Abstract
Adverse childhood experiences (ACEs) are associated with mental health and substance use problems, but lesser known is how they interconnect. The objective of this study was to examine how internalizing and externalizing symptoms mediate the association of ACEs with prescription opioid misuse in order to understand how ACEs interconnect with mental health and substance use problems. Adults aged 18 or older from the National Epidemiological Survey on Alcohol and Related Conditions Wave 3 (NESARC-III) conducted in 2012–2013 were included (N = 36,309). The prescription opioid misuse outcomes examined include prescription opioid misuse status, early-onset status of prescription opioid misuse, frequency of past-year prescription opioid misuse, and opioid use disorder. A natural effect model and regression analyses were used to conduct the mediation analyses. We found that respondents with higher ACE scores had greater odds of reporting past-year and lifetime prescription opioid misuse and DSM-V-diagnosed opioid use disorder as well as early onset of prescription opioid misuse (AORs range from 1.06 to 1.12). These associations are partially mediated by internalizing and externalizing symptoms. The findings suggest that internalizing and externalizing symptoms may be potential pathways through which ACEs are associated with prescription opioid misuse. Our results underscore the importance of preventing ACEs and reducing risk for internalizing and externalizing symptoms after exposure, which may reduce later prescription opioid misuse.
Keywords: Prescription opioid misuse, Adverse childhood experiences, Internalizing symptoms, Externalizing symptoms, Mediation
1. Introduction
Since 1999, the number of overdose deaths involving opioids has increased dramatically. In 2017, 47,600 people died from drug overdose involving an opioid (Seth et al., 2018b), and the rate of deaths involving prescription opioids was five times higher than that in 1999 (Seth et al., 2018a). According to a nationally representative survey conducted in 2017, about 10.3 million adults aged 18 years or older misused prescription opioids, and 1.6 million had an opioid use disorder (OUD) (Center for Behavioral Health Statistics and Quality, 2018). The associated economic costs of prescription opioid misuse and overdose are substantial. The total estimated economic burden was $78.5 billion in 2013, which is attributed to healthcare and substance abuse treatment costs, lost productivity, and criminal justice costs (Florence et al., 2016). Collectively, these findings underscore the urgent need to address factors that contribute to prescription opioid misuse and overdose.
An area with promise to prevent prescription opioid misuse is the body of work focused on preventing and addressing Adverse Childhood Experiences (ACEs)-a collection of potentially, traumatic experiences that occur during the first 18 years of life. ACEs often include exposure to abuse (emotional, physical, sexual), neglect (emotional and physical) and household challenges (domestic violence, substance abuse, mental illness, parental separation or divorce and incarcerated family member) (Felitti et al., 1998; Merrick et al., 2018). While some degree of childhood adversity is a normal and essential part of human development, chronic or repeated exposure to ACEs can result in a toxic stress response that derails optimal development by producing changes in gene expression, brain function, immune function, and coping strategies adopted (Shonkoff and Garner, 2012), which may contribute to an individual’s risk for substance misuse, including prescription opioid misuse (Norman et al., 2011).
Previous studies demonstrate robust associations between ACEs and substance misuse (Dube et al., 2003; Orsi et al., 2018). Specifically, childhood emotional abuse, physical abuse, and sexual abuse are associated with high risk of using/misusing substances in later adolescence and adulthood. In studies that focused specifically on opioid misuse, the prevalence of childhood trauma was high among people with OUD (Conroy et al., 2009). ACEs were also associated with early onset for opioid misuse among patients with OUD (Stein et al., 2017). However, the number of studies focused on the association between ACEs and prescription opioid misuse and the pathways between these experiences is limited. One previous study documented the associations between ACEs and prescription opioid misuse (Austin and Shanahan, 2018), identifying ACEs as a risk factor that predict prescription opioid misuse. However, the pathways through which ACEs are associated with prescription opioid misuse remain unclear. Mediation analyses are needed to understand these pathways to uncover the underlying mechanisms which can inform more specific public health interventions based on different mechanisms.
Previous studies have shown the association of internalizing (depression, anxiety, and traumatic distress) and externalizing (aggression, delinquency, and hyperactivity) symptoms with both ACEs and prescription opioid misuse (Hunt et al., 2017; Martins et al., 2012; Young et al., 2012). However, few studies have explicitly tested whether internalizing and externalizing symptoms mediate the relationship between ACEs and opioid misuse. In addition, understanding this mediated association is important for designing or identifying existing interventions that address internalizing and externalizing symptoms for those exposed to ACEs to prevent them from misusing prescription opioids later.
Given these connections, this study focuses on internalizing and externalizing symptoms as potential mediators of the association between ACEs and prescription opioid misuse. Internalizing and externalizing symptoms may mediate the association between ACEs and prescription opioid misuse in different ways and may uncover important implications for interventions. For example, the potential pathway through which internalizing symptoms mediate the association between ACEs and prescription opioid misuse is based on the self-medication hypothesis that prescription opioids are used to alleviate distress and anxiety disorders (Garland et al., 2015; Martins et al., 2012). Only one study has examined this potential pathway using the National Longitudinal Study of Adolescent to Adult Health data, which found that distress and anxiety disorders did not mediate the association between ACEs and prescription opioid misuse (Austin and Shanahan, 2018). The authors thought one explanation for this finding was the inadequate measures for anxiety disorders that only captured symptoms in a short time frame (the past week). Externalizing symptoms may also mediate the association between ACEs and prescription opioid misuse based on the sensation seeking hypothesis that prescription opioids are used to meet the need for excitement (Boyd et al., 2006; Horvath et al., 2004; Young et al., 2012). Sensation seeking was defined as a trait of seeking varied, novel, complex and intense sensations and experiences (Zuckerman and Kuhlman, 2000) and was linked to externalizing symptoms (Joireman et al., 2003; Wilson and Scarpa, 2011). We therefore hypothesized that some individuals exposed to ACEs were likely to develop externalizing symptoms and misused prescription opioids for sensation seeking. Past studies demonstrate that externalizing symptoms, such as aggression, are associated with substance use/misuse (Colder et al., 2013). However, to our knowledge, no research has examined how externalizing symptoms mediate the association between ACEs and prescription opioid misuse.
