Abstract
Coronavirus disease 2019 (COVID-19) has caused a devastating global pandemic and continues to overwhelm the health-care facilities and shatter the economies of countries worldwide. Although it primarily affects the lungs, it shares a strong interplay with the cardiovascular system. The presence of underlying cardiovascular disease and its risk factors (diabetes, hypertension) predispose the patients to increased severity and mortality associated with COVID-19. On the other hand, COVID-19 itself leads to various cardiovascular complications, which increase its associated morbidity and mortality in affected patients. It is, therefore, prudent to review the rapidly evolving data in this field and understand the mechanisms behind the cardiovascular involvement of this lethal disease.
Keywords: Coronavirus, COVID-19, Cardiovascular disease, Complications, Comorbidities
Introduction
The whole world is facing an unprecedented challenge of stopping a virus called “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” that has caused havoc affecting more than 180 countries worldwide and caused numerous deaths across the globe.1 World Health Organization has named the new and lethal disease caused by SARS-CoV-2 as “COVID-19” (Coronavirus Disease 2019).2 Over a short span of fewer than five months, COVID-19 has affected nearly 2.8 million people worldwide and caused more than 1,95,000 deaths globally (as on 25th April 2020). India recorded its first case on 30th January 2020 and 500th case on 25th March, but since then, there has been an upsurge of cases, and as on 25th April, India has recorded more than 25,000 cases and more than 785 deaths as a result of this deadly viral disease.
COVID-19 is primarily a respiratory disease; however, observational studies have shown a strong interplay between COVID-19 and cardiovascular disease (CVD).3,4 COVID-19 tends to be more severe in patients with established CVD or those with risk factors of CVD such as diabetes mellitus (DM) and hypertension (HTN).3, 4, 5 Furthermore, it has been seen that COVID-19 provokes myocardial injury and dysfunction by various mechanisms (Fig. 1), significantly increasing the morbidity and mortality.3 Because India is the world capital of DM, HTN, and CVD, and is getting to terms with bracing the imminent impact of COVID-19, it becomes essential for us to know how to mitigate the risk of this lethal disease among the most vulnerable of our population. However, for that, it is crucial for us to know and understand the various ways and mechanisms of cardiac injuries caused by this novel coronavirus infection.
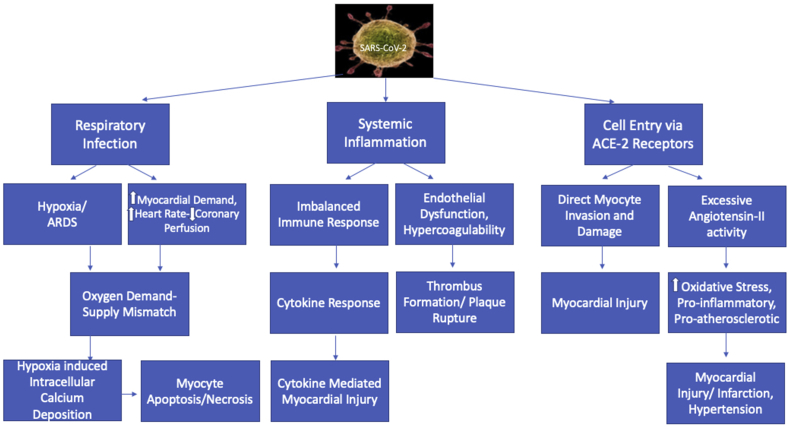
Fig. 1.
Mechanisms behind cardiovascular injury associated with COVID-19. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ARDS = acute respiratory distress syndrome; ACE-2 = angiotensin-converting enzyme-2.
