Abstract
The beginning of plant cultivation is one of the most important cultural transitions in human history1–4. Based on molecular markers showing the genetic similarities between domesticated plants and wild relatives, south-western Amazonia has been proposed as one of the early centres of plant domestication4–6. However, the nature of the early human occupation and the history of plant cultivation in south-western Amazonia are still little understood. Here, we document the cultivation of Cucurbita at ca. 10,250 cal yr BP, Manihot at ca. 10,350 cal yr BP and Zea mays at ca. 6,850 cal yr BP in the Llanos de Moxos. We show that, starting ca. 10,850 cal yr BP, pre-Columbians created an anthropic landscape made of approximately 4,700 artificial forest islands within a treeless seasonally flooded savannah. Our results confirm the Llanos de Moxos as a hotspot for early plant cultivation and demonstrate that ever since their arrival, humans have caused a profound alteration of Amazonian landscapes, with lasting repercussions for habitat heterogeneity and species conservation.
Recent genetic and archaeological evidence suggests the existence of at least four independent centres of early Holocene domestication, two in the Old World and two in the New World (Mesoamerica and northern South America)1. However, the closest wild ancestors of several globally important domesticated cultigens occur in south-western (SW) Amazonia. These include the wild ancestors of manioc (Manihot esculenta) M. e. subsp. flabellifolia7; squash (Cucurbita maxima spp. maxima) C. m. spp. andreana8; peach palm (Bactris gasipaes)9; jack bean (Canavalia plagiosperma) C. piperi4 and chili peppers’ (Capsicum baccatum var. pendulum) C. baccatum var. baccatum10. This suggests that SW Amazonia could be the fifth global early Holocene centre of domestication. However, with the exception of Calathea sp. phytoliths, possibly representing lerén (C. alluoia), recently documented in the upper Madeira11, archaeological evidence has not been found for early plant cultivation in SW Amazonia. Our research fills this gap with new data from 61 early and mid-Holocene archaeological sites that we refer to as forest islands (FIs)12–14 because they now occur as patches of forest surrounded by savannah.
Mapping of forest islands
Using remote sensing data, we mapped 6643 FIs in the Llanos de Moxos (LM). The average size of FIs is 0.5 ha (min: 0.05 ha; max: 16 ha; SD 0.65 ha). We surveyed 82 of these, which represents ca. 1.2% of all sites. We took column sediment samples in all of them and carried out archaeological excavations in four. Out of the total 82 sites sampled, 83 including Monte Castelo in Brazil14, we classified 64 sites as anthropic, based on the presence of deep dark sediments rich in organic matter, charcoal, and burned earth frequently associated with shell and bone fragments (Fig. 1). The FIs surveyed are between ~0.5 m and 3 m high. The proportion of anthropic vs. natural sites suggests the existence of at least 4700 anthropic FIs in the LM (Fig. ED1). This is probably far less than the original amount built in the early and mid-Holocene, as during the transition to the late Holocene, most of the rivers in the SW part of the LM became very active and many of the pre-existing soils and potential archaeological sites were covered by alluvial deposits, sometimes up to 5 m thick15. This explains the modern distribution of FIs and why 48% of the 6643 FIs we mapped are concentrated in a relatively small area in the north-western LM (Fig. ED1), where the landscape did not change notably during this period. Anthropic FIs are mostly located in interfluvial settings covered by seasonally flooded savannahs, and they account for an estimated 24 km2 of forested area and 1,000 km of forest/savannah ecotone.
Figure 1.
Forest islands mapped in the LM. Numbers associated with middens are ages expressed in median cal yr BP from the deepest anthropic datable layer at each site (Table ED1). Insets a, b, d and e identify the four areas that were surveyed to estimate the total number of anthropic FIs in the LM (Fig. ED1). The square in inset c identifies the study area; the shaded area in inset c depicts Greater Amazonia.
Sixty-six AMS 14C dates from 31 archaeological sites (Table S1) bracket the human occupation of FIs throughout the Holocene between ca.10,850 and 2,300 cal yr BP. Except for three sites in the north-eastern LM dated at ca. 2,350, 2,350 and 4,100 cal yr BP, the remaining dated sites were established between the early and mid-Holocene.
Evidence of early plant cultivation
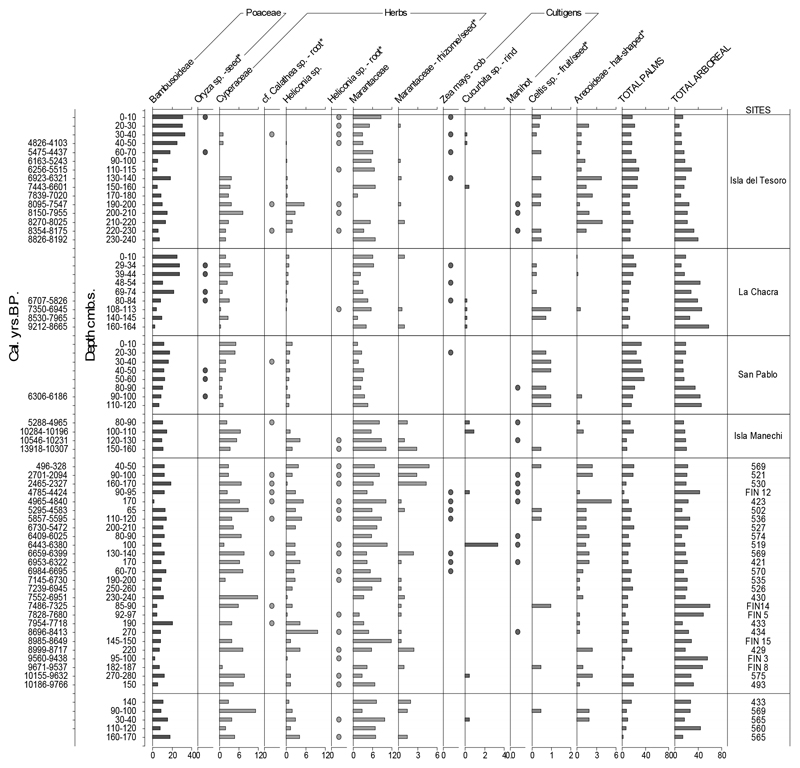
We analysed phytoliths from the radiocarbon-dated profiles of 30 FIs (Figs. 2, 3). The earliest evidence of Manihot is a heart-shaped phytolith16 documented at the Isla Manechi dated ca. 10,350 cal yr BP and in Isla del Tesoro dated ca. 8,250 cal yr BP (Fig. 1). Scalloped sphere phytoliths derived from the rind of Cucurbita sp. were identified in layers dated to ca. 10,250 cal yr BP in Isla Manechi and ca. 9,850 cal yr BP in Site 575. We have identified wavy-top rondel phytoliths, produced in the cob of maize17, dated ca. 6,850 cal yr BP (Site 570) and ca. 6,700 cal yr BP (Site 421). We detected the early presence of Marantaceae Calathea sp. rhizome phytoliths at ca. 8,275 cal yr BP (Isla del Tesoro), before ca. 7,800 cal yr BP (Site 433) and at ca. 7,400 cal yr BP (Site FIN 14). Other Marantaceae, Cyperaceae, Phenakospermum