Although previous studies have concluded that ACEs are risk factors for prescription opioid misuse, the pathways through which ACEs are associated with prescription opioid misuse are not clearly identified. This study aimed to address the following research gaps: 1) studying the associations between ACEs and prescription opioid misuse; 2) examining how internalizing symptoms and externalizing symptoms mediate this association by examining multiple prescription opioid misuse outcomes. In the present study, we hypothesize that 1) having a history of ACEs is associated with prescription opioid misuse in adulthood; 2) both internalizing and externalizing symptoms are positively associated with ACEs and prescription opioid misuse; 3) internalizing symptoms and externalizing symptoms mediate the association.
2. Methods
2.1. Data and study sample
We used data from Wave 3 of the National Epidemiological Survey on Alcohol and Related Conditions (NESARC-III) conducted in 2012–2013 by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The NESARC-III is a nationally representative sample of the non-institutionalized adult population 18 years and older in the United States (N = 36,309). Multistage probability sampling is used to randomly select respondents from the U.S. adult population (Grant et al., 2015). This study was deemed exempt for review from the Institutional Review Board of Indiana University.
2.2. Measures
2.2.1. Outcome variables
The six prescription opioid misuse outcomes examined include prescription opioid misuse status (past-year and lifetime), early-onset status of prescription opioid misuse, opioid use disorder (past-year and lifetime), and frequency of past-year prescription opioid misuse. These measures have been used in previous literature (Arterberry et al., 2016).
2.2.1.1. Prescription opioid misuse status.
NESARC respondents were asked about their misuse of prescription opioids. We used two variables to measure prescription opioid misuse status: lifetime (ever before) and past-year (in the past 12 months) misuse. Both variables are binary. In the survey, for instance, respondents were asked “Have you ever used any of these painkillers, for example… methadone, codeine, Demerol, Vicodin, Oxycontin, opium, oxy, Percocet, Dilaudid, Percodan, morphine that you may have used on your own - that is, either without a doctor’s prescription; in greater amounts, more often, or longer than prescribed; or for a reason other than a doctor said you should use them?”
2.2.1.2. Early-onset status of prescription opioid misuse.
Previous studies using NESARC utilized 25th percentile of the onset age to define the cutoff for early-onset status for substance use (equal or younger than age 17). Based on this, we created a binary early onset variable based on this cutoff coded “1” for early onset and “0” otherwise (Arterberry et al., 2016; Lin et al., 2016).
2.2.1.3. Opioid use disorder.
Past-year OUD and lifetime OUD status variables were created by NESARC-III based on validated DSM-V criteria. OUD was assessed based on the NIAAA Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5), which was designed to assess the diagnostic definitions in the DSM-V. The disorder diagnosis has been validated (Grant et al., 2015; Grant et al., 2016). Both variables are binary.
2.2.1.4. Frequency of past-year prescription opioid misuse.
The frequency of prescription opioid misuse in the past 12 months is coded with a 0–10 scale: 0 = never, 1 = once a year, 2 = 2 times a year, 3 = 3–6 times a year, 4 = 7–11 times a year, 5 = once a month, 6 = 2–3 times a month, 7 = 1–2 times a week, 8 = 3–4 times a week, 9 = nearly every day, and 10 = every day. This variable was treated as continuous.
2.2.2. Primary independent variable
The ACEs variable serves as the primary independent variable. The ACE score variable was created based on twenty-nine questions regarding ten ACEs categories (see Appendix A): emotional abuse, physical abuse, sexual abuse, physical neglect, emotional neglect, household physical violence, household substance abuse, incarcerated household member, household mental illness, and parental separation or divorce. Following the same method that a previous study used for ACEs coding, questions were collapsed for each ACE category, and exposure was determined. Respondents were coded as a “1” if they were exposed to that category of ACE, as specified in the previous study (Dong et al., 2004). We then summed the number of ACEs categories each respondent was exposed to (score ranged from 0 to 10).
2.2.3. Mediator variable
In the present study, the mediator variables are internalizing symptoms and externalizing symptoms. Global Appraisal of Individual Needs - Shorter Screener (GAIN-SS) provides a list of items that can be used to identify individuals who have lifetime internalizing or externalizing symptoms. The reliability of GAIN-SS has been validated (Dennis et al., 2006). We chose items from NESARC-III based on the GAIN-SS manual and further conducted factor analysis using the NESAR-III data to ensure the validity of the selected items. Fourteen items measuring internalizing symptoms and fourteen items measuring externalizing symptoms (see Appendix B) were included. Cronbach’s alpha coefficients suggest that our scales for internalizing (α = 0.80) and externalizing (α = 0.76) symptoms are reliable. Reports of yes are coded as 1, or 0 otherwise and summed. Each respondent received a total score for lifetime internalizing symptoms (score ranged from 0 to 14) and externalizing symptoms respectively (score ranged from 0 to 14). Both of the variables are continuous.
