Abstract
Background
Several observational studies have reported elevated baseline D-dimer levels in patients hospitalized for moderate to severe coronavirus disease 2019 (COVID-19). These elevated baseline D-dimer levels have been associated with disease severity and mortality in retrospective cohorts.
Objectives
To review current available data on the association between D-Dimer levels and mortality in patients admitted to hospital for COVID-19.
Methods
We performed a systematic review of published studies using MEDLINE and EMBASE through 13 April 2020. Two authors independently screened all records and extracted the outcomes. A random effects model was used to estimate the standardized mean difference (SMD) with 95% confidence intervals (CI).
Results
Six original studies enrolling 1355 hospitalized patients with moderate to critical COVID-19 (391 in the non-survivor group and 964 in the survivor group) were considered for the final pooled analysis. When pooling together the results of these studies, D-Dimer levels were found to be higher in non-survivors than in-survivors. The SMD in D-Dimer levels between non-survivors and survivors was 3.59 μg/L (95% CI 2.79–4.40 μg/L), and the Z-score for overall effect was 8.74 (P < 0.00001), with a high heterogeneity across studies (I2 = 95%).
Conclusions
Despite high heterogeneity across included studies, the present pooled analysis indicates that D-Dimer levels are significantly associated with the risk of mortality in COVID-19 patients. Early integration of D-Dimer testing, which is a rapid, inexpensive, and easily accessible biological test, can be useful to better risk stratification and management of COVID-19 patients.
Keywords: Coronavirus disease 2019, SARS-CoV-2, D-Dimer, biomarker, Mortality
Introduction
A cluster of pneumonia cases of unknown origin was first reported in December 2019 in the city of Wuhan, Hubei, China. This disease, now named coronavirus disease 2019 (COVID-19), rapidly spreads from Wuhan to all countries around the world. On 30 January 2020, the outbreak of COVID-19 was declared as a Public Health Emergency of International Concern by the WHO, then as a global pandemic on 12 March 2020. Up to 18 April 2020, there were 2,313,897 confirmed cases of COVID-19 and 159,033 COVID-19-related deaths worldwide.
Huang et al. first reported the clinical features and outcomes of 41 COVID-19 patients admitted to a single Wuhan hospital [1]. Subsequently, several retrospective cohorts analyzed the clinical characteristic and the course of COVID-19 in patients suffering from mild to critical forms [2], [3], [4], [5], [6], [7], [8]. While most of COVID-19 patients suffer from mild to moderate symptoms and do not require hospitalization, 15–20% of patients progress to severe pneumonia resulting in hypoxia and respiratory failure which necessitate supportive care for critical illness and supplemental nasal oxygen, and 5% of them progress to acute respiratory distress syndrome (ARDS) or multiple organ failure (MOF) requiring admittance to an intensive care unit (ICU) with mechanical ventilation or extracorporeal membrane oxygenation (ECMO) [9].
Risk factors for developing ARDS and MOF throughout the course of COVID-19 are not fully understood yet. Due to their limited capacity, health care systems are facing an unprecedented critical crisis worldwide. Early optimized care may improve prognosis in patients at high risk for developing the most severe forms, however early identification of poor prognosis and risk stratification remains challenging. Therefore, reliable biomarkers or risk assessment models allowing to earlier predict poor prognosis are warranted to help physicians in decision making regarding the most appropriate therapeutic approach to further provide the best possible care for each individual patient.
D-dimers are degradation products of cross-linked fibrin produced when plasmin cleaves fibrin to break down clots. D-dimer testing is rapid, reproducible, inexpensive, and easily accessible. Early retrospective cohort studies of COVID-19 patients admitted to hospital reported increased baseline D-dimer levels in 36% to 43.6% of cases, [1], [2], [3], [4] particularly in patients admitted to ICU. In a recent pooled analysis of 4 studies including 553 patients, baseline D-Dimer levels were associated with COVID-19 severity [10]. While a retrospective cohort of 183 severe COVID-19 patients suggested that in the most severe cases high D-dimer levels could be related to disseminated intravascular coagulation (DIC) [11], two recent prospective studies aiming to characterize the coagulation profile of COVID-19 ARDS patients with standard and viscoelastic coagulation tests reported a pro-coagulant profile without evidence of DIC [12], [13].
