DEL prevalence has been well characterised for most large world populations, and DEL is encountered in some patients and blood donors. They appear Rh-negative on routine blood group serology, although they are Rh-positive. Their DEL phenotype has many known molecular causes. The DEL prevalence varies greatly: 1 in 5 for China among individuals who are Rh-negative by routine serology, but only 1 in 500 for the US and EU. DEL red cells can boost pre-existing anti-D in transfusion recipients. Patients with certain DEL variants can develop anti-D, but patients with the Asian type DEL do not receive RhIg during pregnancy in China and could be transfused Rh-positive. We propose to evaluate cost benefit studies for donor typing, given that a molecular DEL screen could replace some serology testing. Safer procedures for the same or lower costs than current serology should be embraced, regardless of how safe current serology might be perceived.
Okubo et al.1 identified Japanese donors in 1984 who typed Rh-negative on routine serology although their red cells bound anti-D. Because these researchers could elute anti-D, they named the phenotype Del (D eluate). In 2001, we discovered the molecular cause was represented by a large variety of variants (alleles) of the RHD gene2. Since 2005, the phenotype has been called DEL3.
The current count for DEL alleles causing a DEL phenotype exceeds 43, and researchers continue to find rarer DEL variants4. Among the DEL alleles originally observed2, one DEL variant stands out for its practical relevance worldwide. The allele is scientifically called RHD:c.1227G>A2, also known as RHD*DEL1, and its phenotype is commonly referred to as Asian type DEL5,6. Researchers quickly recognised that the Asian type DEL is rather common among Rh-negative individuals in all East Asian populations7,8.
DEL PREVALENCE AND POPULATIONS
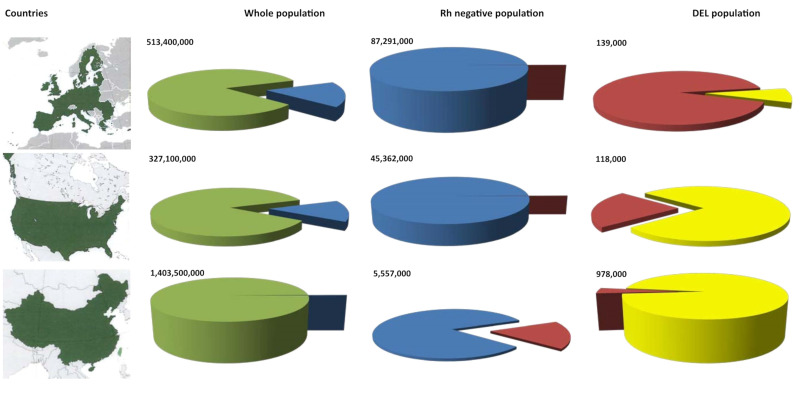
The prevalence of Rh-negative and DEL varies greatly among large populations (Figure 1). Rh-negative is relatively common in the EU (17%) and US (13.9%), but DEL is rare (0.26% or less among Rh-negative)9. Rh-negative is rare in China (0.4%), whereas DEL is relatively common (17.5% among Rh-negative)10. Among them, 96%, and thus almost 1 million people in China, carry the Asian-type DEL (Figure 1, yellow). A similar situation is seen in the East Asian populations of Korea11,12 and Japan13. While DEL frequencies need to be the focus of further studies in the large Southeast and South Asian populations14, reference to the gnomAD database confirms that the Asian-type DEL is rare in South Asia15.
Figure 1.
Prevalence of DEL and Rh-negative in 3 major populations
Greater than 83% are Rh-positive (green) in any large population, as shown for the EU (upper panels), US (middle panels), and China (lower panels). Among Rh-negative (blue), a small fraction represents DEL phenotypes (red, middle column of the pie charts). One distinct DEL variant, the Asian-type DEL (yellow), represents almost all DEL in China, but is also prevalent in the US. Numbers indicate inhabitants for the whole population, and Rh-negative and DEL individuals. Maps reproduced with permission by Creative Commons Attribution-ShareAlike 3.0 from Wikipedia.
