Abstract
Climate and landscape change are drivers of species range shifts and biodiversity loss; understanding how they facilitate and sustain invasions has been empirically challenging. Winter severity is decreasing with climate change and is a predicted mechanism of contemporary and future range shifts. For example, white-tailed deer (Odocoileus virginianus) expansion is a continental phenomenon across the Nearctic with ecological consequences for entire biotic communities. We capitalized on recent temporal variation in winter severity to examine spatial and temporal dynamics of invasive deer distribution in the Nearctic boreal forest. We hypothesized deer distribution would decrease in severe winters reflecting historical climate constraints, and remain more static in moderate winters reflecting recent climate. Further, we predicted that regardless of winter severity, deer distribution would persist and be best explained by early seral forage subsidies from extensive landscape change via resource extraction. We applied dynamic occupancy models in time, and species distribution models in space, to data from 62 camera traps sampled over 3 years in northeastern Alberta, Canada. Deer distribution shrank more markedly in severe winters but rebounded each spring regardless of winter severity. Deer distribution was best explained by anthropogenic landscape features assumed to provide early seral vegetation subsidy, accounting for natural landcover. We conclude that deer dynamics in the northern boreal forest are influenced both by landscape change across space and winter severity through time, the latter expected to further decrease with climate change. We contend that the combined influence of these two drivers is likely pervasive for many species, with changing resources offsetting or augmenting physiological limitations.
Subject terms: Boreal ecology, Conservation biology, Invasive species
Introduction
The twin spectres of climate change and landscape change loom behind the human domination of global ecosystems and biodiversity loss in the Anthropocene1,2. Climate change and landscape change likely interact to affect biotic communities3,4, but interactions are difficult to quantify, as they can be additive, antagonistic, or synergistic5. Moreover, species distributions vary in time and space with habitat availability, community assembly, abiotic and climatic variables, and a host of other factors. Identifying the main drivers of a species’ distribution is the goal of niche ecology6 and a requisite for conservation plans to curtail biodiversity loss, so understanding the relative contributions of landscape and weather variability to changes in population size and distribution is a key ecological pursuit.
For mammals, responses to climate – and the weather it manifests over small temporal scales – are often rooted in changing energetic demands7,8. Likewise, mammal responses to landscape change can trace proximal causes to metabolic costs9. Direct habitat loss and isolation incurred by habitat fragmentation are two commonly cited mechanisms of biodiversity loss10,11, but disturbing established vegetation communities can create resource subsidies for some species – a notable mechanism of species invasions12. In the North American boreal forest, for example, anthropogenic disturbance replaces mature forest with early seral vegetation that benefits some mammal species over others13,14. Balancing energy intake and metabolic demands is particularly important for boreal species, where winters can be severe and food supplies ephemeral15,16. The interplay between landscape change and severe weather should therefore be expected to be particularly important in this cold, energetically costly, nutritionally poor biome.
We examined the roles of landscape change – defined here as anthropogenic landscape features – and variable winter severity in distribution dynamics of invasive white-tailed deer (Odocoileus virginianus, hereafter “deer”) in the western Canadian boreal forest. Deer have expanded markedly across the continent since European colonization17, affecting forest structure, community composition, biodiversity, ecosystem dynamics, and predator-prey dynamics18. In the vast northern boreal forests, deer expansion has resulted in woodland caribou (Rangifer tarandus) declines19,20 through apparent competition21,22. The alarming and ongoing caribou declines23 and expected suite of ancillary effects of deer invasion24 prompt a need to understand the ecological factors sustaining deer invasion.
As ruminants white-tailed deer rely greatly on sufficient nutritious forage25,26. In mature boreal forests early seral vegetation is limited to regenerating disturbances from fire, insects, and canopy caps13. Thus, deer metabolic demands may have eclipsed energy derived limited forage, likely restricting deer distribution from the boreal25,27,28. In winter when snow is deep deer incur great metabolic demands from movement stressing them energetically; they experience starvation, and pre-season stores and available forage control this starvation, otherwise mortality ensues25. With ongoing climate change, winters globally have become less severe29, and evidence suggests this is true of boreal regions as well, with less snow (Fig. S1) and less extreme temperatures30. At the same time the landscape footprint of forest harvesting, energy exploration and extraction, and transportation infrastructure has generated a spatially extensive array of early seral vegetation, smaller in patch size but much more widely dispersed, and greater in overall area, than natural disturbance regimes31,32.
In the boreal forests of Alberta, Canada, provincial-scale aerial deer survey and snowtracking data modelled with remotely sensed landcover data suggest climate change drives white-tailed deer invasion whereas landscape change plays a secondary role27,33. However, processes notoriously differ across scales34,35, and the landscape-scale processes facilitating invasion remain unknown. Empirical estimation of landscape-scale deer distribution dynamics across winters of differing severity, and of deer spatial distribution relative to anthropogenic footprint, would greatly help elucidate the relative influence of landscape and climate change on deer invasion.
To that end we examined deer spatiotemporal dynamics in Alberta’s northeastern boreal forest using camera trapping – an increasingly popular ecological research tool36,37 – analyzed using spatially explicit, hierarchical models for repeat occurrence data38, and species distribution models39. Climate change is a long-term trend, thus responses to climate change are concomitantly lengthy to document. In the immediate term, response to extreme weather events40 that mirror historical and contemporary climate is a useful proxy for predicted climate change effects on biota41,42. We capitalized upon marked variation in boreal winter weather severity over three years, representative of historically severe and contemporary moderate winters (Fig. S1), to test two hypotheses: (1) Deer distribution fluctuates in time, contracting markedly in response to in severe winters typical of 20th-century climate, but rebounds in spring, leaving relatively stable annual distributions; (2) Deer spatial distribution is positively related to anthropogenic features creating early seral vegetation subsidies, both annually and in the winter season. Understanding the influences of both landscape disturbance and energetically costly severe weather in sustaining invasive populations is a critical endeavour, as these processes are expected to rapidly increase with global change.