In the present study, we reviewed all current available data on the association between D-Dimer levels and mortality in COVID-19 patients in order to assess the potential of D-dimer testing for predicting outcomes in patients admitted to hospital.
Methods
We performed a comprehensive literature search of published studies from all languages using MEDLINE and EMBASE through 13 April 2020. The following keywords (MeSH terms) were used: ‘D-Dimer’ AND (‘Coronavirus disease 2019’ OR ‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘novel corona’ OR ‘2019-ncov’). We also screened pre-print articles from arXiv.
Studies were eligible to be included in the meta-analysis if they met the following inclusion criteria:
-
•
original cohort study;
-
•
written in English language;
-
•
reporting separately D-Dimers levels in hospitalized COVID-19 patients with fatal outcome and in those without fatal outcome.
No restriction was made on the patient disease severity (i.e. COVID-19 patients with moderate, severe or critical disease). Comments, letters to the editor, editorial, reviews, case reports and basic science studies were excluded.
Two authors (M.S. and C.F.) independently screened all records identified through database searching for study eligibility based on title and abstracts. The agreement between the reviewers for study selection was assessed using the kappa statistic [14]. Any discrepancies were resolved by consensus after discussion between the two authors or upon consensus from a third author (D.F.). The Newcastle–Ottawa scale was used to assess the studies quality and risk of bias [15].
Data were independently extracted by two authors [including first author's last name, publication year, study design, country, study population, study period, sample size, sex, age, mortality rate, and means and standard deviations (SD) of D-Dimer in COVID-19 hospitalized patients with fatal outcome and in those without fatal outcome] using a standardized data extraction form.
Continuous data were represented by mean, SD and standardized mean difference (SMD) with 95% confidence intervals (CI). For studies reporting medians and interquartile ranges (IQR), means and SD were estimated according to the algorithm proposed by Hozo et al. [16] Heterogeneity among studies was assessed by the I2 statistic, I2 > 50% representing high degree of heterogeneity. A random-effects model was used in order to take into account within-study and between-study variance. Visualization of funnel plots was used to assess for publication bias. A P-value < 0.05 was considered as statistically significant. All statistical analyses were performed using the Cochrane's Review Manager software (RevMan, version 5.3, Copenhagen, Denmark).
1. Results
A total of 26 records were identified through database searching, of which 20 were excluded because they were editorial (n = 1), review article (n = 1), basic science study (n = 1), they were written in foreign (Chinese) language (n = 5), they did not assess mortality as an outcome or they did not reported D-dimer levels separately in COVID-19 patients with fatal outcome and in those without fatal outcome (n = 12).
Finally, 6 original studies enrolling a total of 1355 patients hospitalized for moderate to critical COVID-19 (391 in the non-survivor group, and 964 in the survivor group) were considered for the final pooled analysis. [5], [6], [7], [11], [17], [18] The agreement between authors for study selection was 100% (kappa statistic 1.0).
The main characteristics of included studies are summarized in Table 1 . All studies were retrospective cohorts conducted in China between December 2019 and February 2020 [5], [6], [7], [11], [17]. The duration of follow-up was unclear except in one study [6]. All studies were at high risk of bias according to the Newcastle–Ottawa scale.
Table 1.
Characteristic of the studies included in the metanalysis.
| Reference | Year | Study design | Country | Study population | Study period | Patients, n | Male, n (%) | Age, median (IQR) or mean, years | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [5] | 2020 | Retrospective case series | China | Moderately to severely ill or critically ill patients with confirmed COVID-19 | January 31, 2020–February 12, 2020 | 274 (113 non-survivors and 161 survivors) | 171 (62) | 62 (44–70) | 14.1 |
| Tang et al. (A) [11] | 2020 | Retrospective, single-center cohort study | China | Consecutive patients with confirmed COVID-19 admitted in a single center | January 1, 2020–February 13, 2020 | 183 (21 non-survivors and 162 survivors) | 98 (53.5) | 54.1 ± 16.2 | 11.5 |
| Tang et al. (B) [17] | 2020 | Retrospective, single-center cohort study | China | Consecutive patients with confirmed severe COVID-19 admitted in a single center | January 1, 2020–February 13, 2020 | 449 (134 non-survivors and 315 survivors | 268 (59.6) | 65.1 ± 12 | 29.8 |
| Tu et al. [18] | 2020 | Retrospective, single-center cohort study | China | Consecutive patients with confirmed severe COVID-19 admitted in a single center | January 3, 2020–February 24, 2020 | 174 (25 non-survivors and 149 survivors | 79 (45.5) | NR | 14.4 |
| Wu et al. [6] | 2020 | Retrospective, single-center cohort study | China | Consecutive patients with confirmed COVID-19 admitted in a single center | December 25, 2019–Januray 26, 2020 Follow-up until February 13, 2020 | 201 including 84 patients with ADRS: 44 non-survivors and 40 survivors) | 128 (63.7) | 51 (43–60) | 21.9 |
| Zhou et al. [7] | 2020 | Retrospective, multicenter cohort study | China | Consecutive patients with confirmed COVID-19 admitted in 2 centers | December 29, 2019–Januray 31, 2020 | 191 (54 non-survivors and 137 survivors) | 119 (62) | 56 (46–67) | 28.2 |
ADRS: acute detress respiratory syndrom; COVID-19: Coronavirus Disease 2019; IQR: interquartile range; NR: not reported.
