Abstract
Background:
Interleukin-22 (IL-22) has beneficial effects on inflammation and impaired hepatic regeneration, that characterize AH. F-652 is a recombinant fusion protein of human IL-22 and IgG2-Fc.
Aims:
To assess safety and efficacy signals of F-652 in patients with moderate and severe AH.
Methods:
A phase-2 dose escalating study was carried out. F-652 (10, 30 or 45 μg/kg) administered on day-1 and 7 was tested in 3 patients each with moderate (MELD-scores:11-20) and severe AH (MELD-scores:21-28). Safety was defined by absence of serious adverse events (SAE) and efficacy was assessed by Lille score, changes in MELD score, and serum bilirubin and aminotransferases at days 28 and 42. Three independent propensity matched comparator patient cohorts were used. Plasma extracellular vesicles (EVs) and multiplex serum cytokines were measured to assess inflammation and hepatic regeneration.
Results:
18 patients (9 moderate and 9 severe AH) were enrolled, 66% male, mean age 48 years. The half-life of F-652 following the first dose was 61-85 hours. There were no SAE leading to discontinuation. MELD score, and serum aminotransferases decreased significantly at days 28 and 42 from baseline (p<0.05). Day-7 Lille score was ≤0.45 in 83% patients as compared with 6%,12%, and 56% among the comparator cohorts. EV counts decreased significantly at day-28 (p<0.013). Cytokine inflammatory markers were downregulated, and regeneration markers were upregulated at days 28 and 42.
Conclusions:
F-652 is safe in doses up to 45 μg/kg and associated with high rate of improvement as determined by Lille and MELD scores, reductions in markers of inflammation and increases in markers of hepatic regeneration. This study supports the need for randomized placebo-controlled trials to test efficacy of F-652 in AH.
Keywords: alcohol, steatohepatitis, interleukin-22, stopah, alcoholic liver disease
LAY SUMMARY
This study investigates a potentially new treatment of alcoholic hepatitis. The drug F-652, was found to be free from significant side effects, well tolerated and associated with a high rate of improvement in scores that reflect severity of liver disease. In addition, there were reductions in serum and plasma markers of inflammation and increases in markers of liver regeneration. This study supports the need for randomized placebo-controlled trials to test efficacy of F-652 in patients with severe alcoholic hepatitis.
INTRODUCTION
Alcohol-related liver disease (ALD) is a major cause of morbidity and mortality in the United States and other parts of the world (1–3) and encompasses a clinico-histologic spectrum of disease including fatty liver, alcoholic hepatitis (AH), and alcohol-related cirrhosis (4, 5). AH is a life-threating form of ALD; one in five chronic, heavy drinkers develops AH, a syndrome of progressive inflammatory liver injury (6–8). Severe AH may be associated with 90-day mortality upwards of 40% (6, 9). No new drugs for AH have been successfully developed since corticosteroids were introduced for treatment of severe AH in the early 1970s (10–12). Moreover, corticosteroids are contraindicated in the presence of infection, gastrointestinal bleeding, pancreatitis, and viral hepatitis (11). A recent study of the most commonly used agents in the treatment of AH showed benefit only at 1 month with prednisone and no survival benefit with pentoxifylline. Therefore, there is a need for development of novel agents to treat AH.
IL-22 is a potential agent for the treatment of patients with AH because of its antioxidant, anti-apoptotic, anti-steatotic, anti-microbial, and pro-proliferative effects demonstrated in various experimental systems (13, 14). IL-22 is a member of the IL-10 family of cytokines that control bacterial infection, homeostasis, and tissue repair. F-652 is a recombinant fusion protein consisting of human interleukin 22 (IL-22) and human IgG2 Fc fragments with the same mechanism of action as the native IL-22. However, F-652 has extended and/or improved pharmacokinetic (PK) and pharmacodynamic (PD) properties due to the addition of human Fc fragments at the C-terminal. F-652 is able to protect tissue from damage, inflammation, and infection, and enhances tissue repair by activating STAT3 mediated by IL-22R1 expressed on epithelial cells such as hepatocytes.
Preclinical studies in various animal models have described the protective role of IL-22 in experimental hepatitis. In the Concanavalin A (ConA) and carbon tetrachloride (CCl4)-induced liver injury mouse models, administration of recombinant IL-22 ameliorated the elevated serum levels of alanine-aminotransferase (ALT) and aspartate-aminotransferase (AST). Delivery of IL-22-expressing plasmid in the liver significantly reduced the elevated serum levels of ALT, AST, and apoptotic hepatocyte damage in these models (15). In an AH murine model, administration of IL-22 adenovirus ameliorated the elevation of serum AST and ALT (16). In the high fat diet-induced liver steatosis mouse model, administration of recombinant IL-22 not only reduced hepatic steatosis, but also down-regulated key enzymes for the synthesis of triglyceride and cholesterol, and modulators for de novo lipogenesis (17). The hepatoprotective effect of the F-652 formulation of IL-22 was demonstrated in acute liver injury models including partial hepatectomy (16, 18). F-652 demonstrated a favorable safety profile in acute and long-term (3-month) studies in rats and monkeys (19), and healthy volunteers (20). Given its mechanisms of action, the IL-22 agonist F-652 is an excellent candidate for AH treatment. Thus, the aim of our study was to assess in patients with AH and MELD score between 11-28, the safety and efficacy of the IL-22 agonist (F-652) as determined by improved MELD scores at days 28 and 42.
MATERIALS AND METHODS
Study Design
This was a phase 2a open-label study. The trial was registered on clinicaltrials.gov ( NCT02655510). The study was carried out through the TREAT Consortium (21) and the protocol approved by the Institutional Review Boards. All subjects gave written, informed consent. Data were analyzed at Mayo Clinic, the NIAAA and by Generon. Authors had access to the data and participated in analyses and drafting the manuscript. All authors approved the manuscript and assume full responsibility for data accuracy and completeness.
