Abstract
The role of regulatory T cells (Treg cell) in controlling autoimmune disease is an area of intense study. As such, the characterization and understanding the function of Treg markers has the potential to provide a considerable impact in developing treatments and understanding the pathogenesis of autoimmune diseases. One such inhibitory Treg cell marker that has been recently discovered is T cell immunoglobulin and ITIM domain (TIGIT). In this review, we discuss what is known about the expression and function of TIGIT on Treg cells, and we discuss the relationship between TIGIT expressing Treg cells and different autoimmune diseases such as atopic dermatitis, autoimmune thyroiditis, type 1 diabetes, autoimmune uveitis, aplastic anemia, multiple sclerosis, systemic lupus erythematosus, arthritis, and colitis.
1. Introduction
1.1. Regulatory T cells in autoimmune disease
The “re-discovery” of Tregs in 1995 was marked by the observation that CD25+CD4+ T cells suppress autoimmune disease when transferred to athymic BALB/c nude mice [1]. The FoxP3 gene was linked to Scurfy mice that suffer from fatal autoimmune disease [2], and loss of function mutations in the human FOXP3 gene were connected with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), inflammatory bowel disease, and severe allergy [3–5]. Subsequent study of regulatory T cells (Tregs) has continued to expand to identify additional surface and intracellular markers. Tregs function through the production of soluble factors such as TGF-β, IL-10, IL-35, adenosine, and fibrinogen like 2 (FGL2) [6–9], and contact dependent factors such as CTLA-4, PD-1/PD-L1, LAG3, and TIM3 [7–10]. Single cell analysis is allowing for identification and characterization of multiple Treg subsets found in diverse tissue, and tumor environments [11]. The recently discovered T cell immunoglobulin and ITIM domain (TIGIT) is an inhibitory receptor expressed on Treg cells that have the potential to control autoimmune disease. In this review, we discuss what is known about TIGIT, its role in Treg function and expression, and what has been demonstrated regarding TIGIT+ Tregs with autoimmune diseases such as atopic dermatitis, autoimmune thyroiditis, type 1 diabetes, autoimmune uveitis, aplastic anemia, multiple sclerosis, systemic lupus erythematosus, arthritis, and colitis.
1.2. TIGIT background
TIGIT is also known as WUCAM, VSTM3, or VSIG9 and is part of the poliovirus receptor (PVR) or nectin family of proteins that consists of TIGIT, CD226 (DNAM-1), CD96, CD112R, PVR (CD155), CD112 (PVRL2/nectin-2), and CD113 (PVRL3/nectin-3) [12–16]. TIGIT binds with high affinity to PVR and with lower affinity to PVRL2 and PVRL3, but PVRL3 has not been demonstrated to bind to human TIGIT [12]. Nectins and nectin-like proteins are expressed on the cell surface and homophilic and heterophilic trans-interactions mediate cell adhesion with other cells, cell polarization, tissue organization, and signal transduction [17,18]. The structure of TIGIT includes an extracellular immunoglobulin variable-set (IgV) domain, type 1 transmembrane domain, intracellular domain with a canonical immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoglobulin tyrosine tail (ITT) motif [12]. TIGIT expression has been observed on CD4+ T cells, CD8+ T cells, follicular T cells (Tfh cells), and NK cells [12,19–22].
TIGIT was first reported by Grogan, et al. in 2009 as an inhibitory receptor that suppresses T cell activation by inducing regulatory dendritic cells [12]. Grogan, et al., screened for proteins exclusively expressed by T cells [23] that had an ITIM [24,25], and further demonstrated TIGIT expression on activated human T cells, high affinity binding to PVR whose interaction promotes regulatory dendritic cells in an IL-10 mediated manner [12]. This report just ten years ago initiated the study of TIGIT+ Treg cells in cancer and autoimmunity.
1.3. Regulation of TIGIT expression
TIGIT expression on naïve T cells is undetectable, but is upregulated following activation. The transcription factors that control TIGIT expression include BLIMP1 and low expression of Bach2 in Tfh cells [26]. The transcription factor, Eomes, is expressed on CD8+ T cells from patients with newly diagnosed acute myeloid leukemia, and Eomes positively regulates TIGIT expression [27]. As of the writing of this review, the transcriptional program that controls TIGIT expression is not well understood, and additional work needs to be done to further define how TIGIT expression is regulated in Treg cells.
