Abstract
Potassium K2P (“leak”) channels conduct current across the entire physiological voltage range and carry leak or “background” currents that are, in part, time- and voltage-independent. The activity of K2P channels affects numerous physiological processes, such as cardiac function, pain perception, depression, neuroprotection, and cancer development. We have recently established that, when expressed in Xenopus laevis oocytes, K2P2.1 (TREK-1) channels are activated by several monoterpenes (MTs). Here, we show that, within a few minutes of exposure, other mechano-gated K2P channels, K2P4.1 (TRAAK) and K2P10.1 (TREK-2), are opened by monoterpenes as well (up to an eightfold increase in current). Furthermor\e, carvacrol and cinnamaldehyde robustly enhance currents of the alkaline-sensitive K2P5.1 (up to a 17-fold increase in current). Other members of the K2P potassium channels, K2P17.1, K2P18.1, but not K2P16.1, were also activated by various MTs. Conversely, the activity of members of the acid-sensitive (TASK) K2P channels (K2P3.1 and K2P9.1) was rapidly decreased by monoterpenes. We found that MT selectively decreased the voltage-dependent portion of the current and that current inhibition was reduced with the elevation of external K+ concentration. These findings suggest that penetration of MTs into the outer leaflet of the membrane results in immediate changes at the selectivity filter of members of the TASK channel family. Thus, we suggest MTs as promising new tools for the study of K2P channels’ activity in vitro as well as in vivo.
Keywords: K2P channel, TREK-1, TRAAK, TASK-1, TALK, monoterpenes, leak channels, voltage-dependent current
Introduction
Potassium channels selectively and rapidly enable the movement of K+ ions across biological membranes down the electrochemical K+ gradient at a rate close to that of diffusion (Mackinnon, 2003). Members of the potassium leak channel family are structurally unique among potassium channels since each subunit possesses four transmembrane segments and two pore-forming domains (2P/4TM architecture). As such, these channels are often referred to as two pore-domain K+ or K2P channels (Goldstein et al., 2001; Choe, 2002). These channels conduct current across the entire physiological voltage range and are essential for neurophysiological function, while their activity modulates excitability. It was shown that K2P channels could also increase excitability by supporting high-frequency firing once an action potential threshold is reached (Brickley et al., 2007). It was recently reported that the majority of K2P channels are gated by membrane potential in spite of their lack of a voltage sensor, as the outward current of K+ ions through the selectivity filter was found to open this gate (Schewe et al., 2016). Members of this family may react to membrane stretch, as well as to intracellular and extracellular pH changes, phosphorylation, the activity of various G-protein coupled receptors, and more (Goldstein et al., 2001; Brickley et al., 2007; Demeure et al., 2011; Feliciangeli et al., 2015). K2P channels activity was shown to modulate various important physiological processes such as pain perception (Alloui et al., 2006) and cardiac activity (Ellinghaus et al., 2005; Decher et al., 2017; Schmidt et al., 2017). Human K2P3.1 channels (TASK-1) are expressed mainly in the atria and possess a promising target for atrial fibrillation treatment (Schmidt et al., 2014; Schmidt et al., 2018). A mutation in K2P9.1 (TASK-3) is connected to the Birk–Barel syndrome, mental retardation, and unique dysmorphism syndrome (Barel et al., 2008). Also, the effect of several volatile analgesics is mediated, in part, through their action on K2P2.1 and K2P4.1 (TRAAK) (Franks and Honore, 2004).
Terpenes are a large group of structurally diverse organic chemicals that are mostly produced in plants. Monoterpenes (MTs) are terpenes that are composed of two five-carbon isoprene units. For centuries, MTs have been known for their beneficial effects as antifungal agents (Marei et al., 2012), antibacterial (Garcia et al., 2008), and analgesic (Khalilzadeh et al., 2016) agents. Terpenes have been proposed as remedies for the treatment of pain (Quintans-Junior et al., 2013; Quintans Jde et al., 2013; Guimaraes et al., 2014) and cardiovascular diseases (Magyar et al., 2004; Aydin et al., 2007; Menezes et al., 2010; Peixoto-Neves et al., 2010; Santos et al., 2011), and were shown to possess antitumor, local anesthetic, and anti-ischemic abilities (Koziol et al., 2014).