DEL represents an excellent example of why US regulators cannot base their decisions on clinical data from other health care regions; they must evaluate differences in population structures and their possible consequences for diagnostics and treatment in the US. An EU application for DEL may be adapted to the large variety of DEL found in the EU (Figure 1, red), but may not necessarily fit the Asian-type DEL (Figure 1, yellow), because in the EU (Figure 1, right upper pie chart), at 9%, the Asian-type DEL (Figure 1, yellow) is only a small fraction of all DEL. While DEL remains rare in Europe, a growing proportion of the US population has East Asian ancestry. Therefore, the situation for DEL (Figure 1, right column) in the US, with 77% Asian-type DEL (Figure 1, yellow), is more similar to China than to the EU. This situation has practical implications for the clinical management of DEL donors and DEL patients, and suggests that clinical needs and technical approaches might differ between the US and the EU.
DEL IN BLOOD DONORS
We recently proposed a focussed study to resolve whether DEL is or is not an issue in the US6, possibly by inducing primary anti-D immunisations. Other health care systems have already implemented the technology. One German blood service with 1 million donations per year has routinely typed blood donors with a molecular screen for DEL since 20012,9. The DEL application constituted the first implementation of molecular immunohaematology in routine blood donor testing9,16. In Switzerland, a mandatory molecular DEL screen was introduced in 201317. This is the first implementation of molecular immunohaematology under national regulations and cleared any DEL unit from the Rh-negative inventory nationwide18. Similar blood centre initiatives have been effective in Austria in 200419–21, in Northern Germany since 200916, at the NIH Clinical Center since 201022, and in Brazil in 201223.
Missed weak D phenotypes may be a more serious clinical problem24,25 than possibly that posed by missed DEL phenotypes6. Molecular DEL screening allows the discontinuation of the indirect antiglobulin test in serological D typing9,16,18 and still provides a more reliable detection of any weak D type that may have been missed by serological donor testing with antiglobulin. The reason why some blood centres, and all of those in Switzerland, have moved to molecular DEL testing is not due to any “unsafe” serology. The transition was prompted by the, albeit small, improvement in safety combined with the same or lower costs, thus replacing the costly antiglobulin test. The decision is controversial, however, as researchers in Denmark26 concluded their “serologic RhD typing was safe” and rejected the transition26, while those in Australia27 proposed “to estimate risk as a basis for developing policy regarding molecular RHD typing strategies”27.
RISK BENEFIT ANALYSIS
While US regulators are justifiably cautious about accepting clinical applications based on data from non-US populations, DEL testing may be a special case. There is little disadvantage in screening donors for DEL other than cost. A false negative result would not change the currently accepted clinical practice. A false positive result could cause the unnecessary transfer of true Rh-negative units to the Rh-positive inventory, which would only affect a few units per year in the US at the most and would not put anyone at risk.
Given the absence of risk for transfusion recipients, any benefit, albeit small, should tilt the decision in favour of molecular DEL screening of donors who are Rh-negative by routine blood group serology. Clearly, such routine DEL screening of donors could avoid any secondary anti-D boost by blood products labelled Rh-negative in US patients4,6. Screening may enhance patient safety, even if no “DEL issue”6 is recognised.
COST BENEFIT ANALYSIS
As the risk benefit seems to favour DEL screening, a cost benefit analysis should guide the decision as to whether blood centres should or should not introduce DEL donor screening. Regulations in Germany were modified in 2010 to allow the indirect antiglobulin test to be replaced by a molecular DEL screen, where increased sensitivity of the molecular assay is achieved at no additional cost compared to the traditional test that was previously mandatory for all donations9. At least since 2000, when its 20th Edition was published, the AABB Standards allow the same approach to be adopted in the US: blood centres can meet the requirement for “using a method designed to detect weak D” by applying a DEL screen rather than a serological screen, for example, an indirect antiglobulin test. However, there is still not sufficient evidence available in the US for a nationwide molecular DEL screen to be implemented without a cost benefit analysis6.