1.4. TIGIT Signaling
TIGIT ligation results in recruitment of Grb2 and SHIP1 in NK cells through the tyrosine region [28]. Presumably the ITIM or ITT region in T cells also recruits SHIP1 and/or SHP2 as with PD-1. As such, the human T-cell leukemia virus type 1 (HTLV-1) inhibits SHP-2 to allow for T-cell proliferation despite PD-1 and TIGIT ligation [29]. While Treg studies of downstream TIGIT signaling are lacking, it has been demonstrated that upregulation of TIGIT in Treg cells results in the demethylation of FOXP3 [30].
Another interesting observation that demonstrates a role for the microbiome in TIGIT signaling was made with Fusobacterium nucleatum [31]. F. nucleatum is a common oral anaerobic Gram-negative found in human tumors, adenocarcinoma tumors in particular [32,33]. Using a library of F. nucleatum mutants, the Fap2 protein expressed by F. nucleatum was identified as a ligand for TIGIT and suppresses T cell activity to promote tumor survival [31].
1.5. Mechanism of action
Three mechanisms have been identified through which TIGIT accomplishes immunoregulation (Fig 1). First, TIGIT can act as a ligand for PVR, and with less affinity for PVRL2 and PVRL3, to suppress the denritic cell or antigen presenting cell that expresses PVR. Ligation of PVR with TIGIT results in phosphorylation of PVR, Erk, and p38 promoting a regulatory dendritic cell that secretes IL-10 [12]. Second, TIGIT can act as a receptor on T cells that when ligated with PVR results in a block to T cell priming [12], possibly through downregulation of the TCR [34], and triggers the production of the immunosuppressive molecule, FGL2 [35]. In NK cells, TIGIT phosphorylation recruits Grb2 and β-arrestin which recruits SHIP1 and SHP2 to inhibit PI3K and NF-κB [28,36], while this has not yet been shown in T cells the NK cell work suggests that TIGIT signaling in T cells may work through SHIP1 and SHP2. Finally, TIGIT binds CD226 with higher affinity than PVR binding with CD226 [12,37], to prevent CD226 mediated activation. CD226 is an Ig superfamily member that is an activating receptor when bound by PVR that is disrupted through TIGIT binding to CD226. Therefore, it is through one or more mechanisms that TIGIT may suppress T cell activation to control an autoimmune response that makes TIGIT expressing Treg cells an attractive therapeutic for controlling autoimmune disease.
Figure 1.
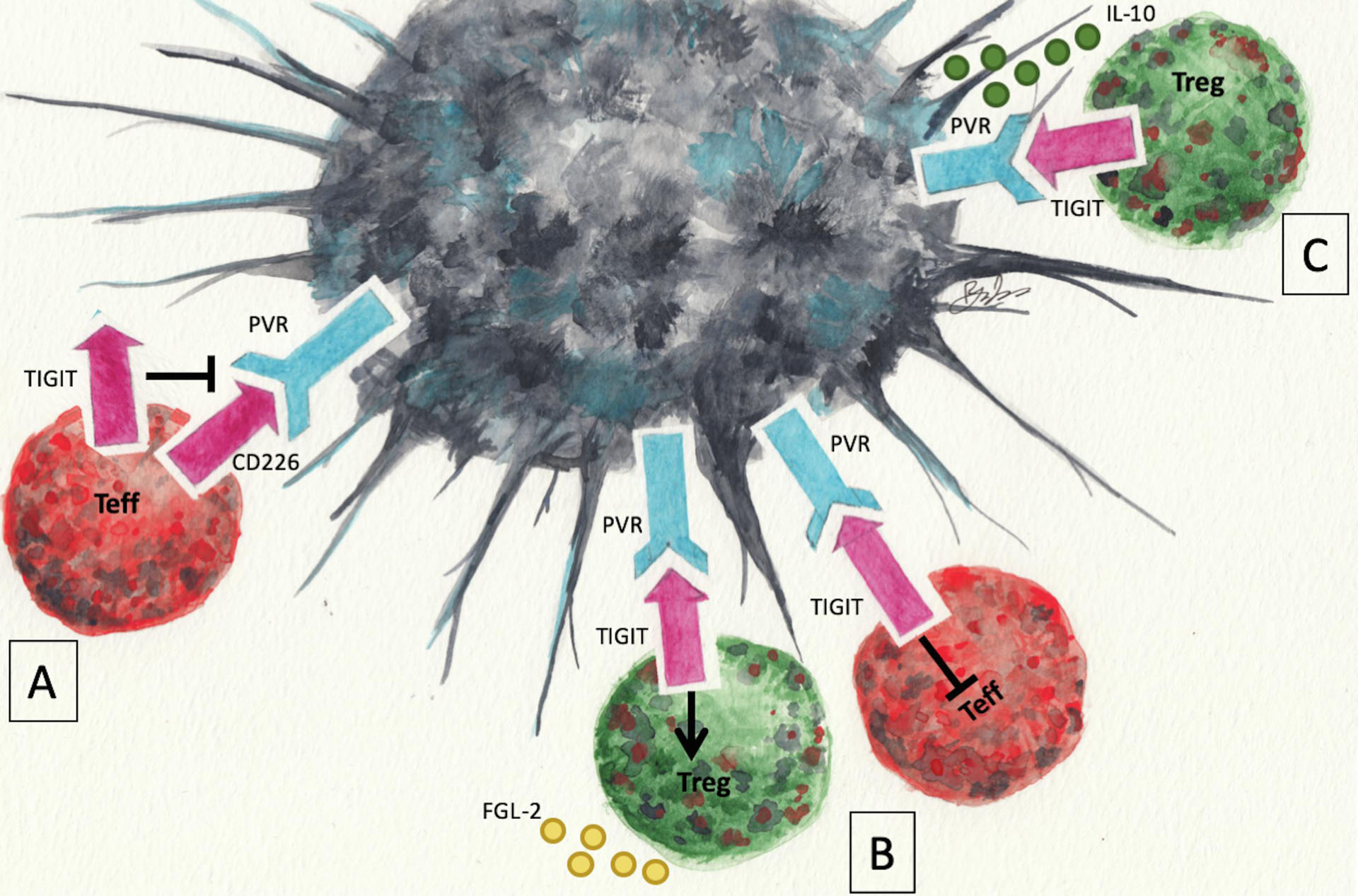
Mechanisms of TIGIT mediated suppression. TIGIT can block CD226-PVR mediated activation of T cells because it binds to CD226 with higher affinity than PVR (A). Ligation of TIGIT with PVR on naïve T cells blocks T cell priming, and triggers the production of the immunosuppressive molecule, FGL2 (B). Ligation of PVR with TIGIT promotes a regulatory dendritic cell that secretes IL-10 (C). Artwork by Ryan E. Lee.
