Abstract
Careful recognition of cutaneous lesions in patients with malignancies may aid in avoiding additional morbidity during end of life care.
Keywords: acinar cell carcinoma, lipase hypersecretion syndrome, neoplastic syndrome, pancreatic panniculitis
Careful recognition of cutaneous lesions in patients with malignancies may aid in avoiding additional morbidity during end of life care.
1. INTRODUCTION
Pancreatic panniculitis is a rare cutaneous manifestation of a variety of pancreatic diseases.1 When seen in conjunction with polyarthritis and eosinophilia, it is termed lipase hypersecretion syndrome (LHS), a paraneoplastic syndrome most commonly seen in the setting of pancreatic acinar cell carcinoma.2 LHS is a neoplastic disorder resulting from exocrine excess, with the release of enzymes into circulation, a phenomenon that has been described as “endocrine‐ization of an exocrine function.”2 First reported by Berner in 1908 who described a “functional acinar cell” malignancy accompanied by this constellation of findings3; the occurrence of these findings has been attributed to the enzymatic properties on distant tissues of high circulating levels of lipase within peripheral circulation.4 A recent review article found that since this initial report, further observations of this pathology have been limited to a few case reports.2 Given this rarity and infrequent presentation, LHS remains a poorly studied subject that may often be initially misdiagnosed or unrecognized2 This is important to consider as peripheral signs of pancreatic malignancies may often precede the development of locoregional effects by weeks,5 thus providing an opportunity for early diagnosis. Our ensuing review will include a case report, followed by an overview of the syndromes’ clinical manifestations, pathophysiology, histological features, and management principles pertinent to surgical care.
2. CASE HISTORY/EXAMINATION
Our patient was a 69‐year‐old man who presented to the hospital with multiple painful nodular lesions on his legs bilaterally. These lesions were first noticed one month prior to presentation on his right lower limb but had progressed over time to now involve both legs and were associated with worsening pain and serous discharge. He was not associated with fevers or chills; however, he did admit to loss of appetite, weight loss, and malaise. Past medical history was significant for hypertension, Hepatitis C, and prostate cancer managed with brachytherapy. Physical examination revealed a 3 × 3 cm tender nodule on anteromedial aspect of right leg draining seropurulent fluid as well as several 1‐2 cm nodules on bilateral legs, hyperpigmented and tender to palpation. (Figures 1 and 2).
Figure 1.
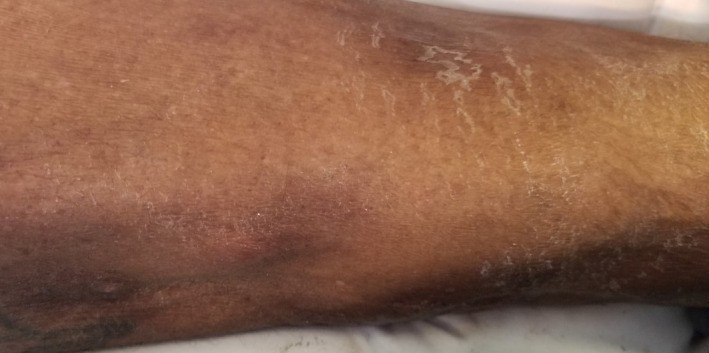
Characteristic extremity nodules of LHS
Figure 2.
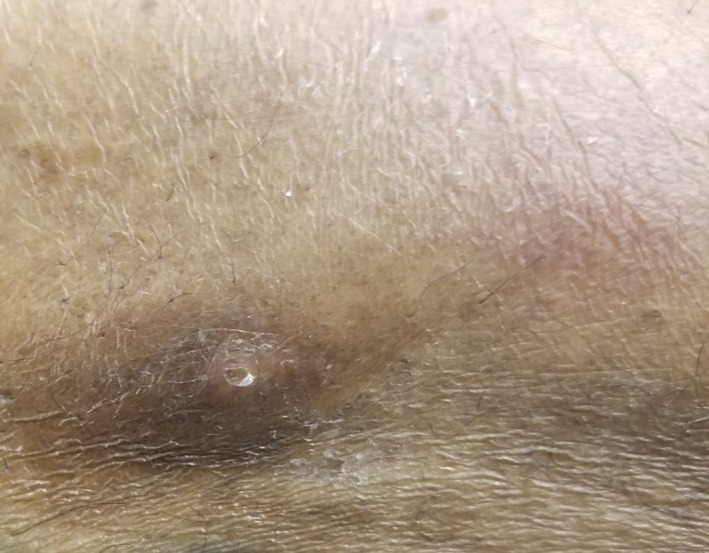
Characteristic extremity nodules of LHS
3. DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
An excisional biopsy of the prominent right leg nodular lesion was performed, and histopathologic findings included subcutaneous enzymatic fat necrosis surrounded by neutrophils and foamy histiocytes and epidermal necrosis consistent with pancreatic panniculitis (Figure 3). This prompted a workup for pancreatic pathology including blood and radiological investigations, upper and lower endoscopies.