Several MTs were found to affect ion channels, both in excitable cells (Oz et al., 2015) and in other tissues (Muruganathan et al., 2017). To name a few, carvacrol and thymol were found to activate and sensitize the murine and human transient receptor potential (TRP) channel TRPV3, and acyclic MTs like citronellol, nerol, and their derivatives were found to modulate the activity of TRPA1 (Ortar et al., 2014). MTs were found to act upon other TRP channels (Xu et al., 2006; Parnas et al., 2009), as well as on voltage-gated ion channels and GABA receptors (Czyzewska and Mozrzymas, 2013; Kawasaki et al., 2013). However, their activity on members of the K2P potassium channels had not yet been studied.
Recently (Arazi et al., 2020), we reported the activation of K2P2.1 by various MTs. Here, we report that MTs activate the other two mechano-gated K2P channels (i.e., K2P4.1 and K2P10.1), in addition to members of other groups of K2P channel families (e.g., TALK, TRESK). Moreover, we found that MTs display remarkable selectivity towards the different K2P channels, and we report that they selectively inhibited the voltage-dependent current of TASK family members.
Methods
Animals
All experiments using animals were performed following the guidelines of the Institutional Animal Care and Use Committee. The project approval number is IL-61-09-2015.
Cloning
Channels were cloned into plasmid pRAT that included a T7 RNA polymerase promoter to enable cRNA synthesis, as well as the 3′-UTR and 5′-UTR sequences of the Xenopus laevis β-actin gene to ensure efficient expression in Xenopus oocytes. Competent Escherichia coli DH5α cells were transformed by heat shock. Plasmid DNA was purified with a Wizard Plus SV Miniprep kit (Promega). Restriction enzyme digestions were performed according to the manufacturer’s instructions (Fermentas or NEB). Point mutations were generated according to the Quickchange site-directed mutagenesis technique (Stratagene) and confirmed by sequencing. cRNA was transcribed in vitro by T7 polymerase using an AmpliCap High Yield Message Maker (Epicentre) kit.
Electrophysiology
Xenopus laevis oocytes were isolated and injected with 20–40 nl of solutions containing 0.3–40 ng cRNA using a 3.5″ Drammond#3-000-203-G/X glass capillary, pulled in a Sutter P97 capillary puller and a Drummond manual oocyte microinjection pipette (3-000-510-X). Whole-cell currents were measured 1–3 days after injection by the two-electrode voltage-clamp technique (GeneClamp 500B, Axon Instruments). Data were filtered at 2 kHz and sampled at 5 kHz with Clampex 9.0 software (Axon Instruments). For two-electrode voltage-clamp experiments, the pipette contained 3M KCl and the bath solution contained (in mM) unless otherwise noted: 4 KCl, 96 NaCl, 1 MgCl2, 0.3 CaCl2, 5 HEPES, pH 7.4 with NaOH (standard solution). All measurements of K2P5.1 and K2P17.1 channels were performed at pH = 9.0. When needed, bath solution sodium ions were isotonically replaced by potassium ions and vice versa. When testing MT activity, the standard bath solution was supplemented with the same concentration of the solvent (ethanol) as of the tested chemical.
Injection of cRNA into oocytes was done in OR-2 solution (in mM: 5 HEPES, 1 MgCl2, 2.5 KCl, 82.5 NaCl, pH = 7.4). Post-injection oocytes were maintained in ND-91 solution (in mM: 5 HEPES, 1 MgCl2, 1.8 CaCl2, 2 KCl, 91 NaCl, pH = 7.4). Specific recording protocols are mentioned in the relevant figure legends. To determine the voltage-dependent fraction of the current, the initial, voltage-independent (instantaneous) current was estimated by fitting the current to an exponential decay slope as the initial currents are masked by the capacitive transient current, as was previously described (Arazi et al., 2020).
Chemicals
Carvacrol (cat#282197), thymol (cat#T0501), p-cymene (cat#C121452), 4-isopropylphenol (cat# 175404), eugenol (cat#E51791, cinnamaldehyde (cat#W228613), menthol (cat#M2772), beta-citronelol (cat# C83201), geraniol (cat#16333, 4-methylcatechole (cat# M34200), and arachidonic acid (cat#A3611) were all purchased from Sigma-Aldrich.