2.2.4. Sociodemographic and other substances misuse/use variables
The sociodemographic control variables include self-reported sex (male/female) at the time of the survey, age, race/ethnicity (non-Hispanic white, non-Hispanic black, Non-Hispanic American Indian, Non-Hispanic Asian, Hispanic), education (less than high school, high school, some college, college, or graduate school), marital status (married or not), region (Northeast, Midwest, South, or West), and employment status (employed or not). Because studies have shown that cigarette and cannabis use are associated with prescription opioid misuse (Fiellin et al., 2013; Griesler et al., 2019; Romberg et al., 2019), we also controlled for lifetime cannabis use (yes/no) and lifetime use of at least 100 cigarettes (yes/no).
2.3. Statistical analyses
To understand the mechanism through which ACEs are associated with prescription opioid misuse, mediation analyses were conducted. There are two mediators in the present study: internalizing symptoms and externalizing symptoms. Fig. 1 provides a depiction of the mediation models. Path c represents direct effects of ACEs on prescription opioid misuse outcomes. Path a and path b together represent indirect effects of ACEs on prescription opioid misuse. We used the natural effect model (NEM) (Valeri and VanderWeele, 2013) to examine the association between ACEs and prescription opioid misuse outcomes, the mediating influences of internalizing and externalizing symptoms, while controlling for socioeconomic variables. The NEM can estimate the indirect effect of ACEs on prescription opioid misuse through internalizing symptoms and through externalizing symptoms. Because there are two mediators, one mediator was controlled for while the mediation effect of the other was estimated. All statistical analyses were conducted using Stata SE 15. The Stata package “paramed” was used to conduct all mediation analyses because it provides correct estimates of standard errors of indirect effect in the logistic regression model; it also allows hypothesis testing for the mediation. One limitation of this package is that it does not allow analysis with complex survey weighting, which prevented this study from conducting weighted analysis.
Fig. 1.
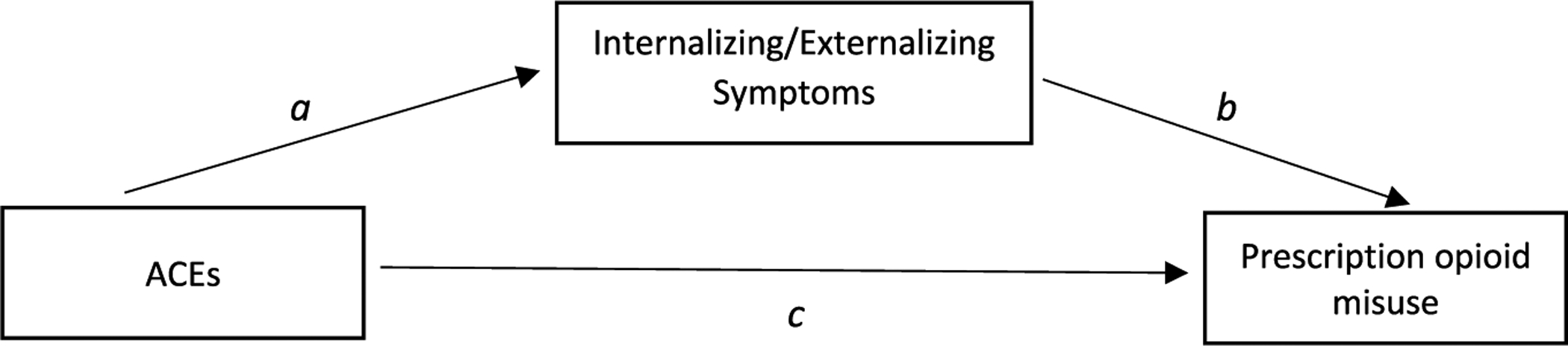
The concept model of the mediation relationship.
3. Results
3.1. Descriptive statistics and direct effect
The descriptive statistics of the sample are reported in Table 1. Table 2 contains the estimates of the direct association between ACEs and prescription opioid misuse, while controlling for internalizing symptoms, externalizing symptoms and sociodemographic and other substance use variables. Adjusted odds ratios (AOR) and beta coefficients (β) are reported for binary outcomes and continuous outcomes, respectively, in this study. Each unit increase in ACE score is directly associated with greater odds of past-year prescription opioid misuse (direct AOR = 1.03, 95% CI = 1.00–1.06), lifetime prescription opioid misuse (direct AOR = 1.04, 95% CI = 1.02–1.06), early onset of prescription opioid misuse (direct AOR = 1.07, 95% CI = 1.03–1.11), having lifetime prescription OUD (direct AOR = 1.06, 95% CI = 1.02–1.10), and higher frequency of prescription opioid misuse (β = 0.01, 95% CI = 0.002–0.02). ACE score was not associated with past-year prescription OUD.
Table 1.
