Abstract
Background
There are many advantages to using cord blood (CB) as a source of therapeutic platelet and plasma derivatives for regenerative medicine. These include availability, universal use, young donor source, and virally safe biological material, rich in tissue regenerative factors.
Materials and methods
We aimed to validate a bioprocess design for the production of cord blood-derived platelet concentrates (CBPC) in a public Cord Blood Bank (CBB). CBPC was defined as a product of 10±5 mL, 1,000±200×109/L total platelets, free of erythrocytes and leukocytes. A total of 300 CB units were centrifuged in two steps to enrich for platelets, in compliance with Good Manufacturing Practice. The samples were tested for the degree of platelet activation present, and the levels of growth factor were analysed to evaluate their potential function. CBPC were then activated after thawing with 10% calcium gluconate to generate platelet gels (CBPG) to treat patients with diabetic foot ulcers.
Results
After processing, 84% of the products fulfilled the acceptance criteria. Final products contained 1,017±149×106 platelets/mL in 10±3mL of plasma. Platelet recovery was 50±9%. The methods described here ensure depletion of white and red blood cells down to a residual concentration of 0.2±0.1×106/mL and 0.03±0.02×106/mL, respectively. Platelets showed low levels of activation during processing, but were significantly activated after thawing, as indicated by an increase in CD62p expression. The growth factors EGF, VEGF, bFGF, PDGF AB/BB and TGF-β1 were at concentrations of 1,706±123 pg/mL; 1,602±227 pg/mL; 314±26 pg/mL; 30±1.5 ng/mL; 24±2 ng/mL (mean±standard error of mean), respectively. For clinical evaluation, a total of 21 CBPG were applied in 3 patients, with no reported adverse events and improvement of ulcers in all of them.
Discussion
We designed and validated a highly reproducible, closed system method to manufacture high quality CBPC suitable for clinical applications using CB units not suitable for transplantation in a public CBB.
Keywords: cord blood plasma, platelet rich plasma, platelet gel, regenerative medicine, cord blood banking
INTRODUCTION
Cord blood (CB) is used as an important source for haematopoietic stem cell transplantation in children and adults with cancer, bone marrow (BM) failure syndromes, haemoglobinopathies and other genetic metabolic disorders1. Cord Blood Banks (CBB) are responsible for the collection, processing, storage and distribution of CB-derived haematopoietic progenitor cells (HPC) for transplantation2. Due to the high number of cells required for engraftment, only a small fraction of the collected donations meet the strict cellular criteria required for clinical use.
Thus, large amounts of donated units are discarded. However, these could be used for other applications whilst also avoiding ethical concerns by utilising donated materials otherwise destined to be discarded as medical waste. In addition, as a source of new medicinal products, these CB units have the advantage that they are an accredited source; they were collected by trained midwives following validated procedures3, hold appropriate informed consent, and fulfill the rigorous quality criteria required for human use. Other advantages are inherent to the nature of the starting material: absence of safety risks for the donor, easily accessible source, very low risk of transmissible infectious diseases, and low immunogenicity2. One promising approach is to use CB components for novel clinical applications. Evidence is already available for the therapeutic efficacy of CB plasma and platelets associated with their anti-inflammatory and regenerative properties4,5. Platelet rich plasma (PRP) has been used as a regenerative product in several applications, with many publications suggesting that platelets may offer beneficial effects on wound healing6. Developing a well-defined, off-the-shelf product from an allogeneic source like CB may contribute to the standardisation of such new therapeutic approaches7. This would also solve issues related to autologous donations, such as problems in obtaining therapeutic products during long-term treatments, or in situations where the plasma or the platelets may have deteriorated due to an underlying disease.
There are a number of reports on the use of CB components, including anti-inflammatory treatment for osteoarthritis5, wound healing enhancer in ocular surface lesions8, part of biological scaffolds to promote tissue regeneration with bedsores9, diabetic foot ulcers (DFU)10, or their use in mucocutaneous lesions related to graft-vs-host disease (GvHD)11.
In order to increase the use of such products for clinical use, we propose to recover and process CB units not suitable for transplantation and to generate an intermediate product, cord blood platelet concentrate (CBPC), as starting material for further development of different medicinal products (eye drops, platelet gel, and other platelet and plasma derivatives). Here, and using the experience of a multicentre standardisation programme carried out by the Italian cord blood network7, we describe and validate a procedure to concentrate platelets in a small volume of plasma with a defined amount of platelets, free of erythrocytes and cells. The objective was to achieve a process of CBPC production compliant with current Good Manufacturing Practice (GMP). To evaluate their biological activity, we assessed the levels of platelet activation and growth factors (GF) present. Finally, we assayed their healing properties in the context of a clinical pilot study for the treatment of DFU.
