Abstract
Ivermectin is an antiparasitic drug that has shown also an effective pharmacological activity towards various infective agents, including viruses. This paper proposes an alternative mechanism of action for this drug that makes it capable of having an antiviral action, also against the novel coronavirus, in addition to the processes already reported in literature.
Keywords: Ivermectin, Antiviral, Ionophore, COVID-19, SARS-CoV-2
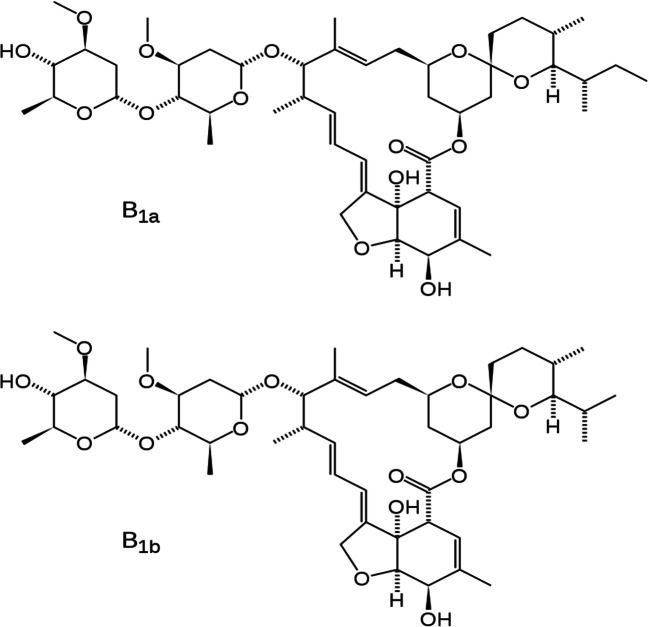
Ivermectin [mixture of 22, 23-dihydroavermectin B1a (80%) and 22, 23-dihydroavermectin B1b (20%)] (Fig. 1) is a macrocyclic lactone with a broad-spectrum antiparasitic pharmacological activity (Gonzalez Canga et al. 2008). It is the safest and most effective semi-synthetic derivative of the entire class of avermectins, discovered in 1975 by Professor Satoshi Ōmura as fermentation products of the actinomycete bacterium Streptomyces avermitilis (Crump 2017) (later reclassified in S. avermectinius (Takahashi et al. 2002)). Its main pharmacodynamics is to bind some channel proteins for chlorine controlled by glutamate, typical of specific classes of invertebrates, causing a greater permeability to this electrolyte: all this causes a hyperpolarization of the cell membrane, blocking inhibitory neurotransmission in neurons and myocytes, resulting in paralysis and death (Geary 2005). Commercialized since 1981, its low cost, its high efficacy and safety, and the marked tropism for helminths (therefore with an almost zero impact on the biochemistry of human beings) have led to its inclusion in the twenty-first World Health Organization's List of Essential Medicines (World Health Organization 2019).
Fig. 1.
Structural formulas of ivermectin compounds
Ivermectin is a versatile drug with unique characteristics, which make it interesting also for basic and applied research (in particular for drug repurposing): it seems to reveal an antibacterial (Lim et al. 2013; Ashraf et al. 2018), antiviral, and anticancer activity (Juarez et al. 2018; Intuyod et al. 2019), besides being potentially useful for the treatment of some chronic pathologies (Ashraf and Prichard 2016; Ventre et al. 2017), result of an action on a wide range of cellular targets.
Regarding its role as an antiviral agent, its efficacy has been demonstrated on several viruses, both in vitro and in vivo. Among the many mechanisms by which it performs its function, the most consolidated one sees ivermectin as an inhibitor of nuclear transport mediated by the importin α/β1 heterodimer, responsible for the translocation of various viral species proteins (HIV-1, SV40), indispensable for their replication (Wagstaff et al. 2011; Wagstaff et al. 2012). This inhibition appears to affect a considerable number of RNA viruses (Jans et al. 2019; Caly et al. 2012), such as Dengue Virus 1-4 (DENV) (Tay et al. 2013), West Nile Virus (WNV) (Yang et al. 2020), Venezuelan Equine Encephalitis Virus (VEEV) (Lundberg et al. 2013), and Influenza (Gotz et al. 2016). In addition, ivermectin has been shown to be effective against the Pseudorabies virus (PRV, with a DNA-based genome), both in vitro and in vivo (Lv et al. 2018), using the same mechanism. Caly et al. (Caly et al. 2020) have recently shown that the drug also inhibits the replication of the SARS-CoV-2 virus in vitro, however not clarifying how it occurs. Since the causative agent of COVID-19 is an RNA virus, it can be reasonably expected an interference with the same proteins and the same molecular processes described above.