2. Autoimmune diseases connected with TIGIT+ Tregs
2.1. Atopic dermatitis
Clinical analysis of 17 patients with atopic dermatitis (AD) and 14 healthy individuals found the number of TIGIT expressing CD4+ T cells was increased in AD patients, but the four most severe AD cases showed a significant reduction in the number of TIGIT+ CD4+ T cells [38]. The increased number of TIGIT+ CD4+ T cells from AD patients may be compensatory because they had a significant reduction in proliferation capacity with anti-CD3 and anti-CD28 stimulation compared with healthy individuals [38]. This study suggests that a reduction in TIGIT+ T cells may contribute to more severe disease.
2.2. Autoimmune thyroiditis
A role for TIGIT+ Treg cells with autoimmune thyroiditis has been reported in humans and mice. It has been demonstrated in mice that OX40L-JAG1 treatment expanded CTLA4+ and TIGIT+ Tregs that alleviated experimental autoimmune thyroiditis, and humanized NSG mice also showed an expansion of the Tregs in the liver [39]. Analysis of thirty autoimmune thyroiditis (AT) patients compared with ten healthy controls revealed a correlation between expression of Fc Receptor Like 3 (FCRL3), an inhibitory receptor, and TIGIT with different subtypes of AT [40]. These mouse and clinical studies suggest that TIGIT+ Tregs function to control autoimmune thyroiditis.
2.3. Diabetes
There have been several observations linking TIGIT expressing Tregs with diabetes in different diabetic mouse models. TIGIT+ Tregs have been identified in the islets of NOD mice [41], and another group found that high-affinity TCR Tregs from the pancreas expressed TIGIT [41]. The S1PR1 agonist, CYM-5442, prevented type 1 diabetes and upregulated Tigit in T cells in the mouse Rip-LCMV T1D model [42]. A sub-immunogenic vaccination with strong insulin mimetopes was effective in preventing the onset of diabetes and induced TIGIT expression in Tregs in a humanized NSG mouse [43].
Importantly, TIGIT+ Tregs have also been shown to be expressed by T cells from human diabetes patients. In a large transcriptional profiling and functional analysis of T cell subsets from type 1 diabetic patients, TIGIT+CD226− Tregs from type 1 diabetic patients are stable and suppressive, compared to TIGIT−CD226+ T cells that were a mixed population of TCM, TEM, and Treg with a decreased suppressive capacity, effector cytokine and IL-10 production [44]. Another study examining microRNA in CD4+ T cells from 23 pre-diabetic patients compared with 29 healthy controls revealed that expression of miR-26a correlates with FoxP3 and TIGIT expression, and the mechanism is through negative regulation of the histone methyltransferase EZH2 whose inhibition correlates with decreased Tregs [44]. These mouse and clinical studies suggest that TIGIT+ Tregs are capable of suppressing type 1 diabetes.