MATERIALS AND METHODS
Sample collection: raw material
Cord blood units were collected in authorised maternity hospitals within the Concordia programme. Our Blood and Tissue Bank (BST) is accredited by FACT-Netcord and also holds the Spanish CAT Foundation certification. Before delivery, mothers signed an informed consent for donation that allows the use of these samples for research and validation purposes. Qualified health care professionals collected CB units while placenta was still in utero using validated procedures3. All samples were transferred from the BST’s authorised Biobank, following local regulations and after approval from the Hospital de la Vall d’Hebron’s ethics committee (ref.: 192/2014).
All processed CB units were selected among those excluded by the quality control criteria for haematopoietic stem cell cryopreservation. The most frequent exclusion criteria were total nucleated cell and total CD34+ cell counts below 1.5×106 and 4×106, respectively. These CB units also had to comply with the following inclusion criteria to be eligible for producing CB PRP: less than 48 hours from collection, >50 mL volume (excluding anticoagulant citrate-phosphate-dextrose, CPD), absence of visible haemolysis, and platelet concentration >150×106/mL. The target product profile (TPP) is shown in Table I.
Table I.
Acceptance criteria for cord blood platelet concentrate manufacturing
| Type of sample | Parameter | Acceptance criteria |
|---|---|---|
| WCB | Time from collection | <44 hours |
| Signed Informed consent | Present | |
| Volume | 75–150 mL (including CPD) | |
| Visible haemolysis | Absence | |
| Platelet count | ≥150×106/mL | |
| CBPC | Volume | 10 (±5) mL |
| Platelet count | 800–1,200×106/mL | |
| Leukocytes | ≤0.5×106/mL | |
| Erythrocytes | ≤0.1×106/mL | |
| Virology | Negative | |
| Haemoculture | Negative | |
| Maternal blood | Virology | Negative |
WCB: whole cord blood; CPD: citrate-phosphate-dextrose.
Cord blood platelet concentrate manufacturing
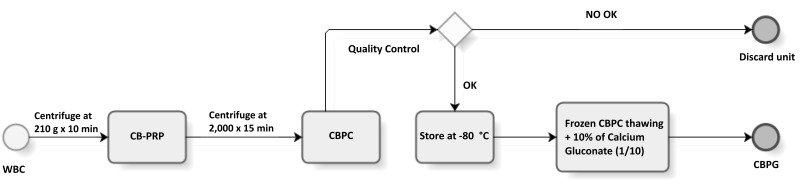
The objective of CB processing was to obtain platelet concentrates (CBPC) within the ranges defined above. A total of 300 CB units were included for processing following a method based on a previously described two-step centrifugation protocol7 (Figure 1). Modifications were made to this protocol, including using an irradiated pre-fabricated kit, and additional sampling for virology testing from CB product to ensure safety, following local laws for medicinal products (2001/83/CE). First, whole cord blood (WCB) was transferred into a 150 mL bag (Fenwal Inc., Lake Zurich, IL, USA) and centrifuged at 210 g for 10 min to isolate a leucocyte poor and platelet rich plasma (PRP). PRP, which is an intermediate product, was transferred to another 150 mL bag using a manual plasma extractor while the pellet containing the majority of nucleated cells and the red blood cell (RBC) fraction was discarded. Then, the PRP was centrifuged at 2,000 g for 15 min, the platelet poor plasma (PPP) was transferred to another 150 mL bag and the platelet pellet was re-suspended in an appropriate volume of PPP (as defined below) to obtain a standard final concentration of 800–1,200×106 platelets/mL in the CBPC (Table I). The appropriate volume of PPP required for resuspension was determined according to the initial platelet count multiplying the PRP volume by a reduction factor (0.25, 0.33, 0.40 and 0.50 for ranges of 150–199, 200–249, 250–299, and >300×106 platelet/mL) to achieve a range of volume of 10±5 mL, and stored in special bags to facilitate clinical application (PRPS Biomed Device SrL, Modena, Italy). CBPC were then stored into sealed security wraps (PRPS000 Biomed Device SrL, Modena, Italy) at −80 °C for subsequent evaluation. All procedures were performed in GMP-compliant facilities.
Figure 1.
Manufacturing flow of cord blood platelet gel (CBPG) for clinical application
WCB: whole cord blood; CB-PRP: cord blood-platelet rich plasma; CBPC: cord blood platelet concentrate; min: minutes.