However, ivermectin could prove to be a powerful antiviral, therefore also useful for a possible treatment of the new coronavirus associated syndrome, even from a new perspective. This could happen assuming its role as an ionophore agent, only hinted in the recent past but never fully described (Juarez et al. 2018). Ionophores are molecules that typically have a hydrophilic pocket which constitutes a specific binding site for one or more ions (usually cations), while its external surface is hydrophobic, allowing the complex thus formed to cross the cell membranes, affecting the hydro-electrolyte balance (Freedman 2012). These chemical species have historically been used to study the mitochondrial respiratory chain and ATP synthesis in eukaryotes (in this case also known as decoupling agents, such as 2, 4-dinitrophenol), and their antibiotic activity has long been appreciated (Bakker 1979). It is also hypothesized their role as antiviral drugs (Krenn et al. 2009; Sandler et al. 2020) and anticancer chemotherapeutic agents (Kaushik et al. 2018). Thinking of the structure of two of the most important ionophores, monensin A and valinomycin, respectively a polyether and a depsipeptide antibiotic, it is clear that they internally present many oxygen atoms (with related free electron doublets), indispensable for binding cations and transporting them through phospholipidic bilayers.
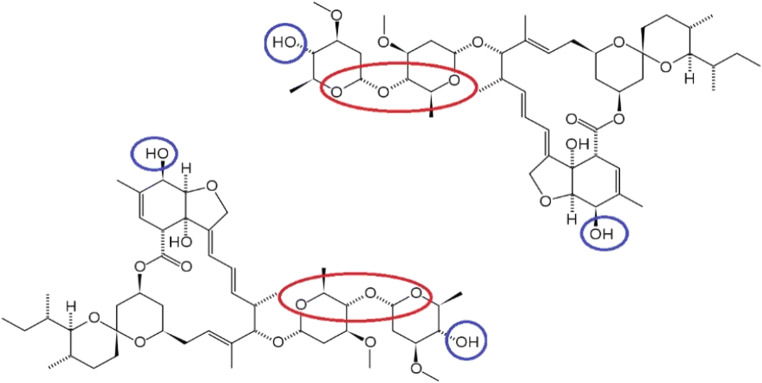
At a first glance, the two structures that make up the ivermectin formula do not have these chemical properties, nor those mentioned above, essential for a compound to be defined as ionophore. However, it can be hypothesized that two ivermectin molecules, reacting with each other in a “head-tail” mode, can create a complex suitable to be considered such (Fig. 2). This interaction could occur spontaneously or be mediated by the binding of the same molecules to some plasma transport proteins, in particular albumin (Klotz et al. 1990), which would have the role of positioning them in the correct way to obtain the proposed configuration.
Fig. 2.