Coronaviruses and mechanism of cardiac injury
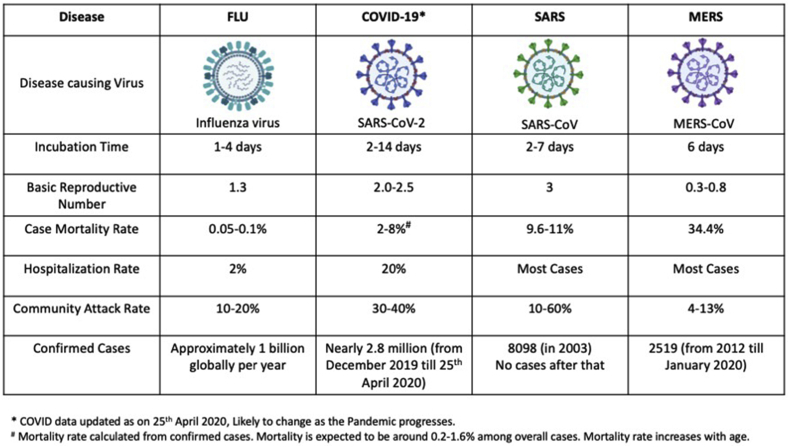
SARS-CoV-2 is a novel single-stranded enveloped RNA virus, probably originating in chrysanthemum bats, with Malayan pangolins (who share 91% nucleotide identity) as intermediate hosts.6 There are seven known human coronaviruses. Four of these seven coronaviruses (229E, OC43, NL63, and HKU1) are benign and cause only self-resolving infections and account for 15–30% of common colds. However, the remaining three have been associated with epidemics of the SARS in 2002 by SARS-CoV, in 2012 by the Middle East respiratory syndrome coronavirus (MERS)-CoV, and now COVID-19, a global pandemic in 2019–2020 by SARS-CoV-2.6 Of all three, COVID-19 appears to have the highest secondary infection rate (Fig. 2). However, mortality seems to be lowest with COVID-19 (2%) compared with SARS (9.6%) and MERS (34.4%).3 All three have been associated with cardiac complications, mainly in the form of myocarditis, heart failure, and arrhythmias.6
Fig. 2.
Epidemiological comparison of respiratory viral infections.
SARS-coronaviruses contain glycosylated spike proteins that bind to the cells expressing angiotensin-converting enzyme 2 (ACE-2) receptors, after activation by transmembrane protease serine 2.3 Alveolar epithelial cells appear to be the primary portal of entry for SARS-CoV-2, explaining its predominantly respiratory symptoms. ACE-2 is expressed mainly by the epithelial cells of the lungs, heart, intestine, kidney, and blood vessels.3 ACE-2 is physiologically distinct from the ACE-1 enzyme. ACE-1 converts angiotensin I to angiotensin II, which then binds to the type 1 angiotensin II receptors. On the other hand, the principal function of ACE-2 is the degradation of angiotensin-II (Ang-II) into angiotensin 1–7 (Ang 1–7), which counteracts the actions of Ang-II.7
Because SARS-CoV-2 binds to the ACE-2 receptor, it, therefore, attenuates the residual ACE-2 activity, thus leading to a state of inappropriately increased Ang-II activity.8 Importantly, Ang-II has a lot of adverse effects. Besides inducing local, as well as systemic HTN and vasoconstriction, Ang-II is proatherosclerotic and causes inflammatory and oxidative organ damage. Locally increased Ang-II also triggers an increase in vascular permeability resulting in pulmonary edema and acute respiratory distress syndrome (ARDS)–like picture.7,8 Thus, by an effect on ACE-2 receptors, SARS-CoV-2 predisposes to acute lung injury, myocardial damage, inflammatory organ damage, and a prothrombotic state, responsible for its varied systemic complications and multiorgan dysfunction. Direct invasion of myocyte by the virus might also result in myocyte necrosis and, eventually, myocardial damage.3 (Fig. 1).
