Abstract
Hyperuricemia is a risk factor for cardiovascular metabolic diseases. However, in the very elderly, the relationship between hyperuricemia and the metabolic syndrome (MetS) is not yet clear. This study was aimed to investigate the potential association between hyperuricemia and MetS in community very elderly in Chengdu. In this cross-sectional study, 1056 very elderly in the community were enrolled. Serum uric acid (SUA), fast plasma glucose, triglycerides and high–density lipoprotein cholesterol were measured, and then MetS components were calculated. Logistic regression models were used to explore risk factors for MetS in the very elderly. Finally, 1035 participants were included in analysis whose ages ranged between 80 and 100 with a mean age of 83.6 ± 3.4 years. The mean SUA level was 356.2 ± 95.0 µmol/L. The estimated prevalence of MetS in the very elderly was 25.0% vs. 21.6% (international diabetes federation (IDF) criteria vs. Chinese guideline), which was significantly higher for women (IDF criteria:17.3% in men vs 33.6% in women, p < 0.001). Logistic regression has found that participants with hyperuricemia (SUA level > 416 µmol/L in men and > 357 µmol/L in women) had a higher risk (IDF criteria: odds ratio (OR): 2.136, 95% confidence interval(CI): 1.525–2.993, p < 0.001. Chinese guideline: OR: 1.769, 95%CI: 1.249–2.503, p = 0.001) of MetS in very elderly Chinese. MetS is common in the community of very elderly Chinese in Chengdu. Hyperuricemia is associated with MetS in general very elderly and lifestyle changing should also be considered in the very elderly.
Subject terms: Cardiovascular diseases, Metabolic syndrome
Introduction
As a worldwide epidemic clinical syndrome nowadays, metabolic syndrome (MetS) is characterized by co-occurrence of abdominal obesity, hyperglycemia, dyslipidemia, and hypertension. It has been shown that MetS is a well-known risk factor for Type 2 diabetes mellitus (DM), and atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality, resulting in an enormous economic burden to society1. A national survey has reported that the prevalence of MetS in Chinese aged from 35 to 74 years was about 10% and 18% in men and women, respectively, in 2000–20012. However, the prevalence of MetS is increasing with social-economic growth and undergoing lifestyle transition. Another recent national survey has demonstrated that the prevalence of MetS in Chinese older than 18 years was 31.0% in men and 36.8% in women3. Meanwhile, China has become an aging society with a proportion of elderly of about 1.6% in 2010 accompanying with great economic achievements. Since ASCVD has already become the first disease burden in China4, MetS with an increasing prevalence has thus become a big public health challenge to prevent ASCVD in China.
Serum uric acid (SUA) is the end product of purine metabolism in humans, which has been reported as a risk factor for hypertension5,6, atrial fibrillation7,8, acute myocardial infarction9, ischemic heart disease10, and stroke11. Although numerous studies12–15 have demonstrated the association between SUA level and MetS in young, middle-aged and elderly population, little is known about the association between them in the very elderly. Moreover, this group has not usually been included in epidemiological or clinical studies, even less as a unique study group. Therefore, this study aimed to investigate the potential association between hyperuricemia and MetS in the very elderly in Southwest China.
Methods
Subjects
This cross-sectional study was designed to detect the cardiovascular and metabolic risk factors in the general community of the very elderly population (≥80 years old) in Chengdu, which was described elsewhere16. From 2013 to 2015, a representative sample of the community very elderly was recruited using of a stratified three-stage cluster sampling design. A total of 1056 very elderly from 20 residential communities were taken according to the registration data from local government16. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Ethics Committee of the Second People’s Hospital of Chengdu. Moreover, all participants gave their informed consent.