Patients
A total of 18 participants were prospectively recruited all through Mayo Clinic Enterprise (10 from Rochester, MN or Mankato Regional Hospital, Manako, MN; 6 Phoenix, AZ; and 2 Jacksonville, FL). Inclusion criteria included the ability to provide written informed consent, age ≥21 years and diagnosis of AH . The NIAAA criteria used to define AH were: 1) history of heavy alcohol use: >40 g/day in females and >60 g/day in males for a minimum period of 6 months; 2) alcohol consumption within 6 weeks of recruitment to study; 3) serum bilirubin >3 mg/dL and AST>ALT, but both <500 U/L; and 4) liver biopsy confirmation, unless criteria 1-3 were met and other differential diagnoses excluded (22). That is, liver biopsy was required when one or more of the possible confounding factors was present including: possible ischemic hepatitis, drug-induced liver injury, recent sepsis, uncertain alcohol assessment and/or atypical laboratory tests (e.g. AST<40 U/L or >500 U/L, AST/ALT ratio <1.5) (22). Potential subjects with organ failure (as defined by hepatic encephalopathy >stage 3; need for ventilator or vasopressor support; renal replacement therapy or creatinine >2.5); hepatocellular carcinoma or other malignancy; MELD <11 or >28 were excluded from the study. Patients on steroids and/or pentoxyfilline were not excluded from the study. Since the presence of current infection is not a contraindication for F-652, infection was not an exclusion criteria.
Subjects with AH and MELD score 11-28 were enrolled. These study subjects were then dichotomized according to the MELD score: 9 subjects in the MELD 11-20 group, and following demonstration of the absence of side effects in this group, 9 patients in the MELD 21-28 group were recruited into the study. The cut-off of 20 points of MELD was used to define moderately severe alcoholic hepatitis (MELD 11-20) and severe AH (21–28) (23, 24). Patients were sequentially assigned into 3 subgroups according to F-652 dose starting with 10 μg/kg, followed by 30 μg/kg and finally, 45 μg/kg. This dose escalation regimen was designed to assess safety and efficacy signals. F-652 was administered as 2 doses by slow intravenous infusion, on day 1 and day 7. The CONSORT flowchart is shown in Figure 1.
Fig. 1. CONSORT Flowchart.
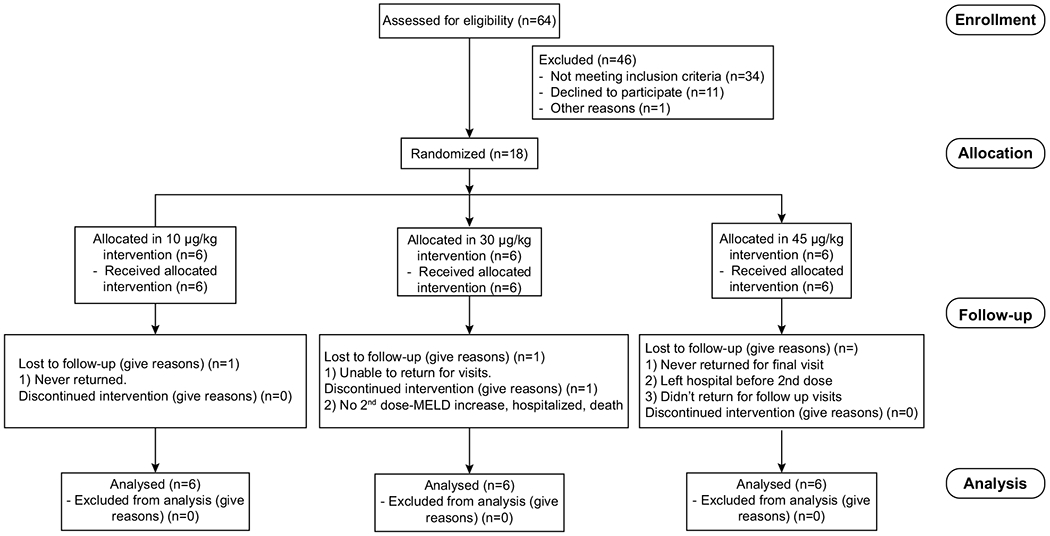
CONSORT flowchart from enrolled patients.
Independent cohorts
We used propensity score matching from independent patient cohorts to provide context for our findings. We used two prospective cohorts, one with AH subjects recruited at Mayo Clinic ( NCT02172898) and another with steroid treated AH subjects recruited at Virginia Commonwealth University and Indiana University ( NCT01968382), as a part of the TREAT consortium. Additionaly, we used another independent retrospective cohort of AH subjects recruited at Mayo Clinic (25). The three cohorts used for comparison with F-652 treatment group were then screened with propensity score matching analysis to eliminate potential selection bias and identify correct controls for the comparison. Matching was done using three variables: MELD score, age and sex, using the MatchIt package on R-studio. In the matched samples, absolute standardized quartile differences in means of those variables were <0.10 to be balanced between subjects in the two comparison groups.
Outcomes
The primary endpoint was safety and tolerability of F-652 as determined by absence of significant adverse events (SAE). We also assessed pharmacokinetics. Secondary endpoints included improvement in MELD score (reduction) at 28 and 42 days. This end-point was based on a prior publication (26), allowing at least 30 days of follow-up since the last dose of the drug. Survival at 42 days; responsiveness by Lille score; and reduction in serum bilirubin, AST and ALT were also secondary endpoints. Lille scores were calculated using original Lille score online calculator (www.lillemodel.com) as published by Mathurin et al (27). Tertiary endpoints were reduction from baseline of non-invasive biomarker extracellular vesicles (EV) count which serves as a surrogate for improvement in inflammation associated with AH (28, 29); and improvement from baseline in cytokine and chemokine profiles using multiplex analyses.
Pharmacokinetics/Pharmacodynamics
To measure F-652 concentrations in plasma by electrochemiluminescence (ECL) immunoassay, anti-IL-22 antibody was captured onto a MSD Standard SECTOR plate for coating. The F-652 in STDs, QCs, controls and samples was captured onto the coated plate. After thorough washing of the wells to remove the unbound protein, human anti-IL-22 biotinylated detection antibody was added to the wells. The conjugate bound to the captured F-652. Excess unbound conjugate was removed by further washing of the wells. After washing of the wells, Streptavidin Sulfo-tag was added to the wells. The Streptavidin Sulfo-tag bound to the biotinylated anti-IL-22/F-652 complex. Excess unbound conjugate was removed by further washing of the wells. Following the incubation of the final reagent, the plate was washed followed by addition of MSD read buffer. The assay plate was then read using a MSD ECL plate reader. The electrochemiluminescence signal generated was relative to the amount of F-652 present in the STDs, QCs, controls and samples tested. The concentrations of the F-652 in controls and samples were back calculated with a non-linear calibration curve established by the F-652 standards (Four Parameter Logistic Fit (4PL), weighting factor: 1/y2) (20).