Secondly, COVID-19 leads to an inflammatory state, which results in a cytokine storm triggered by an uncontrolled and dysfunctional immune response by type 1 and type 2 T helper cells.7 This results in continuous activation and proliferation of various cytokines, which cause apoptosis and necrosis of myocardial cells and also predispose to plaque rupture. Finally, hypoxia-induced excessive intracellular calcium deposition resulting in myocardial cell apoptosis can also lead to myocardial damage associated with COVID-19. There is a state of oxygen supply-demand mismatch caused as a result of infection-induced increased myocardial oxygen demand.3
COVID-19 in patients with underlying CVD
Being cardiologists, it is of great concern to us whether COVID-19 is more common in patients with pre-existing CVD. Evidence from the previous epidemics suggests that SARS (2002) and MERS (2012) were commonly associated with prior cardiac and cerebrovascular abnormalities.9, 10, 11 In SARS, DM and CVD were seen in 11%, and 8% of patients, respectively, and the presence of either of them doubled the mortality.9,10 Similarly, in a systematic analysis involving 637 patients with MERS, the prevalence of DM, HTN, CVD, and obesity was 50%, 50%, 30%, and 16%, respectively.11
Similar trends of an increased presence of cardiovascular comorbidities have been observed in patients with COVID-19. In one of the largest Chinese cohort of 1099 laboratory-confirmed COVID-19 patients (outpatient and inpatient), the prevalence of patients having pre-existing HTN, DM, coronary artery disease (CAD), and cerebrovascular disease was 15%, 7.4%, 2.5%, and 1.4%, respectively.12 Among patients who were intubated or died, the prevalence of these comorbidities increased to 36%, 27%, 9%, and 6%, respectively.12 In another study involving 416 hospitalized patients with COVID-19 from Wuhan, HTN (30.5%), DM (14.4%), and CAD (10.6%) were the three most common comorbidities, followed by cerebrovascular disease (5.3%) and chronic heart failure (4.1%).13 Similarly, in a systemic meta-analysis of 6 Chinese studies, involving 1527 patients, the proportions of HTN, cardiovascular disease and DM in patients with COVID-19 were 17.1%, 16.4%, and 9.7%, respectively. These proportions increased to about two-folds, three-folds, and two-folds, respectively, higher in severe/intensive care unit (ICU) cases than in their non-severe/non-ICU counterparts.14 Interestingly, the CVDs report of the year 2018 from China reveals the prevalence of HTN and DM to be 23.2% and 10.9%, respectively, among the general population.15 Therefore, the prevalence of HTN and diabetes in people infected with the virus was not higher compared with the general population.
Similar trends of comorbidities were observed in an analysis of 355 patients who died of COVID-19 in Italy, with pre-existing HTN, DM, CAD, atrial fibrillation, and cerebrovascular disease recorded in 76%, 35.5%, 30%, 24.5%, and 9.6%, respectively.16 Finally, preliminary data from the United States involving 7162 patients with COVID-19 revealed the presence of DM in 10.9% and prior CVD in 9% of patients.17 It was also revealed that the presence of underlying health conditions such as DM, chronic lung disease, and CVD, were associated with a higher risk of developing severe COVID-19–associated disease than persons without these conditions.17
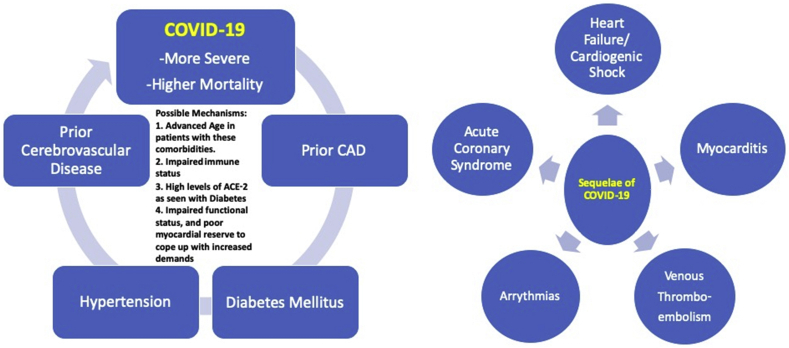
Thus, it is a matter of debate whether the presence of underlying CVD and its risk factors such as DM and HTN predispose a person to a higher risk of developing COVID-19. Still, one thing is clear that they are associated with increased severity and complications of COVID-19 (Fig. 3). The mechanism of this association, however, remains unclear at this time. Possible explanations include the advanced age of patients with these comorbidities, functionally impaired immune system, higher levels of ACE-2 in patients with DM, and patients using anti-hypertensive medications like ACE-inhibitors and ARBs (Ang-II Receptor Blockers).
Fig. 3.
Pictorial diagram showing cardiovascular comorbidities and sequelae of COVID-19. COVID-19 = coronavirus disease-2019; ACE-2 = angiotensin-converting enzyme-2; CAD = coronary artery disease.