Materials and Methods
Study area
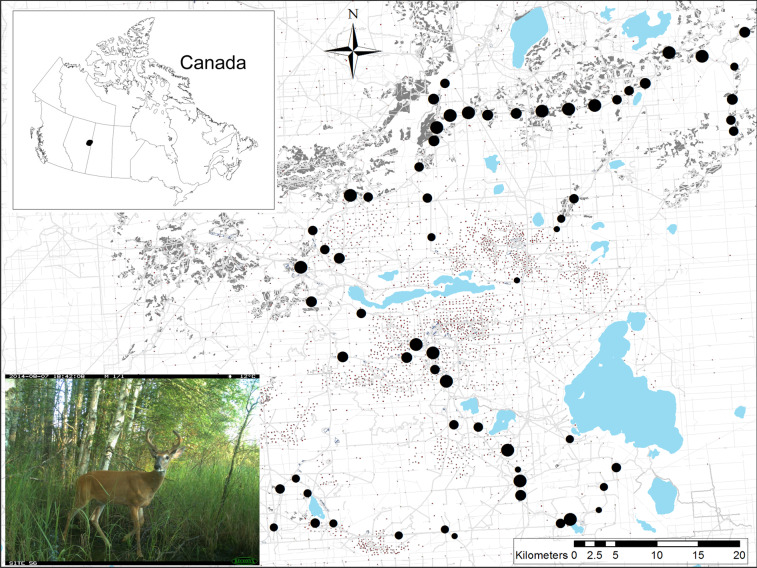
We surveyed white-tailed deer distribution in northeast, Alberta, Canada (Fig. 1), within a boreal forest ecosystem invaded by northward-expanding deer populations in the last few decades. The 3000 km2 study area is a mosaic of white (Picea glauca) and black spruce (Picea mariana), aspen (Populus tremulodies), jack pine (Pinus banksiana), and Ledum groenlandicum-dominated muskeg. Extensive petroleum exploration and extraction features, forest harvesting, roads (car accessible), off-highway vehicle (OHV) trails, and other landscape disturbances are dispersed throughout the study area (Fig. 1).
Figure 1.
White-tailed deer occurrence was surveyed at 62 camera sites (large block dots, scaled to the relative number of observations over three years) in the boreal forest of northeast Alberta, Canada. Anthropogenic footprint is extensively and intensively imposed on this landscape, including forest harvesting cutblocks (grey polygons), well sites (square dots), seismic lines (grey), and roads and trails (dark grey and colored lines). Lakes are in blue. Map was created in ArcGIS v.10 based on Alberta Biodiversity Monitoring Institute Human Footprint data 2010 (abmi.ca) and Government of Alberta water bodies data.
We used Environment Canada weather data (http://climate.weather.gc.ca) for Fort McMurray, Alberta–the closest station with complete data– to measure winter severity in the study region. White-tailed deer are highly susceptible to snow depth, which incurs a substantial metabolic cost leading to overwinter mortality26,43–45, so we used daily mean snow depth (“snow-on-ground”) for the region across the three years of study (Fig. 2). Fluctuations across this period provided a severe snowfall winter typical historically (Fig. S1), a moderate snowfall winter typical of contemporary winters, and one between these extremes.
Figure 2.
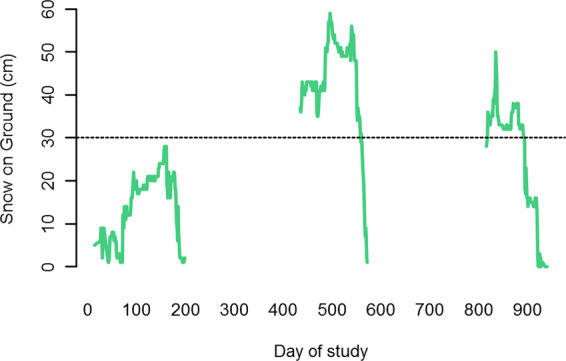
Winter severity as indicated by snow-on-ground, from Environment Canada weather data, Fort McMurray, Alberta, 2011–2014, summarised daily. The 30-year mean maximum snow depth (dashed line) is 30 cm. The winter of 2011–12 exhibited contemporary average snow depth, whereas the winters of 2012–13 and 2013–14 were comparatively severe.
Deer distribution in space and time
We sampled deer occurrence at 62 camera trap sites deployed in a constrained stratified random design46 based on digital forest inventory data, as in14. In a geographic information system (GIS) we reclassified the landscape into landcover categories (Table S1) and overlaid it with 1 × 1-km grid cells. We categorized each grid cell by the most abundant landcover within it, then randomly selected grid cells as sampling sites, such that each category received equal sampling effort. Random selection was constrained to induce a minimum 2-km distance from other sites to maintain independence, and multiple different individuals were indeed identified at adjacent sites. The resulting design produced some sampling site clustering (Fig. 1) induced by the naturally clumped distribution of landcover classes on the landscape, but captured the range of this landscape’s anthropogenic footprint and natural heterogeneity. Cameras were placed on active wildlife trails embedded in forests, a minimum of 200 m from the roads and trails used to access the grid cell. The area is so replete with these linear features (Fig. 1) that although roads and trails are more abundant within 250-m of our sites than within the study landscape, this effect falls away at 500-m radius of quantification, and at the 1-km radius scale of our analysis (Fig. S2) roads are represented in our sample as they exist in the landscape. We obtained permission from government land officers and industry leaseholders to access all sampling areas. We deployed one Reconyx PC900 Hyperfire infra-red remote digital camera (Holmen, WI, USA) at each site for three years: October 2011 - October 2014. We define ‘site’ as the area likely to be used by a deer within a three-month season, centered on the camera detection zone. We assume the study area is the ca. 3000 km2 area encompassing a minimum convex polygon surrounding the camera sites.
Modelling deer occupancy dynamics through time
Modelling serial occurrence data, such as repeated detections via camera traps, is an area of active research without current consensus, and there is much debate about how well many species distribution models actually meet their assumptions36,47–49. To analyze deer distribution across time we used hierarchical occupancy models50 which can be considered as simultaneous generalized linear models (GLMs) of serial detection data, applied to each component of the model, with binomial errors (logistic link). In this model we consider zeroes in serial detection histories (e.g. 1010) as resulting from movement of this vagile animal into and out of the site49,51,52; Variation in p – the probability of detecting that species if present – is attributed mainly to movement in and out of the camera detection zone36,51,52; this is important since deer movement rates are likely to vary seasonally, particularly with snow cover. With variance due to p thus partitioned, we estimate occupancy as the probability of site use within each season. With multi-season models, species distributions can be considered closed during a survey and open to change among them; occupancy dynamics can be modeled through time, providing estimates of local site “extinction” and colonization53. Probabilities of occupancy (ψ), local extinction (ε), and colonization (γ) are estimated via a first-order Markov process in a maximum likelihood framework wherein the probability of occupancy at a site in year t + 1 is contingent on occupancy in year t, and:
ψ1 = probability a site is occupied in year 1
εt = probability an occupied site becomes unoccupied between seasons t and t + 1
γt = probability an unoccupied site becomes occupied between seasons t and t + 1
λt = spatial growth rate or rate of change in occupancy, ψt+1 / ψt
pt,j = probability that deer are detected at a site in survey j of season t (given presence)
We used multi-season occupancy models to estimate the change in deer distribution among seasons spanning three years. We separated continuous camera data into month-long (30.4 day) “secondary” survey periods53. Three secondary surveys comprised a primary sampling season. Deer behaviour varies seasonally, with most variation due to changes associated with mating, parturition, and dispersal54. We therefore classed each “deer season” as three-month periods: rut (autumn, October – December), post-rut (winter, January – March), pre/early fawning (spring, April – June), and post-fawning (summer, July – September). We relax the closure assumption and assume non-Markovian variation in deer site-use among months within a 3-month season primary season38. The full data frame for the study is thus 12 seasons, with 3 repeated surveys within each season, for a total of 36 surveys at each site, each comprised of deer detection-nondetection within the month.