US REGULATORY ASPECTS
On December 3rd, 2018, the US Food and Drug Administration (FDA) approved a Biologics License Application to include an alternative procedure and to label as Rh-positive red cells from DEL phenotype donors who test Rh-negative by licensed serological blood group assays but genotype as D positive using laboratory-developed and validated molecular assays. The Department of Transfusion Medicine at the NIH Clinical Center started labelling red cell units based on a laboratory-developed molecular assay in January 2019. This represents the first time that blood components in the US were permitted to include labelling based on a molecular assay for one of the major ABO or Rh blood group antigens.
DEL IN PATIENTS
Among Rh-negative individuals transfused with Rh-positive red cells, a significant proportion does not develop anti-D. The reasons for this are still not clear, despite decades of research. DEL cannot explain the majority of such non-responders outside of Asia. However, Okubo et al. speculated that “some D negative persons who are non-responders to D may type Del”1, and envisioned the lack of immune response later recognised in some DEL types3,6,24,28. Hence, antenatal RhIg prophylaxis is not recommended for pregnant women with Asian type DEL in China5,28. More research is needed before this recommendation can be transferred to US guidelines or expanded to other DEL variants that cannot be identified unless evaluated at the molecular level.
SUMMARY
Thanks to research, DEL prevalence and its molecular bases are now well characterised. Tools to screen donors and patients are in place and industry are ready to design such tools once either a critical clinical need or a cost benefit is established. Implementation at blood centres worldwide is ongoing, and DEL screening of donors will bring our research to the bedside. Progress in transfusion medicine is incremental and every step, small though this may be, will eventually contribute to patient safety.
ACKNOWLEDGEMENTS
Supported by the Intramural Research Program (project ID Z99 CL999999) of the NIH Clinical Center.
Footnotes
STATEMENT OF DISCLAIMER
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the US Federal Government.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Okubo Y, Yamaguchi H, Tomita T, Nagao N. A D variant, Del? [Letter] Transfusion. 1984;24:542. doi: 10.1046/j.1537-2995.1984.24685066827.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Körmöczi GF, Gassner C, Shao CP, et al. A comprehensive analysis of DEL types: partial DEL individuals are prone to anti-D alloimmunization. Transfusion. 2005;45:1561–7. doi: 10.1111/j.1537-2995.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwon DH, Sandler SG, Flegel WA. DEL phenotype. Immunohematology. 2017;33:125–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Shao CP. Transfusion of RhD-positive blood in “Asia type” DEL recipients. N Engl J Med. 2010;362:472–3. doi: 10.1056/NEJMc0909552. [DOI] [PubMed] [Google Scholar]
- 6.Sandler SG, Flegel WA. Does transfusion of Asian-type DEL red blood cells to D-recipients cause D alloimmunization? Transfusion. 2019;59:2455–8. doi: 10.1111/trf.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao CP, Maas JH, Su YQ, et al. Molecular background of Rh D-positive, D-negative, D(el) and weak D phenotypes in Chinese. Vox Sang. 2002;83:156–61. doi: 10.1046/j.1423-0410.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Wang XD, Shao CP, et al. Molecular basis of DEL phenotype in the Chinese population. BMC Med Genet. 2014;15:54. doi: 10.1186/1471-2350-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegel WA, von Zabern I, Wagner FF. Six years’ experience performing RHD genotyping to confirm D-red blood cell units in Germany for preventing anti-D immunization. Transfusion. 2009;49:465–71. doi: 10.1111/j.1537-2995.2008.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J, Sun AY, Wang XD, et al. Analysis of density and epitopes of D antigen on the surface of erythrocytes from DEL phenotypic individuals carrying the RHD1227A allele. Blood Transfus. 2014;12:244–9. doi: 10.2450/2013.0091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JY, Kim SY, Kim CA, et al. Molecular characterization of D-Korean persons: development of a diagnostic strategy. Transfusion. 2005;45:345–52. doi: 10.1111/j.1537-2995.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- 12.Lüttringhaus TA, Cho D, Ryang DW, Flegel WA. An easy RHD genotyping strategy for D-East Asian persons applied to Korean blood donors. Transfusion. 2006;46:2128–37. doi: 10.1111/j.1537-2995.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara K, Suzuki Y, Sasaki K, et al. Molecular basis for D-Japanese: identification of novel DEL and D-alleles. Vox Sang. 2015;109:359–65. doi: 10.1111/vox.12290. [DOI] [PubMed] [Google Scholar]
- 14.Weinstock C. It is worthwhile filling in the remaining blank spots for blood group antigen frequencies. Blood Transfus. 2014;12:3–6. doi: 10.2450/2013.0192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.gnomAD database 2019. Available from: www.biorxiv.org/content/biorxiv/early/2019/08/13/531210.full.pdf.