Figure 3.
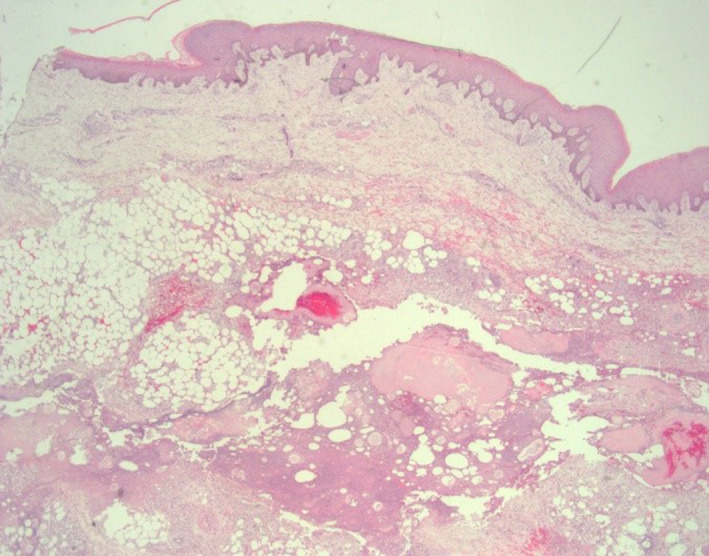
40× magnification of fat necrosis surrounded by neutrophils and foamy histiocytes consistent with pancreatic panniculitis
Pertinent laboratories included serum lipase (5444 IU/L), carbohydrate antigen 19‐9 (CA 19‐9) (44 U/mL), carcinoembryonic antigen (CEA) (0.5 ng/mL).
Computed tomography of the abdomen revealed an 8.7 × 6.2 × 5.7 cm mass arising from the pancreatic head (Figures 4 and 5) causing significant mass effect upon the second portion of the duodenum. Decreased attenuation of the portal vein and the superior mesenteric vein was suggestive of invasion. There was no pancreatic duct, common bile duct, or intrahepatic biliary ductal dilatation. Multiple large hepatic lesions showing peripheral enhancement were noted, with the largest lesion measuring 5.1 cm in greatest dimension consistent with metastatic disease. These suspicious masses in the liver were biopsied percutaneously by interventional radiology in order to obtain a definitive tissue diagnosis and to aid in formulating a therapeutic plan.
Figure 4.
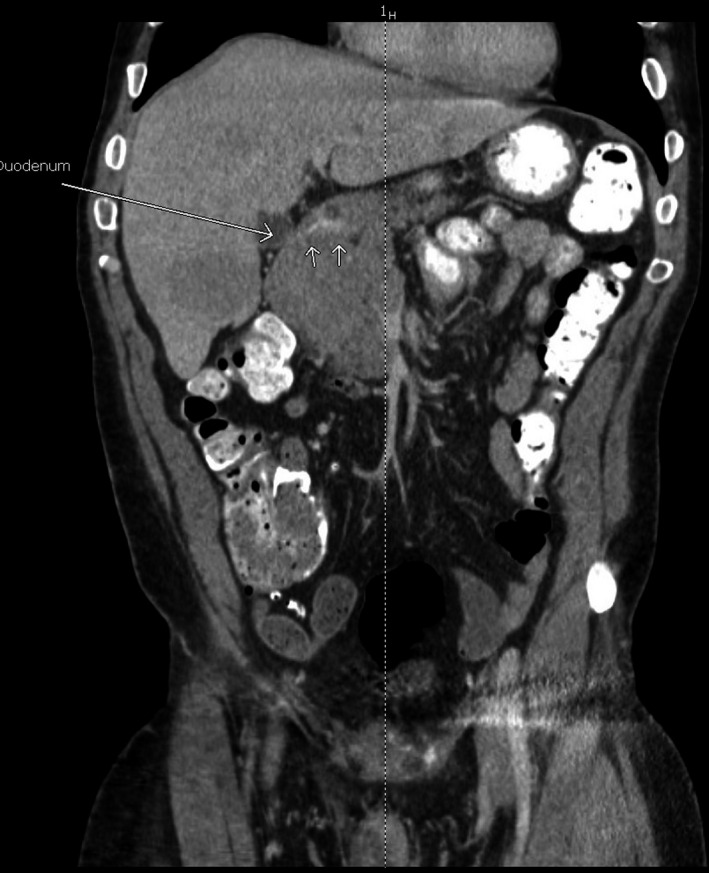
Coronal and axial cuts of an abdominal CT demonstrating pancreatic mass with duodenal invasion
Figure 5.