guianensis, and Heliconia phytoliths have been found in almost all the samples starting from ca. 10,400 cal yr BP (Fig. 2, S2). We identified Oryza seed phytoliths dated at ca. 6,250 cal yr BP (San Pablo); and phytoliths from the epidermis of Celtis seeds from contexts dated to ca. 9,600 cal yr BP (Site FIN8). Hat-shaped and globular echinate phytoliths diagnostic of subfamily Arecoideae17 of the palm family (Arecaceae) are present at ca. 9,975 cal yr BP (site 493). Peach palm (Bactris sp.), a member of this subfamily, is the only palm domesticated in South America, and its domestication likely took place in SW Amazonia6. Bactris produces these hat-shaped phytoliths, but does not produce diagnostic phytoliths at the species level18. Bactris and other arecoid genera growing today in the LM (Astrocaryum, Desmoncus, Geonoma and Socratea), are used for food, building materials and medicine in present Amazonia19. The size of squash phytoliths is well in the range of the domesticated species (table ED2), however, they do not show increase in size over time as would be expected under domestication pressure (Table. ED2)17.The presence of domesticated Cucurbita sp. beginning at ~10,250 cal yr BP in Isla Manechi is the oldest evidence of Cucurbita sp. in Amazonia and coincides with the domestication of several species of Cucurbita across Central 20 and South America21,22 at the very beginning of the Holocene. Further studies analysing larger sample sizes are needed to determine whether the domesticated squash cultivated in the early Holocene was adopted in the LM from other regions or was domesticated in situ. Maize cob phytoliths were documented at site 570 ca. 6,850 cal yr BP representing the oldest evidence, by a few centuries, of maize cultivation in the Amazon basin. As hypothesized by Kistler et al.23, early maize in this and other areas probably represented a partially domesticated variety that later diverged into two South American groups of fully domesticated maize varieties. This early evidence of maize phytoliths is consistent with a temporal gradient of maize dispersal that began in western Amazonia and reached the eastern Amazon by ~4,300 cal yr BP. The early use of Manihot in Isla Manechi in the LM began more than 10,000 years ago, which coincides with the time of estimated molecular divergence from the wild ancestor and with current biogeography of manioc’s closest wild ancestor7,24. It possibly spread later to northern Peru (8,500 cal yr BP), Colombia (7,000 cal yr BP) and Panama (7,600 cal yr BP)5suggesting the bi-directional exchange of cultivars between Amazonia and the Andes beginning since the early Holocene. Our study shows that, as in other regions of Amazonia and Central America, also in the LM the development or arrival of full-blown agricultural societies was a very late phenomenon9, as there is no evidence of land prepared for agriculture in the LM until raised fields and drainage canals were built around 1,500-1,000 years ago25.
Figure 2.
Diagram showing the percentage of most relevant phytolith groups from anthropic forest islands. Dots indicate presence ≤ 1% (for more details see Fig. ED2 and table ED1). *Non-domesticated, edible plants (used as food resources)26. Phytolith percentages are based on a minimum of 200 diagnostic phytoliths counted per slide.
Figure 3.
Photomicrographs of phytolith morphotypes recovered from Isla del Tesoro, La Chacra and Isla Manechi: (a) wavy-top rondel from the cob of maize (Zea mays) (sampleIT190-200); (b) heart-shaped phytolith from the secretory cells of manioc (Manihot) (sample BANR17-UE1-57); (c) scalloped sphere from the rind of squash (Cucurbita sp.) (sample BANR17-UE1-31); (d) double-peaked glume from the seed of rice (Oryza sp.) (sample SM3-116s); (e) flat domed cylinder from the rhizome of Calathea sp. (sample IT30-40); (f) short trough body from the rhizome of Heliconia sp. (sample IT130-140); (g) stippled polygonal body from the seed of Cyperaceae (sample IT150-170); (h) nodular projections with a pointed apex phytolith from the seed of Marantaceae (sampleIT90-100) (i) stippled plate from the seed/fruit of hackberries (Celtis sp.) (sample SM3-69-74). Scale bars = 20μm.
The importance of starch-based foods
Paleoecological studies indicate that the LM was covered by cerrado-like savannah during the early and mid-Holocene15 indicating that plant cultivation in the Neotropics also started in shrub savannahs along with seasonal tropical forest environments4. It does not come as a surprise that phytoliths derived from plants producing underground storage organs constitute an important part of the total phytolith assemblages from the LM’s FIs including Manihot, Calathea and other Marantaceae, Heliconia, Cyperaceae and Phenakospermum. These plants, which are abundant in savannahs, produce carbohydrate-rich foods which, with the exception of some varieties of manioc27, are easy to process and cook. They are nowadays consumed by indigenous groups19 and probably provided a considerable part of the calories consumed by the first inhabitants of the LM. Large herbivores and fish available in the savannahs12,13 would have complemented a mixed economy. The fertile FIs were probably the home gardens where these crops were cultivated. Our data are in agreement with the hypothesis that plants producing underground storage organs were a fundamental part of the diet of human populations colonizing new territories28,29.
