Abstract
A high percentage of subjects diagnosed with alcohol use disorder (AUD) suffer from sleeping difficulties. Lack of sleep could lead AUD patients to relapse or, sometimes, to suicide. Most of the currently prescribed medications to treat this complex problem retain a high risk of side effects and/or dependence. Therefore, the aim of the current clinical trial is to investigate the possibility of the use of a safer treatment, such as the natural health product melatonin, to treat alcohol-related sleeping problems. Sixty treatment-seeking AUD subjects were assigned to melatonin (5 mg) or placebo for 4 weeks of treatment. Change in sleeping quality which is the primary outcome of the study was assessed using the Pittsburgh sleep quality index (PSQI) scale. Linear mixed models were used to statistically analyze the difference in scores before and after 4 weeks of treatment. There was a reduction in the global PSQI score in both groups with no significant drug effect between groups. In conclusion, the use of melatonin (5 mg)/day didn’t differ from placebo in decreasing sleeping problems in a sample of AUD subjects after 4 weeks of treatment. However, higher doses are worth exploring in future research.
Subject terms: Therapeutics, Translational research
Introduction
Over 70% of subjects diagnosed with alcohol use disorder (AUD) suffer from alcohol- induced sleep problems and some studies suggest that the percentage could reach up to 91%1,2. According to The Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) disturbed sleep, or Sleep-Wake disorder, is further subdivided to insomnia, hyper- somnolence, circadian rhythm sleep-wake disorder, restless legs syndrome, narcolepsy, breathing-related sleep disorder, rapid eye movement (REM) disorder, non–rapid eye movement (NREM) sleep arousal disorders, nightmare disorder, and substance or medication-induced sleep disorder2,3.
The reasons explaining why AUD subjects would suffer from sleeping problems could be multifactorial: a genetic component, comorbid or preexisting depression or a general disturbance in the physiological wake-sleep circadian rhythm could all play a role4. Studies show that AUD subjects sleeping problems are present in all drinking stages i.e. active use, early and prolonged abstinence, and even during withdrawal5,6. Chronic alcohol use causes a decrease in the inhibitory response of GABA-A receptors accompanied by an increase in the excitatory activity of glutamate receptors7. Further, when alcohol effects wear off, AUD subjects experience an increase in sleep latency, or the time taken to fall asleep, with a decrease in total sleep duration, (circadian rhythm sleep-wake disorder); this may represent a mechanism by which tolerance to the hypnotic effects of alcohol could be explained8.
Polysomnography (PSG) studies examining sleep patterns in AUD found a persistent increase in light sleep or stage 1, a decrease in slow wave or deep sleep, an increase in vivid dreaming, and a disruption in REM sleep that could last for several months after sobriety9–11. Between 5 to 9 months of continuous abstinence is needed to normalize the time to fall asleep and sleep efficiency12,13, and more than a year is needed to restore normal sleep duration12. Disturbed sleep represents a special concern for AUD patients as it can lead to depression, cardiovascular complications, low quality of life, relapse, vehicle accidents and suicidal ideation4,5,14–17. Treated AUD subjects tend to relapse to alcohol to maintain a good sleep as a self-medication mechanism18. Therefore, no matter how successful AUD treatments are, the persistence of disturbed sleep represents a huge barrier to successful long term abstinence19.
It is not unusual for benzodiazepine receptor agonist medications to be prescribed to treat sleeping problems. Despite their well-known efficiency, benzodiazepine use is associated with impaired cognitive and psychomotor skills, increased risk of falls, dependence and abuse14. These side effects, including alcohol- induced risk of overdose and abuse potential, explains why addiction medicine practitioners avoid the use of benzodiazepines long after detoxification. Therefore, there is a real need for a safer treatment for sleeping problems, especially among patients suffering from substance use disorder (SUD)4.
Melatonin, (N-acetyl-5-methoxytryptamine), is an important hormone secreted by the pineal gland in response to darkness. It binds to melatonin receptors MT1 and MT2 in the suprachiasmatic nucleus and plays a fundamental role in the sleep-wake cycle20. Melatonin levels are usually low during the day and can reach a nocturnal peak up to 80–90 pg/ml, with some individual variability21. In normal individuals, the level of melatonin starts to increase before night time sleep, reaches its highest levels between 2:00 and 4:00 am then starts to decrease again around waking time. This peak melatonin surge is found to be blunted in AUD subjects22,23. In addition, chronic alcohol consumption alters melatonin production and functions, delays the nocturnal melatonin peak rise and decreases melatonin levels in AUD subjects12,23,24.
As a supplement melatonin has low oral bioavailability, a very short half-life (20–30 minutes), and is extensively metabolized by the liver enzymes25. On the market, melatonin is available as an over the counter dietary supplement as tablets, sublingual capsules and in liquid form. It is regulated by the FDA and Health Canada not as a medicinal drug but as a natural health product26,27. It is commonly used by people with sleep difficulties due to night shifts, jet lag, and restless leg syndrome or patients suffering from sleep-wake problems in general28. Most clinical trials show that melatonin significantly improves sleep quality, reduces sleep-onset latency period and the number of night-awakenings, as well as enhancing morning activity as assessed by validated sleep-wake questionnaires and/or PSG29,30.