We ran several competing occupancy models in software Presence55. We tested whether p was constant or varied among seasons or surveys; whether occupancy (ψ), site colonization (γ), and site extinction (ε) were either constant or varied among seasons. We sought to estimate mean differences in ψ among seasons across the study area, and moreover models incorporating landcover classes (q.v.) as covariates of ψ, γ, and ε did not converge due to the data demands of occupancy models and problems with border estimates; occupancy and recolonization often approached 1.0. Therefore we assumed spatial homogeneity in ψ, which should then be interpreted as a seasonal landscape average of the proportion of the area occupied by deer. We ranked competing models via Akaike’s Information Criterion (AIC) in an information-theoretic approach, normalised them as AIC weights (AICw), and calculated evidence ratios (ER) for each variable56. From per-survey estimates of p we calculated the probability of false absence (PFA) within a season as [1-p]3,57.
Modelling deer distribution across space
Species distribution models assume that the repeated occurrence or persistence of a species at a site is related to the environment within some defined area around that site58. As the variable use of a site by a species is often an ecological signal (rather than error) we modelled serial detections and nondetections across space to understand deer response to landscape change. As for the temporal analysis, our dependent data remained as above: the repeated occurrence of deer among 36, one-month survey periods, yielding a 0–36 response variable. Monthly detections serve to minimize effects of temporal heterogeneity in detection rates induced by using all detections, such as changes to detection rates caused by variable movement36. Each month can be considered an independent Bernoulli trial in which the deer was detected (1) or not (0) at a site59.
We quantified the study area landscape using three spatial digital resource inventories. We quantified natural land cover within multiple buffers around each camera site using Alberta Vegetation Inventory (AVI), a digital forest inventory dataset. We quantified the percent of area of polygonal anthropogenic features in these buffers from the Alberta Biodiversity Monitoring Institute (ABMI) 2010 Human Footprint Map Ver 1.1 (ambi.ca), and ABMI linear features layer derived from 2012 SPOT satellite imagery (Table S1). We omitted correlated variables (r > 0.7)60 to prevent multicollinearity. These data were the most contemporary available to match our deer sample available; we did not expect the landscape to change extensively during the 1–2 years separating the datasets. We combined sparsely represented variables (<1–2% of area) into a single, combination variable (Table S1). We rescaled each variable (mean = 0, s.d.=1) to compare effect sizes.
It is challenging to identify the area of habitat around each sampling site that best explains species occurrence (spatial scale), as different processes operate at different spatial scales35,61. One approach is to quantify habitat at multiple spatial scales, run multivariate species distributions models at each scale, and determine the spatial scale at which habitat best explains species occurrence14,62. We modelled the 0–36 months of white-tailed deer occurrence against landscape variables quantified at 20 spatial scales ranging from 250-m to 5000-m radius around each camera site. Each generalized linear model (binomial errors, logit link) in R ver. 3.2.2 (R Foundation for Statistical Computing 2014) regressed deer persistence against all 20 landscape variables (Table S1). We used the step-AIC function in R package MASS; to identify the spatial scale that generated the best-supported model explaining deer occurrence63, plotted as normalised AICw56,64.
We used model selection to weigh support for 30 competing, non-mutually exclusive hypotheses about deer response to natural and anthropogenic landscape features (Table S2). These hypotheses were grouped into candidate sets, with each set assuming deer distribution was driven by (1) natural landcover, (2) nonforested areas, (3) forestry features; (4) petroleum extraction features; (5) petroleum and forestry combined; (6) human access linear features; or (7) combinations of natural features and (3–6). Each hypothesis was represented by a generalised linear model (binomial errors, logit link), and generated an AIC score and normalised AICw, which we ranked to identify the best-supported model among the candidate sets. We tested the best supported model for overdispersion, and calculated the deviance explained by each model65. We used R package boot66 to assess model fit with 10-fold cross validation67, and calculated Moran’s I to test for spatial autocorrelation of model residuals.
This approach allowed us to test hypotheses about annual deer occurrence. However as winter is critical for deer survival25,68, and winter severity is expected to change with climate change, we also asked whether the same landscape factors influenced deer occurrence within winter periods only, repeating this analysis with data from winter months (January-March) yielding a 0–9 response variable (number of winter months over three years in which deer occurred at a site).
Research ethics
All research was permitted by the Government of Alberta, Ministry of Environment and Parks, Fish & Wildlife Division, Collection License 49143.
Animal ethics
This research was reviewed and approved by InnoTech Alberta’s Animal Care and Use Committee (ACUC), permit ACUC0524.frm /clj/IO.II.02. No animals were handled in this phase of the study.
Results
White-tailed deer were identified in 112,648 of 141,140 animal images captured by the camera trap survey, spread across the study area, and are now by far the most prevalent large mammal in this system (Fig. 1). Winter severity was markedly different among the three years (Fig. 2). The 30-year mean annual snow depth (snow on ground) –measured in February, when snow is deepest –at the Fort McMurray weather station is 30 cm69. Snow depth exceeded 30 cm in 0 days in 2011–2012; 125 days in 2012–2013; and 73 days in 2013–2014. We interpreted these as mild, severe, and moderately severe winters, corresponding to snow-on-ground variability over the last century (Fig. S1).
Deer occupancy dynamics through time
The probability of deer site extinction and colonization varied seasonally, and the probability of detecting deer given their occurrence at a site (p) varied monthly; this model carried 100% of the AIC weight among the set of competing candidate models of detectability (Table S3). Estimated p was lowest in late winter and spring and highest in summer (Fig. 3), providing support for the prediction that deer yard in winter and p, being affected by movement, reflects this lower movement rate. PFA approached 0 in most seasons and at worst was 0.03, indicating that deer were detected at sites where they occurred and lending confidence to our estimates of occupancy.