Preparation of Compounds
Compounds delivered as powders were dissolved into stock solutions (4–6 M) in 100% ethanol. Compounds delivered as liquid oils (6.5–7.5 M) were diluted 1:1 with ethanol to form stock solutions and were kept at −20°C for up to two weeks. Just before testing, stock solutions were diluted in the bath solution to the desired concentration, and diluted compounds were vigorously vortexed until completely dissolved. All solutions were supplemented with ethanol to a final concentration of 0.1% (v/v) (confirmed not to harm the oocytes). The pH was corrected to 7.4 ± 0.05 using NaOH or HCl.
Statistical Analysis
Data were expressed as the mean ± standard error of the mean (SEM) and analyzed and presented using Microsoft Excel 2016. Groups of two paired data sets were analyzed using a Wilcoxon Signed Ranks test and groups of two unpaired data sets were analyzed using Mann–Whitney U test with IBM SPSS Statistics ver. 20 software. Values were considered to be significantly different when the z-value was ≤0.05 (*), ≤0.01 (**), or ≤0.001 (***). All experiments were repeated with at least five oocytes.
Results
Activation of Mechano-Gated Channels by Monoterpenes
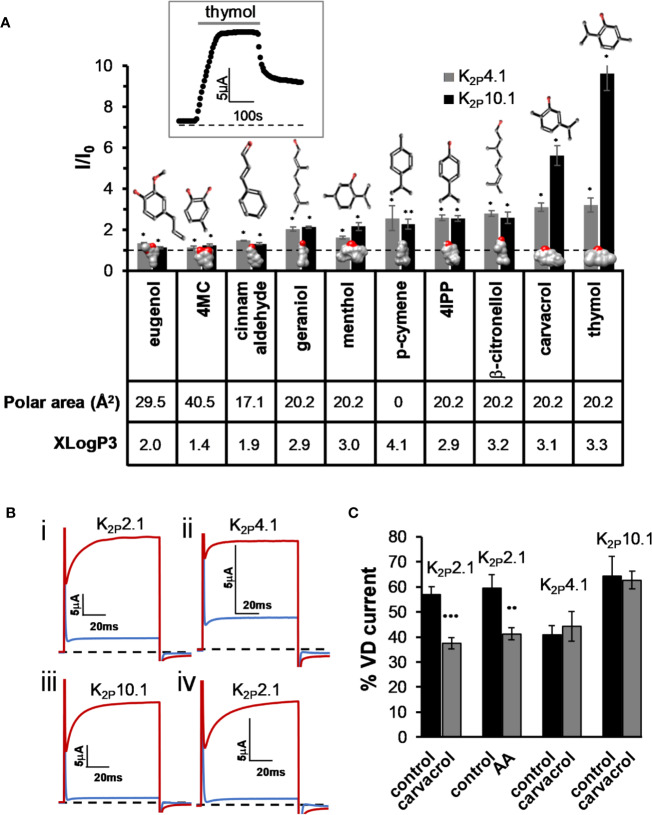
As we have recently reported (Arazi et al., 2020), the activity of K2P2.1 is modulated by various MTs. K2P2.1 is a member of the mechano-gated K2P channel clade that includes K2P4.1 (TRAAK) and K2P10.1 (TREK-2). We, therefore, investigated whether MTs modulate all mechano-gated K2P channels. An external application of MTs resulted in the increase in currents of K2P10.1 channels by seven compounds and in the increase in currents of K2P4.1 channels by eight of the tested compounds, although to lower levels ( Figure 1A ). As was found for K2P2.1 (Arazi et al., 2020), the phenol-containing compounds (carvacrol and thymol) were more potent in opening both channels, while linear compounds and compounds containing no hydroxyl group were less effective ( Figure 1A ). Under standard testing conditions, currents of most K2P channels are composed of two components: an instantaneous “leak” current (voltage-independent, VI) and a voltage-dependent (VD) current (Schewe et al., 2016), as demonstrated in Figure 1B . We, thus, looked at whether MTs affect the voltage sensitivity of the channels by looking at the change in the proportion of the two current components. For K2P4.1 and K2P10.1 channels, no change in voltage dependency was detected ( Figure 1C ). However, for K2P2.1 channels, voltage dependency was reduced during carvacrol application, as was previously reported (Arazi et al., 2020). As expected (Schewe et al., 2016), arachidonic acid had a similar effect to carvacrol ( Figure 1C ).
Figure 1.