Implication for biodiversity
Our results show that inland savannahs were a key region for the early occupation of Neotropics and that they began to be transformed by the arrival of very early human settlers. FIs are entirely anthropic. However, their formation is not only an incidental effect of food-waste dumping, but it can also be seen as an active process of niche construction30. These accumulative middens also constitute fertility hotspots amid poor savannah soils, since: i) they became loci for the accumulation of nutrients that come from gathering activities in the surrounding savannah, and ii) they remained above the water level during the wet season12. It is only after 4,000 years BP, when the old and infertile soils of the south of the LM were covered with fertile alluvium deposited by the Río Grande, that agriculture in the savannahs was facilitated31. Overall, the construction of pre-Columbian FIs, which became key structures in the landscape32,33, increased forest patchiness (Fig. ED3, ED4a) and probably contributed to maintaining landscape-scale species richness in this Ramsar threatened biome. Nowadays, these anthropic FIs are preferential feeding and roosting sites for many species of birds, including the endemic and critically endangered blue-throated macaw (Ara glaucogularis)34. Taken together, our data show that the earliest inhabitants of the LM were not just tropical hunter-gatherers, but colonizers who had engaged in plant cultivation since the early Holocene, thus opening up the possibility that they already had a mixed economy when they arrived in the region. The thousands of keystone structures represented by FIs show that the human footprint on Amazonia is not just restricted to large-scale transformations by late Holocene farming groups9,35, but is rooted in the earliest human dispersal into this region and has lasting implications for habitat heterogeneity and biodiversity conservation.
Methods
Mapping of FIs
FIs were mapped by visual scanning of high-resolution satellite imagery of Esri ArcGIS basemaps (Fig. ED5). When the identification of FIs was not straightforward due to cloud cover or poor resolution, TanDemX and SRTM digital elevation models were used as complementary resources. Patches of forest were classified as FIs when they had a round shape and were completely or partially surrounded by savannah (n = 4,341); or they had an irregular shape but were relatively small (<400 m in diameter) and completely surrounded by savannah (n = 2,304). For each FI, the following attributes were recorded: diameter; shape (perfectly round, almost round, elongated or irregular); location (along paleochannel, along modern river, seasonally flooded savannah, border between seasonally flooded savannah and upland, along a drainage stream, upland surrounded by bushes); presence of other earthworks within ~500 meters; and whether or not FIs were established over fluvial deposits or uplands. The latter attribute is partly redundant with “Location” but sometimes fluvial deposits are not connected to paleochannels (as in the case of old crevasse splays or old meander belts where paleochannels have been infilled) or the FIs are located along a paleochannel with completely eroded levees and the FIs have clearly been built after the erosion of the levees.
Selection of survey areas
Four survey areas were selected in different regions of the LM to ground-truth FIs and evaluate their natural or anthropic origin. The four areas (Fig. 1) were selected based on differences in soil, landcover, hydrology, and accessibility by car. These four areas cover all the different eco-regions identified in the LM36,37. These areas belonged to organizations (area a -see inset in Fig. 1- in the Barba Azul Nature Reserve) or ranchers (b, c, and d) who granted us permission to conduct surveys. In total, we surveyed 21 FIs in area a, 22 in b, 13 in d, and 17 in e. Nine other FIs were surveyed outside of these four areas.
Criteria for identification of anthropic FIs
Several anthropic FIs in the LM have been excavated 12–14. These excavations have revealed thick strata of sediments rich in organic matter, charcoal, burnt earth, and fragmented animal bones and shells; they also have revealed human burials. The clear difference between the sediments found in the anthropic FIs and the soil types found in the LM38–41 makes the field identification of FIs relatively straightforward. In the present work, the FIs surveyed have been classified as anthropic when thick layers of organic-rich sediments contained at least two archaeological materials such as charcoal, burnt earth, animal bones, and shells.
Sampling of FIs
Sampling of undisturbed material was performed at regular intervals in the four sites where archaeological excavations were conducted: Isla del Tesoro (SM1), La Chacra (SM3), San Pablo (SM4), and Isla Manechi (Fig. ED6). The rest of the sites were sampled using an auger soil sampler. The stratigraphy of the recovered cores was described in the field and sampling was carried out only where stratigraphic changes were detected in the field (Fig. ED7). The deepest sample with evidence of charcoal was always sampled. After extraction, cores were inspected to avoid contamination and check that the soil section sampled show no evidence of soil mixing (i.e. root penetration and invertebrate burrowing were absent). The excess of material has been cut off with a knife and only the inner, uncontaminated part of the extracted samples has been stored in plastic bags. Samples have been air-dried in Bolivia before being shipped. Charcoal fragments for 14C have been collected in situ, enveloped in aluminium foil and stored in plastic bags.
Phytolith processing and identification
Phytoliths were extracted from sediments following Lombardo et al.42. Phytoliths were identified and counted using a Zeiss Axioscope 40 light microscope at 500× magnification. Phytolith identifications were made using published material for the Neotropics17,43–46 and by direct comparison with the phytolith reference collection of the Archaeobotany and Palaeoecology Laboratory in the Department of Archaeology of the University of Exeter. A minimum of 200 diagnostic phytoliths were counted per slide. A full scan of the slides was performed to detect the presence of squash, manioc and maize. Phytolith assemblages in SW Amazonia have been studied in modern soils46 and 29 paleosols from the early and late Holocene15 in different natural environments and land covers. In none of these natural contexts have phytoliths of Manihot or Curcubita been found, strongly suggesting that phytoliths of these two genera found in FIs are the direct result of human activity and not of the chance occurrence of wild relatives on these FIs
Radiocarbon dates
The deepest recoverable sample of charcoal from 32 sites was dated in order to establish the minimum age of site foundation. For the four sites that were excavated, 35 samples from different depths were dated in order to establish periods of occupation and abandonment. The complete dataset and code used to calibrate all of radiocarbon dates are available respectively in Table ED1 and Supplementary Information. Radiocarbon dates from the studied sites were calibrated using SHCAL1347. For Isla del Tesoro, Isla Manechi and La Chacra, where stratigraphically ordered ages were available, we run a series of Bayesian age-depth models using the P_Sequence command in OxCal 4.348 with default settings. Each model was stratigraphically constrained by the youngest age in the profile and the deepest section reached in each site (Fig. ED8). Ages of undated samples were estimated using the command Date within the model.