2.4. Aplastic anemia
Using a combination of mouse studies and clinical samples, it has been demonstrated that TIGIT+ Tregs improved the red blood cell count in an aplastic anemia model [45]. This group further showed that the long, non-coding RNA, MEG3 functions to absorb miR-23a that reduces TIGIT expression, resulting in upregulation of TIGIT and reduced expansion of Th1 and Th17 cells [46]. These studies suggest that TIGIT+ Tregs suppress aplastic anemia by suppressing Th1 and Th17 cells, and identify the lncRNA, MEG3, as a positive regulator of TIGIT.
2.5. Autoimmune uveitis
A study examining TIGIT+ Tregs from 50 autoimmune uveitis patients compared with ten control subjects revealed a positive correlation in the number of TIGIT+ Tregs with patients in remission [47]. This clinical study suggests that TIGIT+ Tregs contribute to remission of autoimmne uveitis. As of the time of writing of this report, not other studies involving TIGIT and uveitis have been published. Therefore, additional study of TIGIT expressing Tregs in the pathogenesis of autoimmune uveitis is necessary.
While the above discussed autoimmune diseases show a positive correlation with TIGIT expressing Tregs and autoimmune disease, there are reports of a correlation with a reduction in TIGIT expressing Tregs with autoimmune diseases with TIGIT+ Tregs, as discussed below.
2.6. Multiple Sclerosis
In mice, antigen specific immunotherapy in experimental autoimmune encephalomyelitis induces TIGIT expressing T cells [48]. In contrast, it has been reported that TIGIT expressing CD4 cells is lower in multiple sclerosis (MS) patients with a sample size of 57 MS patients and 19 healthy controls [49]. However, the TIGIT signaling pathway was found to be active in another report that included nine MS patients and seven healthy controls with the observation that TIGIT stimulation of PBMCs from MS patients reduces Th1 differentiation [50]. These studies suggest TIGIT-expressing Tregs are involved in suppression of MS. In mice, TIGIT+ Tregs are associated with suppression of EAE. In MS patients with active disease the low number of TIGIT+ Tregs suggests their absence may contribute to disease.
2.7. Colitis
In a dextran sulfate sodium mouse model of colitis, estrogen receptor β activation was effective in reducing disease and increased the number of infiltrating TIGIT+ Tregs [51]. However, CyTOF analysis of PBMCs from 33 ulcerative colitis patients compared with 14 healthy donors revealed an increase in TIGIT expression on NK and γδ T cells, but was decreased in CD4+ T cells [52]. In these studies, the discrepancy between mouse and patient observations could be due to differences in the state of disease, but suggests a defect in TIGIT expression on CD4+ T cells could contribute to disease.
2.8. Lupus
It has been demonstrated that recombinant TIGIT-Ig is an effective prevention and treatment for murine lupus [53]. However, a study that included 49 systemic lupus erythematosus (SLE) patients and 22 healthy controls found that patients with renal manifestations had a decreased frequency of TIGIT+ T cells [54]. In contrast, another study that included 50 SLE patients and 27 healthy controls found that TIGIT-expressing CD4 T cells are significantly elevated in SLE patients with high anti-dsDNA antibodies, high anti-Sm antibodies, and high levels of urine microalbumin compared with controls [55]. Another larger study including 94 SLE patients and 64 healthy controls also found the number of TIGIT+ CD4 T cells to be elevated in SLE patients and TIGIT+ CD4 T cells have less activation potential compared with TIGIT− CD4 T cells [56]. While the first study discussed above suggests that a decrease in TIGIT+ T cells is related to disease, the conflicting observations in the second two studies that find an increase in TIGIT+ T cells could be due to differences in sample sizes and could be compensatory to keep the disease controlled.
2.9. Arthritis
Mouse models show TIGIT overexpression reduces the severity of rheumatoid arthritis (RA) [57]. However, there is a contradiction in the literature regarding TIGIT expression on T cells as a correlation with disease activity. Two groups analyzed PBMCs from over 70 RA patients that were being treated with various therapies and one found a correlation with TIGIT expression and rheumatoid arthritis [58], but the second group found no correlation with TIGIT expression [59]. Both studies also compared expression with the severity of disease and found opposite correlations. These observations suggest there may be differences between geographic regions, ethnicities, and treatments. Since both studies analyzed PBMCs and not the lymphocytes at the site of inflammation, another group analyzed the CD4 T cells from synovial fluid of RA patients and found a positive correlation with TIGIT expression and disease [60].