Studies show that patients suffering from mood disorders, attention deficit hyperactivity disorder (ADHD), or schizophrenia, could use melatonin as an adjuvant treatment for their insomnia symptoms during acute phases of illness. In addition, the use of melatonin has proved to be helpful in the prevention of relapse among patients diagnosed with a stabilized psychiatric condition who complain of poor sleep quality31. It is not unusual to prescribe melatonin with or without other sleep medications for AUD subjects in medical institutions. However, there is currently no clear data showing efficacy of this approach. Therefore, this double- blinded randomized placebo-controlled pilot study was conducted to explore the efficacy of melatonin to treat sleeping problems in AUD subjects. To our knowledge, this is the first RCT using melatonin alone in AUD patients.
Results
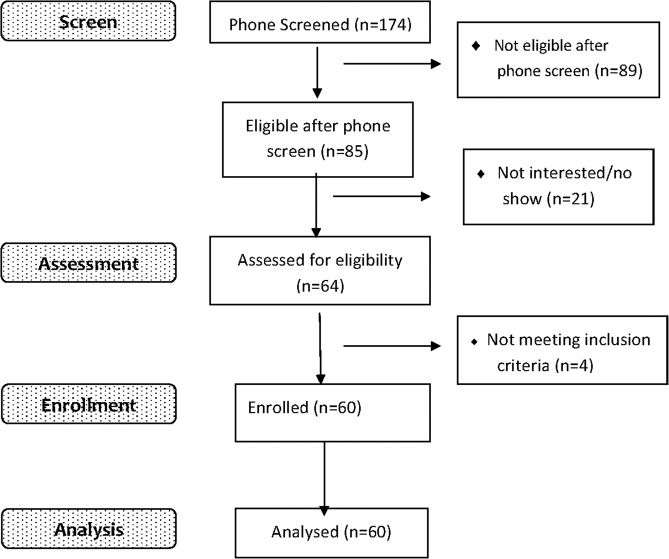
Our sample included 60 treatment-seeking AUD participants as shown in Fig. 1. All subjects were randomly allocated into the melatonin group (n = 30) or placebo group (n = 30). There were 46 males (76.7%) and 14 females (23.3%). 75% of our sample were Caucasian, 3.3% were Black/African, 1.7% Asian and the rest of the sample were from mixed races. The mean Pittsburgh sleep quality index (PSQI) score (±SD) collected at baseline was 12.33 (2.93). Mean BDI and BAI (±SD) were 17.38 (8.44) and 15.43 (11.13) at baseline, respectively. The AUDIT score at baseline was 25.83 (±8.37). Detailed demographics for each group are shown in Table 1.
Figure 1.
CONSORT Flow Chart.
Table 1.
Melatonin/ Placebo groups’ demographics.
| Demographics | Melatonin | Placebo |
|---|---|---|
| N = 30 | N = 30 | |
| Sex (n,%) | ||
| Male | 23, 76.7% | 23, 76.7% |
| Female | 7, 23.3% | 7, 23.3% |
| Age range | ||
| 19–25 | 0, 0.0% | 1, 3.3% |
| 26–40 | 10, 33.3% | 11, 36.7% |
| 41–60 | 16, 53.3% | 17, 56.7% |
| >60 | 4, 13.3% | 1, 3.3% |
| Marital status | ||
| Single | 14, 46.7% | 17, 56.7% |
| Married | 3, 10.0% | 7, 23.3% |
| Divorced/separated | 12, 40.0% | 6, 20.0% |
| Widowed | 1, 3.3% | 0, 0.0% |
| Employment | ||
| Full time (>35 hr/wk) | 4, 13.3% | 7, 23.3% |
| Short-term disability | 3, 10.0% | 3, 10.0% |
| Self-employed | 3, 10.0% | 3, 10.0% |
| Welfare | 3, 10.0% | 0, 0.0% |
| Student | 1, 3.3% | 1, 3.3% |
| Part time(<35 hr/wk) | 1, 3.3% | 4, 13.3% |
| Long-term disability | 3, 10.0% | 3, 10.0% |
| Not employed | 7, 23.3% | 10, 33.3% |
| Retired | 5, 16.7% | 0, 0.0% |
| Highest completed education | ||
| Part of high school | 0, 0.0% | 2, 6.7% |
| High school | 10, 33.3% | 6, 20.0% |
| College | 12, 40.0% | 7, 23.3% |
| University | 7, 23.3% | 14, 46.7% |
| Graduate degree | 1, 3.3% | 1, 3.3% |
| Ethnicity | ||
| Black or African American | 1, 3.3% | 1, 3.3% |
| Asian | 0, 0.0% | 1, 3.3% |
| Caucasian | 24, 80.0% | 21, 70.0% |
| Mixed races | 5, 16.7% | 7, 23.3% |
| Smokers | 16, 53.3% | 14, 46.7% |
| Non smokers | 14, 46.7% | 16, 53.3% |
| AUD severity | ||
| Mild (2-3) | 1, 3.3% | 1, 3.3% |
| Moderate (4-5) | 2, 6.7% | 1, 3.3% |
| Severe (>6) | 27, 90.0% | 28, 93.3% |
| BDI mean at baseline (±SD) | 16.30 (7.05) | 18.47 (9.64) |
| BAI mean at baseline (±SD) | 14.53 (10.08) | 16.33 (12.20) |
| AUDIT score mean at baseline (±SD) | 24.53 (8.67) | 27.13 (7.99) |
| Global PSQI score mean baseline (±SD) | 12.97 (2.28) | 11.70 (3.37) |
Main findings
The results of pill counting and self-reported study medication logs were obtained from (n = 56) as 3 participants dropped out the study and 1 participant was excluded in the middle of the study due to not following the study procedures. The results showed 75% adherence to study medication (i.e. 75% took all the pills). The results of self-reported daily use of alcohol (n = 56) showed that 78.6% (n = 44) were successful in abstaining from alcohol during the 4 weeks of the study while 12 subjects (21.4%) consumed alcohol during the study. Among those who consumed alcohol during the study (n = 6) were from the placebo group and (n = 6) subjects were from the melatonin group. Melatonin was overall well tolerated, where mild to moderate side effects were reported and resolved over the course of the treatment. Irritability (n = 1), and weakness and dizziness (n = 1) were reported in the melatonin group while daytime sleepiness (n = 1), rash (n = 1) and vomiting (n = 1) were reported in the placebo group. No severe side effects were reported at all.