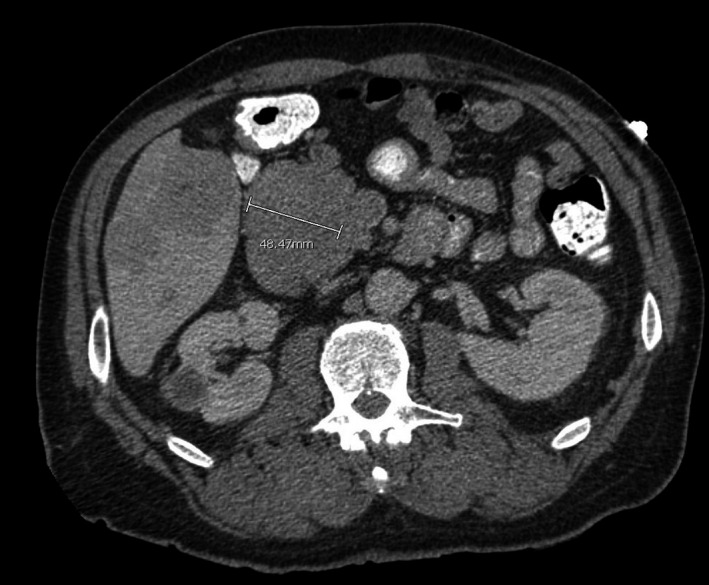
Coronal and axial cuts of an abdominal CT demonstrating pancreatic mass with duodenal invasion
An upper endoscopy was performed, and a large (approximately 5 cm) duodenal mass with ulceration extending from the bulb toward the second portion of the duodenum was seen and biopsied (Figure 6).
Figure 6.
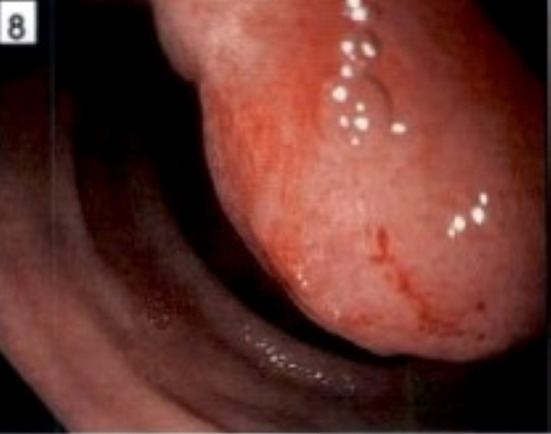
Endoscopic image of pancreatic mass with duodenal invasion
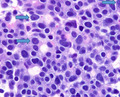
Duodenal and liver biopsy results were concordant with the diagnosis of pancreatic acinar cell carcinoma (see Figures 7, 8, 9). Factors that supported this diagnosis included the strong diffuse cytoplasmic expression of alpha1‐antichymotrypsin, alpha‐1 antitrypsin and chymotrypsin and negative CD‐56 and chromogranin. Synaptophysin immunostaining showed patchy weak expression. Glypican‐3 was negative. Periodic acid‐Schiff (PAS) stain with diastase was noncontributory. After a thorough multidisciplinary team workup including pathology, a diagnosis of lipase hypersecretion syndrome secondary to Stage 4 pancreatic acinar cell carcinoma was made.