The median age of patients ranged from 51 to 62 years. Most of patients (58.6%) were men. The mortality rate varied from 11.5 to 29.8% (Table 1). Of note, most studies excluded patients who were still hospitalized at the end of the study period (i.e. neither recovered nor died).
Chen et al. [5] compared the baseline clinical and biological characteristics of 113 hospitalized COVID-19 patients who died with those of 161 hospitalized COVID-19 patients who recovered. D-Dimer levels were higher in non-survivors (median 4.6 μg/mL; IQR 1.3–21.0 μg/mL) compared to survivors (median 0.6 μg/mL; IQR 0.3–1.3 μg/mL).
Tang et al. (A) [11] compared several coagulation parameters, including the values of D-Dimer in 21 hospitalized COVID-19 patients who died with those of 162 hospitalized COVID-19 patients who recovered (n = 78) or were still hospitalized (n = 84) at time of study. D-dimer values were significantly higher in non-survivors (median 2.12 μg/mL; IQR: 0.77–5.27 μg/mL) compared to survivors (median 0.61 μg/mL; IQR: 0.35–1.29 μg/mL; P < 0.001).
The same authors [Tang et al. (B)] [17] reported baseline clinical and biological characteristics of 134 severe hospitalized COVID-19 patients who died and those of 315 severe hospitalized COVID-19 patients still alive at time of the analysis. D-Dimer levels were significantly higher in non-survivors (median 4.7 μg/mL; IQR 1.42–21.0 μg/mL) compared to survivors (median 1.47 μg/mL; IQR 0.78–4.16 μg/mL; P < 0.001) [5]. Multivariate analysis found a significant association between D-Dimer levels and the 28-days mortality [Odds ratio (OR) 1.058; 95% CI 1.028–1.090; P < 0.001].
Tu et al. [18] reported clinic-laboratory characteristics of 25 fatal cases of COVID-19 patients and those of 149 COVID-19 hospitalized patients discharged at time of analysis. D-Dimer levels were significantly higher in non-survivors (median 3.306 μg/mL; IQR 1.79–7.512 μg/mL) compared to survivors (median 0.66 μg/mL; IQR 0.37–1.108 μg/mL; P < 0.001).
Wu et al. [6] compared baseline clinical and biological characteristics of 44 COVID-19 patients with ARDS who died to those of 40 COVID-19 patients with ARDS who survived among 201 patients hospitalized for COVID-19. D-Dimer levels were significantly higher in non-survivors (median 3.95 μg/mL; IQR 1.15–10.96 μg/mL) compared to survivors (median 0.49 μg/mL; IQR 0.31–1.18 μg/mL; P = 0.001).
Finally, Zhou et al. [7] investigated baseline clinical and biological characteristics of 54 hospitalized patients with COVID-19 who died and 137 hospitalized patients with COVID-19 who survived. D-dimer values were significantly increased in non-survivors (median 5.2 μg/mL; IQR: 1.5–21.1 μg/mL) than in survivors (median 0.6 μg/mL; IQ: 0.3–1.0 μg/mL; P < 0.001).
When pooling together the results from these 6 studies, D-Dimer levels were found to be higher in non-survivors than in-survivors. The SMD in D-Dimer levels between non-survivors and survivors was 3.59 μg/L (95% CI 2.79–4.40 μg/L) (Fig. 1 ), and the Z-score for overall effect was 8.74 (P < 0.00001), with a high heterogeneity across studies (I2 = 95%).