Possible interaction mechanism between two ivermectin molecules
As it can be seen, in this way, an internal cavity is formed: the oxygen atoms (indicated in red), now present in greater number, work as Lewis bases and could therefore coordinate a series of cations (Lewis acids). On the other hand, the –OH groups are highlighted in blue and they could have a decisive role in the stabilization of the new structure, with the establishment of chemical bonds between these functional groups: one or more –O– bridges (however, it is difficult the formation of ether bonds, since acid catalysis at high temperature is not possible under normal conditions, both in vitro and in vivo) or more probably hydrogen bonds could be formed, even among more molecular complexes of this type. However, the formation of other weak and strong interactions of various kinds cannot be excluded. Otherwise, specific cations could bind the two molecules in the proposed way, creating themselves the final structure and stabilizing it: there are examples already known in literature (Abbott et al. 1979). The external part of the complex, then, would already have in itself all the hydrophobic characteristics necessary to carry ions through the viral membrane. As a consequence, it would be determined an ionic imbalance between the external and internal environment, with the recall of water and consequent osmotic lysis. This would allow to neutralize the virus at an early stage of the infection, before; therefore, it can adhere to the host cells and enter it to exploit their biochemical machinery for the production of other viral particles. However, this hypothesis would concern only viruses without a proteic capsid, a structure that shows a certain resistance to osmotic pressure, even if to a lesser extent than a bacterial, fungal, or plant cell wall (Cordova et al. 2003). The new coronavirus is one of these, presenting only a phospholipid envelope in defense of the genetic material, where its few proteins are inserted and which it acquires in the act of exiting the infected cells (Sigrist et al. 2020). This unconventional electrolyte uptake mode could also affect the potential of the viral membrane, threatening its integrity and functionality. The same goes for the viral proteins present here. Furthermore, the concentration variation of some cations, thus determined, could inhibit some key enzymes in the viral replication, such as RNA-dependent RNA polymerases (RdRp) (te Velthuis et al. 2010), already used as pharmacological targets.
Another indication in favor of a possible ionophore role for ivermectin comes from the analysis of molecular similarity that can be carried out through the Drugbank database (www.drugbank.ca). By setting a minimum similarity threshold for ivermectin equal to 0.7, about 14 results are obtained. Among the various selected molecules, the majority of which have antiparasitic and antibiotic activity (already not only on the market but also in the study and experimentation phase), a compound that has high structural similarity is nystatin (score of 0.72), an antimycotic drug with an ionophoric activity at the plasma membrane level, where it forms channels (Yamasaki et al. 2011; Stillwell 2016; Rang 2015).
Immediately afterwards, with a slightly lower similarity, it can be find amphotericin B and natamycin, all pharmacological molecules of assured ionophoric activity (score of 0.71 and 0.706, respectively) (Stillwell 2016; Rang 2015; Ramos 1989; Ikehara et al. 1986).
In conclusion, pending computational simulations and chemical-physical laboratory analysis, this hypothesis could be applied to other known pharmacological molecules, in order to identify compounds with probable ionophore nature to be used in research and clinical practice.
Authors’ contributions
All research phases (idea, drafting of the paper, and proofreading) were conducted by the only author, ER.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbott BJ, Fukuda DS, Dorman DE, Occolowitz JL, Debono M, Farhner L. Microbial transformation of A23187, a divalent cation ionophore antibiotic. Antimicrob Agents Chemother. 1979;16(6):808–812. doi: 10.1128/AAC.16.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Prichard R. Ivermectin exhibits potent anti-mitotic activity. Vet Parasitol. 2016;226:1–4. doi: 10.1016/j.vetpar.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Ashraf S, Chaudhry U, Raza A, Ghosh D, Zhao X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob Resist Infect Control. 2018;7:27. doi: 10.1186/s13756-018-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker EP. Ionophore Antibiotics. In: Hahn F.E. (eds) Mechanism of action of antibacterial agents. Antibiotics. 1979;5:1. [Google Scholar]