Data collection and measurement
Previously well-trained physicians and nurses collected data from the participants with a questionnaire-based face to face interview during clinical visits, which was described elsewhere16. Basic demographic characteristics, past medical history and lifestyle risk factors of participants were obtained from a standardized questionnaire during the interview with participating investigators. The body mass index (BMI) was defined as weight in kilograms divided by the square of the height in meters. The blood pressure (BP) was measured three times in a sitting position by using a standardized automatic electronic sphygmomanometer (HEM-7300, Omron, Kyoto, Japan) and average values were calculated and used in the analysis. Fasting blood samples were collected in the morning after at least 8 hours of fasting for all participants. SUA and other biochemical parameters, such as fast plasma glucose(FPG), total cholesterol(TC), triglycerides(TG), and low–density lipoprotein cholesterol(LDL-C) were analyzed enzymatically on an auto-analyzer (AU5421 Chemistry Analyzer, Beckman, Brea, California, United States) in the central laboratory.
Finally, the estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation modified for the Chinese population: eGFR = 186 × serum creatinine−1.154 × Age−0.203 × 0.742 (if female).
Definitions
hyperuricemia was defined as SUA level >416 µmol/L (7.0 mg/dL) in men and >357 µmol/L (6.0 mg/dl) in women17.
MetS, according to the Consensus Worldwide Definition from the international diabetes federation (IDF)18 and the Chinese guideline for dyslipidemia management19, was defined as follows, respectively:
MetS defined by IDF: abdominal obesity with ethnic-specific waist circumference (WC) cut-points (for Chinese: ≥ 90 cm in men and ≥ 80 cm in women) and fulfills two items of the following: TG ≥ 150 mg/dL (1.7 mmol/L) or treatment for hypertriglycerides, HDL cholesterol <40 mg/dL (1.03 mmol/L) in men or <50 mg/dL (1.29 mmol/L) in women or treatment for low HDL, FPG ≥ 100 mg/dL (5.6 mmol/L) or previously diagnosed type 2 diabetes, and BP ≥ 130/85 mmHg or treatment for hypertension.
MetS defined in the Chinese guideline: MetS should fulfill any three or more of the following criteria: abdominal obesity (WC ≥ 90 cm in men and ≥85 cm in women), fasting TG ≥ 150 mg/dL (1.7 mmol/L), fasting HDL-C < 40 mg/dL (1.0 mmol/L), FPG ≥ 110 mg/dL (6.10 mmol/L) or a 2 hour blood glucose after glycemic load ≥140 mg/dL (7.80 mmol/L) or anti-diabetic treatment, and BP ≥ 130/85 mmHg or anti-hypertensive treatment.
Statistical analysis
SPSS software (Version 22.0, SPSS Inc, Chicago, IL) were used to performed the statistical analyses. Continuous variables with normal distribution are expressed as mean ± standard deviation and skewed continuous variables expressed as median (interquartile range). Frequencies are presented as percentages with 95% confidence interval (95%CI). ANOVA or Kruskal-Wallis test was used to compare the continuous variables between groups, whereas x2 test was applied to compare frequencies. Logistic regression models were used to evaluate the potential association between hyperuricemia and MetS. The receiver operating characteristic (ROC) curve analysis was employed to evaluate the efficiency of SUA level in predicting MetS. A two-sided p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 1035 participants with a mean age of 83.6 ± 3.4 years were enrolled in the final statistical analysis, of which 52.6% were men with a mean age of 83.6 ± 3.3 years. The overall mean SUA level was 356.2 ± 95.0 µmol/L with a mean level of 374.2 ± 94.5 µmol/L in men and 335.2 ± 89.3 µmol/L in women (p < 0.001). According to both sets of criteria explained above, participants with MetS had a significantly higher mean SUA level (IDF criteria: 375.8 ± 90.8 vs. 349.5 ± 95.6 µmol/L, p < 0.001; Chinese guideline: 375.2 ± 88.8 vs. 350.7 ± 96.0 µmol/L, p < 0.001). The overall mean level of eGFR was 58.4 ± 13.9 ml/(min∙1.73m2) (men vs. women: 61.8 ± 14.1 vs. 54.6 ± 12.6 ml/(min∙1.73m2), p < 0.001). As shown in Table 1, participants with more MetS components were more likely to be abdominal obese with higher levels of WC, BMI, SBP, DBP, FBG, TC, TG, LDL and SUA, but a lower level of HDL.