Activation of K2P4.1 and K2P10.1 by monoterpenes. (A) Activation of K2P4.1 and K2P10.1. Oocyte membrane potential was held at −80 mV and pulsed to +25 mV for 75 ms with 5 s interpulse intervals. All MTs were applied at the same concentration (0.3 mM), and currents were measured after 4 min (mean ± S.E., n = 5–10). Polar area (Å2) and octanol–water partition coefficient (logP) prediction (XLogP3) were obtained from PubChem (Kim et al., 2016). 2D structures and the coordinates for the 3D structures of the terpenes were obtained from ChemSpider. 3D models were performed with the UCSF Chimera package (Pettersen et al., 2004). Oxygen molecules are colored red. The dashed line represents no change from the initial current. Inset- currents of a representative oocyte expressing K2P10.1 before, during and after thymol application. (B) Currents at 60 mV before (in red) and during (in blue) application of carvacrol (i–iii) or arachidonic acid (AA) (iv), on K2P2,1 (i, iv), K2P4.1 (ii), and K2P10.1 (iii) (C). Fraction of voltage-dependent current (in %) before (black) and after (gray) application of 0.3 mM carvacrol or arachidonic acid (AA, 100 µM). A fit of the current (at 60 mV) to an exponential decay slope was used to identify the initial current (mean ± S.E., n = 6–9).
Activation of TALK and TRESK Channels
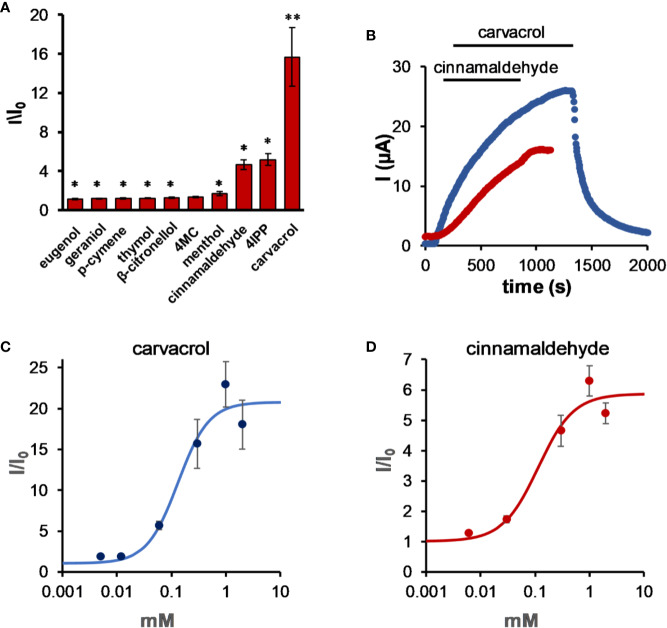
The activity of members of the TALK clade of potassium channels (K2P5.1, K2P16.1, and K2P17.1; TASK-2, TALK-1, and TALK-2, respectively) is sensitive to external pH, as these channels are activated at an alkaline pH (Decher et al., 2001; Girard et al., 2001). K2P5.1 (TASK-2) is expressed mostly at the tubular epithelial and is involved in pathological conditions such as Balkan endemic nephropathy (BEN) (Toncheva et al., 2014; Reed et al., 2016). While most tested compounds had almost no effect on this channel, carvacrol and cinnamaldehyde ( Figure 2 ) activated it by up to 17-fold (15.7 ± 3.0, n = 7 and 4.7 ± 0.5, n = 5, respectively, 0.3 mM for both compounds), although at different rates ( Figure 2B ). While activation by carvacrol was reversible, activation by cinnamaldehyde was not, even after a 5-min wash. It should be noted that irreversible activation of TRPA1 channels by cinnamaldehyde was previously reported (Macpherson et al., 2007). The minimal concentration that showed substantial activation of the channel was 12 µM for carvacrol (albeit not statistically significant) (1.9 ± 0.1-fold, n = 5, Figure 2C ) and 30 µM for cinnamaldehyde (2.8 ± 0.3-fold, n = 5; statistically significant).
Figure 2.