Extracellular vesicles quantification
Blinded plasma samples were used for isolation and analysis. EVs were isolated from 700-800 μL of individual plasma samples. Samples were diluted 1:1 with sterile Dulbecco’s phosphate-buffered saline (D-PBS) to reduce viscosity and centrifuged at 2000g (Allegra 6R GH3.8) to remove cell debris. The pellet was resuspended in sterile D-PBS and subjected to sequential ultracentrifugation at 12000g and twice at 110,000g to collect the pellet. Pellet was finally re-suspended to 100 μL volume in sterile D-PBS. Ten μL was used for nanoparticle size distribution and concentration with a NanoSight NS300 (Malvern Instruments, Malvern, UK) equipped with a monochromatic 488-nm laser, an sCMOS camera, and a syringe pump. Several dilutions were assayed for each sample to fit with the optimal range of the instrument (10-40 particles/frame). Three consecutive 30-second videos with a rate of 20 frames per second were recorded for each sample and processed using the NTA 3.0 (build 0064) software (Malvern Instruments). EV counts were normalized to 1 ml volume of plasma.
Multiplex analyses
Serum samples were used for multiplex analyses using BioLegend LEGENDplex bead-based immunoassay (BioLegend, CA)(30). Capture beads were conjugated to antibodies specific to particular analytes. Multiple predefined capture bead sets were incubated with the serum samples. A biotinylated detection antibody cocktail, supplied by the manufacturer, was added leading to formation of cature bead-analyte-detection antibody sandwiches. Finally, streptavidin-phycoerythrin (SA-PE) was added, which on binding to biotinylated detection antibodies, provided flourescent signal intensities in proportion to the amount of analyte present in the sample. This signal was quantified using flow cytometry technique, and concentrations of analytes were determined using data analysis software.
Statistical Analyses
Results were expressed as the mean ± SD from three or more independent experiments. Two-tailed Student’s t-test or ANOVA was used to test the statistical significance between groups as appropriate. A P-value of less than 0.05 was considered as statistically significant. The moderate AH group (MELD 11–20; n=9); severe AH group (MELD 21–28; n=9); and the composite group (combined moderate and severe AH groups, n=18) were analyzed separately. Lille score response was compared between the treated groups and independent cohorts. Successful treatment was defined a priori as an improvement (decrease) in MELD score >25%. Given that mortality in patients with AH is upwards of 40%, it was anticipated that not more than 25% in the control arm would have a reduction in the MELD score of >25%. Based on preliminary results, it was anticipated that 80% of patients in the F-652 arm would have a reduction in MELD score >25%. With a dichotomous endpoint (yes/no) of reduction in MELD score by >25%, with 25% in control arm and 75% in treatment arm reaching the endpoint, a sample size of 18 patients (in a 1:1 randomization of 9 in each arm) would have a 75% power of detecting differences at an alpha of 0.05.
RESULTS
Subjects
This phase 2a, open-label, dose-escalation study was initiated in January 2016 and fully enrolled by August 2018. A total of 18 subjects meeting inclusion criteria were recruited and were administered F-652. Nine subjects were enrolled in the moderate AH (MELD 11-20) group and 9 in the severe AH (MELD 21-28) group. F-652 was administered as a slow intravenous infusion on days 1 and 7. Out of all participants, 66% were male. Average age of the cohort was 48 years. Most subjects were Caucasian (n=17). Baseline ALT levels were 77.4 ± 10.4 IU/L and AST levels were 141.7 ± 18.5 IU/L. A total of 4/18 (22.2%) patients were on concomitant steroids (1 at the MELD 11-20 group and 3 at the MELD 21-28 group) and none of the patients had evidence of infection at the time of enrollment. Baseline characteristics of study groups are summarized in Table 1.
Table 1.
Summary demographics and baseline characteristics of enrolled subjects.
| MELD 11-20 group (n=9) | MELD 21-28 group (n=9) | |
|---|---|---|
| Age (years) | 51.5 ± 9.7 | 46.8 ± 9.9 |
| Female (%) | 22.2% (2/9) | 44.4% (4/9) |
| Race | 100% White (9/9) | 89.9% white (8/9), 11.1% American Indian (1/9) |
| Alcohol consumption (g/day) | 175 ± 91.5 | 145.5 ± 102.5 |
| Hospitalized at screening (%) | 7/9 (77.8%) | 6/9 (66.7%) |
| Days hospitalized prior to 1st dose | 4.2 ± 2.7 | 2.1 ± 2.0 |
| Steroids use prior to first dose (%) | 11.1% (1/9) | 33.3% (3/9) |
| Baseline infection | 0% (0/9) | 0% (0/9) |
| WBC count (x109) | 9.4 ± 6.9 | 10.7 ± 5.6 |
| Creatinine (mg/dL) | 0.81 ± 0.32 | 0.77 ± 0.19 |
| AST (IU/L) | 151.5 ± 83.5 | 130.7 ± 67.0 |
| ALT (IU/L) | 84.1 ± 38.0 | 66.5 ± 47.2 |
| Bilirubin (mg/dL) | 6.8 ± 3.8 | 14.5 ± 8.6 |
| Bilirubin at day 7 (mg/dL) | 4.2 ± 3.8 | 6.1 ± 2.5 |
| Albumin (g/dL) | 2.8 ± 0.5 | 3.0 ± 0.7 |
| INR | 1.4 ± 0.3 | 2.0 ± 0.3 |
| C-reactive protein (g/dL) | 31.6 ± 27.2 | 12.4 ± 8.0 |
| MELD | 17.3 ± 2.3 | 23.8 ± 2.7 |
| Hepatic encephalopathy (%) | 2/9 (22.2%) | 1/9 (11.1%) |
| Known cirrhosis (%) | 3/9 (33.3%) | 9/9 (100%) |
| Liver biopsy (%) | 2/9 (22.2%) | 1/9 (11.1%) |
Data are presented as mean ± SD, or percentages.