Descriptive Statistics: National Epidemiological Survey on Alcohol and Related Conditions, 2012–2013 (n = 36,309)
| Variable | n (%) |
|---|---|
| Past-year opioid misuse | |
| Misused | 1,579 (4.38%) |
| Not misused | 34,509 (95.62%) |
| Lifetime opioid misuse | |
| Misused | 4,090 (11.28%) |
| Not misused | 32,167 (88.72%) |
| Early-onset opioid misuse | |
| Yes | 1,003 (2.78%) |
| No | 35,118 (97.22%) |
| Past-year OUD | |
| Yes | 330 (0.91%) |
| No | 35,979 (99.09%) |
| Lifetime OUD | |
| Yes | 688 (1.89%) |
| No | 35,621 (98.11%) |
| Opioid misuse frequency | 0.25 (1.35)a |
| ACE score | 1.26 (1.75)a |
| Internalizing symptoms | 2.24 (3.44)a |
| Externalizing symptoms | 1.09 (1.75)a |
| Sex | |
| Female | 20,447 (56.31%) |
| Male | 15,862 (43.69%) |
| Age | 45.63 (17.53)a |
| Race/ethnicity | |
| Non-Hispanic white | 19,194 (52.86%) |
| Non-Hispanic black | 7,766 (21.39%) |
| American Indian | 511 (1.41%) |
| Non-Hispanic Asian | 1,801 (4.96%) |
| Hispanic | 7,037 (19.38%) |
| Education | |
| Less than high school | 5,490 (15.12%) |
| High school | 9,799 (26.99%) |
| Some college | 12,105 (33.34%) |
| College | 5,889 (16.22%) |
| Graduate school | 3,026 (8.33%) |
| Marital status | |
| Married | 14,482 (39.89%) |
| Not married | 21,827 (60.11%) |
| Region | |
| Northeast | 5,180 (14.27%) |
| Midwest | 7,566 (20.84%) |
| South | 14,532 (40.02%) |
| West | 9,031 (24.87%) |
| Employment | |
| Unemployed | 2,557 (7.04%) |
| Employed | 33,752 (92.96%) |
| Cannabis use | |
| Used | 11,272 (31.10%) |
| Not used | 24, 971 (68.90%) |
| Tobacco use | |
| Used | 14, 778 (40.75%) |
| Not used | 21,491 (59.25%) |
Mean (standard deviation) are calculated for continuous variables.
Table 2.
Direct Effect of ACEs on Prescription Opioid Misuse: National Epidemiological Survey on Alcohol and Related Conditions, 2012–2013
| Variable | Prescription opioid misuse outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Past-year opioid misuse | Lifetime opioid misuse | Early onset of opioid misuse | Past-year OUD | Lifetime OUD | Opioid misuse frequency | |||||||
| AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | β | (95% CI) | |
| ACE score | 1.03 | (1.00, 1.06) | 1.04 | (1.02, 1.06) | 1.07 | (1.03, 1.10) | 1.05 | (0.99, 1.10) | 1.06 | (1.02, 1.10) | 0.01 | (0.002, 0.02) |
| Internalizing symptoms | 1.06 | (1.04, 1.07) | 1.05 | (1.04, 1.06) | 1.04 | (1.02, 1.06) | 1.14 | (1.11, 1.17) | 1.13 | (1.11, 1.15) | 0.02 | (0.02, 0.03) |
| Externalizing symptoms | 1.13 | (1.10, 1.16) | 1.16 | (1.14, 1.18) | 1.20 | (1.17, 1.24) | 1.16 | (1.11, 1.22) | 1.19 | (1.15, 1.23) | 0.07 | (0.06, 0.08) |
| Sex: female | 1.04 | (0.93, 1.16) | 0.91 | (0.85, 0.98) | 0.86 | (0.75, 0.99) | 0.97 | (0.77, 1.23) | 0.98 | (0.82, 1.15) | 0.02 | (−0.01, 0.05) |
| Age | 0.99 | (0.99, 1.00) | 0.99 | (0.99, 1.00) | 0.96 | (0.95, 0.96) | 1.00 | (0.99, 1.01) | 0.99 | (0.99, 1.00) | 0.001 | (0.001, 0.002) |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) | ||||||
| Non-Hispanic black | 1.06 | (0.92, 1.21) | 0.66 | (0.60, 0.73) | 0.52 | (0.43, 0.63) | 0.96 | (0.72, 1.28) | 0.51 | (0.40, 0.64) | 0.04 | (−0.002, 0.07) |
| American Indian | 0.90 | (0.61, 1.31) | 0.69 | (0.53, 0.91) | 0.45 | (0.26, 0.78) | 0.83 | (0.40, 1.75) | 0.72 | (0.43, 1.21) | −0.04 | (−0.16, 0.08) |
| Non-Hispanic Asian | 0.62 | (0.43, 0.90) | 0.60 | (0.48, 0.75) | 0.51 | (0.32, 0.82) | 0.38 | (0.12, 1.21) | 0.39 | (0.19, 0.80) | −0.04 | (−0.11, 0.02) |
| Hispanic | 0.88 | (0.75, 1.03) | 0.67 | (0.60, 0.74) | 0.60 | (0.49, 0.73) | 0.85 | (0.61, 1.20) | 0.51 | (0.39, 0.66) | −0.03 | (−0.07, 0.01) |
| Education | ||||||||||||
| Less than high school | (Ret) | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) | ||||||