Cardiovascular sequelae of COVID-19
Data from the MERS, SARS, and influenza epidemics provide enough evidence that viral infections can cause acute and chronic cardiovascular injury.18,19 Acutely, COVID-19 has been shown to trigger acute coronary syndromes (ACSs), myocarditis, arrhythmias, acute onset heart failure, and venous thromboembolism (VTE) (Fig. 3). The overall incidence of acute cardiac injury caused by COVID-19 ranges from 7 to 28% among hospitalized patients.1,13,20,21 The mechanisms include direct myocyte injury via the ACE-2 receptors, infection-induced proinflammatory state, and cytokine storm, and subsequently increased metabolic demand in the background of lung injury induced hypoxia (Fig. 1). All these mechanisms have been explained in detail previously. COVID-19 may exacerbate underlying CVD and may also give rise to new cardiovascular injuries.20 Evidence is now available, supporting the fact that the risk of developing cardiovascular complications is more among patients who have pre-existing CVD compared with those who do not.20 In a retrospective analysis of 187 confirmed patients with COVID-19, those with underlying CVD were more likely to exhibit elevation of troponin T levels compared with the patients without CVD (36 [54.5%] vs. 16 [13.2%]).20
Myocardial injury
Myocardial injury, as defined by an increased troponin level, can occur due to myocardial ischemia/infarction or non-ischemic myocardial injury (myocarditis). In a study involving 416 hospitalized Chinese patients with COVID-19, approximately 20% of patients had cardiac injury defined as high-sensitivity cardiac troponin I (hs-TNI) higher than the 99% percentile upper reference limit. Patients with elevated hs-TNI were elderly, had more comorbidities, and also had higher levels of leukocytes, N-terminal pro-brain natriuretic peptides, C-reactive protein, and procalcitonin, compared with those without elevated hs-TNI.13 Another study by Guo et al20 showed that 28% of the total of 187 hospitalized patients with COVID-19 had evidence of acute myocardial injury in the form of elevated troponin T levels. A meta-analysis of 4 studies involving 341 patients, showed significantly higher cardiac troponin I levels in those with severe COVID-19 infection compared with those without (standardized mean difference: 25.6 ng/L; 95% confidence interval [CI]: 6.8–44.5 ng/L).22
Initial reports from China indicated that there are two patterns of myocardial injury with COVID-19—Primary or secondary myocardial injury. In a retrospective study of 191 patients with COVID-19 by Zhou et al23, the incidence of elevation in hs-cTnI (>28 pg/mL) was 17%, and it was significantly higher among non-survivors (46% versus 1%, p < 0.001). Furthermore, the elevation of this biomarker was noted to be a predictor of in-hospital death (univariable odds ratio: 80.07, 95% CI: [10.34–620.36], p < 0.0001). Interestingly, it was demonstrated that four days after onset of symptom, median hs-cTnI levels were 8.8 pg/mL in non-survivors vs. 2.5 pg/mL in survivors. During follow-up, the median hs-cTnI among survivors did not change significantly (2.5–4.4 pg/mL). In contrast, it kept on increasing among non-survivors with the most abrupt increase noted beyond Day 16 after disease onset and reached up to 290.6 pg/mL on Day 22. Notably, the median time to death from symptom onset was 18.5 days (interquartile range = 15–20 days).22 The trends in the rise of hs-cTnI were parallel to the increase of inflammatory biomarkers (interleukin-6, D-dimer, ferritin, and lactate dehydrogenase). These results led us to believe that cardiac insult occurs secondary to the cytokine storm or secondary hemophagocytic lympho-histiocytosis rather than an isolated primary myocardial injury.23 Another implication of this study is that we should repeat troponin estimation frequently during hospitalization. Besides initial triaging, it provides useful prognostic information and also helps to decide which patient requires more intensive care.
