Am. Chem. Soc. 115, 11606–11607 (1993). [Google Scholar]
- 43.Gerwick WH & Fenical W Ichthyotoxic and cytotoxic metabolites of the tropical brown alga Stypopodium zonale (Lamouroux) papenfuss. J. Org. Chem. 46, 22–27 (1981). [Google Scholar]
- 44.King-Smith E, Zwick CR III & Renata H Applications of oxygenases in the chemoenzymatic total synthesis of complex natural products. Biochemistry 57, 403–412 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Loskot SA, Romney DK, Arnold FH & Stoltz BM Enantioselective total synthesis of nigelladine A via late-stage C–H oxidation enabled by an engineered P450 enzyme. J. Am. Chem. Soc. 139, 10196–10199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowell AN, et al. Chemoenzymatic total synthesis and structural diversification of tylactone-based macrolide antibiotics through late-stage polyketide assembly, tailoring, and C−H functionalization. J. Am. Chem. Soc. 139, 7913–7920 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latham J, Henry J-M, Sharif HH, Menon BRK, Shepherd SA & Micklefield J Integrated catalysis opens new arylation pathways via regiodivergent enzymatic C–H activation. Nature Commun. 7, 11873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durak JJ, Payne JT & Lewis JC Late-stage diversification of biologically-active molecules via chemoenzymatic C–H functionalization. ACS Catal. 6, 1451–1454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulcitki V, Harghel P & Ungur N Unusual cyclic terpenoids with terminal pendant prenyl moieties: from occurrence to synthesis. Nat. Prod. Rep. 31, 1686–1720 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Domingo V, Arteaga JF, Quilez del Moral JF & Barrero AF Unusually cyclized triterpenes: occurrence, biosynthesis and chemical synthesis. Nat. Prod. Rep. 26, 115–134 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full experimental details, information about enzyme variants, and Supplementary Tables 1–9 are available in the Supplementary Information. Any additional information is available from the corresponding author upon request.