Figure 3.
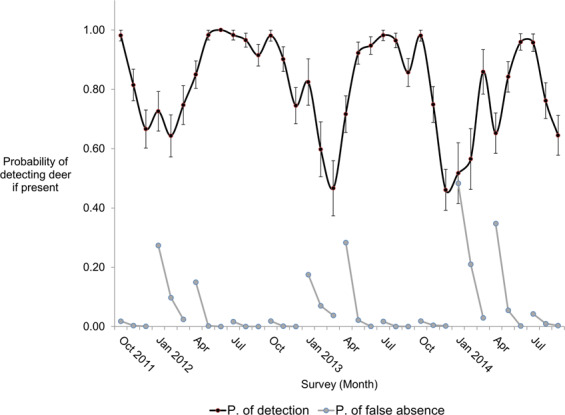
The probability of detecting deer via cameras (black line) varied monthly. The probability of false absence (blue lines) within each season approached 0.0 for most seasons and at worst (spring 2014) was 3%. Bars represent standard errors of estimates.
Deer occupancy fluctuated widely among seasons (Fig. 4). Deer occupied nearly the entire study area at the onset of autumn 2011. Occupancy dropped slightly in the first mild winter, then rebounded in the following spring. Occupancy remained mostly high and stable through the summer and autumn, and then dropped precipitously in the severe second winter of 2012. Deer occupancy again rebounded in spring 2013. This same pattern repeated through the third, moderately severe winter of 2013–2014 (Fig. 4).
Figure 4.
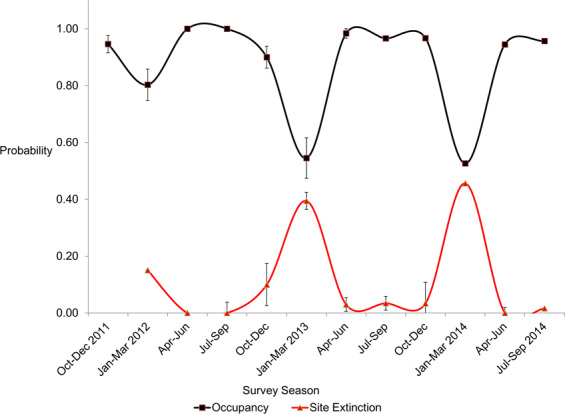
The probability of white-tailed deer occupancy (black line) and the probability of site extinction (red line) varied among seasons. Site extinction was greatest in the two severe winters.
These occupancy dynamics were driven (in part) by the probability of site extinction (ε) (Fig. 4). Estimated ε was low (0.15, SE = 0.05) in the mild 2012 winter, but greater in the severe 2013 winter (ε = 0.39, SE = 0.07). About 40% of sites were emptied of deer in the winter, and this dynamic repeated in the third year (ε = 0.46, SE = 0.07). Occupancy dynamics were also driven by the probability of site colonization (γ) (Fig. 5). After the 2012 mild winter, all empty sites were recolonized (γ = 1.0, SE = 0.00). After the severe 2013 winter, the landscape was again fully recolonized (γ = 1.0, SE = 0.00); after the moderately severe 2014 winter, site recolonization was again very high (γ = 0.88, SE = 0.06). Colonization was difficult to estimate in summer and fall because most sites were occupied; standard errors were large due to difficulties in model convergence and parameter estimation at border conditions (e.g. 1 or 0).
Figure 5.
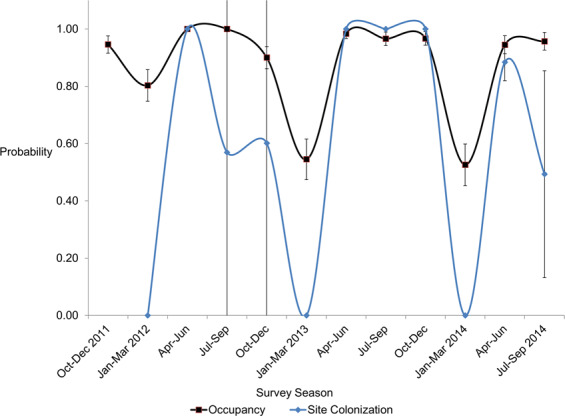
The probability of white-tailed deer occupancy (black line) and site colonization (blue line) varied among seasons and was high in all three spring periods. Bars represent standard errors.
Distribution dynamics can be synthesized as a spatial expansion parameter or “spatial growth rate” (λ), analogous to population growth rate53. Distribution is stable if λ = 1.0, shrinking if λ <1, and expanding if λ> 153. Deer λ declined more markedly in the severe winters of 2013 (λ = 0.61; SE = 0.07) and 2015 (λ = 0.54, SE = 0.07) than the mild winter of 2012 (λ = 0.8, SE = 0.05) (Fig. 6). However, deer distribution rebounded dramatically in both subsequent springs (λ = 1.8, SE = 0.24), returning to a stable distribution (λ = 1.0, SE = 0.03) in each summer and fall season – despite winter severity.
Figure 6.
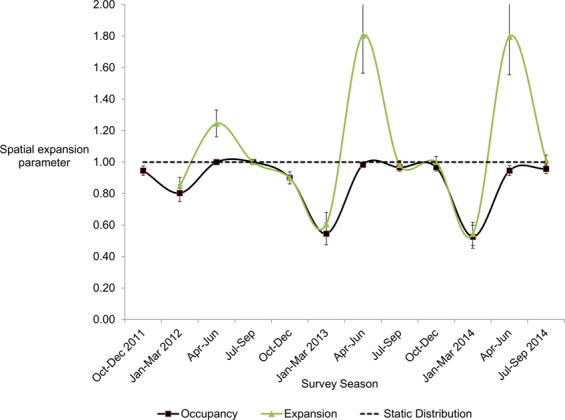
The spatial expansion parameter of boreal white-tailed deer varied among seasons and was >1 in all three spring periods. Bars represent standard errors of estimates; the dashed line represents zero spatial growth and a stable distribution.