Product safety was evaluated by serology for infectious disease markers in maternal and CB samples (for HIV-1/2, HCV, HBs and HBc, CMV, HTLV I–II and Trypanosoma cruzi antibodies, Treponema pallidium, and nucleic acid testing for HIV, HBV and HCV). For sterility testing, a mixed sample from PPP and residual erythrocyte bag was used to determine the presence of aerobic and anaerobic bacteria, and fungi (BacTalert, Biomerieux Inc., Durham, UK). The final product was also characterised for cell counts and a blood sample from a residual bag used for RBC immunophenotyping (ABO and Rh blood group). Cell count was performed using a haematology analyser validated for the CBB activity (LH750 model, Beckman Coulter Inc., USA). Acceptance criteria for the final product are detailed in Table I.
In vitro evaluation of cord blood platelet concentrate
Validation of the manufacturing process was performed by determining platelet recovery, leukocyte and erythrocyte contamination, level of platelet activation, and GF content.
Platelet activation by flow cytometry
As a part of the validation of CBPC manufacturing, the activation of preserved platelets was demonstrated before and after freezing. To do this, five CB units were assayed at different stages of the manufacturing process, using flow cytometry for assessing the platelet activation phenotype of samples from: whole CB (WCB), PRP, PC before freezing and after thawing to analyse platelets surface and platelet activation levels of CD41aPE+ CD62pAPC+ positive and negative control IgG isotype (Beckton Dickinson, USA) markers antibody12. Platelets were used a positive control, which was activated with its own thrombin in the presence of anticoagulant.
Growth factor measurements by Luminex
The next validation step consisted of the determination of platelet-derived GF content in platelet releasates of CB. After thawing at 37 °C in a waterbath, the unit was activated using 10% calcium gluconate (1/10). To generate platelet releasates, clots were consolidated in approximately 10 min. Samples were then kept at room temperature for one hour and subsequently centrifuged at 5,000 g for 15 min. Supernatant was collected to measure EGF, VEGF, bFGF, PDGFAB/BB and TGFβ1 in multiplex (R&D Systems, Abingdon, UK) using a Luminex 100IS analyser (Luminex Corp., Austin, TX, USA) following the manufacturer’s instructions.
Clinical evaluation of cord blood platelet concentrate
Preparation of platelet gel
For clinical application, 21 units of CBPC meeting the acceptance criteria were used. After the quarantine period of 2 weeks to discard transmissible diseases, units were released for clinical use. Upon request, released CBPC were thawed at 37 °C in a water bath, and then activated using 10% calcium gluconate 1: 3 vol: vol. A platelet gel was formed in approximately 10 min. This investigational product, called Cord Blood Platelet Gel (CBPG), could then be used for application to a DFU. After application to the side of the ulcer, a hydrophobic dressing (Mepilex®Lite, Molnlycke Health Care, Sweden) was used to prevent product absorption.
Clinical trial
A pilot clinical trial was conducted in order to demonstrate safety and efficacy as part of product validation. To this end, we obtained the approval from the Spanish Medicines Agency (AEMPS) and from the Ethics Committee of the Hospital de la Santa Creu I Sant Pau (HSP), (EC ref.: 15/043; EudraCT: 2015-000510-22; clinicaltrials.gov identifier: NCT02389010). The study had an open-label, two-arm, randomised design and was conducted at the HSP Barcelona, Spain, in patients with DFU. The inclusion criteria were an ulcer classified as at least Wagner II stage13, and adult diabetics (>18 years) with certain clinical and laboratory parameters, without tumours or osteomyelitis. The primary objective of the study was evaluation of safety and the secondary objective was to evaluate efficacy by measuring reduction of the ulcer area. The experimental treatment was applied topically twice a week for one month (8 applications in total). The control arm was the standard treatment consisting of cleaning with Povidone-Iodine (Topionic®, Barcelona, Spain) and “wound discharge”, and use of a shoe inner sole was adapted to the ulcer. Both arms received 8 treatment visits after screening and one follow-up visit at month 4. The visit consisted of wound cleaning, photographic documentation with measurements, and recording in a Data Collection Logbook of: stage (according to Wagner), size of ulcer, if there was tunneling, presence of necrotic, granulation tissue, exudate, and evaluation of pain. In addition, laboratory analysis was performed before starting treatment, and at 1-month and 4-month evaluations.
We randomised 11 patients: 6 to CBPG (3 withdrawn) and 5 to control arm (2 withdrawn). The study was terminated before completion of the complete sample size due to slow enrolment.