Figure 7.
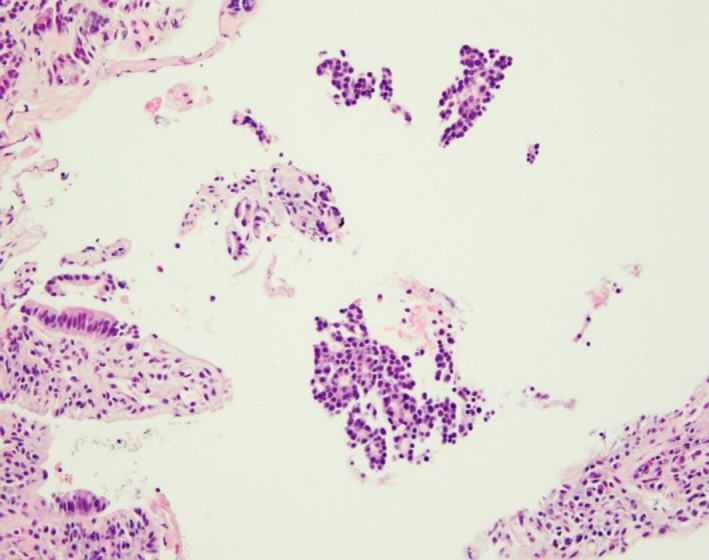
100× magnification of duodenal biopsy with collections of carcinoma with uniform cells and tubular formations
Figure 8.
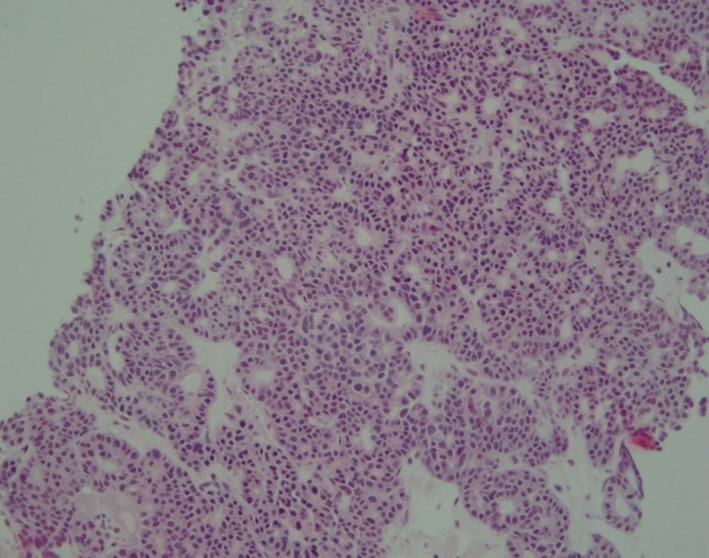
100× magnification of metastatic carcinoma in liver composed of high‐grade carcinoma forming sheets and tubular formations
Figure 9.
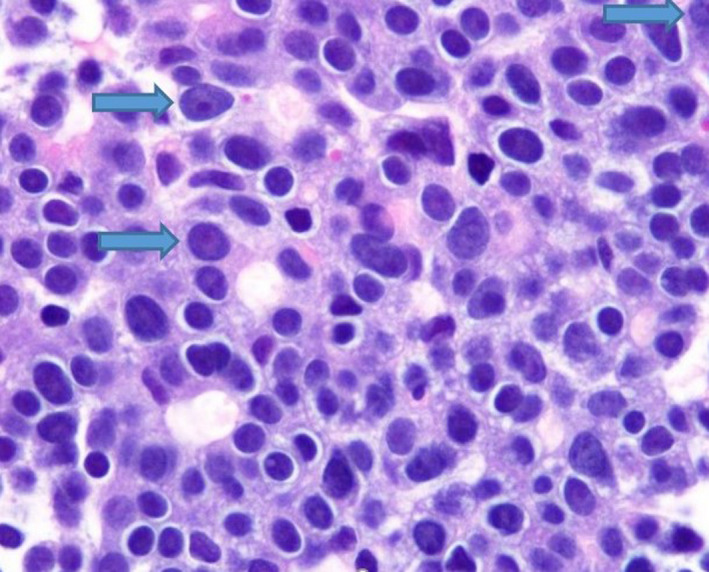
Liver biopsy (1000× magnification) showing fine chromatin, large cherry red nucleoli (arrows) and nuclear pleomorphism
A large portion of his care centered on the management of his chronic nonhealing lower extremity wounds. After serial debridements, washouts, and extensive wound care, he developed multiple lower extremity wounds with the largest measuring 8 × 15 cm in size. These were managed with a combination of routine dressing changes, application of negative pressure therapy, and use of a‐cellular matrix. These wounds were extremely slow to heal due to the patient's overall physiologic condition and did respond to aggressive wound care (Figure 10).