Primary Endpoint: Safety, tolerability and pharmacokinetics
The population for the safety study comprised of all 18 subjects who received at least one dose of F-652. Seventeen adverse events were reported. Pruritus was the most commonly reported AE (n=4). Leukocytosis, arrhythmia, fever and rash each were reported twice. Hematochezia, nausea, diarrhea, hyperbilirubinemia and hypertension were each noted once (Figure 2A). No patient discontinued the drug due to SAE and most AE were qualified as unlikely or unrelated to drug administration. One subject died at day 14 from decompensated end stage liver disease (esophageal variceal bleeding and type 1 hepatorenal syndrome), this subject was assigned to the 30 μg/kg dose, was not on active treatment (only received first dose), and did not receive day 7 dose. Therefore, based on the total lof 18 patients in the study, it was determined that F-652 is safe and tolerated well even by patients with severe AH.
Fig. 2. Safety and Pharmacokinetics.
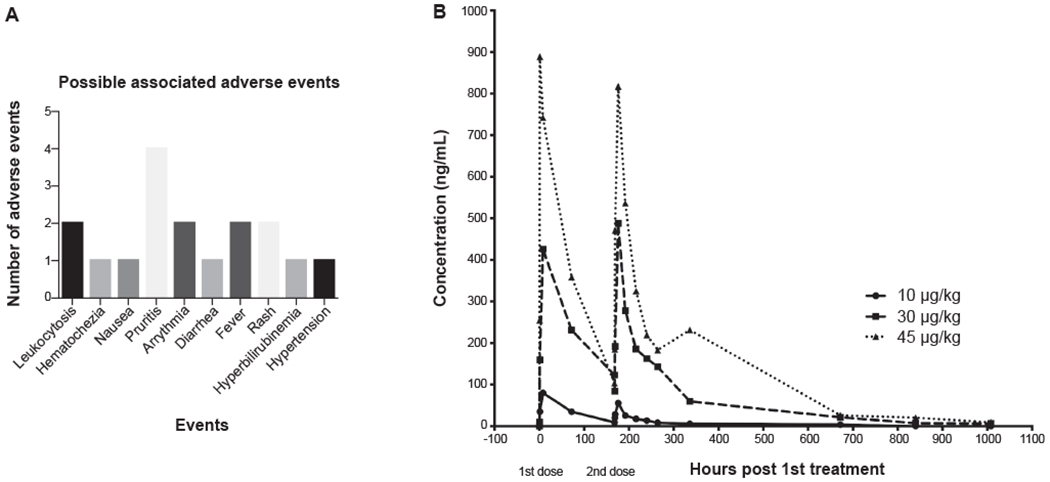
(A) Safety and tolerability of F-652 as determined by absence of significant adverse events (SAE). There were no SAE related to the study drug leading to discontinuation. (B) Pharmacokinetics showing half-life of F-652. The half-life of F-652 following the first dose was 61-85 h.
Pharmacokinetics
The exposure to F-652 increased with dose following both single (AUC0-t, Cmax, and Clast) and multiple doses (AUC0-t, AUC0-inf, Cmax, and Clast). The mean half-life (t1/2) of F-652 calculated on day 7 ranged from 105 to 196 h while the last quantifiable concentration appears to be observed later for the mid and the high doses (Figure 2B). There was no accumulation of F-652 upon multiple dose administrations with a decrease in exposure over repeated dosing for all doses. Overall, F-652 PK parameters following intravenous administration increased in a more than dose-proportional manner over the dosing range of 10 to 45 μg/kg following single and multiple infusions. The mean AUC0-t, Cmax, and Clast following single dose on day 1 and day 7 are provided in the Supplementary Tables 1–3. As expected, increments in F-652 after single or, multiple administered doses result in a greater body disposition of the drug. The F-652 absence of accumulation upon multiple doses for all doses shows an adequate relationship between dose interval and rate of elimination. However, since PK parameters after intravenous administration increases in a more than dose-proportional manner over the doses explored, non-linear pharmacokinetics for F-652 with a nonconstant clearance may be suspected. This fact could in part explain the long terminal elimination half-life durations observed for the mid and high doses. The lack of dose proportionality could be especially relevant in the presence of severe liver dysfunction and/or cirrhosis, implying the need for dose adjustments in those cases. This issue deserves further exploration, since it may have implications concerning the safety and efficacy of the drug depending on the mechanisms involved. In summary, although the PK results demonstrate that F-652 may be safely re-dosed even at shorter intervals, a 7 day infusion interval is probably optimal.
Secondary Endpoints: Improvement of MELD score; survival; responsiveness by Lille score; reduction in biochemical liver profile; and safety and tolerability of F-652
There was a significant reduction in MELD (Δ MELD) in the composite group as well as in subgroups stratified according to disease severity (Table 2). MELD score improved by 6.0 [−8.25, 0.75] points (p=0.0054) by day 28 in the composite group (Figure 3A). An improvement in the MELD score was observed in the group with moderate AH (MELD 11-20) (Figure 3B) but did not reach significance in the group with severe AH (MELD 21-28) (Figure 3C) at day 28. At day 42, an improvement of MELD score by 5.0 [−9.0, −2.0] points (p=0.0003) was observed in the composite group (Figure 3A). This improvement was also observed in the separate moderate and severe AH groups (Figure 3B–C). Subjects with severe AH had larger improvement in the MELD score at day 42 (Table 2). Supplemental Figure 1A represents Δ MELD in subgroups divided according to study medication doses.
Table 2.
Summary of Delta MELD score, bilirubin and aminotransferases in response to F-652.
| Day 0 | Day 28 | p-value | Day 42 | p-value | |
|---|---|---|---|---|---|
| Total Patients | |||||
| Delta MELD (points) | - | −6.0 [−8.25, 0.75] | 0.0054 | −5.0 [−9.0, −2.0] | 0.0003 |
| Delta total bilirubin (mg/dL) | - | −3.7 ± 1.6 | < 0.0001 | −4.6 ± 1.6 | 0.0002 |
| ALT (IU/L) | 77.4 ± 10.4 | 42.46 ± 6.8 | 0.032 | 40.9 ± 7.2 | 0.026 |
| AST (IU/L) | 141.7 ± 18.5 | 77.2 ± 11 | 0.0062 | 79.8 ± 17.6 | 0.0149 |
| Moderate AH (MELD:11-20) | |||||
| Delta MELD (points) | - | −7.0 [−8.0, 0.0] | 0.034 | −3.0 [−10.0, 10.5] | 0.023 |
| Severe AH (MELD: 21-28) | |||||
| Delta MELD (points) | - | −5.0 [−9.0, 3.0] | N.S. | −5.5 [−9.5, −2.25] | 0.0014 |
| EV concentration (particles/mL) | 2.6X1011 ± 1.9X1011 | 4.7X1010 ± 2.1X1010 | 0.0125 | 8.2X1010 ± 3.3X1010 | N.S. |
Fig. 3. Δ MELD score in the total group and subgroups.