Deer distribution across space
Of the 20 different spatial scales we examined, landscape characteristics around a site measured at the 1000-m radius scale best explained deer site persistence both annually (AICw = 0.98) and for winter months (AICw = 0.90), supported by most of the weight of evidence (Fig. S2, S3). White-tailed deer annual persistence was best explained by model 28: upland deciduous forest and anthropogenic features (Table 1). However, not all the variables in this candidate model were significant (i.e. with β coefficients different from 0); as per64 we identified the most parsimonious model by post hoc dropping variables with p> 0.2. The resulting best-supported parsimonious model (AICw = 0.99) with significant β coefficients indicated deer distribution was explained by anthropogenic landscape features deriving from a combination of forest harvesting, oil and gas exploration and development, and transportation infrastructure. Industrial block features, petroleum extraction well sites, and forest harvest cutblocks had large positive effects on deer persistence (Table 1). Deer persistence was negatively associated with off-highway vehicle trails.
Table 1.
Parameter estimates from the best-fit, most parsimonious generalized linear model (binomial errors, log link) of annual deer persistence (number of months of deer presence, out of 36), modelled against landscape features.
| Parameter | β estimate | std. error | t-value | p-value |
|---|---|---|---|---|
| (Intercept) | −0.16 | 0.10 | −1.60 | 0.1090 |
| Upland deciduous forest | 0.03 | 0.00 | 7.07 | <0.0001 |
| Forest harvest blocks | 0.07 | 0.01 | 7.76 | <0.0001 |
| Petroleum well sites | 0.24 | 0.04 | 5.55 | <0.0001 |
| All trails (unpaved roads) | −0.88 | 0.17 | −5.24 | <0.0001 |
| Industrial block features | 0.41 | 0.06 | 7.37 | <0.0001 |
*Null deviance = 465.4 on 60 df; residual deviance = 191.26 on 55 df; AIC = 397.85.
The same relationships were observed in winter, where model 28 (Table S2) was the best supported of the a priori candidate set, which we reduced post hoc by dropping variables with p > 0.02 (Figs. S2, S3). This best supported parsimonious model (AICw = 0.97) suggested deer persistence in winter months was positively related to industrial block features, cutblocks, and well sites, and negatively related to trails (Table 2). Deer’s negative response to trails (β estimate) was twice as great in winter as for annual persistence.
Table 2.
Parameter estimates from the best-fit generalized linear model (binomial errors, log link) of winter deer persistence (number of months of deer presence January-March over 3 winters, 0–9), modelled against landscape features.
| Parameter | β estimate | std. error | t-value | p-value |
|---|---|---|---|---|
| (Intercept) | −1.44 | 0.22 | −6.58 | <0.0001 |
| Upland deciduous forest | 0.02 | 0.01 | 3.75 | 0.0002 |
| Forest harvest blocks | 0.11 | 0.02 | 6.30 | <0.0001 |
| Petroleum well sites | 0.14 | 0.08 | 1.79 | 0.0734 |
| All trails (unpaved roads) | −1.50 | 0.33 | −4.58 | <0.0001 |
| Industrial block features | 0.57 | 0.09 | 6.02 | <0.0001 |
*Null deviance = 295.9 on 60 df; residual deviance = 160.27 on 55 df; AIC = 270.35.
All models fit the observed data relatively well; the best-fit model of annual deer persistence explained 58.9% of the deviance in observed deer detections, with a k-fold (k = 10) validation yielding prediction error of 1.9%. There was no significant spatial autocorrelation in model residuals (Moran’s I = 0.0393, expected = 0.0167, s.d. = 0.030, p = 0.062). The best-fit winter persistence model explained 45.8% of the deviance in the winter deer data and had a prediction error of 6.8%. The convergence of results across models suggests that a combination of upland deciduous forest and anthropogenic features – cutblocks, well sites, trails, and block features – best explained annual and winter white-tailed deer persistence.
Discussion
Landscape change and decreased winter severity typical of contemporary climate change positively affect invasive white-tailed deer landscape-scale distribution in the western Nearctic boreal forest. Disentangling the relative importance of climate and weather on global biodiversity has been the focus of some debate70 and there may be synergies between the two5 that are crucial to understanding mechanisms of biodiversity change, such as biological invasions. Certainly a changing North American climate has led to less severe winters – including the boreal forest – with significant consequences expected for biotic communities8,71–74. We show that the distribution of invasive white-tailed deer did contract more steeply in severe winters – even after accounting for less movement in winter as deer yard up (as movement correlates with estimated p in occupancy models49,75). In severe winters with deep snow on ground, deer distribution dropped nearly 50%, whereas distribution declined only marginally in the mild winter with less snow on ground.
It is an important caveat that we studied only three years across differing weather conditions, and a long-term population analysis will better elucidate responses to climatic trends through time. Rather than wait decades however, because snow conditions varied so markedly across the years–mirroring both mild contemporary winters and severe historical winters–we contend our observations suggest a likely mechanism by which climate change affects deer distributions. Past research at provincial scales using aerial survey data showed winter severity, including snowfall, was a key driver of white-tailed deer invasions27,33. Our examination within a 3000-km2 landscape provides a higher-resolution estimation of seasonal dynamics in relation to snow, as well as response to specific anthropogenic landscape features. Historically, the metabolic costs of moving through deep boreal forest snow likely exceeded energy obtainable from limited available forage25,45,76–78. If metabolic costs exceeds fat stores and new forage deer die. If available forage provides fat stores and winter food to pat these costs, deer live through the winter. Overwinter deer mortality drives seasonal population cycles elsewhere79, but distribution and population in boreal landscapes remain unknown.
Crucially however, regardless of winter severity, deer distribution quickly rebounded each spring. After severe winters in which deer distribution shrank by half, they quickly recolonized almost all empty sites in spring. Photographic analysis showed adults in all sites in this season, indicating recolonization from overwinter refugia; the contribution of reproduction has yet to be examined. Nonetheless deers’ dynamic resiliency defies expectations that two severe winters would significantly decrease boreal deer distribution, and it remains unclear how many severe winters would achieve this reduction. We contend that the spatial association of deer with anthropogenic features implicates early seral vegetation subsidies as a driver of the spring rebound.
Deer persistence – annually, and in especially in winter – was positively related to anthropogenic features, as well as upland forest. The response to upland deciduous forest met predictions as white-tailed deer forage on leaves and stems of woody plants; conifers offer comparatively low nutritional value25,26. Anthropogenic features regenerate into young, early-successional deciduous plants providing abundant deer forage13. This region of the boreal forest has been heavily impacted by resource extraction from multiple sectors, including forest harvesting, transportation, and petroleum extraction – which creates seismic exploration lines, pipelines, well sites, and industrial block features14. Multiple resource sectors in this boreal landscape create a cumulative disturbance of a magnitude and pattern without historic analogue31,32. Each feature removes old or mature forest and resets successional trajectories, collectively increasing the availability of early seral vegetation and providing abundant deer browse. These landscape features are spatially associated with the persistence of white-tailed deer in the boreal landscape.