Linear mixed models to analyze PSQI global score before and after treatment for both groups revealed a significant decrease over the period of the study for both treatments. However, there was no significant drug effect (Table 2; Fig. 2). PSQI subscales showed a significant time effect that was observed for both groups but no significant drug effect of melatonin was shown (Fig. 3). Anxiety and depression scores were collected before and after treatment using BAI and BDI, respectively. There was a significant decrease in BAI and BDI over time, but no significant difference between melatonin and placebo as shown in (Fig. 2; Table 2). This pilot study detected a small effect size of the results: Cohen’s h = 0.27 based on the difference in means of both groups as well as the standard deviation of the placebo group.
Table 2.
A summary of the results showing PSQI Global scores, PSQI subscales, BDI and BAI.
| Variable measure (mean + SEM) | Melatonin group N = 30 | Placebo group N = 30 | F Time*group | P value Time*group | ||
|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |||
| PSQI global score | 12.967 (0.594) | 9.242 (0.611) | 11.700 (0.594) | 8.199 (0.611) | (1,56.338) = 0.058 | 0.81 |
| Comp 1 | 2.167 (0.133) | 1.321(0.138) | 2.0 (0.133) | 1.250 (0.138) | (1,112) = 0.124 | 0.726 |
| Comp 2 | 2.300 (0.165) | 1.551 (0.170) | 2.233 (0.165) | 1.387 (0.170) | (1,55.658) = 0.130 | 0.720 |
| Comp 3 | 2.300 (0.150) | 1.464 (0.155) | 2.133 (0.150) | 1.500 (0.155) | (1,112) = 0.439 | 0.509 |
| Comp 4 | 2.600 (0.217) | 2.143 (0.224) | 2.167 (0.217) | 1.464 (0.224) | (1,112) = 0.309 | 0.579 |
| Comp 5 | 1.900 (0.116) | 1.464 (0.120) | 1.633 (0.116) | 1.393 (0.120) | (1,112) = 0.682 | 0.411 |
| Comp 7 | 1.400 (0.146) | 1.071 (0.151) | 1.533 (0.146) | 1.214 (0.151) | (1,112) = 0.001 | 0.974 |
| BDI | 16.300 (1.653) | 10.042 (1.690) | 18.467 (1.653) | 13.222 (1.690) | (1,56.368) = 0.226 | 0.637 |
| BAI | 14.533 (2.050) | 11.074 (2.097) | 16.333 (2.050) | 13.295 (2.097) | (1,55.934) = 0.024 | 0.877 |
Figure 2.
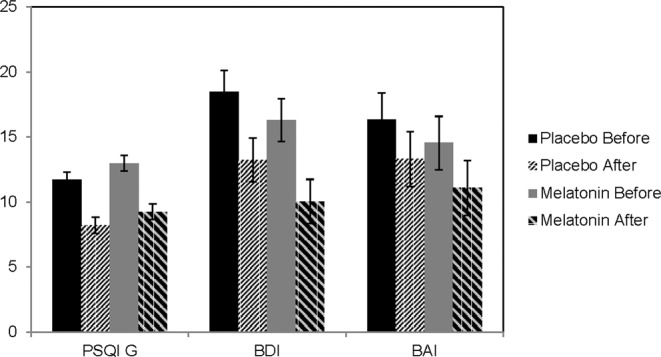
Main outcomes: PSQI, BDI, BAI scores before and after treatment expressed as Mean ± SD. All scores significantly decreased over time for the placebo group from baseline (black bars) to after treatment (2nd pattern bars). Also, the same scores decreased over time for the melatonin group from baseline (grey bars) to after treatment (4thpattern bars). No significant difference between groups was detected (p > 0.05).
Figure 3.

PSQI subscales, (components expressed as Mean ± SD), showed a significant decrease over time for the placebo group from baseline (black bars) to after treatment (2nd pattern bars). Also the scores showed a significant decrease over time for the melatonin group from baseline (grey bars) to after treatment (4thpattern bars). No significant difference between groups was detected (p > 0.05). Component # (6), which is the use of hypnotic sedative medication, is not shown in this figure as it was considered zero for this study.
A list of the concomitant medications and concurrent medical conditions assessed at baseline for all the subjects by a physician is shown in Table 3. Only (17.9%) of participants were not using anti-craving and/or antidepressant medications that would affect sleep. Further analyses of PSQI were conducted excluding all subjects using anti-craving and/or antidepressant medications. PSQI score showed a significant decrease over time with no significant melatonin effect; [F (1, 16 = 0.629); p = 0.439]. Also, secondary analysis for the subjects who maintained complete abstinence from alcohol (78.6%) during the 4 weeks of the study showed a significant time effect where PSQI decreased over time with no drug effect for melatonin [F (1, 86 = 0.031); p = 0.861].