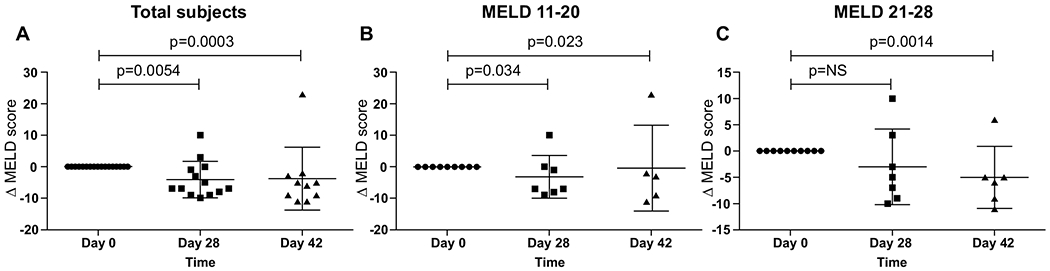
(A) There was a significant reduction in MELD (Δ MELD) in the composite group as well as in subgroups divided according to disease severity (B and C).
In the total cohort, 94.4% (17/18) survived by day 42. Only one subject died at day 14 from decompensated end stage liver disease (esophageal variceal bleeding and type 1 hepatorenal syndrome); this subject was assigned to the 30 μg/kg dose and did not receive day 7 dose. His initial MELD score was 27. Longer follow up of the cohort showed a survival of 88.9% (16/18) by day 90.
Total bilirubin decreased by 3.7 ± 1.6 mg/dL in the composite group by day 28 (p<0.0001), and by −4.6 ± 1.6 mg/dL by day 42 (p=0.0002) (Figure 4A). Supplemental Figure 1B represents changes in total bilirubin in subgroups divided according to study medication doses. Serum CRP levels were reduced non significantly at day 28 (Figure 4B). Leukocyte counts remained similar throughout follow-up (Supplementary Figure 1C). Serum ALT levels were reduced from 77.4 ± 10.4 at baseline to 42.46 ± 6.8 (p=0.032) at day 28 and 40.9 ± 7.2 (p=0.026) at day 42 (Figure 4C). Serum AST similarly decreased from 141.7 ± 18.5 at baseline to 77.2 ± 11 (p=0.0062) at day 28 and 79.8 ± 17.6 (p=0.0149) at day 42 (Figure 4D).
Fig. 4. Clinical parameters at day 0, 28 and 42 of follow-up.
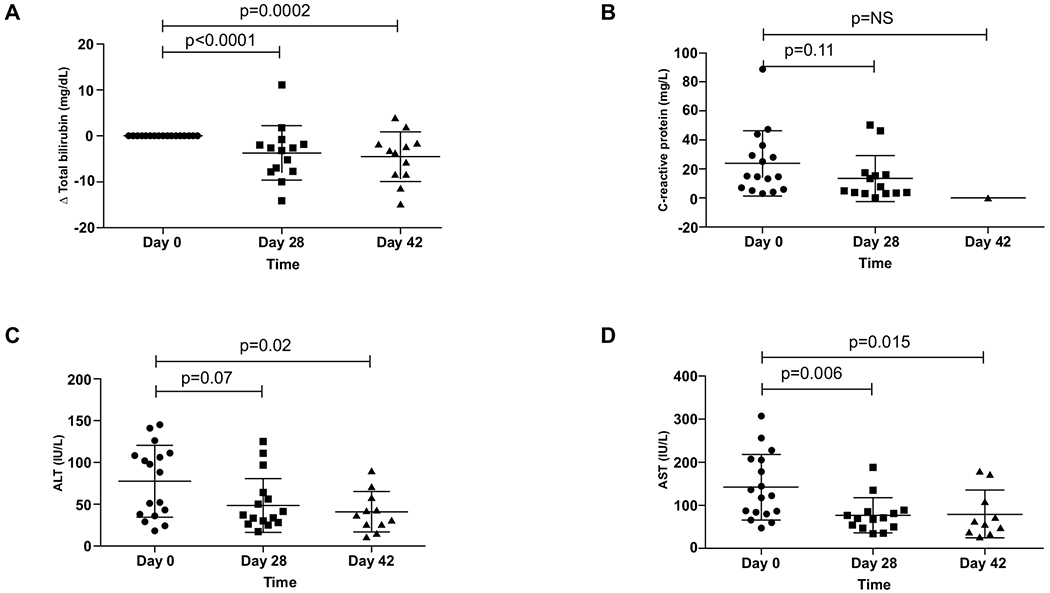
Improvement in (A) Δ total bilirubin, (B) C-reactive protein, (C) ALT, and (D) AST from the entire cohort at days 0, 28 and 42.
Lille score at day 7 was ≤0.45 (traditionally the cut-off to define responders) in 15/18 patients (83%) in the F-652 treatment cohort (Figure 5A). Baseline and day-7 bilirubin are summarized in Table 1. Propensity score matching identified equal number of matching controls from three independent historical cohorts for comparison with F-652 treatment cohort. Supplementary Tables 4 and 5 show matched clinical characteristics of all four groups. This approach successfully matched the F-652 treatment group with the three control groups with regard to independent variables MELD score, sex and age of subjects. For the matched historical AH cohorts, Lille score at day was ≤0.45 in only 12% and 6% in the prospective and retrospective cohorts that included untreated subjects respectively (Figure 5B–C). Lille response was also higher when compared to steroid treated prospective historical cohort (83% vs. 56%) of AH patients (Figure 5D).
Fig. 5. Lille score responders at day 7 for all cohorts.