Table 1.
Characteristics of community very elderly Chinese in Chengdu.
| Number of MetS components | p value | ||||
|---|---|---|---|---|---|
| 0 (n = 72) | 1 (n = 328) | 2 (n = 348) | ≥ 3 (n = 287) | ||
| Age (yrs) | 83.9 ± 3.3 | 83.7 ± 3.5 | 83.6 ± 3.4 | 83.6 ± 3.4 | 0.551 |
| Current smoker, n(%) | 7(9.7) | 42(12.8) | 34(9.8) | 31(10.8) | 0.624 |
| Current drinker, n(%) | 4(5.6) | 27(8.2) | 30(8.6) | 25(8.7) | 0.844 |
| Medical history, n(%) | |||||
| Hypertension | 16(22.2) | 152(46.3) | 200(57.5) | 178(62.0) | <0.001 |
| DM | 2(2.8) | 28(8.5) | 59(17.0) | 88(30.7) | <0.001 |
| Abdominal obesity | 0(0) | 46(14.0) | 235(67.5) | 257(89.5) | <0.001 |
| Medication, n(%) | |||||
| Antihypertensive | 11(15.3) | 129(39.3) | 170(48.9) | 160(55.7) | <0.001 |
| Antidiabetic | 1(1.4) | 18(5.5) | 41(11.8) | 70(24.4) | <0.001 |
| Lipid lowering | 1(1.4) | 22(6.7) | 32(9.2) | 31(10.8) | 0.040 |
| Diuretics | 1(1.4) | 20(6.1) | 26(7.5) | 21(7.3) | 0.237 |
| WC (cm) | 76.9 ± 6.9 | 80.0 ± 9.2 | 89.4 ± 9.0 | 94.1 ± 8.0 | <0.001 |
| BMI (kg/m2) | 19.9 (17.5,21.9) | 20.7 (18.9,23.1) | 23.5 (21.6,25.9) | 24.8 (22.9,27.3) | <0.001 |
| SBP (mmHg) | 120.0 (110.0,123.0) | 142.0 (129.0,157.0) | 148.0 (136.5,164.0) | 151.5 (140.0,166.8) | <0.001 |
| DBP (mmHg) | 69.0 (62.0,72.5) | 73.0 (64.0,81.0) | 74.0 (67.0,82.3) | 76.0 (68.0,83.0) | <0.001 |
| FBG (mmol/L) | 4.80 (4.42,5.24) | 4.90 (4.50,5.27) | 5.10 (4.67,5.80) | 6.00 (5.22,7.56) | <0.001 |
| TC (mmol/L) | 4.64 (4.22,5.23) | 4.77 (4.08,5.36) | 4.94 (4.21,5.56) | 4.88 (4.21,5.66) | 0.018 |
| TG (mmol/L) | 1.04 (0.84,1.30) | 0.99 (0.77,1.27) | 1.17 (0.89,1.48) | 1.76 (1.21,2.29) | <0.001 |
| LDL (mmol/L) | 2.42 (1.98,2.79) | 2.44 (1.95,2.93) | 2.57 (2.06,3.05) | 2.69 (2.26,3.26) | <0.001 |
| HDL (mmol/L) | 1.67 (1.45,1.92) | 1.65 (1.40,1.96) | 1.59 (1.34,1.93) | 1.27 (1.09,1.54) | <0.001 |
| SUA (µmol/L) | 324 (275.8,412.3) | 334.5 (287.8,401.5) | 344.0 (281.0,400.3) | 373.0 (313.0,440.8) | <0.001 |
| Creatinine (μmol/L) | 98.0 (87.0,115.5) | 97.5 (88.8,113.0) | 98.0 (86.0,114.8) | 97.5 (87.0,115.0) | 0.225 |
| e GFR, ml/(min∙1.73m2) | 59.0 (43.0,73.2) | 59.4 (51.0,69.3) | 57.5 (48.9,64.9) | 55.4 (46.1,65.0) | 0.467 |
Data are expressed as mean ± standard deviation for normal distributed continuous variables, median (interquartile range) for skewed continuous variables, or number (percentage) for categorical variables. BMI: body mass index; DBP: diastolic blood pressure; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SUA: serum uric acid; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; WC: Waist circumference.