- 16.Wagner FF. RHD PCR of D-negative blood donors. Transfus Med Hemother. 2013;40:172–81. doi: 10.1159/000351604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crottet SL, Henny C, Meyer S, et al. Implementation of a mandatory donor RHD screening in Switzerland. Transfus Apher Sci. 2014;50:169–74. doi: 10.1016/j.transci.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Henny C, Still F, Lejon Crottet S, et al. Impact of the mandatory donor RHD screening in Switzerland. Vox Sang. 2016;111:56. [Google Scholar]
- 19.Flegel WA, Gabriel C, Gassner W, et al. RHD genotyping of blood donors may avoid anti-D immunization. Blood. 2004;104:739a. [Google Scholar]
- 20.Gassner C, Doescher A, Drnovsek TD, et al. Presence of RHD in serologically D-, C/E+ individuals: a European multicenter study. Transfusion. 2005;45:527–38. doi: 10.1111/j.0041-1132.2004.04211.x. [DOI] [PubMed] [Google Scholar]
- 21.Polin H, Danzer M, Gaszner W, et al. Identification of RHD alleles with the potential of anti-D immunization among seemingly D-blood donors in Upper Austria. Transfusion. 2009;49:676–81. doi: 10.1111/j.1537-2995.2008.02046.x. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava K, Stiles DA, Wagner FF, Flegel WA. Two large deletions extending beyond either end of the RHD gene and their red cell phenotypes. J Hum Genet. 2018;63:27–35. doi: 10.1038/s10038-017-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mota M, Dezan M, Valgueiro MC, et al. RHD allelic identification among D-Brazilian blood donors as a routine test using pools of DNA. J Clin Lab Anal. 2012;26:104–8. doi: 10.1002/jcla.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegel WA. Homing in on D antigen immunogenicity. Transfusion. 2005;45:466–8. doi: 10.1111/j.0041-1132.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 25.Garratty G. How concerned should we be about missing antibodies to low incidence antigens? Transfusion. 2003;43:844–7. doi: 10.1046/j.1537-2995.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- 26.Krog GR, Clausen FB, Berkowicz A, et al. Is current serologic RhD typing of blood donors sufficient for avoiding immunization of recipients? Transfusion. 2011;51:2278–85. doi: 10.1111/j.1537-2995.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 27.Scott SA, Nagl L, Tilley L, et al. The RHD(1227G>A) DEL-associated allele is the most prevalent DEL allele in Australian D-blood donors with C+ and/or E+ phenotypes. Transfusion. 2014;54:2931–40. doi: 10.1111/trf.12701. [DOI] [PubMed] [Google Scholar]
- 28.Shao CP, Xu H, Xu Q, et al. Antenatal Rh prophylaxis is unnecessary for “Asia type” DEL women. Transfus Clin Biol. 2010;17:260–4. doi: 10.1016/j.tracli.2010.07.003. [DOI] [PubMed] [Google Scholar]