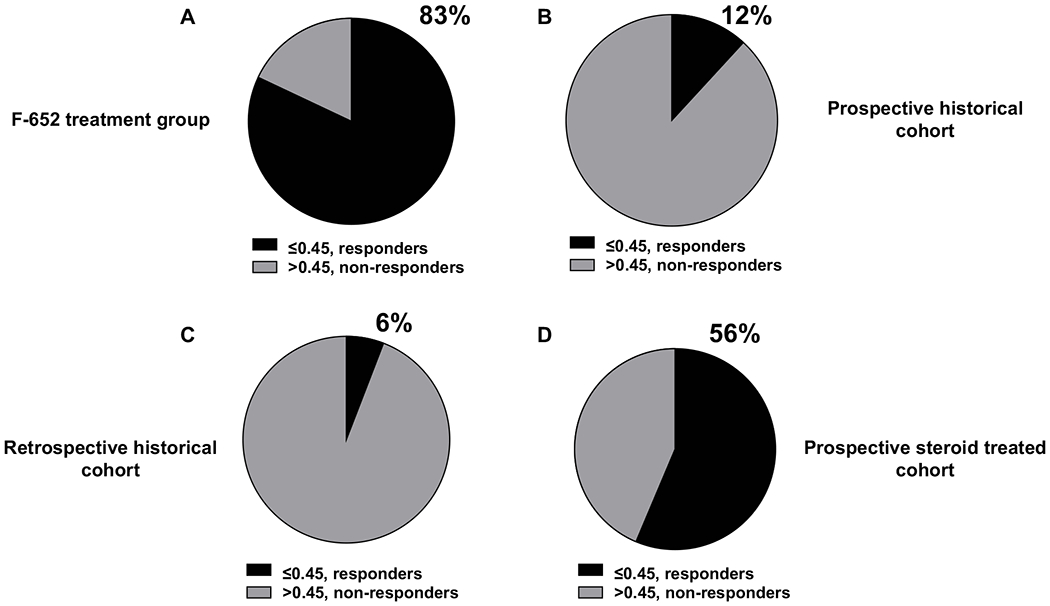
(A) The F-652 treatment cohort had 82% subjects that responded to therapy as evident by Lille score of ≤ 0.45. (B) A prospective historical AH cohort had 12% responders and (C) a retrospective historical AH cohort had 6% responders in comparison. (D) A prospective, steroid treated AH cohort had 56% responders.
Tertiary Endpoints: Reduction in extracellular vesicles count, cytokines and chemokines
We measured extracellular vesicles as a biomarker of alcohol-induced liver injury. Median extracellular vesicles count at baseline were 2.6x1011 ± 1.9x1011 particles/mL of plasma and reduced to 4.7x1010 ± 2.1x1010 (p=0.0001) on day 28, and to 8.2x1010 ± 3.3x1010 on day 42 (p=0.0004) (Figure 6A).
Fig. 6. Extracellular vesicles and cytokine and chemokine profiles.
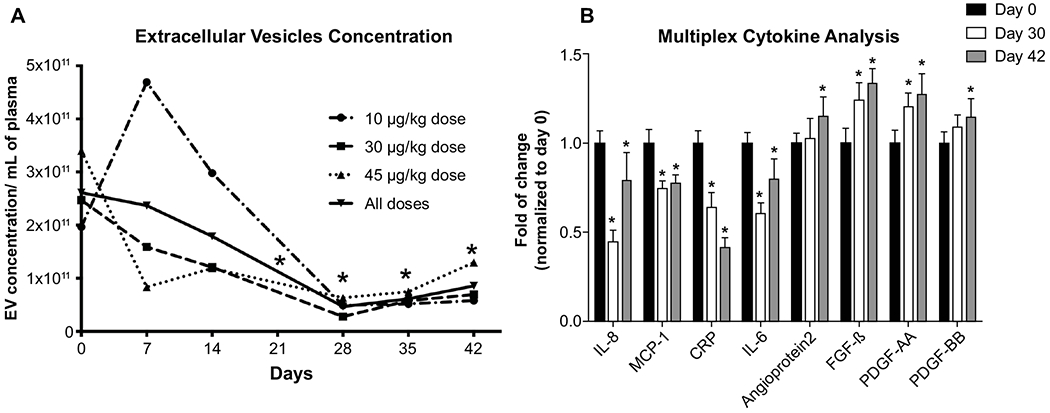
(A) Reduction from baseline in non-invasive biomarker extracellular vesicles (EV) count (*p<0.05 compared to day 0). Reduction in EV counts here acting as surrogate for improvement in inflammation associated with AH. (B) Improvement from baseline in cytokine and chemokine profiles using multiplex analyses at days 28 and 42 (*p<0.05).
By multiplex analyses, serum levels of proinflammatory biomarkers IL-8, MCP-1, CRP and IL-6 decreased at both day 28 and day 42 (Figure 6B and Supplementary Table 6 and 7). Notably, IL-8 decreased from 1345.7 ± 919.7 pg/ml at baseline to 602.3 ± 900.5 pg/ml at day 28 (Δ IL-8= −743.3 pg/ml) and to 1064.3 ± 2128.2 pg/ml at day 42 (Δ IL-8= −281.3 pg/ml); CRP decreased from 536.1 ± 370 ng/ml at baseline to 342.8 ± 447.3 ng/ml at day 28 (Δ CRP= −193.3 ng/ml) and 222 ± 300.7 ng/ml at day 42 (Δ CRP= −314.1 ng/ml). Serum levels of biomarkers of regeneration including Angioprotein-2, FGF-β, PDGF-AA and PDGF-BB increased at day 28 and day 42 (Figure 6B).
DISCUSSION
The present study advances our understanding of AH and provides evidence for F-652, an IL-22 agonist, as a promising agent to treat patients with AH. Our results demonstrated that: 1) F-652 in doses up to 45 μg/kg is safe and is associated with high rate of Lille responsiveness and improvements in MELD score in patients with AH; 2) F-652 administration correlated temporally with reductions in serum and plasma markers of inflammation and increases in markers of regeneration; 3) patients with AH MELD 11–28 receiving F-652 had more than 90% survival by day 42; and 4) though the PK results demonstrate that F-652 may be safely re-dosed even at shorter intervals, a 7 day infusion interval may be optimal.