Estimated prevalence of MetS and its components
The overall estimated prevalence of MetS in the very elderly was 25.0% (17.3% in men vs. 33.6% in women, p < 0.001) according to the IDF criteria, which was significantly higher (p < 0.001) than that of the Chinese guideline (21.6%, 17.8% in men vs. 25.8% in women, p = 0.002). Table 2 describes the prevalence of its components.
Table 2.
Estimated prevalences of metabolic syndrome and its components in community very elderly Chinese in Chengdu.
| IDF criteria | Chinese guideline | |||||
|---|---|---|---|---|---|---|
| Men | Women | p value | Men | Women | p value | |
| MetS (%) | 95 (17.3) | 165 (33.6) | <0.001 | 97 (17.8) | 127 (25.8) | 0.002 |
| Abdominal obesity (%) | 243 (44.5) | 375 (76.2) | <0.001 | 243 (44.5) | 307 (62.4) | <0.001 |
| High blood pressure (%) | 432 (79.1) | 398 (80.9) | 0.484 | 432 (79.1) | 398 (80.9) | 0.484 |
| Hypertriglyceridemia (%) | 106 (19.4) | 127 (25.8) | 0.013 | 106 (19.4) | 127 (25.8) | 0.013 |
| Low HDL cholesterol (%) | 49 (9.0) | 106 (21.5) | <0.001 | 48 (8.8) | 23 (4.7) | 0.012 |
| Hyperglycemia (%) | 191 (34.9) | 154 (31.3) | 0.233 | 135 (24.7) | 109 (22.2) | 0.371 |
HDL: high-density lipoprotein,IDF: international diabetes federation, MetS: metabolic syndrome.
Estimated prevalence of MetS in the very elderly with hyperuricemia
The estimated prevalence of MetS in participants with hyperuricemia was 39.4% vs. 32.9% (p < 0.001) according to the IDF and Chinese guideline, respectively. The estimated prevalence of MetS in very elderly women with hyperuricemia was significantly higher than it was in men according to both the IDF criteria (66.9% vs. 33.1%, p < 0.001) and the Chinese guideline (39.3% vs. 26.6%, p = 0.016).
Relationship between hyperuricemia and MetS
After adjusting for sex, BMI, low-density lipoprotein, eGFR, cigarette smoking, total cholesterol, low-density lipoprotein cholesterol, alcohol consumption and medications for hypertension, DM, and hyperlipidemia, the logistic regression analysis of all cases (Table 3) demonstrated that hyperuricemia was an independent factor for MetS according to both the IDF criteria (odds ratio (OR): 2.136, 95%CI: 1.525–2.993, p < 0.001) and the Chinese guideline (OR: 1.769, 95%CI: 1.249–2.503, p = 0.001) in overall participants (Table 3). Moreover, the same finding was also found between SUA level and MetS for both the IDF criteria (OR: 1.342, 95%CI: 1.102–2.305, p < 0.001) and the Chinese guideline (OR: 1.215, 95%CI: 1.109–2.304, p < 0.001). Also, in subgroup analysis, hyperuricemia is associated with MetS both in very elderly women (OR: 2.324, 95%CI: 1.500–3.601, p < 0.001) and in men (OR: 1.920, 95%CI: 1.128–3.267, p = 0.016). It is noteworthy mentioning that both SUA level and hyperuricemia were also associated with two components of MetS, which were abdominal obesity and hypertriglyceridemia (Table 3).
Table 3.