Linear anthropogenic features (OHV trails) were negatively associated with deer distribution, with almost double the effect size in winter. Linear features expedite travel by wolves80 especially in winter, thereby increasing predation rates on woodland caribou81,82, and we hypothesized the same for deer. We posit deer avoid landscapes with higher densities of these features due to mortality risk. Trails also permit motorized human access, and deer are harvested in this landscape. Recreational access can have a significant effect on ungulate distribution83,84 but surprisingly little research has been devoted to understanding the effect of industrial access on wildlife distribution85.
Across their vast species range, white-tailed deer habitat relationships vary among seasons54,79 and we expected the same in the boreal, with different selection in winter vs. annually. However, selection in winter and annually was concordant, suggesting factors driving boreal deer distribution are consistent: selection of deciduous and early seral forage, and avoidance of anthropogenic linear features. In comparison, provincial-scale back-casting models suggests climate change eclipses landscape change in importance to boreal deer expansion33. The apparent difference between that study and this might be due to assumptions inherent in respective modelling approaches; spatial scale-dependency34,62 from the provincial scale to the landscape scale of our study; or less likely, a change in mechanisms over the last few years. Both views provide insights. Landscape-scale distribution dynamics observed in this study are consistent with the hypothesis that climate and landscape change both support persistence of this invasive species, much as they are synergistic for so many other ecological processes86,87.
Finally, we show how a single camera-trapping array camera can be used to investigate both spatial and temporal mammalian distribution patterns through time, generating insights about the ecological effects of landscape and climate change. We repeat recent appeals for a global network of camera trapping arrays37 to collect biodiversity data across all of Earth’s ecosystems to inform conservation decisions in this era of rapid biodiversity loss.
Conclusions
Mild winters typical of contemporary weather stemming from climate change support persistence of white-tailed deer in Nearctic boreal forests, but even severe winters do not suppress landscape-scale distribution of this invasive ungulate into spring. The positive association between deer persistence and anthropogenic features suggests that resource extraction is supporting white-tailed deer, providing nutritional subsidies that stabilize deer distributions despite fluctuation in winter severity. The expansion of white-tailed deer has been an ongoing continental-scale problem for over a century18,28, leading to species at risk declines in this system, and community changes across the Nearctic. Expansion has occurred despite heavy hunting and a massive increase in urban and rural residential landscapes. The conversion of mature forest, especially conifer forest, into deciduous and early seral vegetation is a primary mechanism of continental expansion. Our research suggests that anthropogenic disturbance stemming from multiple forms of resource extraction supports continued white-tailed deer persistence near this northern range limit. We conclude that mild weather resulting from climate change and landscape change operating in tandem have sustained invasions. Emerging research on other species and systems86–88 is likewise showing that the twin drivers of concurrent landscape and climate change are shifting physical conditions and resource availability, collectively altering species ranges, interactions, and ultimately biodiversity.
Supplementary information
Acknowledgements
At InnoTech Alberta, thanks to project team M. Hiltz, L. Nolan, L.D. Roy, S. Melenka, D. Pan, B.R. Eaton, K. Richardson, K. Tereschyn, S. Eldridge, J. Dennett, J. Watkins, T. Zembal, S. Frey, N. Heim, and W. Fuller. At Alberta Environment, thanks to B. Maile, G. Chapman, C. Dockrill, P. MacMahon, and K. Norstrom–RIP. Thanks to supporters S. Grindal, L. Mayes, T. Such, and S. Geoghegan. F.E.C. Stewart helped with graphics, and the University of Victoria’s ACME Lab provided valuable commentary. This project was primarily funded by Alberta Environment & Parks (AEP), InnoTech Alberta (ITA), Petroleum Technology Alliance of Canada (PTAC)‘s Alberta Upstream Petroleum Research Fund, and MEG Energy. Alberta Conservation Association also contributed additional funds. Alberta Biodiversity Monitoring Institute (ABMI)‘s Caribou Monitoring Unit contributed landscape data. ACB was supported by the Canada Research Chairs program.
Author contributions
J.T.F. conceived the research and the design; J.T.F., L.N. and L.R. created the sampling design; L.N. and L.R. led field data collection; J.T.F. conducted statistical analyses, and J.T.F. and A.C.B. conceived the questions and wrote the paper. All authors reviewed and edited the draft and gave final approval for publication.
Data availability
All data and code used in this research have been uploaded as supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65385-3.
References
- 1.Steffen W, Grinevald J, Crutzen P, McNeill J. The Anthropocene: conceptual and historical perspectives. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2011;369:842–867. doi: 10.1098/rsta.2010.0327. [DOI] [PubMed] [Google Scholar]
- 2.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 3.Brodie JF. Synergistic effects of climate change and agricultural land use on mammals. Frontiers in Ecology and the Environment. 2016;14:20–26. [Google Scholar]
- 4.Kareiva, P. M., Kingsolver, J. G. & Huey, R. B. Biotic interactions and global change. (Sinauer Associates Incorporated, 1993).
- 5.Côté Isabelle M., Darling Emily S., Brown Christopher J. Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B: Biological Sciences. 2016;283(1824):20152592. doi: 10.1098/rspb.2015.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chase, J. M. & Leibold, M. A. Ecological niches: linking classical and contemporary approaches. (University of Chicago Press, 2003).
- 7.Humphries Murray M. Climate Change. 2009. Mammal Ecology as an Indicator of Climate Change; pp. 197–214. [Google Scholar]
- 8.Lawler JJ, et al. Projected climate-induced faunal change in the Western Hemisphere. Ecology. 2009;90:588–597. doi: 10.1890/08-0823.1. [DOI] [PubMed] [Google Scholar]
- 9.Marquet PA, Labra FA, Maurer BA. Metabolic ecology: linking individuals to ecosystems. Ecology. 2004;85:1794–1796. [Google Scholar]
- 10.Fahrig L. Effect of habitat fragmentation on the extinction threshold: a synthesis. Ecological Applications. 2002;12:346–353. [Google Scholar]
- 11.Fahrig Lenore. Effects of Habitat Fragmentation on Biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2003;34(1):487–515. [Google Scholar]
- 12.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. Interactive effects of habitat modification and species invasion on native species decline. Trends in ecology & evolution. 2007;22:489–496. doi: 10.1016/j.tree.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JT, Wilkinson L. The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Review. 2005;35:51–81. [Google Scholar]
- 14.Fisher JT, Burton AC. Wildlife winners and losers in an oil sands landscape. Frontiers in Ecology and the Environment. 2018;16:323–328. [Google Scholar]
- 15.Humphries MM, et al. Expenditure freeze: the metabolic response of small mammals to cold environments. Ecology letters. 2005;8:1326–1333. [Google Scholar]
- 16.Humphries MM, Umbanhowar J, McCann KS. Bioenergetic prediction of climate change impacts on northern mammals. Integrative and Comparative Biology. 2004;44:152–162. doi: 10.1093/icb/44.2.152. [DOI] [PubMed] [Google Scholar]
- 17.Laliberte AS, Ripple WJ. Range contractions of North American carnivores and ungulates. BioScience. 2004;54:123–138. [Google Scholar]
- 18.Côté, S. D., Rooney, T. P., Tremblay, J.-P., Dussault, C. & Waller, D. M. Ecological impacts of deer overabundance. Annual Review of Ecology, Evolution, and Systematics, 113-147 (2004).