Table 3.
Concomitant medications and medical conditions at baseline.
| Melatonin (n, %) | Placebo (n, %) | |
|---|---|---|
| Medications | ||
| Anticoagulants | (2, 6.7%) | (0, 0.0%) |
| Anticonvulsants | (4, 13.3%) | (1, 3.3%) |
| Blood pressure medications | (5, 16.7%) | (3, 10.0%) |
| Sedatives or hypnotics | (0, 0.0%) | (0, 0.0%) |
| Psychotropic medications | (20, 66.7%) | (19, 63.3%) |
| Steroids | (0, 0.0%) | (1, 3.3%) |
| Others | (12, 40.0%) | (16, 53.3%) |
| None | (5, 16.7%) | (4, 13.3%) |
| Medical condition | ||
| Asthma | (4, 13.3%) | (2, 6.7%) |
| Cardiovascular disease | (6, 20.0%) | (1, 3.3%) |
| Chronic kidney disease | (0, 0.0%) | (0, 0.0%) |
| Depression | (20, 66.7%) | (19, 63.3%) |
| Diabetes | (0, 0.0%) | (0, 0.0%) |
| Liver disease | (0, 0.0%) | (1, 3.3%) |
| Migraine | (1, 3.3%) | (3, 10.0%) |
| Seizure disorders | (1, 3.3%) | (4, 13.3%) |
| Hormonal disease | (0, 0.0%) | (0, 0.0%) |
| None | (9, 30.0%) | (7, 23.3%) |
Discussion
The current RCT studied the effect of melatonin 5 mg on sleeping problems in 60 treatment- seeking AUD subjects versus placebo for 4 weeks. PSQI global score and subscales were significantly decreased at the end of the treatment period. Nevertheless, there was no significant drug effect. Further analyses of mood showed a significant time effect where anxiety and depression scores decreased significantly after 4 weeks. However, no drug effect was observed.
Controversy about the effect of a drug is not unusual in the scientific literature. Although some previous studies showed promising effects of melatonin, other placebo-controlled trials showed different results. Our results are consistent with a study among patients diagnosed with Alzheimer’s disease (AD) where melatonin (10 mg) didn’t improve sleep quality vs placebo32. Another study among patients diagnosed with dementia didn’t show a significant effect of melatonin (6 mg) on sleep after 2 weeks of treatment33. Further, pooled data from three different RCTs in 209 AD patients suffering from sleep disturbances revealed no significant difference between melatonin and placebo34. One interpretation is that these results could be explained by a weakening of the effect of melatonin due to the presence of a mental illness that could interfere with the circadian rhythm35,36.
On the other hand, PSQI index improved significantly after 4 weeks of melatonin (3 mg) treatment compared to placebo in a study of 18 patients diagnosed with Parkinson’s disease and sleeping disturbances,. Nevertheless, PSG was not improved. The authors suggested that this discrepancy between PSQI and PSG was due to the small sample size and the complexity and variations of the PSG observations37.
In this study, all subjects were asked to maintain 2–3 weeks of alcohol abstinence before the start of the medication to ensure their adherence to the 4 weeks of abstinence during the study. Abstinence at baseline was verified by Time Line Follow Back (TLFB) for the last 2 weeks. Our sample was in early recovery from alcohol (2 to 8 weeks) at baseline. Our findings are consistent with other studies showing that the persistence of sleep disturbance during early recovery, including increased sleep onset latency, decreased sleep duration, and low sleep efficiency, contributes to the late withdrawal symptoms or the protracted abstinence and the CNS hyper-excitability found in AUD subjects after several weeks of abstinence38–41.
Initial insomnia, or difficulties initiating sleep (PSQI component # 2), and middle insomnia, or interrupted sleep, are the 2 main sleeping problems experienced by individuals with AUD1,42. Therefore, in the current study, it was hypothesized that AUD subjects would benefit from the use of melatonin, as a previous meta-analysis of 15 studies in healthy subjects(n = 1683) demonstrated that melatonin specifically reduced sleep onset latency by 3.9 minutes and enhanced overall sleep duration by 13.7 minutes43. However, in our study there was a decrease in PSQI components over time but that decrease wasn’t accompanied by a significant drug effect. This discrepancy could be due to the fact that participants in the other studies included in the meta-analysis didn’t have any medical or psychological comorbidity that could interfere with the action of melatonin on sleep. Another interpretation is that the damaging effects of alcohol on the wake-sleep cycle could not be reversed with only 4 weeks of treatment, since studies suggest that damage to sleep quality could take more than 5 to 9 months of sobriety to be improved44,45 and it could take up to 14 months to restore normal sleep duration13. This could partially explain the negative results in the present study taking into consideration the short period of abstinence. In this regard, we performed secondary analyses to examine alcohol abstinence effects on sleep. The results for subjects who maintained complete abstinence from alcohol (n = 44, 78.6%) during the 4 weeks of the study showed a significant time effect, whereby PSQI decreased over time with no drug effect (P > 0.05) (data not shown).