Association between hyperuricaemia and metabolic syndrome using logistic regression models in community very elderly Chinese in Chengdu.
| Serum uric acid OR (95% CI) | Hyperuricemia OR (95% CI) | |
|---|---|---|
| Metabolic syndrome (IDF criteria) | 1.342 (1.102–2.305)* | 2.136 (1.525–2.993)* |
| Metabolic syndrome component | ||
| Abdominal Obesity | 1.320 (1.151–2.412)* | 1.833 (1.204–2.792)* |
| High blood pressure | 1.120 (0.898–1.503) | 0.953 (0.615–1.477) |
| Hypertriglyceridemia | 1.303 (1.122–2.205)* | 1.843 (1.301–2.612)* |
| Low HDL cholesterol | 1.352 (1.182–2.967)* | 1.974 (1.292–3.017)* |
| Hyperglycemia | 1.242 (1.156–1.881)* | 1.330 (0.931–1.901) |
| Metabolic syndrome (Chinese guideline) | 1.215 (1.109–2.304)* | 1.769 (1.249–2.503)* |
| Metabolic syndrome component | ||
| Abdominal Obesity | 1.306 (1.138–2.326)* | 1.550 (1.052–2.284)* |
| High blood pressure | 1.120 (0.898–1.503) | 0.953 (0.615–1.477) |
| Hypertriglyceridemia | 1.303 (1.122–2.205)* | 1.843 (1.301–2.612)* |
| Low HDL cholesterol | 1.121 (0.797–1.304) | 1.706 (0.566–2.046) |
| Hyperglycemia | 1.102 (0.898–1.602) | 1.114 (0.754–1.647) |
Adjusted for sex, body mass index, low-density lipoprotein, eGFR, cigarette smoking, total cholesterol, low-density lipoprotein, alcohol consumption and medications for hypertension, diabetes mellitus, and hyperlipidemia. eGFR: estimated glomerular filtration rate. HDL: high-density lipoprotein, IDF: international diabetes federation. *p < 0.05.
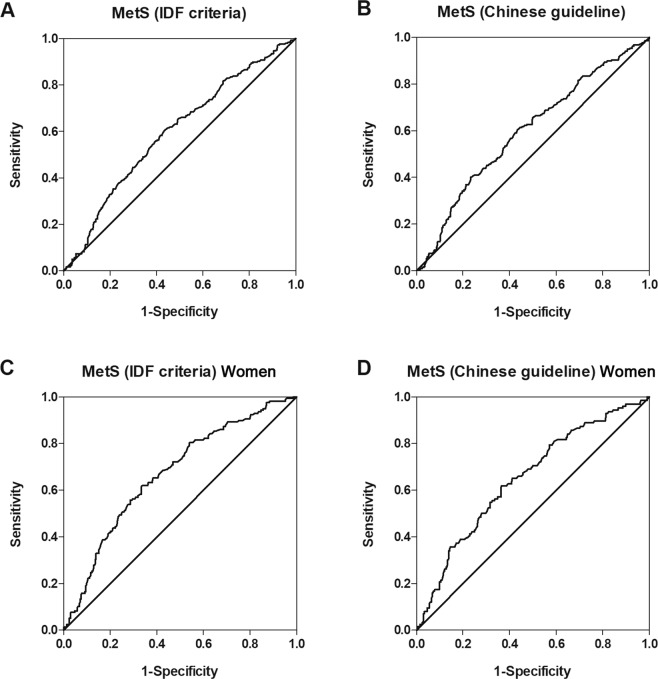
The overall optimal SUA level cutoff point for predicting MetS (IDF criteria) was 351.5 µmol/L (Sensitivity: 60.3%, Specificity: 56.7%) in the elderly and 339.0 µmol/L (Sensitivity: 62.0%, Specificity: 66.3%) in very elderly women, respectively. The overall optimal SUA level cutoff point for predicting MetS (Chinese guideline) in the elderly was 352.5 µmol/L (Sensitivity: 60.4%, Specificity: 56.6%) and 339.0 µmol/L (Sensitivity: 61.9%, Specificity: 63.4%) in very elderly women, respectively (Fig. 1).
Figure 1.