Carvacrol and cinnamaldehyde robustly activate K2P5.1. (A) Activation of K2P5.1 by monoterpenes. Oocyte membrane potential was held at −80mV and pulsed to +25 mV for 75 ms with 5 s interpulse intervals. All MTs were applied at the same concentration (0.3 mM), and currents were measured after 5 min of incubation (mean ± S.E., n = 6–10). (B) Time course for activation by 0.3 mM carvacrol and 0.3 mM cinnamaldehyde for representative oocytes expressing K2P5.1 channels. Currents were measured as in (A). (C) Carvacrol dose-response for K2P5.1 channels (mean ± S.E., n = 5–8) (EC50 = 0.13 ± 0.05 mM). (D) Cinnamaldehyde dose–response for K2P5.1 channels (mean ± S.E., n = 5–8) (EC50 = 0.11 ± 0.07 mM). Currents were measured at 25 mV, as in (A), after incubation for 5 min. *p ≤ 0.05, **p ≤ 0.01.
K2P16.1 and K2P17.1 are expressed predominantly in the pancreas and may be involved in the exocrine secretion of bicarbonate. A gain of function mutation in K2P17.1 was associated with progressive cardiac conduction disorder (Friedrich et al., 2014). While K2P16.1 was not affected by any of the tested MTs (not shown), K2P17.1 was moderately activated by menthol, thymol, β-citronellol, 4MC, and carvacrol ( Figure 3A ). K2P18.1 (TRESK, KCNK18) is unique among other K2P channels by having an extra-long cytoplasmatic domain that is located between the two pore-forming domains (Sano et al., 2003). This channel is expressed in the dorsal root ganglion, trigeminal ganglion neurons, and spinal cord (Sano et al., 2003; Kang and Kim, 2006; Dobler et al., 2007). Mutation in this channel was linked to familial migraine with aurora (Lafreniere et al., 2010). K2P18.1 was opened rapidly and robustly by carvacrol and to a lesser degree by thymol and 4-isopropylphenol (4IPP) ( Figure 3B ). Other compounds displayed mild to no effect on this channel. Since with mechano-gated K2P channels, we observed a reduction in the proportion of the voltage-dependent current as a result of activation by carvacrol ( Figure 1C ), we tested this feature in these channels as well. In K2P5.1 channels, the share of the voltage-dependent current indeed decreased ( Figure 3C ). In contrast, for K2P17.1 channels, the share of the voltage-dependent current did not change. In K2P18.1 channels, where the basal share of the voltage-dependent current was low, currents displayed more sensitivity to voltage after incubation with carvacrol ( Figure 3C ).
Figure 3.
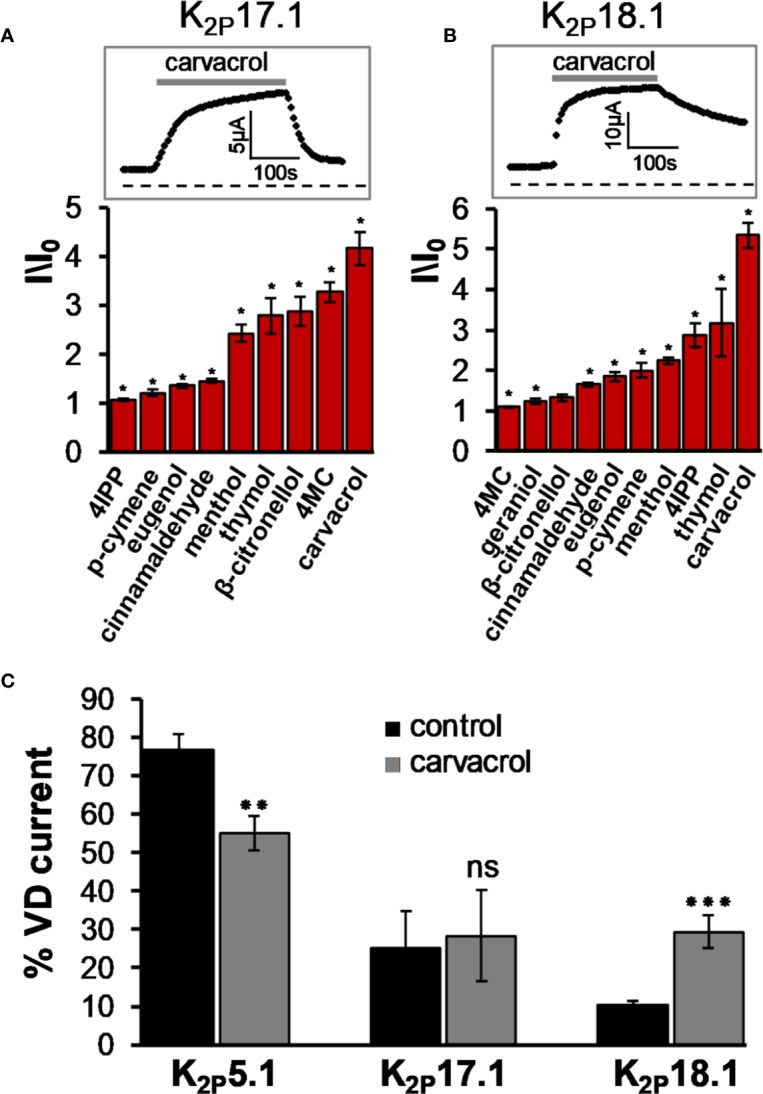