Analysis of the feasibility of cord blood platelet concentrate production
To evaluate the capacity to maintain a defined size of CBPC stock, a retrospective analysis was performed using data from CB units registered in the Programa Concordia. This analysis was based on the calculation of the number of units received from 2014 to 2018 that fulfilled the required volume of >75 mL (including anticoagulant) and a time of collection to reception up to 44 hours. The number of patients with DFU that could potentially require CBPG treatment was based on the yearly report of hospital activity from the Functional Unit of the Diabetic Foot of the HSP. The cost of production was calculated on the basis of expenses for kits, consumables, testing, installations, maintenance, and labour provided by the Finance Department of the BST.
Statistical analysis
Results shown are presented as mean and standard deviation (SD) on cell count, unit volume or standard error of mean (SEM) on assays of platelet activation and GF measurement. U-Mann-Whitney test was used to compare processing stages. Due to low recruitment, non-statistical analysis was applied to compare two arms of the clinical trial, and percentage of size reduction was shown.
RESULTS
Validation of cord blood platelet concentrate manufacturing
Of the WCB selected for processing, 300 fulfilled the acceptance criteria for CBPC production. The mean platelet count in WCB was 240±46×106/mL and final PC product showed a platelet count of 1,017±149×106/mL, resulting in a 50±9% platelet recovery in a final volume of 10±3mL. An almost negligible amount of red and white blood cells were present (see Table II). Following the described protocol, more than 98% of initially measured leukocyte and 99% of the erythrocyte content were depleted from the CBPC.
Table II.
Product validation parameters and results (n=300)
| Parameter | Volume | Platelets ×106 mL | Leukocytes ×106 mL | Erythrocytes ×106 mL |
|---|---|---|---|---|
| WCB | 85 (±14) mL | 240±46 | 11.2±2.8 | 3,1±0.3 |
| CBPC | 10 (±3) mL | 1,017±149 | 0.2±0.1 | 0.03±0.02 |
| Yield | 12±4% | 50±9% | 2±1% | 0.1±0.05% |
WCB: Whole cord blood; CBPC: cord blood platelet concentrates; n: number. Values are expressed as mean±standard deviation.
Amongst the 300 WCB units processed, only 29 units (9.6%) did not fulfill CBPC acceptance criteria, thus demonstrating that the manufacturing procedure was robust. The main reason for failure was visible haemolysis (21 products). In a further 8 units it was due to other factors including volume, platelet counts, erythrocyte and/or leukocyte contamination. In addition, 20 units (6.6%) failed to pass quarantine due to a reactive serology or positive results in microbiological cultures. The remaining 251 (83.7%) units of CBPC met the acceptance criteria. All products used for the clinical application displayed the pre-defined target criteria.
In vitro evaluation of cord blood platelet concentrate
Platelet activation
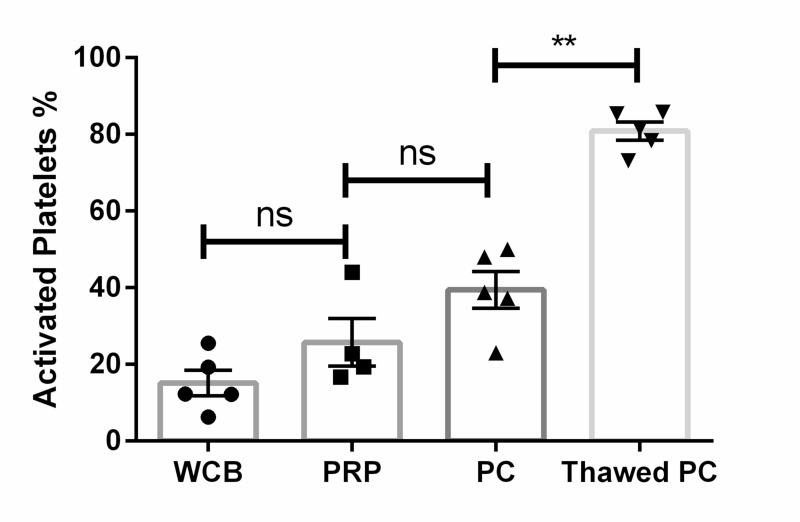
To measure activation, we compared percentage of expression of CD62p at different stages of the manufacturing process. The proportion of CD41a+CD62p+ events was 15%±4 SEM, which rose slightly to 25% ±6 SEM after the first centrifugation step (PRP) and significantly increased to 39%±5 SEM after the second centrifugation step (PC) (p=0.01 with respect to the starting sample). This suggests that loss of GF during CBPC processing was limited. After thawing, 81% of the stored platelets were recovered. These platelets showed high levels of activation (percentage CD62p+ within the CD41a population was 80% ±2 SEM; p=0.0015 with respect to the fresh CBPC) (Figure 2), suggesting the stored CBPC retain the ability to activate. This also suggests a good functionality for GF release. Furthermore, CBPC after thawing were able to form gels after addition of calcium, demonstrating capacity to be that of clinical applications.