To our knowledge this is the first RCT using melatonin (5 mg) taken orally 1 hour before bed time for 4 weeks of treatment in AUD subjects. Our results were not consistent with the results of open-label studies that explored MT1 and MT2 synthetic agonists in AUD subjects. Ramelteon (8 mg) was used 30 minutes before bed time in an open-label study with 5 AUD participants for 4 weeks and showed a decrease in signs of insomnia according to the Insomnia Severity Index and a sleep diary45. Another open-label study showed that Agomelatine, an MT1and MT2 agonist, led to a significant improvement in PSQI score in 9 AUD subjects after 6 weeks of treatment46. However, none of these trials had a control group using placebo which therefore provides weak statistical evidence.
In our sample of patients the average AUDIT score at baseline was 25.83 (±8.37) with the majority meeting severe dependence criteria. PSQI score was high in both groups. A positive correlation between AUD severity and sleep disturbance measured by PSQI(perceived sleep quality factor, and daily disturbance factor) in non-treatment-seeking AUD subjects (N = 295) was shown previously, where higher PSQI scores were correlated with greater severity of AUD.
We hypothesized that melatonin would enhance mood and decrease anxiety more than placebo based on previous research showing that melatonin has significant anxiolytic effects47. Moreover, agomelatine, a melatonin receptor agonist, is used as anti-depressant in some countries. However, while the results of the present study showed an improvement in mood and anxiety over time, there was no significant drug effect on mood scores. These results are consistent with pooled data from eight clinical trials in patients diagnosed with mood disorders, including depression and bipolar disorder, which showed no significant mood improvement following treatment with melatonin48. This review concluded that although the use of melatonin alone did not appear to improve depressive or anxiety symptoms, a synergistic effect may be observed when melatonin is given as add-on therapy combined with other anxiolytic or antidepressant medication.
The current data, showing an improvement in mood over the course of the study irrespective of treatment condition, may reflect enhancements in sleep quality as a result of increasing duration of alcohol abstinence, which in turn may have had a positive effect on decreasing depression and anxiety scores.
Cognitive-behavioral therapy (CBT)-I, which includes cognitive therapy, sleep hygiene, sleep restriction and stimulus control49, is recommended as a 1st line treatment for insomnia in general populations50 and is thought to be effective in treating sleep difficulties in AUD subjects. However, the long therapy duration, commitment and the lack of highly trained professionals could limit CBT-I use in practice51. In this study, CBT sessions were not applied, however all subjects were provided with a sleep hygiene form during the medication visit as a helping tool to be used during the study51. Practicing sleep hygiene techniques is only likely to be successful if used as part of a daily routine, and establishing healthy sleep habits could take a long time to be achieved52,53.
While melatonin is a natural health product, Ramelteon and Tazimelteon, approved by the FDA in 2005 and 2014, respectively, are two synthetic melatonin agonists used for sleep problems. Both are characterized by a higher half-life compared to melatonin; Ramelteon T1/2 is 1 to2.6 hours and Tasimelteon T1/2 is 1.3 to 3.7 hours. In vitro research for receptor binding assays showed that Ramelteon had a greater selectivity and affinity for both MT1 and MT2 receptors and a significantly higher dissociation constant from MT receptor binding sites. Also it showed lesser affinity than melatonin to MT3, the melatonin related enzyme Quinone reductase. Clinically, Ramelteon proved to be 10 times more potent than melatonin at enhancing sleep quality. For instance, it showed better results in shortening sleep onset latency and increasing sleep duration compared to melatonin. Although studies on Tasimelteon have shown that it is a melatonin-receptor agonist at the MT1 and MT2 receptors, it shows higher affinity for the MT2 receptor, which explains its use for non-24 hour sleep-wake disorder in blind subjects. Nevertheless, both Ramelteon and Tazimelteon are much more expensive than melatonin54,55. In this study, natural melatonin was used, although it is worth investigating synthetic melatonin agonists in future research.
The negative findings of the present study are not surprising as the clinical and preclinical findings are still very controversial, but it now seems clear that melatonin likely acts only in helping to fall asleep rather than in increasing sleep time. This could be explained by the very short half-life of melatonin and also by the contrasting/complementary role of melatonin receptors on sleep phases. Preclinical studies showed that MT1 selective stimulation increased rapid eye movement (REM) sleep while MT2 receptors increased non-rapid eye movement (NREM) sleep highlighting an opposite role of both receptors56. Further research investigating melatonergic receptors and different ligands is needed in order to clarify the specific role of each ligand.
There are some limitations of the current study. For instance, only one dose (5 mg) of fast dissolving melatonin tablets was used. It would therefore be worthwhile to explore higher doses and an extended release formulation of melatonin. In addition, in this study melatonin was used as single treatment, while in practice melatonin is prescribed as an adjuvant drug to be taken in combination with other sleeping pills. Therefore, it is worth exploring the different effects on sleep quality when melatonin is used alone versus in conjunction with other sleep aids.
Further limitations of the study are that medication adherence was verified by self-report and pill count at the end of the study, whereas weekly verification may have reduced recall bias; the timing of melatonin administration was not objectively assessed - future research could make use of smart pill bottle technology to record the time of the pill taken; and finally sleep was not objectively assessed and therefore future research would benefit from the use of daily sleep diaries, actigraphy or polysomnography, and assessment of blood levels of melatonin.