2.10. Disease summary
The above observations suggest that in some autoimmune diseases and disease states the TIGIT+ T cell population may be increased in an attempt to keep the disease under control, and in some cases when this population is absent or reduced, this may indicate the disease has progressed into a more severe disease state. While this review is focused on TIGIT-expressing Treg cell mediated expression it should be noted that because TIGIT is expressed on NK cells, Tfh cells, and CD8 T cells it likely that some of the suppressive mechanisms utilized by Treg cells to suppress through TIGIT are shared. Unfortunately, an extensive comparative analysis of the suppressive mechanisms of the various TIGIT-expressing cells has not been done. However, it is likely the extrinsic mechanisms of suppression such as induction of immunosuppressive dendritic cells would be shared between all cell types, but Treg cell specific mechanisms such as FGL2 production may not be shared.
3. Conclusions
A role for TIGIT+ Tregs in some autoimmune diseases is clear, but the heterogeneity of autoimmune diseases, disease status, and geographic variations of patient composition likely contributes to inconsistent observations regarding TIGIT+ Tregs as a definitive marker for the status of autoimmune diseases. Regardless of utilizing TIGIT+ Tregs as a marker for autoimmune disease, it is clear that this Treg cell represents a population of suppressive cells that may be used for the treatment of many different autoimmune diseases. As such, further investigation of TIGIT expression and function on Tregs is necessary.
Acknowledgements:
This work was supported by National Institutes of Health/National Eye Institute grants EY021725 (P30), EY024951 (DJL), EY029240 (DJL), and in part by an unrestricted Research to Prevent Blindness grant (New York, NY, USA). We would also like to thank Ryan E. Lee for the artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: “The authors have declared that no conflict of interest exists.”
References
- 1.Sakaguchi S; Sakaguchi N; Asano M; Itoh M; Toda M Immunologic self-tolerance maintained by activated t cells expressing il-2 receptor alpha-chains (cd25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995, 155, 1151–1164. [PubMed] [Google Scholar]
- 2.Brunkow ME; Jeffery EW; Hjerrild KA; Paeper B; Clark LB; Yasayko SA; Wilkinson JE; Galas D; Ziegler SF; Ramsdell F Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001, 27, 68–73. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL; Christie J; Ramsdell F; Brunkow ME; Ferguson PJ; Whitesell L; Kelly TE; Saulsbury FT; Chance PF; Ochs HD The immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome (ipex) is caused by mutations of foxp3. Nat Genet 2001, 27, 20–21. [DOI] [PubMed] [Google Scholar]
- 4.Chatila TA; Blaeser F; Ho N; Lederman HM; Voulgaropoulos C; Helms C; Bowcock AM Jm2, encoding a fork head-related protein, is mutated in x-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 2000, 106, R75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildin RS; Ramsdell F; Peake J; Faravelli F; Casanova JL; Buist N; Levy-Lahad E; Mazzella M; Goulet O; Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001, 27, 18–20. [DOI] [PubMed] [Google Scholar]
- 6.Shalev I; Liu H; Koscik C; Bartczak A; Javadi M; Wong KM; Maknojia A; He W; Liu MF; Diao J, et al. Targeted deletion of fgl2 leads to impaired regulatory t cell activity and development of autoimmune glomerulonephritis. J Immunol 2008, 180, 249–260. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez-Villar M; Hafler DA Regulatory t cells in autoimmune disease. Nat Immunol 2018, 19, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing JB; Tanaka A; Sakaguchi S Human foxp3(+) regulatory t cell heterogeneity and function in autoimmunity and cancer. Immunity 2019, 50, 302–316. [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ; Lu LF; Rudensky AY Regulatory t cells: Mechanisms of differentiation and function. Annu Rev Immunol 2012, 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco LM; Sage PT; Sharpe AH The pd-1 pathway in tolerance and autoimmunity. Immunol Rev 2010, 236, 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason GM; Lowe K; Melchiotti R; Ellis R; de Rinaldis E; Peakman M; Heck S; Lombardi G; Tree TI Phenotypic complexity of the human regulatory t cell compartment revealed by mass cytometry. J Immunol 2015, 195, 2030–2037. [DOI] [PubMed] [Google Scholar]