Figure 2.
Graphical representation of platelet activation throughout manufacturing processing
Significant differences in percentage of activated platelets between fresh platelet rich plasma (PC) and thawed PC (mean±standard error of mean). Statistical significance assessed by U-Mann-Whitney test.
**p=0.0015; ns: not significant. N=5.
WCB: whole cord blood; PRP: platelet rich plasma.
Growth factor measurements
Determination of GF was performed in platelet releasates obtained from CBPC. The mean platelet count was 1,077×106/mL±122 SD, the EGF had a concentration of 1,706 pg/mL±123 SEM; VEGF −1.602 pg/mL±227 SEM; bFGF −314 pg/mL±26 SEM; PDGF AB/BB −30 ng/mL±1.5 SEM; TGFβ1 −24 ng/mL±2 SEM.
Clinical evaluation of cord blood platelet concentrate
Eleven patients were randomised in the clinical study (6 to CBPG and 5 to standard procedure (STD) (Online Supplementary Table SI). Five of them withdrew due to clinical protocol violation (3 CBPG, 2 STD). From the remaining 6 patients, 3 of them received CBPG, and 3 STD. Clinical outcome of patients receiving STD resulted in one being infected and withdrawing from the study, whilst another had reduction of the ulcer area (62%) at 4 weeks but this was followed by an increase in area at the 4 month follow-up visit (75% of the initial ulcer area remaining at this time point). Finally, the last patient included in the STD arm had good evolution with ulcer closure at 4 weeks and at the 4-month follow-up visit. In contrast, in the experimental treatment (CBPG) arm, all 3 patients improved. In one (BST-10), the ulcer remains open but with a reduction in area to 44% and to 71% at the 4th and 16th week follow-up visit, respectively. The ulcers of the other two patients had completely healed at different time points: patient BST-08 by the 3-week visit and BST-10 at 4 months. There were no reported adverse events in the CBPG treated patients (Online Supplementary Table SI) and signs of efficacy were observed. No exudate, necrotic tissue or pain was reported.
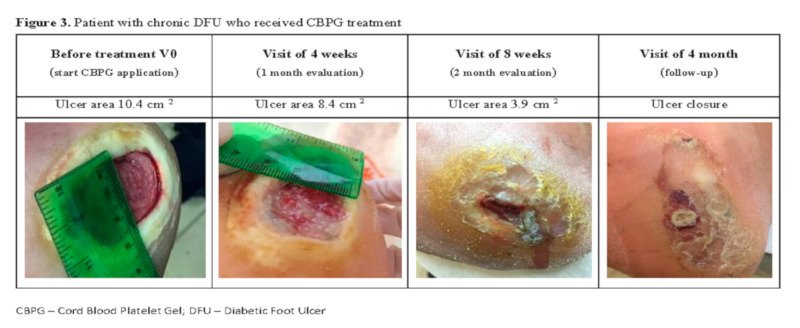
As an example, Figure 3 shows the evolution of the ulcer of patient BST10. This patient suffered from a chronic DFU that failed to heal after different previous treatments, and entered the study in Wagner II stage with 10.4 cm2. The patient was randomly-assigned to CBPG treatment and showed a 20% ulcer area reduction after the first 8 applications (1 month of treatment, two applications per week). The patient continued receiving CBPG and at the two month follow-up visit the ulcer had continued to improve with a remaining area of 37%. CBPG administration was stopped but the ulcer was completely closed by the 4-month follow-up visit, showing the CBPG medicinal product had maintained its effect. No safety issues were reported.
Figure 3.
Patient BST-10 with chronic diabetic foot ulcers (DFU), who received platelet gel (CBPG) treatment
Feasibility of regular production of cord blood platelet concentrate in a public Cord Blood Bank
According to the historical data of Programa Concordia, a median of 4,973 CB units were collected every year in the last 5 years. Of these, 11% fulfill the strict criteria for haematopoietic stem cell transplantation processing. Interestingly, 62% (up to 3,621 units) of the total CB units received would have been eligible for CBPC production according to the volume and time criteria defined in the Materials and methods section (Online Supplementary Table SII). This large number suggests the feasibility of a sustained CBPC production within the environment of a public CBB.