| High school | 0.98 | (0.83, 1.15) | 1.03 | (0.92, 1.16) | 1.09 | (0.88, 1.35) | 0.83 | (0.61, 1.12) | 0.88 | (0.69, 1.12) | −0.05 | (−0.10, −0.01) |
| Some college | 0.90 | (0.77, 1.05) | 1.15 | (1.03. 1.29) | 1.11 | (0.90, 1.37) | 0.67 | (0.49, 0.91) | 0.80 | (0.63, 1.01) | −0.08 | (−0.12, −0.04) |
| College | 0.78 | (0.63, 0.95) | 1.09 | (0.95, 1.26) | 0.94 | (0.71, 1.23) | 0.40 | (0.25, 0.66) | 0.59 | (0.43, 0.82) | −0.13 | (−0.18, −0.08) |
| Graduate school | 0.76 | (0.59, 1.00) | 0.96 | (0.81, 1.14) | 0.90 | (0.62, 1.32) | 0.33 | (0.16, 0.68) | 0.45 | (0.28, 0.72) | −0.12 | (−0.18, −0.06) |
| Married: yes | 0.75 | (0.66, 0.84) | 0.81 | (0.75, 0.87) | 0.86 | (0.74, 1.01) | 0.81 | (0.62, 1.05) | 0.92 | (0.77, 1.10) | −0.06 | (−0.09, −0.03) |
| Region | ||||||||||||
| Northeast | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) | ||||||
| Midwest | 1.31 | (1.10, 1.57) | 1.23 | (1.09, 1.39) | 1.52 | (1.20, 1.93) | 0.96 | (0.67, 1.37) | 0.80 | (0.61, 1.04) | 0.07 | (0.02, 0.12) |
| South | 1.14 | (0.97, 1.36) | 1.17 | (1.04, 1.31) | 1.33 | (1.06, 1.67) | 0.85 | (0.61, 1.18) | 0.90 | (0.71, 1.14) | 0.05 | (0.01, 0.09) |
| West | 1.28 | (1.07, 1.53) | 1.27 | (1.13, 1.44) | 1.49 | (1.17, 1.88) | 0.83 | (0.58, 1.19) | 0.87 | (0.68, 1.12) | 0.06 | (0.01, 0.10) |
| Unemployed: yes | 1.06 | (0.89, 1.27) | 1.11 | (0.98, 1.26) | 1.00 | (0.81, 1.23) | 0.95 | (0.66, 1.36) | 1.13 | (0.88, 1.45) | −0.01 | (−0.07, 0.04) |
| Cannabis use: yes | 2.90 | (2.57, 3.28) | 4.35 | (4.01, 4.71) | 4.20 | (3.54, 5.00) | 2.56 | (1.95, 3.37) | 4.08 | (3.30, 5.04) | 0.23 | (0.20, 0.26) |
| Tobacco use: yes | 1.56 | (1.39, 1.76) | 1.48 | (1.36, 1.60) | 1.76 | (1.51, 2.06) | 1.73 | (1.31, 2.27) | 1.81 | (1.48, 2.21) | 0.11 | (0.08, 0.14) |
| n | 36,034 | 36,201 | 36,066 | 36,211 | 36,211 | 36,022 | ||||||
AOR = adjusted odds ratio; CI = confidence interval
All models were adjusted for internalizing and externalizing symptoms, sex, race/ethnicity, education, marital status, region, employment status, cannabis use, and tobacco use.
3.2. Mediation analysis
Table 3 presents mediation analyses results. The second column of Table 3 demonstrates the estimates of the total effect. The total effect is equal to the summation of the indirect effect and the direct effect. As we have two mediators, total effects were calculated for internalizing symptoms and externalizing symptoms, respectively.
Table 3.
Mediation Effect of Lifetime Externalizing and Internalizing Symptoms on the Association between ACEs and Prescription Opioid Misuse: National Epidemiological Survey on Alcohol and Related Conditions, 2012–2013
| Outcome Variable | Total Effect | Natural Indirect Effect | Proportion Mediated | ||
|---|---|---|---|---|---|
| AOR or β | (95% CI) | AOR or β | (95% CI) | ||
| Mediator: internalizing symptoms | |||||
| Past-year opioid misuse | 1.06 | (1.03, 1.09) | 1.03 | (1.02, 1.03) | 47% |
| Lifetime opioid misuse | 1.07 | (1.05, 1.09) | 1.02 | (1.02, 1.03) | 34% |
| Early onset of opioid misuse | 1.09 | (1.05, 1.12) | 1.02 | (1.01, 1.03) | 22% |
| Past-year OUD | 1.11 | (1.06, 1.17) | 1.06 | (1.05, 1.08) | 58% |
| Lifetime OUD | 1.12 | (1.08, 1.17) | 1.06 | (1.05, 1.07) | 50% |
| Frequency of opioid misusea | 0.02 | (0.01, 0.03) | 0.01 | (0.008, 0.012) | 49% |
| Mediator: externalizing symptoms | |||||
| Past-year opioid misuse | 1.06 | (1.04, 1.09) | 1.03 | (1.02, 1.04) | 50% |
| Lifetime opioid misuse | 1.08 | (1.06, 1.10) | 1.04 | (1.03, 1.04) | 48% |
| Early onset of opioid misuse | 1.12 | (1.08, 1.15) | 1.05 | (1.04, 1.05) | 43% |
| Past-year OUD | 1.09 | (1.03, 1.14) | 1.04 | (1.03, 1.05) | 46% |
| Lifetime OUD | 1.11 | (1.07, 1.15) | 1.04 | (1.04, 1.05) | 43% |
| Frequency of opioid misusea | 0.03 | (0.02, 0.04) | 0.02 | (0.01, 0.02) | 61% |
AOR = adjusted odds ratio; CI = confidence interval.