The other pattern of myocardial injury involves the primary myocardial damage, in which the patients present predominantly with cardiac complaints instead of respiratory symptoms. It can occur secondary to acute myocardial infarction (type 1) in a patient with COVID-19. However, it can also occur as a result of either viral myocarditis or stress-induced cardiomyopathy. The possibility of the latter two entities finds support from a few case reports where patients with COVID-19 have presented primarily with chest pain, elevated cardiac enzymes, and left ventricular dysfunction on echocardiography, along with ST-T changes on electrocardiogram, but with normal coronary angiograms.3,24, 25, 26 In a recently published case report, an otherwise healthy 53-year-old woman developed acute myopericarditis with systolic dysfunction confirmed on cardiac magnetic resonance imaging a week after onset of fever and dry cough owing to COVID-19. Interestingly she did not show any respiratory involvement during the clinical course.25 This case confirmed the direct role of viral myocarditis in the pathogenesis of COVID-19 associated myocardial injury. It also described the pericardial involvement with COVID-19 for the first time. Sporadic autopsy reports in patients with COVID-19 with high viral load have suggested infiltration of myocardium by interstitial mononuclear inflammatory cells.6 However, currently, we do not have enough data to define the role of endomyocardial biopsy and non-invasive imaging modalities such as cardiac PET to aid in the diagnosis of COVID-19–induced myocarditis. Until further data are available, one should have a high index of suspicion for acute myocarditis if a patient with COVID-19 presents with raised cardiac enzymes, new onset or worsening cardiac arrhythmias, and heart failure.
Arrhythmias
Cardiac arrhythmias are a common manifestation in patients with COVID-19. Among 137 hospitalized patients with COVID-19, 7.3% of patients reported having nonspecific palpitations.27 In another retrospective analysis of 138 hospitalized patients, 23 (17%) had cardiac arrhythmias, which were significantly higher among those requiring ICU care (44.4% versus 6.9%, p < 0.001), compared with those who did not.1 The type of arrhythmia, whether atrial or ventricular, was not specified. The possible mechanisms behind the higher prevalence of arrhythmia among patients with COVID-19 include myocardial ischemia/injury, viral infection–induced state of hypoxia, fever, metabolic disarray, neurohormonal dysregulation, and inflammatory stress.28 The use of drugs causing QT prolongation such as chloroquine, hydroxychloroquine, azithromycin, which have been repurposed for use in COVID-19, has also been implicated as a possible cause of ventricular arrhythmias in COVID-19.28 The risk of arrhythmias is more in patients with severe/critical disease and pre-existing CVD/inherited arrhythmia syndromes.28,29 Malignant arrhythmias in the presence of troponin elevation should raise the suspicion of fulminant myocarditis.3 Arrhythmias in the setting of COVID-19 should be treated in the same way as in non-COVID patients.3
Acute heart failure and cardiogenic shock
In a series of 191 patients with COVID-19, acute heart failure occurred in 44 patients (23%). The incidence was significantly higher among non-survivors compared with those who survived (52% versus 21%, p < 0.0001).23 Acute heart failure and cardiogenic shock in COVID-19 cases can occur secondary to exacerbation of pre-existing left ventricular dysfunction, and/or due to new onset myocarditis, ACS, cardiac arrhythmias, and stress-induced cardiomyopathy. Possibility of right heart failure and associated pulmonary HTN should always be kept in view of severe parenchymal lung disease and ARDS. Coronary CT angiogram and echocardiography are valuable non-invasive diagnostic modalities to guide further management.
Thromboembolic complications
Patients with COVID-19 appear to have an increased risk of arterial and VTE because of a state of vascular inflammation, endothelial dysfunction, and hypercoagulability associated with COVID-19 infection.3,30 Immobilization in severe/critical COVID-19 further adds to the risk of developing VTE. Studies have shown elevated D-dimer and fibrin degradation products levels, and a higher incidence of disseminated intravascular coagulation, among non-surviving patients, compared with survivors.3,31 However, there is limited data on the incidence of thrombotic complications associated with COVID-19.30 A small observational study involving 184 patients of COVID-19, admitted in ICU, revealed a 31% incidence of radiologically confirmed thrombotic complications (27% venous and 4% arterial) despite receiving standard doses of thromboprophylaxis.30 Pulmonary embolism was the most frequent thrombotic complication.30 Thromboprophylaxis is thus recommended in all patients with COVID-19 admitted to ICUs.3,30,31 There is no validated regimen for prophylaxis against VTE in patients hospitalized with COVID-19. Standard society guidelines, as for patients with non-COVID, should thus be followed.32 Nevertheless, if a hospitalized patient with COVID-19 demonstrates sudden clinical deterioration as evidenced by hypoxia or hemodynamic instability, the thromboembolic disease should always be considered in the differential diagnosis with a low threshold for using the appropriate diagnostic tests.