- 19.Boutin S, et al. Why are caribou declining in the oil sands? Frontiers in Ecology and the Environment. 2012;10:65–67. [Google Scholar]
- 20.Latham ADM, Latham MC, McCutchen NA, Boutin S. Invading white-tailed deer change wolf-caribou dynamics in northeastern Alberta. The Journal of Wildlife Management. 2011;75:204–212. [Google Scholar]
- 21.Holt RD. Predation, apparent competition, and the structure of prey communities. Theoretical population biology. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 22.DeCesare NJ, Hebblewhite M, Robinson HS, Musiani M. Endangered, apparently: the role of apparent competition in endangered species conservation. Animal Conservation. 2009;13:353–362. doi: 10.1111/j.1469-1795.2009.00328.x. [DOI] [Google Scholar]
- 23.Hervieux D, et al. Widespread declines in woodland caribou (Rangifer tarandus caribou) continue in Alberta. Canadian Journal of Zoology. 2013;91:872–882. doi: 10.1139/cjz-2013-0123. [DOI] [Google Scholar]
- 24.Cardinal E, Martin J-L, Côté SD. Large herbivore effects on songbirds in boreal forests: lessons from deer introduction on Anticosti Island. Ecoscience. 2012;19:38–47. [Google Scholar]
- 25.Hewitt, D. G. In Biology and Management of White-tailed deer Ch. 3, 75–106 (CRC Press, Taylor & Francis Group, 2011).
- 26.Moen Aaron N. Seasonal Changes in Heart Rates, Activity, Metabolism, and Forage Intake of White-Tailed Deer. The Journal of Wildlife Management. 1978;42(4):715. [Google Scholar]
- 27.Dawe KL, Bayne EM, Boutin S. The influence of climate and human land use on the distribution of white-tailed deer, Odocoileus virginianus (Zimmerman, 1780), in the western boreal. Canadian Journal of Zoology. 2014;92:353–363. [Google Scholar]
- 28.Heffelfinger, J. R. In Biology and Management of White-Tailed Deer (ed. D. G. Hewitt) Ch. 1, 3–42 (CRC Press, Taylor & Frances Group, 2011).
- 29.Karl, T. R. & Trenberth, K. E. Modern global climate change. science302, 1719–1723 (2003). [DOI] [PubMed]
- 30.Dawe, K. L. Factors driving range expansion of white-tailed deer, Odocoileus virginianus, in the boreal forest of northern Alberta, Canada, University of Alberta, (2011).
- 31.Pickell PD, Andison DW, Coops NC, Gergel SE, Marshall PL. The spatial patterns of anthropogenic disturbance in the western Canadian boreal forest following oil and gas development. Canadian Journal of Forest Research. 2015;45:732–743. [Google Scholar]
- 32.Pickell PD, Andison DW, Coops NC. Characterizations of anthropogenic disturbance patterns in the mixedwood boreal forest of Alberta, Canada. Forest Ecology and Management. 2013;304:243–253. [Google Scholar]
- 33.Dawe KL, Boutin S, Climate change is the primary driver of white-tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecology and evolution. 2016;6:6435–6451. doi: 10.1002/ece3.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin SA. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology. 1992;73:1943–1967. [Google Scholar]
- 35.Wheatley M, Johnson C. Factors limiting our understanding of ecological scale. Ecological Complexity. 2009;6:150–159. doi: 10.1016/j.ecocom.2008.10.011. [DOI] [Google Scholar]
- 36.Burton AC, et al. Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. Journal of Applied Ecology. 2015;52:675–685. doi: 10.1111/1365-2664.12432. [DOI] [Google Scholar]
- 37.Steenweg Robin, Hebblewhite Mark, Kays Roland, Ahumada Jorge, Fisher Jason T, Burton Cole, Townsend Susan E, Carbone Chris, Rowcliffe J Marcus, Whittington Jesse, Brodie Jedediah, Royle J Andrew, Switalski Adam, Clevenger Anthony P, Heim Nicole, Rich Lindsey N. Scaling-up camera traps: monitoring the planet's biodiversity with networks of remote sensors. Frontiers in Ecology and the Environment. 2016;15(1):26–34. [Google Scholar]
- 38.MacKenzie, D. I. et al. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. (Academic Press, 2006).
- 39.Austin M. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecological modelling. 2002;157:101–118. [Google Scholar]
- 40.Maxwell SL, et al. Conservation implications of ecological responses to extreme weather and climate events. Diversity and Distributions. 2019;25:613–625. [Google Scholar]
- 41.Morellet N, et al. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within E urope. Journal of Animal Ecology. 2013;82:1326–1339. doi: 10.1111/1365-2656.12105. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber, E. & Burger, J. In Biology of marine birds 197–234 (CRC Press, 2001).
- 43.Parker, K. L., Robbins, C. T. & Hanley, T. A. Energy expenditures for locomotion by mule deer and elk. The Journal of Wildlife Management, 474–488 (1984).
- 44.Beier, P. & McCullough, D. R. Factors influencing white-tailed deer activity patterns and habitat use. Wildlife Monographs, 3–51 (1990).
- 45.Ditchkoff, S. S. In Biology and Management of White-Tailed Deer (ed. D. G. Hewitt) Ch. 2, 43–74 (CRC Press, Taylor & Francis Group, 2011).
- 46.Krebs, C. J. Ecological methodology. (Harper & Row New York, 1989).
- 47.Rota, C. T., Fletcher Jr, R. J., Dorazio, R. M. & Betts, M. G. Occupancy estimation and the closure assumption. Journal of Applied Ecology, 10.1111/j.1365-2664.2009.01734.x (2009).