Figure 1.
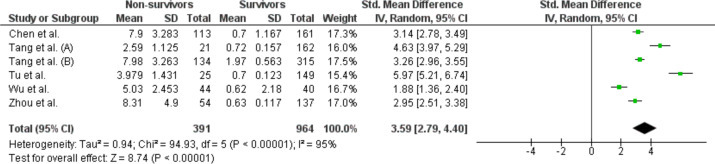
Forest plot of Standard Mean Difference of D-Dimer levels between non-survivors and survivors in the 6 included studies (random effect). Abbreviations: SD: standard deviation; St: Standard; CI: confidence interval.
2. Discussion
Retrospective cohorts early reported increased baseline D-dimer levels in one third of overall COVID-19 patients admitted to hospital [1], [2], [3], [4]. In two studies, significantly higher levels of D-Dimers were observed in small samples of patients with severe disease (i.e. admitted to ICU) compared to those with mild or moderate disease (i.e. not requiring an admission to ICU) [1,4. A pooled analysis of 4 studies (553 patients) found an association between D-Dimer levels and the severity of COVID-19 disease [10].
Our pooled analysis now indicates that D-Dimer levels might be a useful marker for predicting mortality in COVID-19 patients admitted to hospital and suggests that D-Dimer testing on admission might be useful to early stratify patients with COVID-19 admitted to hospital and to further individualize treatment. However, it was not performed on individual patient data and the impact of potential confounders remains unknown.
Interestingly, Zhou et al. investigated numerous potential risk factors for in hospital-mortality in 191 patients hospitalized for COVID-19. In multivariate analysis, a D-dimer level >1 μg/mL was the best predictor of mortality [Odds ratio (OR) 18.42, 95% CI 2.64–128.55; P = 0.0033]. [7] Similarly, Tang et al. [17] reported that D-Dimer levels were significantly associated with 28-days mortality (OR 1.058, 95% CI 1.028–1.090; P < 0.001) in a retrospective cohort of 449 patients with severe COVID-19.
To the best of our knowledge, six risk assessment models (RAM) for predicting mortality among patients with suspected or confirmed COVID-19 have been yet developed. [19], [20], [21], [22], [23], [24] Only three of these RAMs included biological data (C-reactive protein, lactic dehydrogenase, and lymphocyte count). However, none of them included D-dimer levels as a predictor.
More recently, a retrospective study investigated the prognosis and the optimal cut-off values for baseline D-dimer levels to predict mortality in 343 COVID-19 patients. Thirteen deaths occurred during hospitalization. A D-dimer value ≥ 2.0 μg/mL at admission was found to be significantly associated with mortality (Hazard ratio 51.5, 95% CI 12.9–206.7). This cutoff value of 2.0 μg/mL predicts mortality with a C-index of 0.89, a sensitivity of 92.3% and a specificity of 83.3%. [25]
The mechanisms underlying the increase in D-Dimer in COVID-19 patients is not fully understood. In their retrospective cohort, Tang et al. found that 71.6% of non-survivors fulfilled the criteria for sepsis-induced coagulopathy (SIC) compared to 0.6% in survivors. [11] However, 2 recent prospective studies reported a pro-coagulant profile in COVID-19 ARDS patients using standard and viscoelastic coagulation tests without further evidence of DIC, [12], [13] indicating that the increase in D-Dimers levels, which is constantly observed in severe COVID-19 cases, might not be related to DIC. Moreover, in a prospective cohort of 184 patients admitted to ICU for COVID-19 pneumonia, none of the patients developed DIC. [26]
In the most severe cases of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in uncontrolled inflammatory innate and impaired adaptive immune responses, as reflected by increased serum levels of pro-inflammatory cytokines such as IL-6, IL-1β, IL-2, IL-8, IL-17, G-CSF, GM-CSF, IP10, MCP1, MIP1α and TNF [27]. Importantly, there is a crosstalk between inflammation and blood coagulation, the production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, leading to the upregulation of tissue factor (TF) expression on the endothelial cells and monocytes surfaces, further resulting in a procoagulant activity [28]. In addition, autopsy reports of COVID-19 patients found extensive neutrophil infiltration in pulmonary capillaries, with acute capillaritis and fibrin deposition, indicating that neutrophil extracellular traps (NETs) may contribute to organ damage and promote thrombosis [29]. Hypoxia has also been demonstrated to trigger a procoagulant activity through the upregulation of hypoxia-inducible transcription factors that modulate the expression of several coagulation and fibrinolysis factors such as tissue factor pathway inhibitor, TF, and PAI-1 [30]. Accordingly, there is mounting evidence that COVID-19 is associated with a prothrombotic state [12], [13] resulting in an increased risk of venous thromboembolism (VTE). Increased D-Dimer levels might simply reflect this prothrombotic state. Several case reports have documented an association between COVID-19 and the occurrence of pulmonary embolisms (PE) or in situ microvascular thrombosis [31], [32], [33]. In a chinese retrospective study of 81 severe COVID-19 patients admitted to ICU who were not under thromboprophylaxis, the prevalence of VTE reached 25% [34]. More recently, a prospective cohort of 184 patients admitted to ICU for COVID-19 reported a cumulative incidence of VTE of 27%, PE being the most frequent event [26]. Therefore, thromboprophylaxis is required in all hospitalized COVID-19 patients. A call for awareness regarding the need of adapted thromboprophylaxis in COVID-19 patients has been recently published [35].