The Functional Unit of the Diabetic Foot of the HSP treated 137±10 patients per year during 2015–2017 (Report of HSP activity); 24±7 of them had a DFU (Patient Register Database). Following our estimation of production (3,621 units per year), taking into account 8 units per patient, this would require approximately 240 units per year.
Finally, we analysed the direct costs of CBPC production, including consumables, quality controls (cell count), and safety assays (virology, microbiology and fungal detection in CB samples), facilities and equipment, maintenance and labour costs of technicians and supervisors. This initial cost assessment showed that the direct cost of manufacturing a clinical grade CBPC using this validated procedure is € 156.1 ($172.98) (Online Supplementary Table SIII), with €1,248 ($1,383) per treatment course. Such costs are highly competitive compared to the cost of treating the ulcers using alternative therapies, where DFU treatment can reach >$ 3,00014.
DISCUSSION
Wound healing is a clinical condition which still has no satisfactory therapeutic solution, especially in patients with chronic ulcers9,15. We developed this project to answer a clinical need for new products for difficult-to-treat skin wounds. To achieve this, we developed a TPP16.
In this study, we focused on developing a scalable CBPC production protocol according to this defined TPP from CB units that are otherwise discarded for haematologic application at the CBB2,17. We first described a manufacturing process that obtains a product with very low content ofleukocytes and erythrocytes, within a defined range of platelet content. This medicinal product fulfills safety requirements, including negative results for infectious disease markers and microbiological contamination before unit release. In this regard, the GMP-compliant processing of CB platelets was hugely facilitated by the experience and established procedures of the CBB that had successfully completed rigorous accreditation schemes. Thereafter, the product was described in the investigational medicinal product dossier for a clinical trial to test the healing potential of CBPG in DFU patients. The gel consists of platelet-derived factors trapped in coagula, which are continuously released at the wound site after application.
Cord blood platelet concentrate is a new tool for tissue engineering and regenerative medicine applications. Here we also describe the feasibility of CBPC production as well as the versatility of its therapeutic applications based on the possibility to conveniently preserve off-the-shelf products in the CBB to further produce different formulations of CB plasma, according to the desired final use. CBPC offers some advantageous benefits for patients because of: i) its safety; ii) the fact that pregnant donor women were previously evaluated for presence of transmissible diseases; iii) the fact that quality control analysis can be easily carried out before product release; iv) the immediate availability; and v) the unique properties of CB plasma18 due to the presence of angiogenic GF, and immunomodulatory cytokines19 with recognised beneficial effect to wound healing20. Importantly, the possibility of standardising CBPC manufacturing to yield a well-characterised product also provides the chance to reduce product variability; this is in contrast to autologous PRP applications where treatment dose and composition change on an individual basis.
Platelet rich plasma is a well-known biological product typically used in the autologous application settings21,22 for therapeutic purposes. The medical use of platelet GF has been described for eye drops23, platelet gel24,25, and supplements of culture media26,27, and for advanced therapy medicinal products (ATMPs), amongst others. In this sense, there are several recently described clinical applications of autologous PRP, including chronic wound healing10,20, skin and soft tissue repair25, treatment of inflammatory pathologies, and even anti-ageing medicine applications22. However, the clinical application of autologous PRP has some disadvantages, such as the variability in the raw materials and processing protocols, lack of characterisation of the final product applied to the patient, and the contraindication for some patient populations to obtain blood for PRP preparation (haematologic malignancies, elderly patients with limited mobility9). Improvements in wound healing based on platelet properties, after treatment with peripheral blood platelet gel, have been reported elsewhere10,20, although the scientific evidence28 is scarse. Parazzi et al. also showed by proteomic analyses that adult plasma is richer in inflammatory factors compared to CB18. In this regard, the use of CBPC manufactured with the methodology proposed here would easily overcome the aforementioned disadvantages due to the standardisation of platelet content in a defined volume, the validated manufacturing protocol, and its potential universal use.