Extended Data
Fig. ED1.
Estimated number of anthropic FIs. The number was estimated by extending the proportion of anthropic FIs in the surveyed areas (in green) to the portion of the LM with similar physical geography and land cover (polygons). Background image represents the density of FIs, calculated using the Kernel Density tool in Esri ArcGIS.
Fig. ED2.
Detailed percentage phytolith diagram for all the samples analyzed from anthropogenic FIs. *Non-domesticated, edible plants. Phytolith percentages are based on a minimum of 200 diagnostic phytoliths that were counted per slide
Fig. ED3.
Map of all the FIs and platform fields in a north-western subset of the LM. Platform fields are mostly built along paleochannels. Rivers Omi and Yruyañez flow inside old channels of the Beni River. FIs are mostly located in interfluvial areas. The region contains a total of 2428 patches of forest, 955 of which are FIs. Once all the patches of forest within a 2-km buffer of a river, paleoriver and lake are removed, FIs account for 60% of the remaining 1191 patches.
Fig. ED4.
Characteristics of FIs. a) Photo of the anthropic landscape dotted with forest islands in the Barba Azul Nature Reserve; b) Histogram showing the distribution of the diameter of FIs. The left side of the distribution is truncated at 25 m because smaller FIs have not been mapped; c) Photo taken at Site 579, natural FI. Samples are taken every 10 cm, from top left to down right. Material is silt with no organic matter; d) Photo taken at Site 425, anthropogenic FI. Depth of the sample 140 cm; e) Photo taken at Site 430, anthropogenic FI. Depth of the sample 160 cm. Samples in d) and e) are representative of the whole profile in site 425 and 430 respectively (see also Fig. ED7). We performed one core for each FI we visited.
Fig. ED5.
Examples of surveyed FIs as seen in high-resolution satellite imagery of Esri ArcGIS basemap. a-f: FIs classified as anthropic (a, Sm4; b, Isla Manechi; c, Site 575; d, SM3; e, FIN 12; f, Isla del Tesoro); g-i: FIs classified as natural (g, FIN2; h, FIN11; I, Sire 529). Source: ESRI, DigitalGlobe, GeoEye, Earthstar Georaphics,CNES/Airbus DS.
Fig. ED6.
Stratigraphic profile and sampling sites at Isla Manechi. Charcoal fragments for AMS 14C dating were collected during the excavation and yellow triangles indicate their depth. Yellow squares indicate the sampling locations of sediments analyzed for phytoliths, which have been sampled after the excavation from the vertical profile. The dashed line indicates the transition from the ceramic phase to the pre-ceramic one. This transition is characterized by a sharp increase in the compactness of sediments and amount of burnt earths. Values in parenthesis are median calibrated radiocarbon ages BP. A chronological gap of almost 4500 years exists between the two phases (Table ED1). All of these differences make it possible to exclude any contamination of phytoliths coming from the ceramic to the pre-ceramic contexts. For a description of archaeological excavations at Isla del Tesoro (SM1), La Chacra (SM3), and San Pablo (SM4) see Capriles et al.13
Fig. ED7.
Stratigraphic descriptions of cored sites. Radiocarbon dates are included in ovals as median calibrated years before present.
Fig. ED8.
Age-depth models of the modelled profiles. a) and b), Isla del Tesoro; c) Isla Manechi; d) and e) Isla La Chacra. The age-depth models have been produced using OxCal V4.3, code is available in Supplementary Information
Table ED1.
Radiocarbon ages of all the dated sites cited in the text including provenance and calibrations. Stratigraphically ordered dated depths have been modelled using a Bayesian age-depth model (OxCal 4.3’s P_Sequence). The modelled ages for samples BANR17-UE1-53 and BANR17-UE1-64 have been calculated using the Date command. Code is available in Supplementary Information.
| Lab _ Code | Site | 14C BP | 14C Age SD | Unmodelled (BP) 95.4% | Modelled (BP) 95.4% | Median | Material | Depth (cm) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| from | to | from | to | |||||||
| D-AMS-1737 | SM4, San Pablo | 5476 | 35 | 6306 | 6124 | 6239 | Bulk | 58 | ||
| Poz-46397 | SM4, San Pablo | 5190 | 80 | 6178 | 5664 | 5898 | Charcoal | 65 | ||
| D-AMS-1741 | SM4, San Pablo | 5490 | 32 | 6306 | 6186 | 6245 | Bulk | 93 | ||