In conclusion, it is quite common for AUD patients to relapse to drinking alcohol to self-medicate their sleeping problems15. Therefore, it is worth conducting further studies with melatonin using different doses and meticulous follow up in order to find a safe strategy for the treatment of alcohol-related sleeping problems to prevent relapse. The results of the current study showed no significant effect of 4 weeks oral melatonin (5 mg) on attenuation of sleep problems in treatment-seeking AUD subjects.
Methods
Study design
This study is a double blind randomized placebo-controlled trial with two arms. Sixty outpatient treatment-seeking subjects diagnosed with AUD and suffering from sleeping problems were recruited from Addiction Medical Service clinic and other clinics at the Center for Addiction and Mental Health (CAMH) using study posters and staff referrals. Recruitment started January 2017 and the study was closed January 2019. Informed consent was obtained from all individual participants included in the study. Sleeping quality, which is the primary outcome of the study, was assessed using PSQI score which is a validated questionnaire formed of 7 components57. The PSQI is widely used by sleep specialists to evaluate sleep disturbance as a diagnostic tool for sleep problems, and is used in research for assessment of treatment outcomes37,46. The scale is formed of 19 self-rated questions and another 5 questions answered by the bed partner or roommate if present. The scoring of the PSQI is dependent on the 19 self-rated questions, the 5 other questions are used for clinical evaluation purposes only. The 7 components of the questionnaires are each scored on a 0–3 scale. The sum of all 7 component scores forms the global PSQI score, which ranges from 0-21; the higher the score the worse the sleep quality. For this project, component # 6, which records the number of times a sleeping aid was used during the past month, was scored as zero because this overlapped with one of the exclusion criteria. Usually, a PSQI score higher than 5 indicate a sleep problem57. The inclusion criteria for this study were: aged19 years or older, PSQI > 5, meet DSM-5criteria for AUD, not taking a benzodiazepine receptor agonist or any other sleeping pills during the past month. Any subject not fulfilling the inclusion criteria or pregnant, (as verified by a urine test), were excluded from the study. All subjects were assigned randomly to either melatonin (5 mg) or placebo arms. The melatonin tablets used were: Nature’s bounty Melatonin (5 mg), NPN: 80033974, fast dissolving tablets. Study approvals were obtained from The Center for Addiction and Mental Health Research Ethics Board (REB) following all relevant guidelines and regulations (Protocol ID: 099-2016). The study was registered with the clinical studies database ClinialTrials.gov (NCT03043443, registration date: 06/02/2017).
Demographics were collected from all subjects at baseline, including: contact information, concomitant medication, Time Line Follow Back (TLFB)58 for the past 2 weeks regarding the use of nicotine, alcohol drinks/day, caffeine, cannabis and other substances. A single alcohol drink serving contains about 14 grams of ethanol or “pure” alcohol which could be 12 oz. of beer (about 5% alcohol), 8-9 oz. of malt liquor (about 7% alcohol), 1.5 oz. of hard liquor (about 40% alcohol), or 5 oz. of wine (about 12% alcohol). Fagerstrom test for nicotine dependence (FTND)58, Beck Depression Inventory (BDI)59, Beck Anxiety Inventory (BAI)60, AUD criteria according to DSM-561, and Alcohol use disorder identification test (AUDIT)62–63 were also collected at baseline.
After verifying their eligibility, participants were randomized. Subsequently they picked up the medication (Melatonin or placebo), provided in a blister pack with instructions and a sleep hygiene document, and were instructed to take 1 pill every night 1 hour before bedtime for 4 weeks. PSQI score was measured again after 4 weeks of treatment. An online survey was sent after 2 weeks and after 4 weeks of treatment to monitor side effects along with other questionnaires (TLFB, BDI, BAI). All participants were asked to bring back the medication blister pack to do a pill count and check all the missing pills. In this study, participants were compensated for their participation over the course of the study with $85; $20 was provided at the assessment visit and the remainder of the compensation was provided after completion of all the visits and the surveys. All study medications (Melatonin and placebo) were dispensed by the CAMH pharmacy that was also responsible for all randomization as well as blinding procedures (for both researchers and subjects).
The sample size was calculated based on Mixed Effect Models and significance tests, where all tests were two-sided, using a confidence level of 0.05 with power fixed at 80%.
Data analyses
Our primary outcome measure was sleeping problems measured using the PSQI global score at baseline and after 4 weeks of treatment. Secondary outcome measures included the subscales of PSQI which are: (1) subjective quality of sleep; (2) sleep onset latency; (3) sleep duration; (4) sleep efficiency; (5) presence of sleep disturbances; and (7) presence of daytime disturbances, as an indication of daytime alertness. Also, BDI and BAI scores before and after treatment were considered as secondary outcomes. Linear mixed models with subjects as random effects were used to analyze the outcomes. An interaction between time (pre/post treatment) and treatment groups (melatonin/placebo) was used to investigate difference in change in PSQI global score as well as sub-scores, BDI and BAI scores between study groups. All analyses were conducted using SPSS v.24. Associations with p-values of less than 0.05 were considered statistically significant, and all tests were 2-sided. Both sex and age were controlled for in the analysis to limit confounding by these variables.
Study procedures
All the study procedures and methods as well as the study approvals were obtained from The Center for Addiction and Mental Health Research Ethics Board (REB) following all relevant guidelines and regulations (Protocol ID: 099-2016). Also, a detailed description of the study is found in ClinialTrial.gov website ID# NCT03043443/ (registration date: 06/02/2017).