Acute coronary syndrome
As aforementioned, COVID-19 induces a state of the infection-induced intense inflammation and hemodynamic alterations, which is likely to augment the risk of plaque rupture in susceptible individuals.3 However, many investigators across Europe have reported a reduction in patients admitted for ACS after the COVID pandemic began.33, 34, 35 These decreased numbers are more likely secondary to people preferring to stay at home in the wake up of strict travel restrictions, lockdowns, and hospital emergencies being filled with patients with COVID.34 People may have a fear they will catch COVID-19 if they call an ambulance or end up in the emergency department.34 Consequently, the number of patients with delayed presentation and ACS-related complications is bound to be higher. In a recently published report from the province of Bergamo, Italy, there was an increase in late presentations (+25%) of patients with ACS in the province in March 2020, as compared with the monthly average of the previous year.34 Extensive infarctions, heart failure, high coronary thrombus burden, and no reflow were more frequent.34 Similarly, delays in presentation and management of patients with ST-elevation myocardial infarction were recorded in a small observational study in Hongkong, China.36 Thus, early reports have suggested a decrease in hospital admissions for ACS, but this is mainly because of the over occupancy of the health-care system with patients with COVID-19 and fear of contracting infection among patients. There is no apparent reason for a decline in the incidence of ACS.34, 35, 36 However, there appears an increase in the ACS-related complications and subsequent mortality owing to delays in presentation and management of patients with ACS in the COVID-19 era.34,36
COVID-19 also imposes a dilemma in the minds of physicians treating patients with ACS because of closely mimicking conditions.37,38 There is an ever-increasing recognition of COVID-19 associated myocarditis simulating an ST-elevation myocardial infarction presentation, and also of the frequent rise of troponin elevation in patients with COVID-19 which is usually multifactorial.37, 38, 39 Therefore, careful history, examination, and imaging are necessary to differentiate between the true ACS and the conditions aforementioned to allow timely appropriate therapy.
Prognostic implications of cardiac injury associated with COVID-19
Acute myocardial injury (reflected by elevated cardiac enzymes secondary to ischemic/non-ischemic etiology, and also the electrocardiographic and echocardiographic abnormalities), is highly prevalent in patients with COVID-19 and is associated with more severe disease and worse prognosis.3,13,20 In a study involving 416 patients hospitalized with COVID-19, patients with myocardial injury (20%) were more prone to develop ARDS (58.5% vs. 14.7%; P < 0.001) and a higher mortality rate (51.2% vs. 4.5%; P < 0.001) than those without myocardial injury.13 On multivariate analysis, the presence of myocardial injury was the second most robust independent variable associated with mortality, preceded by ARDS, with hazard ratios of 4.26 and 7.89, respectively.13 Another study describing outcomes in 187 patients hospitalized with COVID-19 (43 died; 144 discharged) in Wuhan, China,20 revealed significantly higher mortality in individuals with myocardial injury (elevated troponin T) compared with those without myocardial injury (normal levels of troponin T) (59.6% vs. 8.9%, respectively; P < 0.001). Furthermore, patients with elevated troponin levels showed a higher incidence of complications such as ARDS, malignant ventricular tachyarrhythmias, acute renal injury, and acute coagulopathy. Interestingly, the mortality was highest in those with myocardial injury and underlying CVD (69.4%); however, the mortality was also considerably high in those with myocardial injury but without prior CVD (37.5%). On the contrary, patients with prior CVD without acute myocardial injury had a relatively favorable prognosis (mortality of 13.3%).20 Thus, all these evidence reaffirm that patients with COVID-19 who develop acute myocardial injury have a high risk of major complications and mortality, and the risk is more in patients with prior CVD.