In addition, as set out above, CBPC preserves the content of platelets after thawing, indicating the suitability of the proposed manufacturing protocol to ensure a controlled dose of platelets in the final product for the patient. Thus, our processing protocol yielded CBPC units with at least 800×106 platelets/mL in all cases, demonstrating the reproducibility of our protocol. More importantly, the capacity of those platelets to express activation markers after thawing suggest that GF release, the putative active ingredient, occurs at the moment of application of the medicinal product and not in an uncontrolled manner during processing. Despite this, it is still not clear whether the observed expression of platelet activation marker is a result of the physiological platelet activation triggered by the temperature conditions of preservation29 or because of the platelet membranes breaking after thawing30, or even due to both mechanisms occurring at the same time. In the future, other preservation strategies, such as freeze-drying methods, could be tested to improve presentation. In addition, our analysis of GF levels after activation of CBPC with calcium gluconate supports the preservation of platelet function and showed comparable levels of factors in CBPC compared to the ranges described as reference values31, therefore suggesting that current clinical applications using autologous PRP might potentially be replaced with CBPC.
On the other hand, the very low number ofleukocytes, and the almost complete absence of erythrocytes in our products, assures a low risk of potential immune and inflammatory reactions after allogenic applications, even without HLA or ABO group compatibility, thus enforcing their universal use. In this sense, a safety profile for the use of CBPG has been observed in our pilot clinical trial on DFU patients as a part of product validation and observed product functionality.
Provided that product safety remains a key concern, all the steps of the manufacturing process were designed to be performed in compliance with GMP.
According to these data, it is suggested that allogeneic products be applied on wound healing. Currently, there are several kits commercially available for the preparation of autologous PRP, with prices ranging from $175 to $1,150 US32, excluding the costs of virology and microbiology testing, labour, and product characterisation. Therefore, the cost analysis of the CBPC product presented here shows it to be competitive and suitable for use in public health environments with negligible safety risks. The limitation of our study is the lack of an accurate local cost-to-benefit analysis, which is not feasible at this stage considering the small number of patients that we have treated with CBPG so far. However, encouraging data have been reported by Greppi et al., who showed that the use of allogeneic donor platelet gel generated a 90% reduction in treatment cost vs conventional treatment, and 86% ulcer healing in a series of 11 elderly patients affected by pressure ulcers9.
Our data also assessed the feasibility of a public CBB to regularly produce this therapeutic blood component for clinical use. Furthermore, the full development of this product in the catalogue of a CBB would result in a substantial increase in the efficiency of the CB collection programmes. In our analysis, the current 11% of clinical conversion would increase to 73%, if CBB included the production of CBPC to their routine processing of CB cells for transplantation. In this regard, we propose a new generation of CBBs to be used in other contexts beside transplantation33.
CONCLUSIONS
In conclusion, here we demonstrate the feasibility of obtaining CBPC by implementing GMP protocol using conventional equipment typically present in a CBB. This methodology allows for the large-scale production that is required for conducting future clinical trials to assess efficacy and potential new applications.
Supplementary Information
ACKNOWLEDGEMENTS
First of all, we would like to acknowledge cord blood donors and health care professionals in all hospitals participating in the Programa Concordia BST for making possible the public cord blood programme. We would also like thank the Italian CB Platelet Gel Study Group7; laboratory and technical staff at BST Cell Therapy Service; Bio-Bank for blood samples from CB units; and Dr. Richard Duggleby for help with his critical review of the manuscript. Our laboratory is a member of the Spanish Cell Therapy Network (Red de Terapia Celular, TerCel, expedient No. RD16/0011/0028) and has been acknowledged as a Consolidated Research Group by the Generalitat de Catalunya (ref. 2017SGR719).