Figure 10.
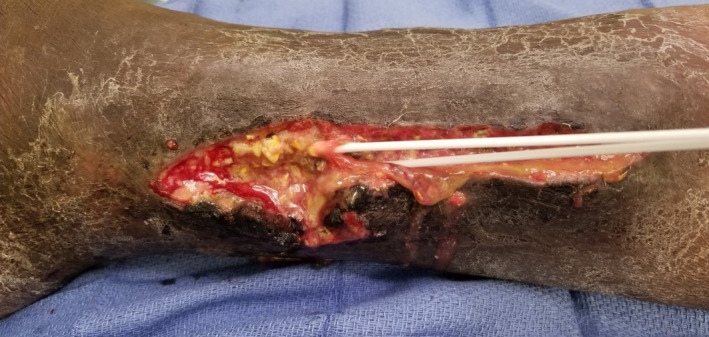
Gross picture of pancreatic panniculitis with characteristic discharge
4. OUTCOME AND FOLLOW‐UP
After a multidisciplinary discussion at tumor board within the Howard University Cancer Center, the patient was offered a management plan centering on palliative care. His poor performance status, however, prohibited the use of chemotherapy. The patient required serial transfusions for chronic gastrointestinal hemorrhage secondary to the pancreatic tumor invasion into the duodenum, and palliative radiotherapy was offered however the family declined. After consideration of his treatment options, the patient and his family had expressed their wish to pursue hospice care with continued palliative treatments largely due to the extremely poor prognosis of his neoplastic condition. He continued to receive appropriate inpatient nutritional support, wound care and management of his comorbid medical conditions with the goal of transitioning his care to a home‐based palliative care system. However, prior to discharge, the patient succumbed to the nature of his end‐stage disease. This represented a total of 2 months overall survival from the time of his diagnosis.
5. DISCUSSION
Acinar cell carcinoma (ACC) is an extremely rare pancreatic tumor comprising 1%‐2% of pancreatic tumors6 with 50% of tumors presenting with metastases at the time of diagnosis and those patients with advanced disease (Stage III and IV) having very poor prognosis.6, 7 Cross‐sectional imaging modalities such as computed tomography and magnetic resonance imaging that are enhanced with contrast agents are often utilized to define the extent of primary lesions and possible metastatic disease.8, 9 An estimated 10%‐15% of ACC’s manifest the neoplastic exocrine disorder termed LHS,10 and typically, this occurs at an advanced stage with most patients having concurrent metastatic disease to the liver.2 Other malignancies that may manifest in this manner include pancreatic islet cell11 and neuroendocrine tumors12 as well as adrenal neuroendocrine carcinoma.4 The abnormally high levels of lipase present in this condition may affect a variety of organs with varying degrees of uncontrolled lipolysis.13 This is most notable in subcutaneous tissues where enzymatic damage to subcutaneous adipocytes results in fat necrosis and a subsequent inflammatory process.1 Resolution of the inflammatory phase is followed by regression of these lesions or formation of fibrocystic nodules.2 Subsequent enlargement of these areas may produce compressive effects manifesting as erythematous or purplish‐brown epithelial discoloration, erosion, and ulceration13 that may have an oily brown discharge.14 Commonly, the lower extremities are afflicted, but lesions have also been described on the trunk and scalp as well.15 A biopsy may be obtained for confirmation with pathognomonic findings described as anucleate “ghost” cells consisting of granular debris with an eosinophilic rim; these “ghost” cells may be calcified and are often surrounded by neutrophils and fat necrosis with accompanying hemorrhage.16
Additionally, lipolysis of periarticular adipose tissue in joints and intramedullary adipose tissue induces intra‐articular inflammation. This results in painful swollen joints,13 typically the knees and ankles17 and in a symmetrical manner.14 This inflammatory tissue may also act as a nidus for infection with cultures typically yielding staphylococcal aureus or epidermis.13 Cross‐sectional imaging of involved joints may demonstrate loss of joint spaces, osteolytic lesions, and periostitis.18