Considerations for prevention and treatment of cardiovascular complications
India, as on 25th April 2020, is witnessing a sharp rise in the cases of COVID-19. In the coming days, we are likely to see an increase in the number of hospitalized patients and, as aforementioned, a high burden of cardiovascular complications. The hospital systems need to ensure preparedness for dealing with a large volume of such patients, many of whom would need ICU care and/or acute cardiac care. Unfortunately, no specific antiviral therapy or vaccines are available for COVID-19. Preventive measures, along with symptomatic supportive treatment, are the best strategies for COVID-19 and its complications at this time. It is crucial to triage the patients according to the status of underlying CVD as the patients with prior CVD, or its risk factors are more prone to develop cardiovascular morbidity and mortality. Hence, appropriate protocols for rapid diagnosis, triaging, isolation, and treatment of patients with COVID-19 with cardiovascular complications should be prepared and well-rehearsed by the hospital administration. As a cardiovascular physician, it is imperative to encourage the use of telemedicine/telehealth for triage and patient management, wherever it is feasible. In-person visits and consultations should be avoided. It is important to cancel elective procedures, i.e., echocardiography, and cardiac catheterization unless they likely to affect outcome meaningfully. Adherence to national/international guidelines for optimal use of personal protective equipment (PPE) is recommended.
American College of Cardiology, in a recent statement, has recommended not to measure troponin levels or natriuretic peptides in patients with COVID-19 unless the diagnosis of acute MI or heart failure is suspected on clinical grounds.39 However, there is evidence that there is a progressive serial increase in both troponin and NT-pro BNP levels during hospitalization in patients who follow a deteriorating clinical course toward death. In contrast, the levels of these biomarkers remain stable and low in those with less severe disease and a more favorable clinical course toward successful treatment and discharge.20 Therefore, frequent and periodic estimation of cardiac biomarkers such as troponin, and NT-pro BNP is warranted in hospitalized patients who have prior CVD or its risk factors, and in those who show early evidence of cardiac injury. Worsening in any of these during serial recording should be regarded as a warning sign for poor outcome, and early and appropriate intervention should be initiated.
The use of ACE-I/ARB in patients with COVID-19 remains controversial. It is concerning because SARS-CoV-2 enters the human cells by binding to ACE-2 receptors, and the increased ACE-2 expression induced by therapy with ACE-I or ARB would aggravate the infection with SARS-CoV-2. However, the available data does not show any difference in outcome among patients treated with or without ACE-I/ARB. On the other hand, a prior study showed a beneficial effect of ACE-I/ARB therapy in patients hospitalized with viral pneumonia.40 The mechanism responsible for this beneficial effect was a reduction in the viral infection–induced pulmonary inflammatory response and cytokine release. Some have attributed this beneficial effect to a compensatory increase in ACE-2.41 Until data from more extensive clinical studies becomes available, it is recommended to continue using ACE-I/ARBs in patients who are at risk for, are being evaluated for, or have COVID-19, a position supported by multiple society guidelines.42
The care of ACS in patients with COVID-19 is challenging. Ideally, all patients with ACS should be managed as per the standard guidelines, but new protocols have to be set for new challenges. Every institution or health-care facility needs to develop its own protocol for the management of patients with ACS during the COVID-19 crisis based on the clinical profile of patients, and the availability of workforce, adequate PPE, and a COVID-19 dedicated cardiac catheterization laboratory with preferably negative pressure ventilation.37,38,43
Conclusion
COVID-19 pandemic has posed a severe health threat on an international scale. Cardiovascular comorbidities are common in patients with severe COVID-19 and are associated with increased mortality. Elevation of cardiac enzymes, suggesting acute cardiac injury, can occur in up to one-fourth of patients with COVID-19 and worsens its outcome. It is crucial to measure cardiac biomarkers such as Troponins and NT-pro BNP serially in hospitalized patients to triage the patients, monitor treatment, and to predict prognosis. Our understanding of this novel coronavirus and its cardiovascular implications is still evolving. Researchers around the world need to share the data continuously at a rapid pace so that we can have a detailed understanding of this novel coronavirus and curb the menace it has caused.