| PSUAMS-4659 | SM4, San Pablo | 6665 | 25 | 7571 | 7441 | 7512 | Charcoal | 150 | ||
| D-AMS-1739 | SM4, San Pablo | 6910 | 30 | 7787 | 7621 | 7696 | Charcoal | 150 | ||
| Poz-46396 | SM4, San Pablo | 7700 | 90 | 8636 | 8218 | 8463 | Charcoal | 197 | ||
| PSUAMS-1450 | SM3, La Chacra | 6030 | 30 | 6935 | 6736 | 6925 | 6734 | 6825 | Charcoal | 100 |
| Poz-38862 | SM3, La Chacra | 5140 | 40 | 5930 | 5733 | 5933 | 5733 | 5825 | Shell | 107 |
| PSUAMS-1563 | SM3, La Chacra | 6650 | 30 | 7566 | 7437 | 7568 | 7441 | 7506 | Charcoal | 121 |
| PSUAMS-1564 | SM3, La Chacra | 7930 | 30 | 8972 | 8590 | 8951 | 8586 | 8672 | Charcoal | 155 |
| Poz-38865 | SM3, La Chacra | 7860 | 50 | 8847 | 8435 | 8682 | 8426 | 8557 | Shell | 236 |
| Poz-38866 | SM3, La Chacra | 7790 | 80 | 8760 | 8383 | 8748 | 8456 | 8590 | Charcoal | 240 |
| Poz-38853 | SM2, San Francisco | 4950 | 40 | 5736 | 5585 | 5638 | Shell | 85 | ||
| Poz-38850 | SM2, San Francisco | 4770 | 60 | 5588 | 5320 | 5465 | Charcoal | 155 | ||
| Poz-38851 | SM2, San Francisco | 5380 | 40 | 6272 | 5994 | 6117 | Shell | 205 | ||
| Poz-38852 | SM2, San Francisco | 5500 | 40 | 6393 | 6128 | 6253 | Charcoal | 205 | ||
| Poz-34228 | SM1, Isla del Tesoro | 345 | 25 | 452 | 304 | 391 | Bone | 15 | ||
| Poz-34229 | SM1, Isla del Tesoro | 3895 | 35 | 4411 | 4153 | 4414 | 4155 | 4292 | Shell | 35 |
| Poz-34230 | SM1, Isla del Tesoro | 4945 | 35 | 5722 | 5586 | 5721 | 5585 | 5629 | Shell | 45 |
| Poz-28854 | SM1, Isla del Tesoro | 3830 | 50 | 4405 | 3986 | 4172 | Shell | 48 | ||
| Poz-28855 | SM1, Isla del Tesoro | 4415 | 35 | 5210 | 4849 | 4937 | Charcoal | 77 | ||
| Poz-22902 | SM1, Isla del Tesoro | 5520 | 40 | 6395 | 6190 | 6280 | Charcoal | 115 | ||
| Poz-34231 | SM1, Isla del Tesoro | 5520 | 40 | 6395 | 6190 | 6295 | 6190 | 6234 | Shell | 115 |
| Poz-24633 | SM1, Isla del Tesoro | 5360 | 40 | 6266 | 5950 | 6096 | Shell | 115 | ||
| D-AMS-1740 | SM1, Isla del Tesoro | 5502 | 30 | 6310 | 6190 | 6309 | 6207 | 6275 | Charcoal | 117 |
| PSUAMS-4658 | SM1, Isla del Tesoro | 5565 | 20 | 6398 | 6281 | 6393 | 6278 | 6305 | Charcoal | 120 |
| Poz-24634 | SM1, Isla del Tesoro | 5505 | 35 | 6388 | 6184 | 6262 | Charcoal | 120 | ||
| Poz-34232 | SM1, Isla del Tesoro | 5460 | 40 | 6304 | 6021 | 6302 | 6205 | 6254 | Shell | 125 |
| Poz-28856 | SM1, Isla del Tesoro | 4480 | 40 | 5284 | 4872 | 5047 | Charcoal | 140 | ||
| Poz-28850 | SM1, Isla del Tesoro | 4495 | 35 | 5288 | 4886 | 5109 | Shell | 140 | ||
| Poz-36136 | SM1, Isla del Tesoro | 5800 | 35 | 6657 | 6453 | 6660 | 6461 | 6563 | Charcoal | 160 |
| D-AMS 032885 | SM1, Isla del Tesoro | 7271 | 40 | 8162 | 7966 | 8151 | 7956 | 8019 | Bulk orqanic | 205 |
| D-AMS 032884 | SM1, Isla del Tesoro | 7447 | 37 | 8348 | 8065 | 8347 | 8174 | 8274 | Bulk orqanic | 225 |
| Poz-34301 | SM1, Isla del Tesoro | 9270 | 60 | 10556 | 10248 | 10573 | 10259 | 10434 | Bulk orqanic | 235 |
| Poz-36135 | SM1, Isla del Tesoro | 9420 | 50 | 10743 | 10433 | 10715 | 10416 | 10574 | Charcoal | 245 |
| BE-4254.1.1 | FIN8_182-187 | 8681 | 22 | 9671 | 9537 | 9582 | Charcoal | 185 | ||
| BE-4253.1.1 | FIN5_92-97 | 6963 | 25 | 7828 | 7680 | 7745 | Shell | 95 | ||
| BE-4250.1.1 | FIN3_95-100 | 8572 | 48 | 9560 | 9438 | 9514 | Charcoal | 97 | ||
| BE-4257.1.1 | FIN15_145-150 | 7997 | 25 | 8985 | 8649 | 8834 | Shell | 147 | ||
| BE-4256.1.1 | FIN14_85-90 | 6552 | 25 | 7486 | 7325 | 7429 | Shell | 87 | ||
| BE-4255.1.1 | FIN12_90-95 | 4092 | 24 | 4785 | 4424 | 4522 | Shell | 92 | ||
| BE-8256.1.1 | BANR17-UE1-5 | 3017 | 21 | 3236 | 3005 | 3319 | 3007 | 3144 | Charcoal | 25 |
| BE-8257.1.1 | BANR17-UE1-20 | 4324 | 22 | 4959 | 4743 | 4957 | 4728 | 4848 | Charcoal | 55 |
| BE-8258.1.1 | BANR17-UE1-31 | 4491 | 23 | 5285 | 4888 | 5288 | 4965 | 5172 | Charcoal | 85 |
| BE-8259.1.1 | BANR17-UE1-38 | 9138 | 24 | 10367 | 10195 | 10284 | 10196 | 10239 | Charcoal | 105 |
| BANR17-UE1-53 | 10546 | 10231 | 10372 | 125 | ||||||
| D-AMS 029221 | BANR17-UE1-57 | 9346 | 41 | 10653 | 10298 | 10664 | 10380 | 10521 | Charcoal | 145 |
| BANR17-UE1-64 | 13918 | 10307 | 10883 | 155 | ||||||
| BE-7663.1.1 | 575_270-280 | 8849 | 50 | 10155 | 9632 | 9848 | Charcoal | 275 | ||
| BE-7671.1.1 | 574_80-90 | 5516 | 62 | 6409 | 6025 | 6273 | Charcoal | 85 | ||
| BE-7667.1.1 | 570_60-70 | 6046 | 48 | 6984 | 6695 | 6844 | Charcoal | 65 | ||