Despite several decades since the introduction of corticosteroids for AH treatment, no other therapies have been found beneficial for this life-threatening condition (12, 31). Furthermore, the effectiveness of corticosteroids has been recently challenged (32). Given mechanisms of action of IL-22 and our preliminary data, we suggest that administration of pharmacological doses of the IL-22 agonist F-652 warrants further evaluation for potential benefit for patients with AH. Notably, F-652 is unlikely to increase the risk for infection since the specific IL-22 receptor is not expressed on immune or hematopoietic cells (20) and IL-22 was shown to attenuate bacterial infection (33). Infection is observed in approximately 25% of patients with severe AH and is major factor contributing to death (34, 35); an early improvement in the liver function is the most important factor contributing to decreased risk of infection, as evidenced by the low incidence of infection in treatment responders as compared with non-responders to corticosteroids (34). Furthermore, in patients with severe AH, prednisolone increases susceptibility to infection and infection-related mortality, especially in steroid non-responders (35). Interestingly, none of the patients (0/18) in this study developed an infection requiring antibiotic treatment during the follow-up period. Thus, it is possible that the beneficial effect of IL-22 may be also related to its anti-microbial function in addition to hepatoprotection and regenerative function. Recently, high levels of IL-22 have been associated with mortality from acute-on-chronic liver failure, probably reflecting the severity of the disease (36).
The efficacy of IL-22 in mitigating liver injury induced by alcohol, carbon tetrachloride (CCl4), or a high-fat diet has been demonstrated in animal models (15, 37–40). The IL-22 agonist’s safety profile and unique biology, which specifically targets epithelial cells, suggests its potential use as monotherapy in patients with AH and MELD scores 11-28. Corticosteroid use is limited by the risk of bacterial infection (35). It has been shown that IL-22 might overcome even the corticosteroid-mediated promotion of infection (41, 42). Therefore, in more severe AH (MELD score >20), IL-22 could theoretically be used alone in a dose of 45 μg/kg or in combination with corticosteroids without increasing risk of infection. Interestingly, although the number of patients in the study was small and follow-up period was only 42 days, the IL-22 agonist was associated with approximately 95% survival. Moreover, a 90-day analysis in this cohort showed a 11.1% mortality (2/18 subjects). If we compare the observed 90-day mortality with historical corticosteroid data, there is a trend towards reduction in mortality using F-652, but the reduction was not statistically significant (27% vs. 11.1%, OR 0.34 [0.08-1.49], p= 0.15) (43). Moreover, the cohort of the STOPAH trial had a 59.6% of steroid-responsiveness per Lille score (32). Similar results were noted with regards to Lille score response, which was much higher in F-652 treated group (83%) when compared with a prospective, corticosteroid treated, and propensity score matched group (56%). This matching was done using MELD, age, and sex as variables to assure appropriate comparability to our F-652 treated cohort. Additionally, we had 2 untreated historic cohorts to compare with F-652 treated cohort from Mayo Clinic. We found only 12% responders in the prospective and 6% responders in the retrospective cohort compared to 82% in the IL-22 group (Supplementary Figure 1D and Supplementary Table 4).
Interestingly, we observed a significantly decreased EV count at day 28 in parallel to the decreased MELD. This effect was consistent in all three doses. EV count has shown to correlate with the severity of the disease (28). Furthermore, the multiplex cytokine analysis showed downregulation of inflammatory markers IL-8, MCP-1, CRP and IL-6; and an upregulation of regeneration markers; angiopoietin2, FGF-β, PDGF-AA and PDGF-BB. This data supports the idea of an anti-inflammatory effect of F-652 while stimulating regeneration, which is a key process to allow recovery of the liver (44), although the role of many of these circulating cytokines in the pathogenesis of AH is unclear. A potential additional advantage of this medication it is that since it is given as infusion and most patients will get both doses while in hospital (day 1 and day 5-7), compliance is unlikely to be an issue in contrast to a 28-day steroid therapy.
Some of the strengths of our study are that it provides evidence of safety and efficacy of a novel drug for use in patients with AH, especially in patients with MELD score ≤20 for whom no drug is currently available. More importantly, it also provides evidence of potential efficacy for use in a sub-set of patients of MELD score 11–28 with infection(s) for whom current agents are contraindicated. The major limitation is that this was not a randomized control study. Thus, it is possible that the improvement noted in the study was related to supportive care (including abstinence) alone. Therefore, a randomized controlled trial is essential comparing F 652 with standard of care before F 652 can be recommended as effective therapy for AH. Additionally, NIAAA criteria were used for the diagnosis of AH and only 3/18 (16.7%) of the patients underwent liver biopsy.
In conclusion, this study provides evidence of F-652, an IL-22 agonist, is safe and is associated with high rate of Lille responsiveness, improvements in MELD score, and higher survival at day 42 than predicted by baseline MELD scores in patients with AH. Additionally, reductions in serum and plasma markers of inflammation and increases in markers of regeneration were observed. A randomized controlled trial to test the efficacy of F-652 in patients with AH is warranted.
Supplementary Material
Acknowledgments
Financial Support Statement: This work was supported by grant(s) NIH DK59615 and AA021171 (VHS), FONDECYT regular #1200227 (JPA). The support of an AASLD/LIFER Clinical and Translational Research Fellowship in Liver Diseases from the AASLD Foundation (to JPA) is also acknowledged. Authors want to thank Amy E. Olofson, RN, for her constant support.
Abbreviations:
- AH
alcoholic hepatitis
- ALD
Alcoholic liver disease
- ALT
Alanino-aminotransferase
- AST
Aspartate-aminotransferase
- AUC
Area under the curve
- ConA
Concanavalin A
- CCl4
Carbon tetrachloride
- D-PBS
Dulbecco’s phosphate-buffered saline
- EV
Extracellular vesicles
- IL-22
Interleukin-22
- NTA
Nanotracking analysis
- PD
Pharmacokinetic
- PK
Pharmacodynamic
- SAE
Significant adverse events
Footnotes
Conflict of Interest: Dr. Chalasani serves as a paid consultant within past 12 months to Abbvie, Coherus, Madrigal, Allergan, Siemens, Genentech, Affimmune, Axovant and NuSirt. Over the last 15 years, he previously served as a consultant to more than 30 companies, primarily in the areas of drug hepatotoxicity and nonalcoholic fatty liver disease. He receives research grant support from Intercept and Exact Sciences. Dr. Shah serves as a consultant to Durect Corporation; Afimmune, Ltd; Vital Therapies, Inc; Enterome; and Novartis Pharmaceuticals. The other authors have nothing to disclose.