All models were adjusted for internalizing and externalizing symptoms, sex, race/ethnicity, education, marital status, region, employment status, cannabis use, and tobacco use.
β coefficient was reported.
The forth column of Table 3 presents the estimates of the indirect effect. Both lifetime internalizing and externalizing symptoms partially mediate the association between ACEs and all prescription opioid misuse outcomes. In particular, internalizing symptoms partially mediate the association between ACEs and past-year prescription opioid misuse (indirect AOR = 1.03, 95% CI = 1.02–1.03, proportion mediated = 47%), lifetime prescription opioid misuse (indirect AOR = 1.02, 95% CI = 1.02–1.03, proportion mediated = 34%), early onset of prescription opioid misuse (indirect AOR = 1.02, 95% CI = 1.01–1.03, proportion mediated = 22%), past-year OUD (indirect AOR = 1.06, 95% CI = 1.05–1.08, proportion mediated = 58%), lifetime OUD (indirect AOR = 1.06, 95% CI = 1.05–1.07, proportion mediated = 50%), and frequency of prescription opioid misuse (indirect β = 0.01, 95% CI = 0.008–0.012, proportion mediated = 49%).
Externalizing symptoms partially mediate the association between ACEs and past-year prescription opioid misuse (indirect AOR = 1.03, 95% CI = 1.02–1.04, proportion mediated = 50%), lifetime prescription opioid misuse (indirect AOR = 1.04, 95% CI = 1.03–1.04, proportion mediated = 48%), early onset of prescription opioid misuse (indirect AOR = 1.05, 95% CI = 1.04–1.05, proportion mediated = 43%), past-year OUD (indirect AOR = 1.04, 95% CI = 1.03–1.05, proportion mediated = 46%), lifetime OUD (indirect AOR = 1.04, 95% CI = 1.04–1.05, proportion mediated = 43%), and frequency of prescription opioid misuse (indirect β = 0.02, 95% CI = 0.01–0.02, proportion mediated = 61%).
4. Discussion
The present study examined the association between ACEs and prescription opioid misuse and whether internalizing symptoms and externalizing symptoms mediate this relationship. To our knowledge, this is the first study to examine the mediation effect of internalizing and externalizing symptoms on the association between ACEs and prescription opioid misuse.
The first hypothesis, that history of ACEs is associated with prescription opioid misuse in adulthood, was supported. Furthermore, among our prescription opioid misuse outcomes, ACEs had the strongest direct effect on early onset of prescription opioid misuse, which is consistent with previous studies on the association of ACEs with substance use/misuse initiation (Dube et al., 2003; Ompad et al., 2005; Stein et al., 2017). This suggests that ACEs play an important role in the early initiation of prescription opioid misuse. Although the odds ratios are not large, preventing ACEs may reduce the risk of early initiation of prescription opioid misuse and thus reduce the risk of future illicit opioid use, prescription opioid injection misuse (Jones et al., 2017), and adverse health outcomes, such as OUD and overdose. Policies, practices, and programs that change conditions for children and families can prevent ACEs from occurring in the first place and may simultaneously mitigate the impact of ACE exposure on later outcomes (e.g., prescription opioid misuse) (Fortson et al., 2016).
In line with the second and third hypotheses, the mediation analysis supports that lifetime internalizing and externalizing symptoms partially mediate the association between ACEs and prescription opioid misuse. Both internalizing and externalizing symptoms were positively associated with ACEs and were positively associated with each of the prescription opioid misuse outcomes. Our finding regarding the mediating role of internalizing symptoms supports the self-medication hypothesis (Colder et al., 2013; Garland et al., 2015; Martins et al., 2012). This hypothesis states that individuals who have experienced ACEs may misuse opioids to cope with internalizing symptoms, such as depression symptoms. Our findings regarding externalizing symptoms reveal another potential pathway between ACEs and prescription opioid misuse and supports the Behavioral Disinhibition Model, which suggests that individuals with behavioral disinhibition, an inability to self-regulate and to inhibit socially undesirable actions (i.e., aggression, delinquency), are often comorbid with substance use/misuse (Iacono et al., 2008; Young et al., 2012).
In general, the mediation effect of externalizing symptoms was larger than that of the internalizing symptoms. This finding suggests that among those individuals exposed to ACEs, externalizing symptoms may play a greater role in predicting prescription opioid misuse. This is consistent with previous findings that the association between externalizing symptoms and substance misuse is stronger than that for internalizing symptoms (Colder et al., 2013; King et al., 2004). Moreover, intervening with children who are exposed to ACEs to prevent externalizing symptoms, such as bullying or aggressive behaviors, may reduce their risk of misusing prescription opioids (David-Ferdon et al., 2016; Pophillat et al., 2016). For instance, a classroom-based program, Life Skills Training (LST), is effective at improving social skills and reducing risk behaviors including drug use and might also be effective at mitigating opioid misuse (David-Ferdon et al., 2016). A combination of LST and Strengthening Families Program (SFP), another evidence based prevention program that aims to improve children’s social skills, was implemented and demonstrated protective effects for prescription opioid misuse into early adulthood (Spoth et al., 2013).