| BE-7675.1.1 | 569_40-50 | 407 | 19 | 496 | 328 | 453 | Charcoal | 45 | ||
| BE-7672,1.1 | 569_130-140 | 5759 | 53 | 6659 | 6399 | 6512 | Charcoal | 135 | ||
| BE-7664.1.1 | 536_110-120 | 4994 | 37 | 5857 | 5595 | 5678 | Charcoal | 115 | ||
| BE-7668.2.1 | 535_190-200 | 6069 | 51 | 7145 | 6730 | 6871 | Charcoal | 195 | ||
| BE-7673.2.1 | 530_160-170 | 2397 | 20 | 2465 | 2327 | 2362 | Charcoal | 165 | ||
| BE-7661.1.1 | 527_200-210 | 5337 | 281 | 6730 | 5472 | 6076 | Charcoal | 205 | ||
| BE-7662.1.1 | 526_250-260 | 6217 | 40 | 7239 | 6945 | 7076 | Charcoal | 255 | ||
| BE-7674.1.1 | 521_90-100 | 2346 | 89 | 2701 | 2094 | 2331 | Charcoal | 95 | ||
| BE-7666.1.1 | 519_100 | 5647 | 22 | 6443 | 6308 | 6368 | Charcoal | 100 | ||
| BE-6164.1.1 | 502 65 | 4365 | 110 | 5295 | 4583 | 4927 | Charcoal | 65 | ||
| BE-6166.1.1 | 493 155 | 8920 | 49 | 10186 | 9766 | 10006 | char/sed | 155 | ||
| BE-6153.1.1 | 490 95-100 | 3796 | 33 | 4237 | 3985 | 4115 | Shell | 97 | ||
| BE-6167.1.1 | 434 270 | 7811 | 57 | 8696 | 8413 | 8543 | char/sed | 270 | ||
| BE-6163.1.1 | 433 175 | 7058 | 50 | 7954 | 7718 | 7852 | char/sed | 175 | ||
| BE-6168.1.1 | 430 235 | 6397 | 130 | 7552 | 6951 | 7269 | chat/sed | 235 | ||
| BE-6158.1.1 | 429 220 | 8028 | 24 | 8999 | 8717 | 8870 | char/sed | 220 | ||
| BE-6157.1.1 | 423 170 | 4365 | 21 | 4965 | 4840 | 4877 | char/sed | 170 | ||
| BE-6159.1.1 | 421 170 | 5875 | 127 | 6953 | 6322 | 6646 | char/sed | 170 | ||
Table ED2.
Length and thickness range and average size of scalloped-sphere phytoliths identified in this study. Based on Piperno et al. (2000), we consider scalloped spheres longer than 72 μm or thicker than 59 μm as coming from domestic varieties of Cucurbita sp.
| Site | Date cal. yr. B.P. |
Length (μm) |
Thickness (μm) |
Domesticated | Depth |
|---|---|---|---|---|---|
| Isla del Tesoro | 7839-7020 | 80,361 | 59,804 | Yes | 170-180 cm |
| 575 | 10155-9632 | 82,524 | 58,318 | Yes | 270-280 cm |
| 72,804 | 65,178 | Yes | |||
| 72,775 | 54,214 | Yes | |||
| 519 | 6443-6380 | 77,884 | 55,212 | Yes | 100 cm |
| 82,418 | 65,752 | Yes | |||
| 75,938 | 54,452 | Yes | |||
| 75,296 | 57,433 | Yes | |||
| FIN-12 | 4785-4424 | 81.19 | 55,162 | Yes | 90-95 cm |
| 80,653 | 60,988 | Yes | |||
| 61,005 | 45,635 | No | |||
| 77,871 | 67,843 | Yes | |||
| La Chacra | 6707-5826 | 83,697 | 59,724 | Yes | 80-84 cm |
| 78,895 | 52,993 | Yes | |||
| 7350-6945 | 75,789 | 56,754 | Yes | 108-113 cm | |
| 8530-7965 | 63,119 | 41,238 | No | 140-145 cm | |
| 9212-8665 | 75,419 | 65,484 | Yes | 160-165 cm | |
| Isla Manechi | 3319-3007 | 74.28 | 52.33 | Yes | 25 cm |
| 72.7 | 57.57 | Yes | |||
| 78.49 | 50.79 | Yes | |||
| 81.34 | 48.89 | Yes | |||
| 70.1 | 45.38 | Yes | |||
| 78.12 | 64.79 | Yes | |||
| 61.29 | 51 | No | |||
| 69.22 | 56.6 | No | |||
| 61.78 | 46.56 | No | |||
| 59.96 | 49.13 | No | |||
| 4957-4728 | 68.67 | 54.95 | No | 55 cm | |
| 57.22 | 40.23 | No | |||
| 70.53 | 49.5 | No | |||
| 63.21 | 49.51 | No | |||
| 70.59 | 44.29 | No | |||
| 81.74 | 66.85 | Yes | |||
| 72.76 | 58.61 | Yes | |||
| 5288-4965 | 74.27 | 57.12 | Yes | 85 cm | |
| 74.53 | 45.13 | Yes | |||
| 58.98 | 44.15 | No | |||
| 81.95 | 56.8 | Yes | |||
| 68.1 | 59.06 | No | |||
| 66.84 | 55.37 | No | |||
| 76.91 | 49.26 | Yes | |||
| 74.69 | 47.15 | Yes | |||
| 84.72 | 60.19 | Yes | |||
| 10284-10196 | 78,173 | 66,108 | Yes | 105 cm | |
| 84,996 | 67,836 | Yes | |||
| 72,601 | 54,422 | Yes | |||
| 64,201 | 50,158 | No | |||
| 84,858 | 64,479 | Yes | |||
| 68.44 | 57.37 | No | |||
| 81.82 | 60.62 | Yes | |||
| 86.81 | 63.17 | Yes | |||
| 10664-10380 | 60.46 | 51.16 | No | 145 cm |
Supplementary Material
Supplementary Information is available for this paper.
Acknowledgments
We acknowledge the support of the Bolivian Ministerio de Culturas y Turismo, the Gobierno Autónomo Departamental del Beni, and the owners of the properties where the study sites are located: J.P. Llapiz, F. Boheme, J. Rivero, O. Sikuajara, F. Velasco and T. Boorsma from the Barba Azul Natural Reserve. We thank L. Rodrigues, N. Zihlmann, G.P. Fernández and L.M. Ortega for participation during fieldwork, as well as E. Canal-Beeby, M. Madella, D. Mckey, J Carson, Mario González and Silvia Tin for their support at different stages of this work.