- 12.Yu X; Harden K; Gonzalez LC; Francesco M; Chiang E; Irving B; Tom I; Ivelja S; Refino CJ; Clark H, et al. The surface protein tigit suppresses t cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nature immunology 2009, 10, 48–57. [DOI] [PubMed] [Google Scholar]
- 13.Stengel KF; Harden-Bowles K; Yu X; Rouge L; Yin J; Comps-Agrar L; Wiesmann C; Bazan JF; Eaton DL; Grogan JL Structure of tigit immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A 2012, 109, 5399–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y; Paniccia A; Schulick AC; Chen W; Koenig MR; Byers JT; Yao S; Bevers S; Edil BH Identification of cd112r as a novel checkpoint for human t cells. J Exp Med 2016, 213, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J; Qian X; Chen Z; Xu X; Gao F; Zhang S; Zhang R; Qi J; Gao GF; Yan J Crystal structure of cell adhesion molecule nectin-2/cd112 and its binding to immune receptor dnam-1/cd226. J Immunol 2012, 188, 5511–5520. [DOI] [PubMed] [Google Scholar]
- 16.Tahara-Hanaoka S; Shibuya K; Kai H; Miyamoto A; Morikawa Y; Ohkochi N; Honda S; Shibuya A Tumor rejection by the poliovirus receptor family ligands of the dnam-1 (cd226) receptor. Blood 2006, 107, 1491–1496. [DOI] [PubMed] [Google Scholar]
- 17.Fabre S; Reymond N; Cocchi F; Menotti L; Dubreuil P; Campadelli-Fiume G; Lopez M Prominent role of the ig-like v domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted c-c’-c”-d beta-strands of the nectin1 v domain. J Biol Chem 2002, 277, 27006–27013. [DOI] [PubMed] [Google Scholar]
- 18.Sakisaka T; Takai Y Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol 2004, 16, 513–521. [DOI] [PubMed] [Google Scholar]
- 19.Johnston RJ; Comps-Agrar L; Hackney J; Yu X; Huseni M; Yang Y; Park S; Javinal V; Chiu H; Irving B, et al. The immunoreceptor tigit regulates antitumor and antiviral cd8(+) t cell effector function. Cancer Cell 2014, 26, 923–937. [DOI] [PubMed] [Google Scholar]
- 20.Wu H; Chen Y; Liu H; Xu LL; Teuscher P; Wang S; Lu S; Dent AL Follicular regulatory t cells repress cytokine production by follicular helper t cells and optimize igg responses in mice. Eur J Immunol 2016, 46, 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boles KS; Vermi W; Facchetti F; Fuchs A; Wilson TJ; Diacovo TG; Cella M; Colonna M A novel molecular interaction for the adhesion of follicular cd4 t cells to follicular dc. Eur J Immunol 2009, 39, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanietsky N; Simic H; Arapovic J; Toporik A; Levy O; Novik A; Levine Z; Beiman M; Dassa L; Achdout H, et al. The interaction of tigit with pvr and pvrl2 inhibits human nk cell cytotoxicity. Proc Natl Acad Sci U S A 2009, 106, 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas AR; Baldwin D; Ma Y; Ouyang W; Gurney A; Martin F; Fong S; van Lookeren Campagne M; Godowski P; Williams PM, et al. Immune response in silico (iris): Immune-specific genes identified from a compendium of microarray expression data. Genes Immun 2005, 6, 319–331. [DOI] [PubMed] [Google Scholar]
- 24.Burshtyn DN; Yang W; Yi T; Long EO A novel phosphotyrosine motif with a critical amino acid at position −2 for the sh2 domain-mediated activation of the tyrosine phosphatase shp-1. J Biol Chem 1997, 272, 13066–13072. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwada M; Giallourakis CC; Pan PY; Rothman PB Immunoreceptor tyrosine-based inhibitory motif of the il-4 receptor associates with sh2-containing phosphatases and regulates il-4-induced proliferation. J Immunol 2001, 167, 6382–6387. [DOI] [PubMed] [Google Scholar]
- 26.Lahmann A; Kuhrau J; Fuhrmann F; Heinrich F; Bauer L; Durek P; Mashreghi MF; Hutloff A Bach2 controls t follicular helper cells by direct repression of bcl-6. J Immunol 2019, 202, 2229–2239. [DOI] [PubMed] [Google Scholar]
- 27.Jia B; Zhao C; Rakszawski KL; Claxton DF; Ehmann WC; Rybka WB; Mineishi S; Wang M; Shike H; Bayerl MG, et al. Eomes(+)t-bet(low) cd8(+) t cells are functionally impaired and are associated with poor clinical outcome in patients with acute myeloid leukemia. Cancer Res 2019, 79, 1635–1645. [DOI] [PubMed] [Google Scholar]