Acknowledgements
We would like to acknowledge the support of CAMH Addiction Medical Service staff and the Translational Addiction Research Laboratory staff for their support. We would like also to extend our thanks to Dr. Leanne Trick for her efforts in reviewing the manuscript. This study was funded by Center of addiction and mental health (CAMH) funds.
Author contributions
M.G. and B.L.F. designed the project procedures. D.L., J.S. and B.L.F. conducted the medical examination; M.G. conducted the clinical trial, subject recruitment, data analysis, manuscript preparation and analyzed the results. All authors reviewed the manuscript. B.L.F. secured funding and provided supervision over the project.
Data availability
All additional data, research protocol, and information on materials used in this investigation will be made readily available upon request as allowed by the governing review boards of the involved research institutions.
Competing interests
Dr. Le Foll has/will received some in-kind donation of cannabis product from Canopy and Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. Dr. Le Foll has/will perform research with industry funding obtained from Canopy, Bioprojet, ACS and Alkermes. Dr. Le Foll has received in kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. The other authors declared no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhabenko N, Wojnar M, Brower KJ. Prevalence and correlates of insomnia in a polish sample of alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2012;36:1600–1607. doi: 10.1111/j.1530-0277.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int. J. Mol. Sci. 2014;15:15924–15950. doi: 10.3390/ijms150915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds CF, 3rd, O’Hara R. DSM-5 sleep-wake disorders classification: overview for use in clinical practice. Am. J. Psychiatry. 2013;170:1099–1101. doi: 10.1176/appi.ajp.2013.13010058. [DOI] [PubMed] [Google Scholar]
- 4.Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49:417–427. doi: 10.1016/j.alcohol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol. Clin. Exp. Res. 1999;23:1044–1051. doi: 10.1111/j.1530-0277.1999.tb04223.x. [DOI] [PubMed] [Google Scholar]
- 6.Brower KJ, Perron BE. Prevalence and correlates of withdrawal-related insomnia among adults with alcohol dependence: results from a national survey. Am. J. addictions. 2010;19:238–244. doi: 10.1111/j.1521-0391.2010.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knapp CM, Ciraulo DA, Datta S. Mechanisms underlying sleep-wake disturbances in alcoholism: focus on the cholinergic pedunculopontine tegmentum. Behavioural brain Res. 2014;274:291–301. doi: 10.1016/j.bbr.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addiction Sci. Clin. Pract. 2016;11:9. doi: 10.1186/s13722-016-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gann H, et al. Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol. psychiatry. 2001;50:383–390. doi: 10.1016/S0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- 10.Gillin JC, et al. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch. Gen. psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- 11.Colrain IM, Turlington S, Baker FC. Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep. 2009;32:1341–1352. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol. Clin. Exp. Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- 13.Williams HL, Rundell OH., Jr. Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol. Clin. Exp. Res. 1981;5:318–325. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 14.Pigeon WR, Bishop TM, Marcus JA. Advances in the management of insomnia. F1000prime Rep. 2014;6:48. doi: 10.12703/P6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am. J. Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr. psychiatry Rep. 2015;17:554. doi: 10.1007/s11920-015-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaghan RC, et al. Alcohol- or drug-use disorders and motor vehicle accident mortality: a retrospective cohort study. Accident; Anal. Prev. 2013;53:149–155. doi: 10.1016/j.aap.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J. Clin. sleep. medicine: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2007;3:24–32. [PubMed] [Google Scholar]
- 19.Miller MB, Donahue ML, Carey KB, Scott-Sheldon LAJ. Insomnia treatment in the context of alcohol use disorder: A systematic review and meta-analysis. Drug. alcohol. dependence. 2017;181:200–207. doi: 10.1016/j.drugalcdep.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doghramji K. Melatonin and its receptors: A new class of sleep-promoting agents. J. Clin. Sleep. Med. 2007;3:S17–S23. doi: 10.5664/jcsm.26932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS one. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou JN, Liu RY, van Heerikhuize J, Hofman MA, Swaab DF. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J. pineal Res. 2003;34:11–16. doi: 10.1034/j.1600-079X.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol. psychiatry. 2003;54:1437–1443. doi: 10.1016/S0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 24.Peres R, et al. Ethanol consumption and pineal melatonin daily profile in rats. Addiction Biol. 2011;16:580–590. doi: 10.1111/j.1369-1600.2011.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan V, et al. Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Therapeutic Adv. neurological Disord. 2011;4:297–317. doi: 10.1177/1756285611406166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCIH. Melatonin: In Depth, https://nccih.nih.gov/health/melatonin (2015).
- 27.MayoClinic. Drugs and Supplements Melatonin (N-acetyl-5-methoxytryptamine), http://www.mayoclinic.org/drugs-supplements/melatonin/background/hrb-20059770?p=1 (2013).