Potential limitations of the present study should be acknowledged. First, our analysis included only observational retrospective studies conducted in a single country, which may have resulted in biases inherent to such studies. Selected studies were heterogeneous in terms of study design, study population, outcome definitions, and length of follow-up. Second, we converted non-normally distributed statistics (i.e. median and range) to normally distributed statistics (i.e. mean and SD), which may also have introduced some bias in the results. Finally, the amount of included studies and their sample size were limited.
In conclusion, evidence has emerged that D-Dimer testing in combination with clinical factors or other biomarkers might be useful to early stratify patients with COVID-19 admitted to hospital and to further individualize treatment. However, it seems premature to use D-Dimer alone to guide clinical decision-making. Further large prospective studies to validate the prognosis performances of D-Dimer and to determine the best cutoff value to be used are warranted.
Author contributions
M. Sakka, J.M. Connors, D. Farge and C. Frere designed the study and collected the data. C. Frere performed the statistical analysis. M. Sakka, J.M. Connors, D. Farge and C. Frere drafted and revised the manuscript. Other authors contributed to critical of intellectual content, and final approval.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:368. doi: 10.1136/bmj.m1091. m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Palliat Med. 2020;9:428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G., Favaloro E.J. D-dimer is associated with severity of Coronavirus Disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGinn T., Wyer P.C., Newman T.B., Keitz S., Leipzig R. For GG, Evidence-Based Medicine Teaching Tips Working group. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic) CMAJ. 2004;171:1369–1373. doi: 10.1503/cmaj.1031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M. Ottawa Health Research Institute; 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu W.-J., Cao J., Yu L., Hu X., Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J., Hungerford D., Chen H., Abrams S.T., Li S., Wang G. medRxiv Cold Spring Harbor Laboratory Press; 2020. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. 2020.03.28.20045997. [Google Scholar]
- 20.Caramelo F., Ferreira N., Oliveiros B. medRxiv Cold Spring Harbor Laboratory Press; 2020. Estimation of risk factors for COVID-19 mortality - preliminary results. 2020.02.24.20027268. [Google Scholar]
- 21.Lu J., Hu S., Fan R., Liu Z., Yin X., Wang Q. medRxiv Cold Spring Harbor Laboratory Press; 2020. ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. 2020.02.20.20025510. [Google Scholar]
- 22.Yan L., Zhang H.-T., Goncalves J., Xiao Y., Wang M., Guo Y. medRxiv Cold Spring Harbor Laboratory Press; 2020. A machine learning-based model for survival prediction in patients with severe COVID-19 infection. 2020.02.27.20028027. [Google Scholar]
- 23.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers DAMPJ, Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. pii: S0049-3848(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto T., Suzuki K. The Role of Gap Junction-Mediated Endothelial Cell–Cell Interaction in the Crosstalk between Inflammation and Blood Coagulation. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18112254. pii: E2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020:217. doi: 10.1084/jem.20200652. pii: e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N., Zhao Y.-Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y., Wang X., Yang P., Zhang S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiology. 2020;2:e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.101111/jth14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