This study also found that the mediation effects of internalizing symptoms and externalizing symptoms on opioids outcomes were heterogeneous. For example, internalizing symptoms played a relatively smaller role in mediating the early onset of prescription opioids, and a larger role in mediating both past-year and lifetime OUD. This suggests that interventions to prevent internalizing symptoms may have differential effects on different prescription opioid misuse outcomes. In contrast, externalizing symptoms had a much larger effect on early onset of prescription opioids than internalizing symptoms, which implies that efforts to prevent externalizing symptoms may be more effective in reducing the chance of early onset of prescription opioid misuse than internalizing symptoms. The heterogeneity implies that different approaches to preventing internalizing and externalizing symptoms may lead to different effects on prescription opioid misuse outcomes. Nevertheless, it remains important to prevent ACEs given the direct relationship between ACEs and opioid misuse.
There were limitations of the present study. First, we were not able to determine the temporal sequence of internalizing and externalizing symptoms, because respondents were asked whether those symptoms ever happened during their lifetime. It is possible that individuals may experience ACEs, misuse prescription opioids and internalizing and externalizing symptoms simultaneously. It is also possible that internalizing or externalizing symptoms developed after the onset of prescription opioid misuse for some individuals as a result of their misuse or life factors. Future longitudinal research to disentangle the temporal relationships among the ACEs, internalizing and externalizing symptoms and prescription opioid misuse are warranted. Second, given the complex statistical analyses, survey weights were not used in this study, and as such, our results may not yield national estimates, which limit generalizability. Third, NESARC-III was conducted in 2012–2013 and therefore our findings may not fully reflect the more recent opioid epidemic (e.g., rapid increase in overdose deaths involving synthetic opioids such as illicitly manufactured fentanyl since 2013). In addition, we used a conservative criterion (Dong et al., 2004; Roos et al., 2013) to establish ACE exposure. While this resulted in a lower ACE score, it may provide a truer sense of exposure to trauma. Despite these limitations, this study still provides important findings to inform the development of public health interventions.
5. Conclusions
Efforts to support safe, stable, and nurturing relationships and environments that can prevent ACEs may also provide an upstream approach to reducing risk for internalizing and externalizing symptoms and prescription opioid misuse (Centers for Disease Control and Prevention, 2014). In addition, interventions that prevent or treat mental health symptoms (e.g., trauma-focused cognitive behavioral therapy, multisystemic therapy) (Cary and McMillen, 2012; van der Stouwe et al., 2014) may reduce the risk of misusing prescription opioids for those already exposed to ACEs. Taken together, these findings highlight the importance of preventing and mitigating the impact of ACEs as a component of the public health strategy for addressing the opioid overdose epidemic.
Supplementary Material
Financial disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of competing interest
The authors have no competing interests or conflicts to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2020.106034.
References
- Arterberry BJ, Horbal SR, Buu A, Lin HC, 2016. The effects of alcohol, cannabis, and cigarette use on the initiation, reinitiation and persistence of non-medical use of opioids, sedatives, and tranquilizers in adults. Drug Alcohol Depend 159, 86–92. 10.1016/j.drugalcdep.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Austin AE, Shanahan ME, 2018. Association of childhood abuse and neglect with prescription opioid misuse: examination of mediation by adolescent depressive symptoms and pain. Child Youth Serv. Rev 86, 84–93. [Google Scholar]
- Boyd CJ, McCabe SE, Cranford JA, Young A, 2006. Adolescents’ Motivations to Abuse Prescription Medications. 118(6). Pediatrics, pp. 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary CE, McMillen JC, 2012. The data behind the dissemination: a systematic review of trauma-focused cognitive behavioral therapy for use with children and youth. Child Youth Serv. Rev 34 (4), 748–757. [Google Scholar]
- Center for Behavioral Health Statistics and Quality, 2018. 2017. National Survey on Drug Use and Health: Detailed Tables (Rockville, MD: ). [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Essentials for Childhood: Steps to Create Safe, Stable, Nurturing Relationships and Environments National Center for Injury Prevention and Control, Atlanta, GA. [Google Scholar]
- Colder CR, Scalco M, Trucco EM, Read JP, Lengua LJ, Wieczorek WF, Hawk LW Jr., 2013. Prospective associations of internalizing and externalizing problems and their co-occurrence with early adolescent substance use. J. Abnorm. Child Psychol 41 (4), 667–677. 10.1007/s10802-012-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy E, Degenhardt L, Mattick RP, Nelson EC, 2009. Child maltreatment as a risk factor for opioid dependence: comparison of family characteristics and type and severity of child maltreatment with a matched control group. Child Abuse Negl 33 (6), 343–352. 10.1016/j.chiabu.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Ferdon C, Vivolo-Kantor AM, Dahlberg LL, Marshall KJ, Rainford N, Hall JE, 2016. A Comprehensive Technical Package for the Prevention of Youth Violence and Associated Risk Behaviors National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Dennis ML, Chan YF, Funk RR, 2006. Development and validation of the GAIN short screener (GSS) for internalizing, externalizing and substance use disorders and crime/violence problems among adolescents and adults. Am. J. Addict 15 (Suppl. 1), 80–91. 10.1080/10550490601006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Giles WH, 2004. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl 28 (7), 771–784. 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF, 2003. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111 (3), 564–572. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fiellin LE, Tetrault JM, Becker WC, Fiellin DA, Hoff RA, 2013. Previous use of alcohol, cigarettes, and marijuana and subsequent abuse of prescription opioids in young adults. J. Adolesc. Health 52 (2), 158–163. 