Footnotes
AUTHORSHIP CONTRIBUTIONS
SQ, DS conceived the initial study; DS, LR designed and performed data analysis of experimental in vitro assays; DS, SQ, EF, LR prepared documentation for both Regulatory Authority and Local Ethics Committee approvals; DS, LR, MC, EV designed and performed CBPC scale-up manufacturing and quality control batch release; DS, RC, ET, JG, JRE designed clinical trial and participated in patient recruitment, treatment and follow-up; DS, LR, JV manuscript writing that was revised and edited. All Authors discussed and revised the final version of the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. 2009;44:621–6. doi: 10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- 2.Page KM, Mendizabal A, Betz-Stablein B, et al. Optimizing donor selection for public cord blood banking: influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion. 2014;54:340–52. doi: 10.1111/trf.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirchia G, Rebulla P, Mozzi F, et al. A quality system for placental blood banking. Bone Marrow Transplant. 1998;21(Suppl 3):S43–7. [PubMed] [Google Scholar]
- 4.Stokhuijzen E, Koornneef JM, Nota B, et al. Differences between platelets derived from neonatal cord blood and adult peripheral blood assessed by mass spectrometry. J Proteome Res. 2017;16:3575. doi: 10.1021/acs.jproteome.7b00298. [DOI] [PubMed] [Google Scholar]
- 5.Kavadar G, Demircioglu DT, Celik MY, Emre TY. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: a randomized prospective study. J Phys Ther Sci. 2015;27:3863–7. doi: 10.1589/jpts.27.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–68. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 7.Rebulla P, Pupella S, Santodirocco M, et al. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14:73–9. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versura P, Profazio V, Buzzi M, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–8. doi: 10.1097/ICO.0b013e3182580762. [DOI] [PubMed] [Google Scholar]
- 9.Greppi N, Mazzucco L, Galetti G, et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals. 2011;39:73–80. doi: 10.1016/j.biologicals.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Driver VR, Hanft J, Fylling CP, Beriou JM Autologel diabetic foot ulcer study group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–70. [PubMed] [Google Scholar]
- 11.Picardi A, Lanti A, Cudillo L, et al. Platelet gel for treatment of mucocutaneous lesions related to graft-vs-host disease after allogeneic hematopoietic stem cell transplant. Transfusion. 2010;50:501–6. doi: 10.1111/j.1537-2995.2009.02439.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz G, Rothe G, Ruf A, et al. European Working Group on Clinical Cell Analysis: consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost. 1998;79:885–96. [PubMed] [Google Scholar]
- 13.Smith RG. Validation of Wagner’s classification: a literature review. Ostomy Wound Manage. 2003;49:54–62. [PubMed] [Google Scholar]
- 14.Montiel-Jarquín ÁJ, García Villaseñor A, Castillo Rodríguez C, et al. Costes directos de atención médica del pie diabético en el segundo nivel de atención médica. Rev Chil Cirugía. 2017;69:118–23. [Google Scholar]
- 15.Tadini G, Guez S, Pezzani L, et al. Preliminary evaluation of cord blood platelet gel for the treatment of skin lesions in children with dystrophic epidermolysis bullosa. Blood Transfus. 2015;13:153–8. doi: 10.2450/2014.0160-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandyopadhyay A. Target product profile: a planning tool for the drug development. MOJ Bioequiv Availab. 2017;3:111–2. [Google Scholar]
- 17.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of Cord-Blood Transplantation from Related and Unrelated Donors. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 18.Parazzi V, Lavazza C, Boldrin V, et al. Extensive characterization of platelet gel releasate from cord blood in regenerative medicine. Cell Transplant. 2015;24:2573–84. doi: 10.3727/096368915X687471. [DOI] [PubMed] [Google Scholar]
- 19.Cox ST, Laza-Briviesca R, Pearson H, et al. Umbilical cord blood plasma contains soluble NKG2D ligands that mediate loss of natural killer cell function and cytotoxicity. Eur J Immunol. 2015;45:2324–34. doi: 10.1002/eji.201444990. [DOI] [PubMed] [Google Scholar]
- 20.Fréchette J-P, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–9. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 21.Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 22.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–39. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 23.Yoon KC. Use of umbilical cord serum in ophthalmology. Chonnam Med J. 2014;50:82–85. doi: 10.4068/cmj.2014.50.3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21:549–54. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 25.Everts PAM, Knape JTA, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 26.Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–87. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 27.Romanov YA, Balashova EE, Volgina NE, et al. Human umbilical cord blood serum: effective substitute of fetal bovine serum for culturing of human multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2017;162:528–33. doi: 10.1007/s10517-017-3654-9. [DOI] [PubMed] [Google Scholar]
- 28.Giannaccare G, Versura P, Buzzi M, et al. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56:595–604. doi: 10.1016/j.transci.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Textor J. Platelet-rich plasma (PRP) as a therapeutic agent: platelet biology, growth factors and a review of the literature. Springer; Berlin, Heidelberg: 2014. pp. 61–94. [Google Scholar]
- 30.Reid TJ, LaRussa VF, Esteban G, et al. Cooling and freezing damage platelet membrane integrity. Cryobiology. 1999;38:209–24. doi: 10.1006/cryo.1999.2164. [DOI] [PubMed] [Google Scholar]
- 31.Strandberg G, Sellberg F, Sommar P, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57:1058–65. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 32.Akhundov K, Pietramaggiori G, Waselle L, et al. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann Burns Fire Disasters. 2012;25:207–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Querol S, Samarkanova D. Rapid review: next generation of cord blood banks; transplantation and beyond. Transfusion. 2019;59:3048–3050. doi: 10.1111/trf.15466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.