- 48.Banks-Leite C, et al. Assessing the utility of statistical adjustments for imperfect detection in tropical conservation science. Journal of Applied Ecology. 2014;51:849–859. doi: 10.1111/1365-2664.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart, F. E., Fisher, J. T., Burton, A. C. & Volpe, J. P. Species occurrence data reflect the magnitude of animal movements better than the proximity of animal space use. Ecosphere 9 (2018).
- 50.MacKenzie, D. I. et al. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. (Elsevier, 2017).
- 51.Efford MG, Dawson DK. Occupancy in continuous habitat. Ecosphere. 2012;3:1–15. [Google Scholar]
- 52.Neilson, E. W., Avgar, T., Burton, A. C., Broadley, K. & Boutin, S. Animal movement affects interpretation of occupancy models from camera‐trap surveys of unmarked animals. Ecosphere9 (2018).
- 53.MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AB. Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology. 2003;84:2200–2207. [Google Scholar]
- 54.DeYoung, R. W., Miller, K. V. & Hewitt, D. White-tailed deer behavior. Biology and management of white-tailed deer, 311–351 (2011).
- 55.PRESENCE- Software to estimate patch occupancy and related parameters. (USGS-PWRC, Patuxent Wildlife Research Centre, Laurel, MD, USA., 2006).
- 56.Burnham, K. P. & Anderson, D. R. Model selection and multi-model inference: a practical information-theoretic approach. (Springer Verlag, 2002).
- 57.Long, R. A., MacKay, P., Ray, J. & Zielinski, W. Noninvasive survey methods for carnivores. (Island Press, 2008).
- 58.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 59.Faraway, J. J. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. (Chapman and Hall/CRC, 2016).
- 60.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 61.Kotliar, N. B. & Wiens, J. A. Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos, 253–260 (1990).
- 62.Fisher JT, Anholt B, Volpe JP. Body mass explains characteristic scales of habitat selection in terrestrial mammals. Ecology and Evolution. 2011;1:517–528. doi: 10.1002/ece3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamashita T, Yamashita K, Kamimura R. A stepwise AIC method for variable selection in linear regression. Communications in Statistics—Theory and Methods. 2007;36:2395–2403. [Google Scholar]
- 64.Anderson, D. R. Model based inference in the life sciences: a primer on evidence. (Springerverlag New York, 2008).
- 65.Zuur, A., Ieno, E. N. & Smith, G. M. Analysing ecological data. (Springer Science & Business Media, 2007).
- 66.Canty, A. & Ripley, B. boot: Bootstrap R (S-Plus) functions. R package version1 (2012).
- 67.Zhang, P. Model selection via multifold cross validation. The Annals of Statistics, 299-313 (1993).
- 68.DeYoung, C. A. In Biology and management of white-tailed deer 160–193 (CRC Press, 2011).
- 69.Environment Canada. Canadian Climate Normals 1981–2010 Station Data, 2015).
- 70.Maxwell SL, Fuller RA, Brooks TM, Watson JE. Biodiversity: The ravages of guns, nets and bulldozers. Nature. 2016;536:143–145. doi: 10.1038/536143a. [DOI] [PubMed] [Google Scholar]
- 71.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486:52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 72.Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 73.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends in ecology & evolution. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Lawler JJ. Climate change adaptation strategies for resource management and conservation planning. Annals of the New York Academy of Sciences. 2009;1162:79–98. doi: 10.1111/j.1749-6632.2009.04147.x. [DOI] [PubMed] [Google Scholar]
- 75.Neilson EW, Avgar T, Burton AC, Broadley K, Boutin S. Animal movement affects interpretation of occupancy models from camera-trap surveys of unmarked animals. Ecosphere. 2018;9:e02092–n/a. doi: 10.1002/ecs2.2092. [DOI] [Google Scholar]
- 76.DelGiudice GD, Mech LD, Kunkel KE, Gese EM, Seal US. Seasonal patterns of weight, hematology, and serum characteristics of free-ranging female white-tailed deer in Minnesota. Canadian Journal of Zoology. 1992;70:974–983. [Google Scholar]
- 77.Delgiudice, G. D., Mech, L. D. & Seal, U. S. Effects of winter undernutrition on body composition and physiological profiles of white-tailed deer. The Journal of wildlife management, 539–550 (1990).
- 78.Delgiudice, G. D., Riggs, M. R., Joly, P. & Pan, W. Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota. The Journal of wildlife management, 698–717 (2002).
- 79.DeYoung, C. A. In Biology and Management of White-tailed deer (ed. D. G. Hewitt) Ch. 5, 147–180 (CRC Press, Taylor & Francis Group, 2011).
- 80.Dickie, M., Serrouya, R., McNay, R. S. & Boutin, S. Faster and farther: wolf movement on linear features and implications for hunting behaviour. Journal of Applied Ecology (2016).
- 81.McKenzie HW, Merrill EH, Spiteri RJ, Lewis MA. How linear features alter predator movement and the functional response. Interface focus. 2012;2:205–216. doi: 10.1098/rsfs.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whittington J, et al. Caribou encounters with wolves increase near roads and trails: a time-to-event approach. Journal of Applied Ecology. 2011;48:1535–1542. doi: 10.1111/j.1365-2664.2011.02043.x. [DOI] [Google Scholar]
- 83.Rogala, J. K. et al. Human Activity Differentially Redistributes Large Mammals in the Canadian Rockies National Parks. Ecology and Society16, 10.5751/es-04251-160316 (2011).
- 84.Muhly TB, Semeniuk C, Massolo A, Hickman L, Musiani M. Human activity helps prey win the predator-prey space race. PloS one. 2011;6:e17050. doi: 10.1371/journal.pone.0017050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trombulak SC, Frissell CA. Review of ecological effects of roads on terrestrial and aquatic communities. Conservation biology. 2000;14:18–30. [Google Scholar]
- 86.Opdam P, Wascher D. Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biological Conservation. 2004;117:285–297. doi: 10.1016/j.biocon.2003.12.008. [DOI] [Google Scholar]
- 87.Travis J. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270:467–473. doi: 10.1098/rspb.2002.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heim Nicole, Fisher Jason T., Clevenger Anthony, Paczkowski John, Volpe John. Cumulative effects of climate and landscape change drive spatial distribution of Rocky Mountain wolverine (Gulo guloL.) Ecology and Evolution. 2017;7(21):8903–8914. doi: 10.1002/ece3.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code used in this research have been uploaded as supplementary material.