ROC curves of SUA level predicting MetS. (A) SUA level predicting MetS according to the IDF criteria in the very elderly. The AUC value was 0.622 (95%CI: 0.581–0.663, p < 0.001). (B) SUA level predicting MetS according to the Chinese guideline in the very elderly. The AUC value was 0.606 (95%CI: 0.563–0.649, p < 0.001). (C) SUA level predicting MetS according to the IDF criteria in very elderly women. The AUC value was 0.669 (95%CI: 0618–0.721, p < 0.001). (D) SUA level predicting MetS according to the Chinese guideline in very elderly women. The AUC value was 0.650 (95%CI: 0.594–0.706, p < 0.001). AUC: area under curve, CI: confidence interval, IDF: international diabetes federation, MetS: metabolic syndrome, ROC: receiver operating characteristic, SUA: serum uric acid.
The area under curve (AUC) values for SUA level predicting MetS were 0.622 (95%CI: 0.581–0.663, p < 0.001) and 0.606 (95%CI: 0.563–0.649, p < 0.001) according to the IDF criteria and the Chinese guideline, respectively (Fig. 1). Likewise, the AUC values for SUA level predicting MetS in the very elderly women were 0.669 (95%CI: 0618–0.721, p < 0.001) and 0.650 (95%CI: 0.594–0.706, p < 0.001) according to the IDF criteria and the Chinese guideline, respectively (Fig. 1).
Discussion
The main findings of this study are as follows: 1) MetS is common in the very elderly in Chengdu, especially in very elderly women. 2) hyperuricemia was generally associated with MetS in the very elderly in Southwest China.
MetS in the very elderly
Because of the dramatic changes in the environment and rapid westernization of lifestyles such as more saturated fat and sugar consumption and less physical activity, the prevalence of MetS and ASCVD has been increasing rapidly during the past decades in China2,3. In addition, it has been shown that the prevalence of MetS is age-dependent. As per other studies in middle-aged and elderly Chinese 14,20, Korean21, and Japanese22, our findings show that the prevalence of MetS is remarkably high in the general community very elderly as well as in very elderly with hyperuricemia. The potential causes could be the clustering of more MetS components with aging in the very elderly (such as hypertension and abdominal obesity), decreased physical activities, and chilly and oil-rich local food style, which result in obesity and considerably dyslipidemia23.
Furthermore, following other studies20,24, this study also demonstrates that MetS is more common in very elderly women and those with hyperuricemia. Previous analysis has already showed a significantly higher prevalence of abdominal obesity and hypertriglyceridemia in very elderly women than it is in men23 and increased WC and extreme obesity increase more risk of MetS in women than in men25. Although the difference in the definition of MetS between the IDF criteria and the Chinese guideline results in the variation of the estimated prevalence of MetS without surprising, we could not ignore the fact that the prevalence of MetS is unexpectedly high in the very elderly residents in Chengdu, especially in very elderly women.
Association between hyperuricemia and MetS
Elevated SUA level and hyperuricemia have already been reported to be associated with MetS and its components in middle-aged or elderly populations in other areas of China14,15. For the first time, this study describes the association between hyperuricemia and MetS in the community very elderly in Southwest China. The potential mechanisms for this association between hyperuricemia and MetS include inflammation and oxidative stress induced by SUA in adipocytes, which cause abnormal adipocytokine secretion and resulting in insulin resistance and the development of MetS26,27.
Previous studies14,15,22,28 establish that there is a gender difference in the prevalence of MetS. Furthermore, some studies14,15,22,29 show that the association between hyperuricemia and MetS is more evident in the middle-aged and elderly women. Following these findings, sub-analyses of our study find that there is a greater OR value of hyperuricemia for MetS in very elderly women than in men but without significant sex interaction. Therefore, whether hyperuricemia could differently contribute to the development of MetS in very elderly women and men, it still needs further prospective studies to clarify that.
In this very elderly population, hyperuricemia is found to be associated with abdominal obesity and dyslipidemia while not with hyperglycemia, which supports the results of some other studies15,28. One possible explanation may be that glucose can competitively inhibit SUA reabsorption and enhance its excretion at the same anatomical position in the gut and that SUA reduces after the onset of diabetes30,31.