As many of these patients present with late‐stage disease, thereby precluding them from surgical resection, much of their care is directed toward symptom relief and palliation. Successful chemotherapy regimens mentioned have used FOLFIRINOX.19 Successful elimination of liver metastases with chemoembolization using a regimen of mitomycin, DSM450, cisplatin, and Lipiodol has also been noted to have drastic improvement in LHS symptoms.20 For infected lesions, following drainage, tetracyclines may be a potential antibiotic choice as they have been shown to decrease lipase secretion21 with resolution of subcutaneous lesions in one case.22 As in our patient, there may be a resulting huge wound burden for which care can be a source of significant pain, discomfort, and metabolic demand in patients who are already burdened by malnourishment and supraphysiologic demands. In this context, it is important for caregivers to recognize the nature, character, and consistency of discharge from foci of pancreatic panniculitis which may frequently be misdiagnosed as infectious or inflammatory nodules (Figures 1, 2, and 10). Appropriate recognition of these lesions is crucial as this can guide diagnostic approach toward punch biopsies as opposed to excisional biopsies, thus avoiding additional morbidity during end of life care. We utilized several approaches for optimizing wound care with the use of negative pressure wound therapy and noncrosslinked wound scaffold matrices to augment healing. In the overall clinical setting, these results were highly promising and serve as a potential guide for similar cases.
In conclusion, lipase hypersecretion syndrome is a rare entity associated with various pancreatic diseases. It should be considered in suspicious lesions that present diagnostic challenges. Early recognition can help initiation of appropriate multidisciplinary care for adequate symptomatic relief and reduced morbidity.
CONFLICT OF INTEREST
The authors declare that they have no competing interests and there are no relevant financial disclosures to report.
AUTHOR CONTRIBUTION
Wasay Nizam: made substantial contributions to conception, design, data acquisition and interpretation of the study and involved in initial drafting of the manuscript and critically revised the final manuscript. Adil Aijaz Shah, Fareed Rajack, and Asa Ramdath: made substantial contributions to conception and design of the study and involved in initial drafting of the manuscript and critically revised the final manuscript. Tammey Naab: made substantial contributions to conception and design of the study and involved in initial drafting of the manuscript and critically revised the final manuscript for important intellectual content. Mallory Williams: made substantial contributions to conception and design of the study, acquisition, analysis, and interpretation of data and involved in initial drafting of the manuscript and critically revised the final manuscript for important intellectual content. All authors: reviewed the final manuscript and give approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and critically reviewed and provided feedback to the entire manuscript. All authors: read and approved the final manuscript.
CONSENT
Written informed consent was obtained from the patient's family prior to submission.