- 28.Liu S; Zhang H; Li M; Hu D; Li C; Ge B; Jin B; Fan Z Recruitment of grb2 and ship1 by the itt-like motif of tigit suppresses granule polarization and cytotoxicity of nk cells. Cell Death Differ 2013, 20, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinosada H; Yasunaga JI; Shimura K; Miyazato P; Onishi C; Iyoda T; Inaba K; Matsuoka M Htlv-1 bzip factor enhances t-cell proliferation by impeding the suppressive signaling of co-inhibitory receptors. PLoS Pathog 2017, 13, e1006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y; Maksimovic J; Naselli G; Qian J; Chopin M; Blewitt ME; Oshlack A; Harrison LC Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by foxp3 in human regulatory t cells. Blood 2013, 122, 2823–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gur C; Ibrahim Y; Isaacson B; Yamin R; Abed J; Gamliel M; Enk J; Bar-On Y; Stanietsky-Kaynan N; Coppenhagen-Glazer S, et al. Binding of the fap2 protein of fusobacterium nucleatum to human inhibitory receptor tigit protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellarin M; Warren RL; Freeman JD; Dreolini L; Krzywinski M; Strauss J; Barnes R; Watson P; Allen-Vercoe E; Moore RA, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012, 22, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostic AD; Gevers D; Pedamallu CS; Michaud M; Duke F; Earl AM; Ojesina AI; Jung J; Bass AJ; Tabernero J, et al. Genomic analysis identifies association of fusobacterium with colorectal carcinoma. Genome Res 2012, 22, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joller N; Hafler JP; Brynedal B; Kassam N; Spoerl S; Levin SD; Sharpe AH; Kuchroo VK Cutting edge: Tigit has t cell-intrinsic inhibitory functions. J Immunol 2011, 186, 1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joller N; Lozano E; Burkett PR; Patel B; Xiao S; Zhu C; Xia J; Tan TG; Sefik E; Yajnik V, et al. Treg cells expressing the coinhibitory molecule tigit selectively inhibit proinflammatory th1 and th17 cell responses. Immunity 2014, 40, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M; Xia P; Du Y; Liu S; Huang G; Chen J; Zhang H; Hou N; Cheng X; Zhou L, et al. T-cell immunoglobulin and itim domain (tigit) receptor/poliovirus receptor (pvr) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem 2014, 289, 17647–17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin SD; Taft DW; Brandt CS; Bucher C; Howard ED; Chadwick EM; Johnston J; Hammond A; Bontadelli K; Ardourel D, et al. Vstm3 is a member of the cd28 family and an important modulator of t-cell function. Eur J Immunol 2011, 41, 902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurita M; Yoshihara Y; Ishiuji Y; Chihara M; Ishiji T; Asahina A; Yanaba K Expression of t-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain on cd4(+) t cells in patients with atopic dermatitis. J Dermatol 2019, 46, 37–42. [DOI] [PubMed] [Google Scholar]
- 39.Kumar P; Lele SS; Ragothaman VK; Raghunathan D; Epstein AL; Chiba S; Prabhakar BS Ox40l-jag1-induced expansion of lineage-stable regulatory t cells involves noncanonical nf-kappab signaling. J Immunol 2019, 203, 3225–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanic M; Tokic S; Suver-Stevic M; Glavas-Obrovac L Expression of tigit and fcrl3 is altered in t cells from patients with distinct patterns of chronic autoimmune thyroiditis. Exp Clin Endocrinol Diabetes 2019, 127, 281–288. [DOI] [PubMed] [Google Scholar]
- 41.Spence A; Purtha W; Tam J; Dong S; Kim Y; Ju CH; Sterling T; Nakayama M; Robinson WH; Bluestone JA, et al. Revealing the specificity of regulatory t cells in murine autoimmune diabetes. Proc Natl Acad Sci U S A 2018, 115, 5265–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marro BS; Ware BC; Zak J; de la Torre JC; Rosen H; Oldstone MB Progression of type 1 diabetes from the prediabetic stage is controlled by interferon-alpha signaling. Proc Natl Acad Sci U S A 2017, 114, 3708–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serr I; Furst RW; Achenbach P; Scherm MG; Gokmen F; Haupt F; Sedlmeier EM; Knopff A; Shultz L; Willis RA, et al. Type 1 diabetes vaccine candidates promote human foxp3(+)treg induction in humanized mice. Nat Commun 2016, 7, 10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuhrman CA; Yeh WI; Seay HR; Saikumar Lakshmi P; Chopra G; Zhang L; Perry DJ; McClymont SA; Yadav M; Lopez MC, et al. Divergent phenotypes of human regulatory t cells expressing the receptors tigit and cd226. J Immunol 2015, 195, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T; Wang J; Zhou X; Liang R; Bai Q; Yang L; Gu H; Gao G; Dong B; Zhu H, et al. Increased expression of tigit on cd4+ t cells ameliorates immune-mediated bone marrow failure of aplastic anemia. J Cell Biochem 2014, 115, 1918–1927. [DOI] [PubMed] [Google Scholar]
