There is limited information about COVID-19 infection and its treatment during pregnancy. It has been proven that pneumonia during pregnancy is associated with increased maternal and fetal, morbidity and mortality [1]. We want to share our experience about this subject.
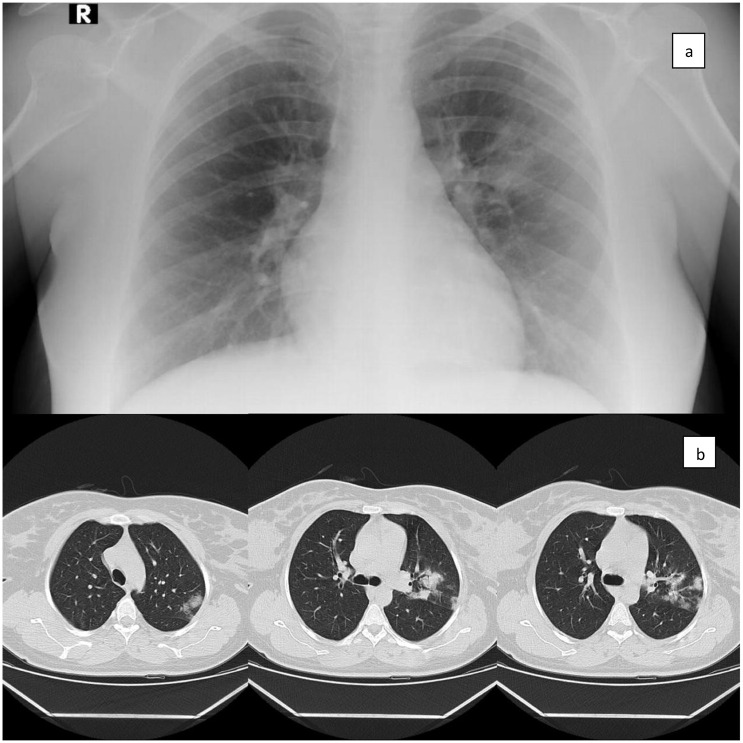
A 38-week-old pregnant woman, twenty-five years old, weighing 90 kg and 168 cm in height, was evaluated for cesarean delivery. Informed consent to publish the case was obtained and preoperative preparations started. There was no medical treatment she used regularly. She had complaints of cough and shortness of breath that started recently. On the examination of the patient, decreased lung sounds in the left side was observed. There were infiltration areas on the preoperative chest x-ray (Fig. 1 ). Upon suspicion of clinical findings in terms of COVID-19, Real-time Polymerase Chain Reaction (RT-PCR) test was performed and the patient's test result was positive. Hydroxychloroquine, oseltamivir and azithromycin triple therapy protocol was planned for COVID-19 medical treatment. It was decided to end the pregnancy by cesarean delivery. No abnormal findings were observed in preoperative blood tests and electrocardiogram.
Fig. 1.
a; Preoperative chest x-ray, b; After surgery, before the medical treatment for Covid-19, Chest CT with patchy consolidations on the lung.
The first values after monitoring were recorded as pulse: 88/min, blood pressure: 140/90 mmHg, and sPO2: 96%. In the study of Chen et al. [2] evaluating 17 cases, epidural anesthesia was reported in 14 cases and general anesthesia in 3 cases. It was stated that 12 of the patients who underwent epidural anesthesia developed prominent hypotension. In a case reported by Xia et al. [3], spinal anesthesia was performed and it was reported to be completed safely. Based on these two reports, we preferred to apply spinal anesthesia. In order to avoid possible hypotension, after the case was taken to the operation room, we applied approximately 500 ml of iv hydration and spinal anesthesia with 10 mg of bupivacaine.
In the 5th minute of the surgery, the birth of a 2900-gram male baby took place. After delivery, 5 mg oxytocin was given intravenously. The baby's 1st minute APGAR score was 9, the 5th minute APGAR score was 10, and the first peripheral oxygen saturation was 78.
During the operation, 50 ml of urine output and approximately 500 ml of surgical bleeding were observed. When the surgery was completed, the level of spinal anesthesia was determined to be at the level of T10. No hypotension was observed in the case. Spinal anesthesia time was at the expected level. There was no headache after spinal.
After cesarean, cranial computed tomography (CT) was ordered. Chest CT showed patchy consolidations on the lung (Fig. 1). Hydroxychloroquine, oseltamivir and azithromycin treatment was applied for the COVID-19 infection of the patient after surgery. On the 10th day of her treatment, the patient was discharged in a healthy state.
RT-PCR control of the baby after birth was negative. There was no evidence of infection in the 10-day follow-up of the baby. During the 15-day follow-up of the personnel who participated in the surgery, no evidence of infection was detected.
In conclusion, patients infected with SARS-CoV-2 may need surgical procedures, whether associated with their current condition or not. Protection of personnel at risk and other patients should be a primary objective and it is important to plan the appropriate environment for surgery in advance. We provided a successful anesthetic management in this case, where we applied spinal anesthesia with cesarean delivery. We believe that regional anesthesia techniques can be applied in order to avoid aerosol producing procedures while making anesthesia preferences.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors declare that they have no funding support.
References
- 1.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R., Zhang Y., Huang L., Cheng B.H., Xia Z.Y., Meng Q.T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020 Mar 16 doi: 10.1007/s12630-020-01630-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. 17 March 2020. Emergency caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Advance Access Publication Date: [DOI] [PMC free article] [PubMed] [Google Scholar]