Nizam W, Shah AA, Rajack F, Ramdath A, Naab T, Williams M. Lipase hypersecretion syndrome: A rare cutaneous manifestation of advanced pancreatic acinar cell carcinoma. Clin Case Rep. 2020;8:905–910. 10.1002/ccr3.2785
REFERENCES
- 1. Zundler S, Strobel D, Manger B, Neurath MF, Wildner D. Pancreatic panniculitis and polyarthritis. Curr Rheumatol Rep. 2017;19(10):62. [DOI] [PubMed] [Google Scholar]
- 2. Taskin OC, Adsay V. Lipase hypersecretion syndrome: a distinct form of paraneoplastic syndrome specific to pancreatic acinar carcinomas. Semin Diagn Pathol. 2019;36(4):240‐245. [DOI] [PubMed] [Google Scholar]
- 3. Berner OC. Subkutane fettgewebsnekrose. Virchows Arch. 1908;193(3):510‐518. [Google Scholar]
- 4. Guanziroli E, Colombo A, Coggi A, Gianotti R, Marzano AV. Pancreatic panniculitis: the “bright” side of the moon in solid cancer patients. BMC Gastroenterol. 2018;18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laureano A, Mestre T, Ricardo L, Rodrigues AM, Cardoso J. Pancreatic panniculitis ‐ a cutaneous manifestation of acute pancreatitis. J Dermatol Case Rep. 2014;8(1):35‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunetti O, Aprile G, Marchetti P, et al. Systemic chemotherapy for advanced rare pancreatic histotype tumors: a retrospective multicenter analysis. Pancreas. 2018;47(6):759‐771. [DOI] [PubMed] [Google Scholar]
- 7. Romano G, Agrusa A, Galia M, et al. Whipple’s pancreaticoduodenectomy: surgical technique and perioperative clinical outcomes in a single center. Int J Surg. 2015;21:S68‐71. [DOI] [PubMed] [Google Scholar]
- 8. Galia M, Albano D, Picone D, et al. Imaging features of pancreatic metastases: a comparison with pancreatic ductal adenocarcinoma. Clin Imaging. 2018;51:76‐82. [DOI] [PubMed] [Google Scholar]
- 9. Agnello F, Burgio MD, Picone D, et al. Magnetic resonance imaging of the cirrhotic liver in the era of gadoxetic acid. World J Gastroenterol. 2016;22(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16(9):815‐837. [DOI] [PubMed] [Google Scholar]
- 11. Millns JL, Evans HL, Winkelmann RK. Association of islet cell carcinoma of the pancreas with subcutaneous fat necrosis. Am J Dermatopathol. 1979;1(3):273‐809. [DOI] [PubMed] [Google Scholar]
- 12. Preiss JC, Faiss S, Loddenkemper C, Zeitz M, Duchmann R. Pancreatic panniculitis in an 88‐year‐old man with neuroendocrine carcinoma. Digestion. 2002;66(3):193‐196. [DOI] [PubMed] [Google Scholar]
- 13. Good AE, Schnitzer B, Kawanishi H, Demetropoulos KC, Rapp R. Acinar pancreatic tumor with metastatic fat necrosis. Am J Dig Dis. 1976;21(11):978‐987. [DOI] [PubMed] [Google Scholar]
- 14. Braun A, Franke I, Tüting T, Gaffal E. Pancreatic panniculitis with polyarthritis (PPP syndrome). JDDG J Dtsch Dermatol Ges. 2019;17(5):546‐547. [DOI] [PubMed] [Google Scholar]
- 15. Bruno CM, Pricoco GS, Bellinvia S, Amaradio MD, Cantone D, Polosa R. Necrotizing Panniculitis as an Uncommon Manifestation of Acute Pancreatitis. Eur J Case Rep Intern Med. 2017;4(3):000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr. 2013;148(4):419‐425. [PubMed] [Google Scholar]
- 17. Singh S, Gorouhi F, Konia T, Burrall B. Pancreatic acinar cell carcinoma‐induced panniculitis. JAAD Case Rep. 2018;4(7):719‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang DJ, Lee SJ, Choo HJ, Her M, Yoon HK. Pancreatitis, panniculitis, and polyarthritis (PPP) syndrome: MRI features of intraosseous fat necrosis involving the feet and knees. Skeletal Radiol. 2017;46(2):279‐285. [DOI] [PubMed] [Google Scholar]
- 19. Callata‐Carhuapoma HR, Pato Cour E, Garcia‐Paredes B, et al. Pancreatic acinar cell carcinoma with bilateral ovarian metastases, panniculitis and polyarthritis treated with FOLFIRINOX chemotherapy regimen. A case report and review of the literature. Pancreatology. 2015;15(4):440‐444. [DOI] [PubMed] [Google Scholar]
- 20. Hudson‐Peacock MJ, Regnard CF, Farr PM. Liquefying panniculitis associated with acinous carcinoma of the pancreas responding to octreotide. J R Soc Med. 1994;87(6):361‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shalita AR, Wheatley V. Inhibition of pancreatic lipase by tetracyclines. J Invest Dermatol. 1970;54(5):413‐415. [DOI] [PubMed] [Google Scholar]
- 22. Chan HL. Panniculitis (Rothmann‐Makai), with good response to tetracycline. Br J Dermatol. 1975;92(3):351‐355. [DOI] [PubMed] [Google Scholar]