- 46.Wang J; Liu X; Hao C; Lu Y; Duan X; Liang R; Gao G; Zhang T Meg3 modulates tigit expression and cd4 + t cell activation through absorbing mir-23a. Mol Cell Biochem 2019, 454, 67–76. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert RM; Zhang X; Sampson RD; Ehrenstein MR; Nguyen DX; Chaudhry M; Mein C; Mahmud N; Galatowicz G; Tomkins-Netzer O, et al. Clinical remission of sight-threatening non-infectious uveitis is characterized by an upregulation of peripheral t-regulatory cell polarized towards t-bet and tigit. Front Immunol 2018, 9, 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burton BR; Britton GJ; Fang H; Verhagen J; Smithers B; Sabatos-Peyton CA; Carney LJ; Gough J; Strobel S; Wraith DC Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun 2014, 5, 4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavon I; Heli C; Brill L; Charbit H; Vaknin-Dembinsky A Blood levels of co-inhibitory-receptors: A biomarker of disease prognosis in multiple sclerosis. Front Immunol 2019, 10, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucca LE; Axisa PP; Singer ER; Nolan NM; Dominguez-Villar M; Hafler DA Tigit signaling restores suppressor function of th1 tregs. JCI Insight 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo D; Liu X; Zeng C; Cheng L; Song G; Hou X; Zhu L; Zou K Estrogen receptor beta activation ameliorates dss-induced chronic colitis by inhibiting inflammation and promoting treg differentiation. International immunopharmacology 2019, 77, 105971. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs S; Sawas N; Staedler N; Schubert DA; D’Andrea A; Zeiser R; Piali L; Hruz P; Frei AP High-dimensional single-cell proteomics analysis identifies immune checkpoint signatures and therapeutic targets in ulcerative colitis. Eur J Immunol 2019, 49, 462–475. [DOI] [PubMed] [Google Scholar]
- 53.Liu S; Sun L; Wang C; Cui Y; Ling Y; Li T; Lin F; Fu W; Ding M; Zhang S, et al. Treatment of murine lupus with tigit-ig. Clin Immunol 2019, 203, 72–80. [DOI] [PubMed] [Google Scholar]
- 54.Zhou H; Li B; Li J; Wu T; Jin X; Yuan R; Shi P; Zhou Y; Li L; Yu F Dysregulated t cell activation and aberrant cytokine expression profile in systemic lupus erythematosus. Mediators Inflamm 2019, 2019, 8450947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Q; Ye J; Zeng L; Li X; Fang L; Ju B; Huang Z; Li J Elevated expression of tigit on cd3(+)cd4(+) t cells correlates with disease activity in systemic lupus erythematosus. Allergy Asthma Clin Immunol 2017, 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao L; Hou H; Wu S; Zhou Y; Wang J; Yu J; Wu X; Lu Y; Mao L; Bosco MJ, et al. Tigit signalling pathway negatively regulates cd4(+) t-cell responses in systemic lupus erythematosus. Immunology 2017, 151, 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W; Dong Y; Wu C; Ma Y; Jin Y; Ji Y Tigit overexpression diminishes the function of cd4 t cells and ameliorates the severity of rheumatoid arthritis in mouse models. Exp Cell Res 2016, 340, 132–138. [DOI] [PubMed] [Google Scholar]
- 58.Luo Q; Deng Z; Xu C; Zeng L; Ye J; Li X; Guo Y; Huang Z; Li J Elevated expression of immunoreceptor tyrosine-based inhibitory motif (tigit) on t lymphocytes is correlated with disease activity in rheumatoid arthritis. Med Sci Monit 2017, 23, 1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M; Liu Y; Mo B; Xue Y; Ye C; Jiang Y; Bi X; Liu M; Wu Y; Wang J, et al. Helios but not cd226, tigit and foxp3 is a potential marker for cd4(+) treg cells in patients with rheumatoid arthritis. Cell Physiol Biochem 2019, 52, 1178–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greisen SR; Yan Y; Hansen AS; Veno MT; Nyengaard JR; Moestrup SK; Hvid M; Freeman GJ; Kjems J; Deleuran B Extracellular vesicles transfer the receptor programmed death-1 in rheumatoid arthritis. Front Immunol 2017, 8, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]