K2P17.1 and K2P18.1 are activated by monoterpenes. (A), (B) Activation of K2P17.1 (A) and K2P18.1 (B) by monoterpenes. Oocyte membrane potential was held at −80 mV and pulsed to +25 mV for 75 ms with 5 s interpulse intervals. All MTs were applied at the concentration of 0.3 mM, and currents were measured 4 min after application of the indicated monoterpene (mean ± S.E., n = 5–10). Insets: currents of representative oocytes expressing K2P17.1 (A) and K2P18.1 (B) during application of 0.3 mM carvacrol. (C) Fraction of voltage-dependent (VD) current (in %) under control conditions and after application of 0.3 mM carvacrol for three channel types, as indicated. Oocytes were held at −80mV, and currents were measured at 30 mV. A fit of the results to an exponential decay slope was used to identify the initial current (mean ± S.E., n = 6–9). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ns, not significant.
Acid-Sensitive K2P3.1 and K2P9.1 (TASK) Channels Are Inhibited by Monoterpenes
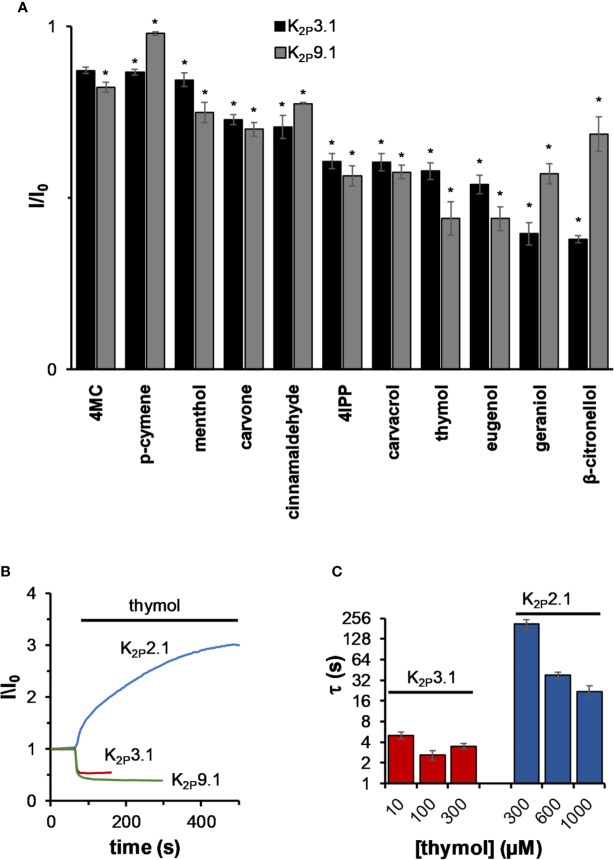
K2P3.1 and K2P9.1 (TASK-1 and TASK-3, respectively) channels are expressed in the pancreas and placenta and to a lesser degree in the brain, heart, and kidneys (Duprat et al., 1997; Kim et al., 2000). Unlike all other tested K2P channels, current levels of K2P3.1 and K2P9.1 decreased by all tested MTs ( Figure 4A ). In contrast to mechano-gated channels, which were affected mostly by cyclic phenolic compounds like thymol and carvacrol, TASK channels were affected mostly by linear MTs such as β-citronellol and geraniol ( Figure 4A ). Inhibition was rapid (e.g. τ = 3.8 ± 0.2, 6.2 ± 1.2, and 5.6 ± 1.5 s for thymol, β-citronellol, and carvacrol, respectively, n = 7–11) and within the solution change rate in our system (τ = 7.7 ± 1.0 s, n = 5). When using thymol, a monoterpene that affects both channel types, it was obvious that the inhibition rate was remarkably faster than that observed for activation of mechano-gated K2P channels ( Figure 4B ). To make sure that the differences in rates were not merely a result of a difference in affinities (Kinhibition of K2P3.1 by thymol is 20 ± 5 µM and Kactivation of K2P2.1 by thymol is 290 ± 50 µM, not shown), we measured the current change rates at three different concentrations for each channel ( Figure 4C ). As expected, no measurable change in the rate of K2P3.1 channels inhibition was observed, while the activation rate for K2P2.1 channels was much slower at all concentrations.