- 28.Herxheimer, A. & Petrie, K. J. Melatonin for the prevention and treatment of jet lag. The Cochrane database of systematic reviews, CD001520, 10.1002/14651858.CD001520 (2002). [DOI] [PubMed]
- 29.Suresh Kumar PN, Andrade C, Bhakta SG, Singh NM. Melatonin in schizophrenic outpatients with insomnia: a double-blind, placebo-controlled study. J. Clin. psychiatry. 2007;68:237–241. doi: 10.4088/jcp.v68n0208. [DOI] [PubMed] [Google Scholar]
- 30.Asayama K, et al. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J. Nippon. Med. Sch. 2003;70:334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- 31.Geoffroy PA, Micoulaud Franchi JA, Lopez R, Schroder CM. & membres du consensus Melatonine, S. The use of melatonin in adult psychiatric disorders: Expert recommendations by the French institute of medical research on sleep (SFRMS) L’Encephale. 2019;45:413–423. doi: 10.1016/j.encep.2019.04.068. [DOI] [PubMed] [Google Scholar]
- 32.Gehrman PR, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. The. Am. J. geriatric psychiatry: Off. J. Am. Assoc. Geriatric Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int. J. geriatric psychiatry. 2002;17:1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 34.McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in Alzheimer’s disease. Cochrane database Syst. reviews, CD009178. 2014 doi: 10.1002/14651858.CD009178.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Sivertsen B, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom. Med. 2012;74:758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 36.Jansson-Frojmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 2008;64:443–449. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros CA, et al. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J. Neurol. 2007;254:459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- 38.Begleiter H. Brain dysfunction and alcoholism: problems and prospects. Alcohol. Clin. Exp. Res. 1981;5:264–266. doi: 10.1111/j.1530-0277.1981.tb04900.x. [DOI] [PubMed] [Google Scholar]
- 39.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alling C, et al. Studies on duration of a late recovery period after chronic abuse of ethanol. A cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr. Scand. 1982;66:384–397. doi: 10.1111/j.1600-0447.1982.tb06720.x. [DOI] [PubMed] [Google Scholar]
- 41.Kolla BP, et al. The course of sleep disturbances in early alcohol recovery: an observational cohort study. Am. J. addictions. 2014;23:21–26. doi: 10.1111/j.1521-0391.2013.12056.x. [DOI] [PubMed] [Google Scholar]
- 42.Curry SJ, Keller PA, Orleans CT, Fiore MC. The role of health care systems in increased tobacco cessation. Annu. Rev. public. health. 2008;29:411–428. doi: 10.1146/annurev.publhealth.29.020907.090934. [DOI] [PubMed] [Google Scholar]
- 43.Brzezinski A, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep. Med. Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addiction Biol. 2003;8:455–462. doi: 10.1080/13556210310001646439. [DOI] [PubMed] [Google Scholar]
- 45.Brower KJ, Conroy DA, Kurth ME, Anderson BJ, Stein MD. Ramelteon and improved insomnia in alcohol-dependent patients: a case series. J. Clin. sleep. medicine: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2011;7:274–275. doi: 10.5664/JCSM.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosshans M, Mutschler J, Luderer M, Mann K, Kiefer F. Agomelatine is effective in reducing insomnia in abstinent alcohol-dependent patients. Clin. Neuropharmacol. 2014;37:6–8. doi: 10.1097/WNF.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 47.Acil M, et al. Perioperative effects of melatonin and midazolam premedication on sedation, orientation, anxiety scores and psychomotor performance. Eur. J. anaesthesiology. 2004;21:553–557. doi: 10.1097/00003643-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 48.De Crescenzo F, et al. Melatonin as a treatment for mood disorders: a systematic review. Acta Psychiatr. Scand. 2017;136:549–558. doi: 10.1111/acps.12755. [DOI] [PubMed] [Google Scholar]
- 49.Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J. addictive Dis. 2007;26:41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep. Med. Rev. 2003;7:215–225. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- 51.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–1132. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaku A, et al. Randomized controlled trial on the effects of a combined sleep hygiene education and behavioral approach program on sleep quality in workers with insomnia. Ind. health. 2012;50:52–59. doi: 10.2486/indhealth.MS1318. [DOI] [PubMed] [Google Scholar]
- 53.Taylor DJ, Schmidt-Nowara W, Jessop CA, Ahearn J. Sleep restriction therapy and hypnotic withdrawal versus sleep hygiene education in hypnotic using patients with insomnia. J. Clin. sleep. medicine: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2010;6:169–175. [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J. Clin. sleep. medicine: JCSM: Off. Publ. Am. Acad. Sleep. Med. 2008;4:456–461. [PMC free article] [PubMed] [Google Scholar]
- 55.Johnsa JD, Neville MW. Tasimelteon: a melatonin receptor agonist for non-24-hour sleep-wake disorder. Ann. pharmacotherapy. 2014;48:1636–1641. doi: 10.1177/1060028014550476. [DOI] [PubMed] [Google Scholar]
- 56.Gobbi G, Comai S. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. J. pineal Res. 2019;66:e12544. doi: 10.1111/jpi.12544. [DOI] [PubMed] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug. alcohol. dependence. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 59.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. personality Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 60.Steer RA, Rissmiller DJ, Ranieri WF, Beck AT. Structure of the computer-assisted Beck Anxiety Inventory with psychiatric inpatients. J. personality Assess. 1993;60:532–542. doi: 10.1207/s15327752jpa6003_10. [DOI] [PubMed] [Google Scholar]
- 61.Grant BF, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saunders JB, Aasland OG, Babor TF. e. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88:791–803. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 63.Hartwell EE, Bujarski S, Glasner-Edwards S, Ray LA. The Association of Alcohol Severity and Sleep Quality in Problem Drinkers. Alcohol. Alcohol. 2015;50:536–541. doi: 10.1093/alcalc/agv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All additional data, research protocol, and information on materials used in this investigation will be made readily available upon request as allowed by the governing review boards of the involved research institutions.