It is well known that kidneys play a key role in SUA elimination, including glomerular filtration, tubular reabsorption, secretion and resorption32. However, renal function decreases with ageing even in apparently healthy individuals, but yet less in individuals with different chronic cardiovascular diseases33. Therefore, renal function can influence on the SUA level. Similar to the eGFR level in very elderly Chinese in CKD-EPI study34, the mean eGFR value in this study was around 60 ml/(min∙1.73m2), which might decrease SUA elimination and increase the SUA level. Taking this into consideration, it would not be surprising that the SUA level in this study is a little higher than that in the previous study with about 2000 Chinese elderly residents35.
The ability of SUA level for predicting a four year MetS incidence in general elderly Italian with an AUC value of 0.647 has been verified previously29. Similar to the results of that study, the AUC value in our research also indicates that the predictive ability of SUA for MetS is reasonable in the very elderly population in Chengdu.
In conclusion, the prevalence of MetS in the very elderly in Chengdu is relatively high and hyperuricemia is associated with the risk of MetS. On the other hand, it implicates that more efficient prevention strategies for cardiovascular and metabolic disease, including all MetS components and hyperuricemia, are urgently needed for middle-aged and elderly residents, especially for women36.
Study limitations
Several limitations to be addressed in this study. First, this cross-sectional study could not reveal a causal relationship between hyperuricemia and MetS. Second, in this study, a two-hour postprandial glucose measurement was not performed, which might cause an underestimation of MetS. Third, the SUA level was a single measurement value, and previous dietary factors (i.e., drinks and meals) were not considered before measurement. Finally, our study could only describe epidemiological distribution in the very elderly in southwest China. Therefore, our findings may limit its generalizability to the very elderly population in other areas of China. Further prospective longitudinal studies are needed to investigate the possible role of SUA in MetS in the very elderly36.
Acknowledgements
We appreciate all participants for their participation in this study. This research was supported by the Science and Technology Bureau of Chengdu, Sichuan, China (contracts: 2019-YF05–00523-SN, 11PPYB034SF−289).
Author contributions
G.H. and T.J.Z. had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. T.J.Z., G.H. and J.B.X. contributed to the study concept and design, data analysis and interpretation, and drafting of the manuscript. L.C., H.X.L., X.Q.Y. and J.W. contributed to the data analysis and interpretation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gang Huang and Junbo Xu.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Gu D, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, et al. Metabolic Syndrome Among Adults in China The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2017;102:507–515. doi: 10.1210/jc.2016-2477. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Internet]. China: WHO statistical profile. http://www.who.int/gho/countries/chn.pdf?ua=1(2012) (Date of access: 05/10/2019)
- 5.Cicero AFG, et al. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: data from the Brisighella Heart Study. J. Hypertens. 2019;37:728–731. doi: 10.1097/HJH.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 6.Kuwabara M, et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension from Prehypertension: A 5-Year Japanese Cohort Study. Hypertension. 2018;71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2014;11:1102–1108. doi: 10.1016/j.hrthm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Huang G, et al. Hyperuricemia is associated with atrial fibrillation prevalence in very elderly - a community based study in Chengdu, China. Sci. Rep. 2018;8:12403. doi: 10.1038/s41598-018-30321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae MH, et al. Serum uric acid as an independent and incremental prognostic marker in addition to n-terminal pro-b-type natriuretic peptide in patients with acute myocardial infarction. Circ. J. 2011;75:1440–1447. doi: 10.1253/circj.cj-10-0952. [DOI] [PubMed] [Google Scholar]
- 10.Li M, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016;6:19520. doi: 10.1038/srep19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazza A, et al. Predictors of stroke mortality in elderly people from the general population: The CArdiovascular STudy in the ELderly. Eur. J. Epidemiol. 2001;17:1097–1104. doi: 10.1023/a:1021216713504. [DOI] [PubMed] [Google Scholar]
- 12.Yadav D, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean ruralcohort. Atherosclerosis. 2015;241:271–277. doi: 10.1016/j.atherosclerosis.2015.04.797. [DOI] [PubMed] [Google Scholar]
- 13.Kamei K, et al. Associations Between Serum Uric Acid Levels and the Incidence of Nonfatal Stroke: A Nationwide Community-Based Cohort Study. Clin. Exp. Nephrol. 2017;21:497–503. doi: 10.1007/s10157-016-1311-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population. Clin. Chim. Acta. 2018;480:119–125. doi: 10.1016/j.cca.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, et al. Association between Serum Uric Acid Level and Metabolic Syndrome and Its Sex Difference in a Chinese Community Elderly Population. Int. J. Endocrinol. 2014;2014:754678. doi: 10.1155/2014/754678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang G, et al. Hyperuricemia is associated with cardiovascular diseases clustering among very elderly women - a community based study in Chengdu, China. Sci. Rep. 2017;7:996. doi: 10.1038/s41598-017-01042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014;26:186–191. doi: 10.1097/BOR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html. (Date of access: 05/10/2019) (2006).