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020 Mar 18 doi: 10.1016/j.jacc.2020.03.031. pii: S0735-1097(20)34637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci A.S., Lane H.C., Redfield R.R. Covid-19: navigating the uncharted. N Engl J Med. 2020 Mar 26;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Team TNCPERE The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) -China, 2020 (J) China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M., Safavi-Naeini P., Solomon S.D. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuster G.M., Pfister O., Burkard T. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020 Mar 20 doi: 10.1093/eurheartj/ehaa235. pii: ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.W.M., Ng C.K., Chan Y.H. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 11.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;25 doi: 10.1001/jamacardio.2020.0950. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 Mar 12 doi: 10.1016/j.ijid.2020.03.017. pii: S1201-9712(20)30136-3. [DOI] [Google Scholar]
- 15.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 Mar 11 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;23 doi: 10.1001/jama.2020.4683. Published online March. [DOI] [PubMed] [Google Scholar]
- 17.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, february 12–march 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.lhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q., Zhou L., Sun X. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017 Aug 22;7:9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;27 doi: 10.1001/jamacardio.2020.1017. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rali A.S., Sauer A.J. COVID-19 pandemic and cardiovascular disease. US Cardiol. 2020;14:e01. [Google Scholar]
- 22.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. PMID: 32171076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H., Ma F., Wei X. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. March. 2020;16 doi: 10.1093/eurheartj/ehaa190. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;27 doi: 10.1001/jamacardio.2020.1096. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection [published online ahead of print, 2020 Apr 8] Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa286. ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C.-I., Postema P.G., Arbelo E. Heart Rhythm; 2020. SARS-CoV-2, COVID-19 and Inherited Arrhythmia Syndromes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui H., Zhang Y., Yang X. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. MedRxiv. 2020 [epub ahead of press] [Google Scholar]
- 30.Li Lei, Li Ranran, Wu Zhixiong. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Ann Intensive Care. 2020;10:45. doi: 10.1186/s13613-020-00661-z. Published online 2020 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang N., Li D., Wang X. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020 Apr;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witt D.M., Nieuwlaat R., Clark N.P. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–3291. doi: 10.1182/bloodadvances.2018024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood S. The mystery of the missing STEMIs during the COVID-19 pandemic. tctMD. April 2, 2020 https://www.tctmd.com/news/mystery-missing-stemis-during-covid-19- pandemic [Google Scholar]
- 34.Roffi M., Guagliumi G., Ibanez B. 2020. The Obstacle Course of Reperfusion for STEMI in the COVID-19 Pandemics. [published online ahead of print, 2020 Apr 21]. Circulation, 10.1161/CIRCULATIONAHA.120.047523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage [published online ahead of print, 2020 Apr 16] Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa314. ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tam C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmud E., Dauerman H.L., Welt F.G. Management of Acute Myocardial Infarction during the COVID-19 Pandemic. Cathet Cardiovasc Interv. 2020 doi: 10.1002/ccd.28946. [published online ahead of print, 2020 Apr 20], 10.1002/ccd.28946. [DOI] [PubMed] [Google Scholar]
- 38.Daniels M.J., Cohen M.G., Bavry A.A., Kumbhani D.J. Reperfusion of STEMI in the COVID-19 era - business as usual? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047122. [published online ahead of print, 2020 Apr 13], 10.1161/CIRCULATIONAHA.120.047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Cardiology: Troponin and BNP use in COVID-19. Available online at: https://www.acc.org/latest-in- cardiology/articles/2020/03/18/15/25/troponin- and-bnp-use-in-covid19. Last accessed April 25 2020.
- 40.Henry C., Zaizafoun M., Stock E. Impact of angiotensin- converting enzyme inhibitors and statins on viral pneumonia. SAVE Proc. 2018;31:419–423. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system Blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. Published online April 03. [DOI] [PubMed] [Google Scholar]
- 43.Fgp Welt, Shah P.B., Aronow H.D. Catheterization laboratory considerations during the coronavirus (COVID 19) pandemic: a joint statement from the American College of Cardiology (acc) interventional council and the society of cardiovascular angiography and intervention (scai) J Am Coll Cardiol. 2020 Mar 16 doi: 10.1016/j.jacc.2020.03.021. pii: S0735-1097(20)34566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]