Figure 4.
The effect of monoterpenes on acid-sensitive K2P channels. (A) Inhibition of K2P3,1 and K2P9.1 currents. Currents were measured before and after 2 in incubation with the indicated MT (mean ± S.E., n = 5–10). All MTs were applied at a concentration of 0.3 mM. (B) Normalized currents during 0.3 mM thymol application for representative oocytes expressing either K2P2.1, K2P3.1, and K2P9.1. (C) The time constant (τ) of current changes during the application of thymol at different concentrations for K2P2.1 or K2P3.1 (mean ± S.E., n = 6–10). *p ≤ 0.05.
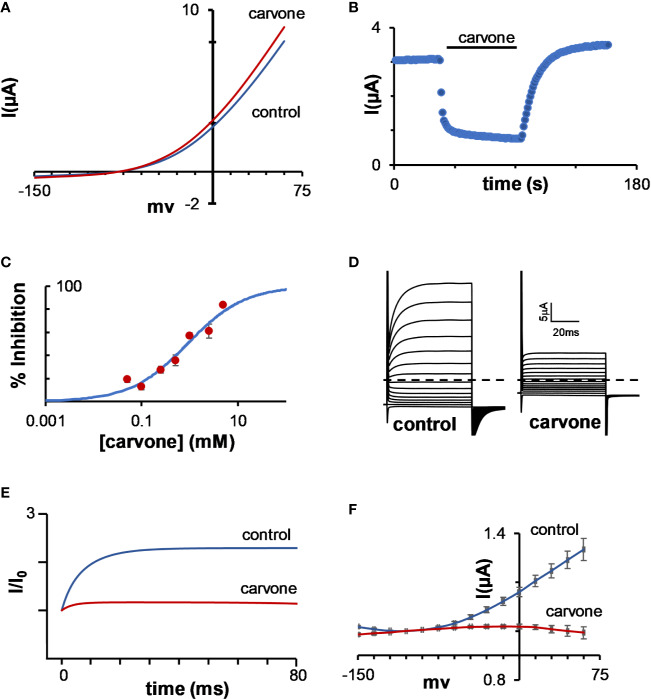
To further examine the unique activity of MTs on TASK channels, we tested the activity of carvone on K2P3.1 channels as a model, as this monoterpene had no activity on K2P2.1 channels ( Figure 5A ), while readily and rapidly ( Figure 5B ) decreasing K2P3.1 channel currents with a Kinhibition of 0.90 ± 0.16 mM ( Figure 5C ). As in most K2P channels, when held at −80 mV, K2P3.1 currents are comprised of two components: an instantaneous, voltage-independent (VI), and a time- and voltage-dependent component (VD) ( Figure 5D , control). As was observed with other MTs, the VD component of the current was dramatically reduced due to application of carvone ( Figures 5D, E ). The inhibition of the VD currents resulted, as anticipated, in the disappearance of K2P3.1 channels tail currents ( Figure 5F ).
Figure 5.
Inhibition of K2P3.1 by carvone. (A) Current–voltage relationship of a representative oocyte expressing K2P2.1, before and after application of 1mM carvone. (B) The current of a representative oocyte expressing K2P3.1 during incubation with 1 mM carvone. Oocyte membrane potential was held at −80 mV and pulsed to +25 mV for 75 ms with 1 s interpulse intervals. (C) Carvone dose–response for K2P3.1 channels (mean ± S.E., n = 6–10) (Kinhibition = 0.90 ± 0.16 mM). (D) Currents of a representative oocyte expressing K2P3.1 channels before and during incubation with 1 mM carvone at 20 mM potassium at the bath. The oocyte was held at −80 mV, then at −135 mV for 30 ms, and then pulsed from −150 mV to 60 mV in 15 mV intervals. The dashed line represents zero current. (E) Currents at 60 mV of a representative oocyte before and during incubation with 1 mM carvone. Currents were normalized to the initial current. A fit of the results to an exponential decay slope was used to identify the initial current. (F) Tail analysis of currents before and during incubation with 1 mM carvone at external potassium concentration of 100 mM (mean ± S.E., n = 6). For each oocyte, currents were normalized to the current at −105 mV.