REFERENCES
- 1.Paula H, Asrani SK, Boetticher NC, Pedersen R, Shah VH, Kim WR. Alcoholic liver disease-related mortality in the United States: 1980-2003. Am J Gastroenterol 2010;105:1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic Liver Disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R, Alexander G, Armstrong I, Baker A, Bhala N, Camps-Walsh G, Cramp ME, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. The Lancet 2018;391:1097–1107. [DOI] [PubMed] [Google Scholar]
- 4.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–2769. [DOI] [PubMed] [Google Scholar]
- 5.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol 2011;54:760–764. [DOI] [PubMed] [Google Scholar]
- 7.Shah ND, Ventura-Cots M, Abraldes JG, Alboraie M, Alfadhli A, Argemi J, Badia-Aranda E, et al. Alcohol-Related Liver Disease Is Rarely Detected at Early Stages Compared With Liver Diseases of Other Etiologies Worldwide. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehrawat T, Jindal A, Kohli P, Thour A, Kaur J, Sachdev A, Gupta Y. Utility and Limitations of Glycated Hemoglobin (HbA1c) in Patients with Liver Cirrhosis as Compared with Oral Glucose Tolerance Test for Diagnosis of Diabetes. Diabetes Ther 2018;9:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014;12:555–564; quiz e531-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology 1978;74:169–173. [PubMed] [Google Scholar]
- 11.Carithers RL Jr., Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med 1989;110:685–690. [DOI] [PubMed] [Google Scholar]
- 12.Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of alcoholic hepatitis. Lancet Gastroenterology and Hepatology In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 2013;28 Suppl 1:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004;39:1332–1342. [DOI] [PubMed] [Google Scholar]
- 16.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 2010;52:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol 2010;53:339–347. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Xie G, Mao Y, Zhou K, Ren R, Zhao Q, Wang H, et al. Enhanced Regeneration and Hepatoprotective Effects of Interleukin 22 Fusion Protein on a Predamaged Liver Undergoing Partial Hepatectomy. J Immunol Res 2018;2018:5241526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Zhong S, Pai R, Rae J, Sukumaran S, Stefanich EG, Lutman J, et al. Nonclinical safety assessment of a human interleukin-22FC IG fusion protein demonstrates in vitro to in vivo and cross-species translatability. Pharmacol Res Perspect 2018;6:e00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang KY, Lickliter J, Huang ZH, Xian ZS, Chen HY, Huang C, Xiao C, et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol 2019;16:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lourens S, Sunjaya DB, Singal A, Liangpunsakul S, Puri P, Sanyal A, Ren X, et al. Acute Alcoholic Hepatitis: Natural History and Predictors of Mortality Using a Multicenter Prospective Study. Mayo Clin Proc Innov Qual Outcomes 2017;1:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018;113:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. Alcohol-related liver disease: Areas of consensus, unmet needs and opportunities for further study. J Hepatol 2019;70:521–530. [DOI] [PubMed] [Google Scholar]
- 25.Peeraphatdit TB, Kamath PS, Karpyak VM, Davis B, Desai V, Liangpunsakul S, Sanyal A, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41:353–358. [DOI] [PubMed] [Google Scholar]
- 27.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–1354. [DOI] [PubMed] [Google Scholar]
- 28.Arab JP, Verma V, Martin-Mateos RM, Simonetto D, Kamath PS, Gores GJ, Shah VH, et al. Extracellular Vesicle C16 Ceramide and S1P Content in Alcoholic Hepatitis Correlates with Disease Severity and Resolution. Gastroenterology 2018;154:1120. [Google Scholar]
- 29.Sehrawat T, Arab JP, Simonetto D, Sanyal AJ, Chalasani NP, Gores GJ, Kamath PS, et al. Circulating Extracellular Vesicles and Sphingolipids Cargo are Highly Accurate Novel Biomarkers for Diagnosis of Alcoholic Hepatitis. Gastroenterology 2019;156.31462210 [Google Scholar]
- 30.Lehmann JS, Zhao A, Sun B, Jiang W, Ji S. Multiplex Cytokine Profiling of Stimulated Mouse Splenocytes Using a Cytometric Bead-based Immunoassay Platform. J Vis Exp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 32.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–1628. [DOI] [PubMed] [Google Scholar]
- 33.Zheng M, Horne W, McAleer JP, Pociask D, Eddens T, Good M, Gao B, et al. Therapeutic Role of Interleukin 22 in Experimental Intra-abdominal Klebsiella pneumoniae Infection in Mice. Infect Immun 2016;84:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009;137:541–548. [DOI] [PubMed] [Google Scholar]
- 35.Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, Forrest EH, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017;152:1068–1077 e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarzkopf K, Ruschenbaum S, Barat S, Cai C, Mucke MM, Fitting D, Weigert A, et al. IL-22 and IL-22-Binding Protein Are Associated With Development of and Mortality From Acute-on-Chronic Liver Failure. Hepatol Commun 2019;3:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol 2004;1:43–49. [PubMed] [Google Scholar]
- 38.Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, et al. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol 2015;21:1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci Rep 2016;6:28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park O, Ki SH, Xu M, Wang H, Feng D, Tam J, Osei-Hyiaman D, et al. Biologically active, high levels of interleukin-22 inhibit hepatic gluconeogenesis but do not affect obesity and its metabolic consequences. Cell Biosci 2015;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao B, Shah VH. Combination therapy: New hope for alcoholic hepatitis? Clin Res Hepatol Gastroenterol 2015;39 Suppl 1:S7–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M, Chang B, Mathews S, Gao B. New drug targets for alcoholic liver disease. Hepatol Int 2014;8 Suppl 2:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, O’Grady JG, et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018;155:458–468 e458. [DOI] [PubMed] [Google Scholar]
- 44.Singal AK, Louvet A, Shah VH, Kamath PS. Grand Rounds: Alcoholic Hepatitis. J Hepatol 2018;69:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