Funding
This work was supported by Swiss National Science Foundation grant nos. 200020-141277/1 and P300P2_158459/1; Marie Skłodowska-Curie Actions EU Project 703045; PAST project funded by the European Research Council (ERC), grant agreement No. ERC_Cog 616179; National Geographic Society grant HJ-074ER-17; TerraSAR-X/TanDEM-X mission, grant no. DEM_OTHER1040; AHRC-FAPESP MoU research grant HERCA, reference AH/S001662/1
Footnotes
Author contributions: U.L. designed the research. U.L., J.M.C., J. RP. and H.V. conducted the fieldwork. J.M.C. and J.RP conducted the archaeological excavations, U.L. conducted the GIS mapping and analyses. U.L., L.H., J.RP., and J.I. carried out the phytoliths analyses. U.L. and J.I. wrote the paper with the help of all authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are included in the paper or in the Extended Data. Code used for 14C calibration in OxCal is available in Supplementary Information.
Reprints and permissions information is available at www.nature.com/reprints
References
- 1.Larson G, et al. Current perspectives and the future of domestication studies. Proceedings of the National Academy of Sciences. 2014;111:6139–6146. doi: 10.1073/pnas.1323964111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zohary D, Hopf M. Domestication of plants in the Old World: the origin and spread of cultivated plants in West Asia, Europe and the Nile Valley. Oxford University Press; 2000. [Google Scholar]
- 3.Zeder MA, Bradley DG, Smith BD, Emshwiller E. Documenting domestication: new genetic and archaeological paradigms. Univ of California Press; 2006. [Google Scholar]
- 4.Piperno DR, Pearsall DM. The origins of agriculture in the lowland Neotropics. 1998. [Google Scholar]
- 5.Piperno DR. The Origins of Plant Cultivation and Domestication in the New World Tropics: Patterns, Process, and New Developments. Current Anthropology. 2011;52:S453–S470. doi: 10.1086/659998. [DOI] [Google Scholar]
- 6.Clement CR, de Cristo-Araújo M, d’Eeckenbrugge GC, Alves Pereira A, Picanço-Rodrigues D. Origin and Domestication of Native Amazonian Crops. Diversity. 2010;2:72–106. doi: 10.3390/d2010072. [DOI] [Google Scholar]
- 7.Olsen KM, Schaal BA. Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am J Bot. 2001;88:131–142. [PubMed] [Google Scholar]
- 8.Sanjur OI, Piperno DR, Andres TC, Wessel-Beaver L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: Implications for crop plant evolution and areas of origin. Proceedings of the National Academy of Sciences. 2002;99:535. doi: 10.1073/pnas.012577299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement CR, et al. The domestication of Amazonia before European conquest. Proceedings of the Royal Society of London B: Biological Sciences. 2015;282 doi: 10.1098/rspb.2015.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaldaferro MA, Barboza GE, Acosta MC. Evolutionary history of the chili pepper Capsicum baccatum L. (Solanaceae): domestication in South America and natural diversification in the Seasonally Dry Tropical Forests. Biological Journal of the Linnean Society. 2018;124:466–478. doi: 10.1093/biolinnean/bly062. %J Biological Journal of the Linnean Society. [DOI] [Google Scholar]
- 11.Watling J, et al. Direct archaeological evidence for Southwestern Amazonia as an early plant domestication and food production centre. PLoS ONE. 2018;13:e0199868. doi: 10.1371/journal.pone.0199868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombardo U, et al. Early and Middle Holocene Hunter-Gatherer Occupations in Western Amazonia: The Hidden Shell Middens. PLoS ONE. 2013;8:e72746. doi: 10.1371/journal.pone.0072746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capriles JM, et al. Persistent Early to Middle Holocene tropical foraging in southwestern Amazonia. Science Advances. 2019;5 doi: 10.1126/sciadv.aav5449. eaav5449, %J Science Advances. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilbert L, et al. Evidence for mid-Holocene rice domestication in the Americas. Nature Ecology & Evolution. 2017;1:1693–1698. doi: 10.1038/s41559-017-0322-4. [DOI] [PubMed] [Google Scholar]
- 15.Lombardo U, et al. Holocene land cover change in south-western Amazonia inferred from paleoflood archives. Global and Planetary Change. 2019;174:105–114. doi: 10.1016/j.gloplacha.2019.01.008. [DOI] [Google Scholar]
- 16.Chandler-Ezell K, Pearsall DM, Zeidler JA. Root and tuber phytoliths and starch grains document manioc (Manihot esculenta) arrowroot (Maranta arundinacea) and llerén (Calathea sp.) at the real alto site Ecuador. Economic Botany. 2006;60:103–120. [Google Scholar]
- 17.Piperno DR. Phytoliths. AltaMira Press; 2006. [Google Scholar]
- 18.Morcote-Ríos G, Bernal R, Raz L. Phytoliths as a tool for archaeobotanical, palaeobotanical and palaeoecological studies in Amazonian palms. Botanical Journal of the Linnean Society. 2016;182:348–360. doi: 10.1111/boj.12438. %J Botanical Journal of the Linnean Society. [DOI] [Google Scholar]
- 19.Hanelt P, Buttner R, Mansfeld R. Mansfeld's Encyclopedia of Agricultural and Horticultural Crops (except Ornamentals) Springer; 2001. [Google Scholar]
- 20.Smith BD. The Initial Domestication of Cucurbita pepo in the Americas 10,000 Years Ago. Science. 1997;276:932–934. doi: 10.1126/science.276.5314.932. %J Science. [DOI] [Google Scholar]
- 21.Piperno DR, Stothert KE. Phytolith Evidence for Early Holocene Cucurbita Domestication in Southwest Ecuador. Science. 2003;299:1054–1057. doi: 10.1126/science.1080365. [DOI] [PubMed] [Google Scholar]