10.1016/j.jadohealth.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, Xu L, 2016. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med. Care 54 (10), 901–906. 10.1097/mlr.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortson BL, Klevens J, Merrick MT, Gilbert LK, Alexander SP, 2016. Preventing Child Abuse and Neglect: A Technical Package for Policy, Norm, and Programmatic Activities National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Garland EL, Hanley AW, Thomas EA, Knoll P, Ferraro J, 2015. Low dispositional mindfulness predicts self-medication of negative emotion with prescription opioids. J. Addict. Med 9 (1), 61–67. 10.1097/adm.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, Hasin DS, 2015. The alcohol use disorder and associated disabilities interview schedule-5 (AUDADIS-5): reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend 148, 27–33. 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Hasin DS, 2016. Epidemiology of DSM-5 drug use disorder: results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry 73 (1), 39–47. 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesler PC, Hu MC, Wall MM, Kandel DB, 2019. Nonmedical prescription opioid use by parents and adolescents in the US. Pediatrics 143 (3). 10.1542/peds.2018-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath LS, Milich R, Lynam D, Leukefeld C, Clayton R, 2004. Sensation Seeking and Substance Use: A Cross-Lagged Panel Design. 2 Individual Differences Research, pp. 3. [Google Scholar]
- Hunt TK, Slack KS, Berger LM, 2017. Adverse childhood experiences and behavioral problems in middle childhood. Child Abuse Negl 67, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M, 2008. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu. Rev. Clin. Psychol 4, 325–348. 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Joireman J, Anderson J, Strathman A, 2003. The aggression paradox: understanding links among aggression, sensation seeking, and the consideration of future consequences. J. Pers. Soc. Psychol 84 (6), 1287–1302. 10.1037/0022-3514.84.6.1287. [DOI] [PubMed] [Google Scholar]
- Jones CM, Christensen A, Gladden RM, 2017. Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004–2013. Drug Alcohol Depend 176, 89–95. 10.1016/j.drugalcdep.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99 (12), 1548–1559. 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Lin HC, Jester JM, Buu A, 2016. The relationships of cigarette and alcohol use with the initiation, reinitiation, and persistence of Cannabis use. J Stud Alcohol Drugs 77 (1), 113–120. [DOI] [PubMed] [Google Scholar]
- Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL, 2012. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the national epidemiologic study on alcohol and related conditions. Psychol. Med 42 (6), 1261–1272. 10.1017/s0033291711002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, Guinn AS, 2018. Prevalence of adverse childhood experiences from the 2011–2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr 172 (11), 1038–1044. 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF, 2011. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend 119 (3), 216–223. 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ompad DC, Ikeda RM, Shah N, Fuller CM, Bailey S, Morse E, Vlahov D, 2005. Childhood sexual abuse and age at initiation of injection drug use. Am. J. Public Health 95 (4), 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi R, Yuma-Guerrero P, Sergi K, Pena AA, Shillington AM, 2018. Drug overdose and child maltreatment across the United States’ rural-urban continuum. Child Abuse Negl 86, 358–367. 10.1016/j.chiabu.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Pophillat E, Rooney RM, Nesa M, Davis MC, Baughman N, Hassan S, Kane RT, 2016. Preventing internalizing problems in 6–8 year old children: a universal school-based program. Front. Psychol 7, 1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg AR, Miller Lo EJ, Barton AA, Xiao H, Vallone DM, Hair EC, 2019. Cigarette smoking, prescription opioid use and misuse among young adults: an exploratory analysis. Prev. Med 129, 105845 10.1016/j.ypmed.2019.105845. [DOI] [PubMed] [Google Scholar]
- Roos LE, Mota N, Afifi TO, Katz LY, Distasio J, Sareen J, 2013. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am. J. Public Health 103 (Suppl. 2), S275–S281. 10.2105/ajph.2013.301323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, Haegerich TM, 2018a. Quantifying the epidemic of prescription opioid overdose deaths. Am. J. Public Health 108 (4), 500–502. 10.2105/ajph.2017.304265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018b. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. MMWR Morb. Mortal. Wkly Rep 67 (12), 349–358. 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, 2012. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129 (1), e232–e246. 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M, Feinberg M, 2013. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am. J. Public Health 103 (4), 665–672. 10.2105/AJPH.2012.301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Conti MT, Kenney S, Anderson BJ, Flori JN, Risi MM, Bailey GL, 2017. Adverse childhood experience effects on opioid use initiation, injection drug use, and overdose among persons with opioid use disorder. Drug Alcohol Depend 179, 325–329. 10.1016/j.drugalcdep.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stouwe T, Asscher JJ, Stams GJJ, Deković M, van der Laan PH, 2014. The effectiveness of multisystemic therapy (MST): a meta-analysis. Clin. Psychol. Rev 34 (6), 468–481. [DOI] [PubMed] [Google Scholar]
- Valeri L, VanderWeele TJ, 2013. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 18 (2), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LC, Scarpa A, 2011. The link between sensation seeking and aggression: a meta-analytic review. Aggress. Behav 37 (1), 81–90. 10.1002/ab.20369. [DOI] [PubMed] [Google Scholar]
- Young A, McCabe SE, Cranford JA, Ross-Durow P, Boyd CJ, 2012. Nonmedical use of prescription opioids among adolescents: subtypes baed on motivation for use. J. Addict. Dis 31 (4), 332–341. 10.1080/10550887.2012.735564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, 2000. Personality and risk-taking: common biosocial factors. J. Pers 68 (6), 999–1029. 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