- Caly L, Wagstaff KM, Jans DA. Nuclear trafficking of proteins from RNA viruses: potential target for anti-virals? Antiviral research. 2012;95:202–206. doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;3:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Deserno M, Gelbart WM, Ben-Shaul A. Osmotic shock and the strength of viral capsids. Biophys J. 2003;85(1):70–74. doi: 10.1016/S0006-3495(03)74455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J Antibiot (Tokyo). 2017;70(5):495–505. doi: 10.1038/ja.2017.11. [DOI] [PubMed] [Google Scholar]
- Freedman JC (2012) Chapter 4 - Ionophores in planar lipid bilayers. In: Sperelakis N (ed) Cell Physiology Source Book, 4th edn. Academic Press, pp 61–66 ISBN 9780123877383
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21(11):530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez Canga A, et al. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz V, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138. doi: 10.1038/srep23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara T, Yamaguchi H, Hosokawa K, Yonezu T, Miyamoto H. Effects of nystatin on intracellular contents and membrane transport of alkali cations, and cell volume in HeLa cells. J Membr Biol. 1986;90(3):231–240. doi: 10.1007/BF01870129. [DOI] [PubMed] [Google Scholar]
- Intuyod K, Hahnvajanawong C, Pinlaor P, Pinlaor S. Anti-parasitic drug ivermectin exhibits potent anticancer activity against gemcitabine-resistant cholangiocarcinoma in vitro. Anticancer Res. 2019;39(9):4837–4843. doi: 10.21873/anticanres.13669. [DOI] [PubMed] [Google Scholar]
- Jans DA, Martin AJ, Wagstaff KM. Inhibitors of nuclear transport. Curr Opin Cell Biol. 2019;58:50–60. doi: 10.1016/j.ceb.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res. 2018;8(2):317–331. [PMC free article] [PubMed] [Google Scholar]
- Kaushik V, Yakisich JS, Kumar A, Azad N, Iyer AKV. Ionophores: Potential use as anticancer drugs and chemosensitizers. Cancers (Basel) 2018;10(10):pii: E360. doi: 10.3390/cancers10100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz U, Ogbuokiri JE, Okonkwo PO. Ivermectin binds avidly to plasma proteins. Eur J Clin Pharmacol. 1990;39(6):607–608. doi: 10.1007/BF00316107. [DOI] [PubMed] [Google Scholar]
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 2009;83(1):58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LE, Vilchèze C, Ng C, Jacobs WR, Jr, Ramón-García S, Thompson CJ. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob Agents Chemother. 2013;57(2):1040–1046. doi: 10.1128/AAC.01696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, Jans DA, Kehn-Hall K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 2013;100(3):662–672. doi: 10.1016/j.antiviral.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Lv C, Liu W, Wang B, Dang R, Qiu L, Ren J, Yan C, Yang Z, Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55–62. doi: 10.1016/j.antiviral.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Ramos H. Attias de Murciano A, Cohen BE, Bolard J. The polyene antibiotic amphotericin B acts as a Ca2+ ionophore in sterol-containing liposomes. Biochim Biophys Acta. 1989;982(2):303–306. doi: 10.1016/0005-2736(89)90069-2. [DOI] [PubMed] [Google Scholar]
- Rang PH. Rang and Dale’s pharmacology. In: Dale, Maureen M, Flower RJ, Rod J, editors. 1945-, Henderson, G. (Graeme) (Eighth ed.). [United Kingdom] 2015. [Google Scholar]
- Sandler ZJ, Vu MN, Menachery VD, Mounce BC (2020) Novel ionophores active against La Crosse virus identified through rapid antiviral screening. bioRxiv doi. 10.1101/2020.01.21.914929 [DOI] [PMC free article] [PubMed]
- Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W (2016) Chapter 19 - Membrane Transport. In: Stillwell W (ed) An introduction to biological membranes, 2nd edn. Elsevier, pp 423–451 ISBN 9780444637727
- Takahashi Y, Matsumoto A, Seino A, Ueno J, Iwai Y, Omura S. Streptomyces avermectinius sp. nov., an avermectin-producing strain. Int J Syst Evol Microbiol. 2002;52(Pt 6):2163–2168. doi: 10.1099/00207713-52-6-2163. [DOI] [PubMed] [Google Scholar]
- Tay MY, et al. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin. Antiviral Res. 2013;99(3):301–306. doi: 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre E, Rozières A, Lenief V, Albert F, Rossio P, Laoubi L, Dombrowicz D, Staels B, Ulmann L, Julia V, Vial E, Jomard A, Hacini-Rachinel F, Nicolas JF, Vocanson M. Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72(8):1212–1221. doi: 10.1111/all.13118. [DOI] [PubMed] [Google Scholar]
- Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. Journal of biomolecular screening. 2011;16(2):192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin alpha/beta mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. The Biochemical journal. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- Yamasaki M, Tamura N, Nakamura K, Sasaki N, Murakami M, Rajapakshage W, Kumara B, Tamura Y, Lim SY, Ohta H, Takiguchi M. Effects and mechanisms of action of polyene macrolide antibiotic nystatin on Babesia gibsoni in vitro. J Parasitol. 2011;97(6):1190–1192. doi: 10.1645/GE-2799.1. [DOI] [PubMed] [Google Scholar]
- Yang SNY, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]