- 19.Joint committee for guideline revision 2016 Chinese guidelines for the management of dyslipidemia in adults. J. Geriatr. Cardiol. 2018;15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J. Am. Coll. Cardiol. 2006;47:1588–1594. doi: 10.1016/j.jacc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Ko Y, Kwak C, Yim ES. Gender differences in metabolic syndrome components among the Korean 66-year-old population with metabolic syndrome. BMC Geriatr. 2016;16:27. doi: 10.1186/s12877-016-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumiyoshi H, et al. Association of Uric Acid with Incident Metabolic Syndrome in a Japanese General Population. Int. Heart J. 2019;60:830–835. doi: 10.1536/ihj.18-444. [DOI] [PubMed] [Google Scholar]
- 23.Huang G, et al. Hyperuricemia is associated with cardiovascular diseases clustering among very elderly women - a community based study in Chengdu, China. Sci. Rep. 2017;7:996. doi: 10.1038/s41598-017-01042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IY, et al. Women with Metabolic Syndrome and General Obesity Are at a Higher Risk for Significant Hyperuricemia Compared to Men. J. Clin. Med. 2019;8:pii: E837. doi: 10.3390/jcm8060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007;293:C584–596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 28.He X, et al. Prevalence and clinical profile of metabolic syndrome in longevity study from Guangxi Zhuang Autonomous Region, China. BMC Geriatr. 2017;17:169. doi: 10.1186/s12877-017-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cicero AFG, et al. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018;8:11529. doi: 10.1038/s41598-018-29955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin KC, Tsai ST, Lin HY, Chou P. Different progressions of hyperglycemia and diabetes among hyperuricemic men women in the Kinmen study. J. Rheumatol. 2004;31:1159–1165. [PubMed] [Google Scholar]
- 31.Nan H, et al. Serum uric acid and incident diabetes in Mauritian and Creole populations. Diabetes Res Clin. Pract. 2008;80:321–327. doi: 10.1016/j.diabres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Méndez Landa CE. Renal Effects of Hyperuricemia. Contrib. Nephrol. 2018;192:8–16. doi: 10.1159/000484273. [DOI] [PubMed] [Google Scholar]
- 33.Denic A, Glassock RJ, Rule AD. Structural and Functional Changes with the Aging Kidney. Adv. Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, et al. A retrospective analysis of kidney function and risk factors by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in elderly Chinese patients. Ren. Fail. 2015;37:1323–1328. doi: 10.3109/0886022X.2015.1068513. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, et al. Association between Serum Uric Acid Level and Metabolic Syndrome and Its Sex Difference in a Chinese Community Elderly Population. Int. J. Endocrinol. 2014;2014:754678. doi: 10.1155/2014/754678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghi C, Cicero AFG. Uric acid and early prevention of vascular diseases: Women under the looking glass. Int. J. Cardiol. 2018;272:314–315. doi: 10.1016/j.ijcard.2018.07.121. [DOI] [PubMed] [Google Scholar]