- 22.Dillehay TD, Piperno DR. In: The Cambridge World Prehistory. Renfrew C, Bahn P, editors. Cambridge University Press; 2014. pp. 970–985. [Google Scholar]
- 23.Kistler L, et al. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science. 2018;362:1309–1313. doi: 10.1126/science.aav0207. %J Science. [DOI] [PubMed] [Google Scholar]
- 24.Rival L, McKey D. Domestication and diversity in manioc (Manihot esculenta Crantz ssp. esculenta, Euphorbiaceae) Current Anthropology. 2008;49:1119–1128. [Google Scholar]
- 25.Rodrigues L, Lombardo U, Veit H. Design of pre-Columbian raised fields in the Llanos de Moxos, Bolivian Amazon: Differential adaptations to the local environment? Journal of Archaeological Science: Reports. 2018 [Google Scholar]
- 26.Junqueira AB, Shepard GH, Clement CRJEB. Secondary Forests on Anthropogenic Soils of the Middle Madeira River: Valuation, Local Knowledge, and Landscape Domestication in Brazilian Amazonia. 2011;65:85–99. doi: 10.1007/s12231-010-9138-8. [DOI] [Google Scholar]
- 27.McKey D, Cavagnaro TR, Cliff J, Gleadow RJC. Chemical ecology in coupled human and natural systems: people, manioc, multitrophic interactions and global change. 2010;20:109–133. doi: 10.1007/s00049-010-0047-1. [DOI] [Google Scholar]
- 28.Jones M. The Evolution of Hominin Diets. Springer; 2009. pp. 171–180. [Google Scholar]
- 29.Aceituno FJ, Loaiza N. The origins and early development of plant food production and farming in Colombian tropical forests. Journal of Anthropological Archaeology. 2018;49:161–172. doi: 10.1016/j.jaa.2017.12.007. [DOI] [Google Scholar]
- 30.Smith BD. General patterns of niche construction and the management of 'wild' plant and animal resources by small-scale pre-industrial societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:836–848. doi: 10.1098/rstb.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombardo U, May J-H, Veit H. Mid- to late-Holocene fluvial activity behind pre-Columbian social complexity in the southwestern Amazon basin. The Holocene. 2012;22:1035–1045. doi: 10.1177/0959683612437872. [DOI] [Google Scholar]
- 32.Manning AD, Fischer J, Lindenmayer DB. Scattered trees are keystone structures – Implications for conservation. Biological Conservation. 2006;132:311–321. doi: 10.1016/j.biocon.2006.04.023. [DOI] [Google Scholar]
- 33.Tews J, et al. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography. 2004;31:79–92. doi: 10.1046/j.0305-0270.2003.00994.x. [DOI] [Google Scholar]
- 34.Berkunsky I, et al. Assessing the use of forest islands by parrot species in a neotropical savanna. Avian Conservation and Ecology. 2015;10:11. [Google Scholar]
- 35.Prümers H, Jaimes Betancourt C. 100 años de investigación arqueológica en los Llanos de Mojos. Arqueoantropológicas. 2014;4:11–53. [Google Scholar]
- 36.Lombardo U, Canal-Beeby E, Veit H. Eco-archaeological regions in the Bolivian Amazon: Linking pre-Columbian earthworks and environmental diversity. Geographica Helvetica. 2011;66:173–182. [Google Scholar]
- 37.Langstroth RP. Biogeography of the Llanos de Moxos: natural and anthropogenic determinants. Geographica Helvetica. 2011;66:183–192. [Google Scholar]
- 38.Lombardo U, Denier S, Veit H. Soil properties and pre-Columbian settlement patterns in the Monumental Mounds Region of the Llanos de Moxos, Bolivian Amazon. SOIL. 2015;1:65–81. doi: 10.5194/soil-1-65-2015. [DOI] [Google Scholar]
- 39.Rodrigues L, Lombardo U, Canal Beeby E, Veit H. Linking soil properties and pre-Columbian agricultural strategies in the Bolivian lowlands: The case of raised fields in Exaltación. Quaternary International. 2017;437(Part B):143–155. doi: 10.1016/j.quaint.2015.11.091. [DOI] [Google Scholar]
- 40.Boixadera J, Poch RM, García-González MT, Vizcayno C. Hydromorphic and clay-related processes in soils from the Llanos de Moxos (northern Bolivia) Catena. 2003;54:403–424. doi: 10.1016/s0341-8162(03)00134-6. [DOI] [Google Scholar]
- 41.Hanagarth W. Acerca de la geoecología de las sabanas del Beni en el noreste de Bolivia. Instituto de ecología; 1993. [Google Scholar]
- 42.Lombardo U, Ruiz-Pérez J, Madella M. Sonication improves the efficiency, efficacy and safety of phytolith extraction. Review of Palaeobotany and Palynology. 2016;235:1–5. doi: 10.1016/j.revpalbo.2016.09.008. [DOI] [Google Scholar]
- 43.Piperno DR. Identifying crop plants with phytoliths (and starch grains) in Central and South America: A review and an update of the evidence. Quaternary International. 2009;193:146–159. doi: 10.1016/j.quaint.2007.11.011. [DOI] [Google Scholar]
- 44.Iriarte J. Assessing the feasibility of identifying maize through the analysis of cross-shaped size and three-dimensional morphology of phytoliths in the grasslands of southeastern South America. Journal of Archaeological Science. 2003;30:1085–1094. [Google Scholar]
- 45.Watling J, et al. Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions II: Southwestern Amazonian forests. Review of Palaeobotany and Palynology. 2016;226:30–43. doi: 10.1016/j.revpalbo.2015.12.002. [DOI] [Google Scholar]
- 46.Dickau R, et al. Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions. Review of Palaeobotany and Palynology. 2013;193:15–37. doi: 10.1016/j.revpalbo.2013.01.004. [DOI] [Google Scholar]
- 47.Hogg AG, et al. SHCal13 Southern Hemisphere Calibration, 0–50,000 Years cal BP. Radiocarbon. 2013;55:1889–1903. [Google Scholar]
- 48.Bronk Ramsey C. Bayesian Analysis of Radiocarbon Dates. Radiocarbon. 2009;51:337–360. doi: 10.1017/S0033822200033865. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.