External K+ concentration is known to affect the open probability of the selectivity filter gate of potassium channels (Hille, 2001) and, in particular, that of K2P channels (Zilberberg et al., 2001). We, thus, tested the effect of external K+ on carvone-induced current inhibition. The concentration of external K+ had a profound effect on K2P3.1 currents. A significant current decrease was observed under low (0 and 4 mM) external K+ concentration ( Figure 6A ). At all external potassium concentrations, carvone reduced K2P3.1 currents ( Figures 6B, C ). Currents at 0 mM (no added potassium) external K+ were too low to allow further accurate analysis. When we analyzed the effect of carvone on each current component (VD or VI) at three external K+ concentrations, we found that the VD current was almost completely eliminated at all concentrations, while the VI current was only mildly affected ( Figures 6C–G ). At 60 mV, inhibition was reduced by high external K+ ( Figure 6C ).
Figure 6.
The effect of external K+ on the inhibition of the voltage-dependent current in K2P3.1. (A, B) Steady-state current–voltage relationships for oocytes expressing K2P3.1 at four external potassium concentrations (0, 4, 20, and 100 mM) under control conditions (A) or after incubation with 1 mM carvone (B). Oocytes were held at −80 mV, pulsed to −135 mV for 30 ms, and then pulsed from −150 mV to 60 mV in 15 mV voltage intervals (mean ± S.E., n = 6–9). (C) The fraction of inhibited current due to carvone application of the total current (Total) and its components: the voltage-independent (VI) and the voltage-dependent (VD) currents. Currents at 60 mV were tested at three external potassium concentrations (4, 20, and 100 mM) (mean ± S.E., n = 6–9). (D–G) Current–voltage relationships for oocytes expressing K2P3.1 channels at three different external potassium concentrations, as indicated (mean ± S.E., n = 6–9). Currents were measured as in (A). The voltage-independent (D, E) and the voltage-dependent (F, G) fractions of the current were calculated as in Figure 1C and are presented individually. Measurements were performed before (D, F) and after (E, G) application of 1 mM carvone. *p ≤ 0.05, ns, not significant.
Discussion
In this study, we used an exogenous expression system to measure the impact of MTs on the activity of various human K2P channels. MTs were found to affect various types of ion channels at high micromolar to millimolar concentrations (Lauritzen et al., 2005; Joca et al., 2012; Johnson et al., 2012; Brohawn et al., 2014; Pham et al., 2015; Bavi et al., 2016; Cabanos et al., 2017; Li Fraine et al., 2017), comparable to the concentrations that were found here to affect K2P channels. Mechano-gated K2P4.1 and K2P10.1 were activated mainly by the cyclic aromatic phenolic MTs, carvacrol, and thymol, with the latter being the most effective. This is in accordance with our finding that the same MTs are the best activators of the other mechano-gated K2P channel, K2P2.1 (Arazi et al., 2020). Unlike in K2P2.1 channels, the voltage dependency of the current did not change as a result of MT activation. Our findings suggest that for best activation of mechano-gated K2P channels, terpenes should be moderately hydrophobic (XLogP3 ~ 3, as is the case for carvacrol and thymol) and to be able to penetrate, yet not become embedded in, the bilayer due to the presence of a small polar area. Also, a phenol moiety was necessary to obtain high channel-stimulating activity. We believe that such molecules are embedded into the outer leaflet of the bilayer and perturbed its structure and/or curvature. Less hydrophobic and more polar molecules (a polar area larger than 20 Å2 and XLogP value lower than 3) will stay in the polar area of the outer leaflet, while more hydrophobic molecules will sink deeper into the bilayer. We showed that for K2P2.1, the cytoplasmic carboxyl-terminal of the channel is needed for the activity of MTs (Arazi et al., 2020). It is yet to be determined whether this is the mechanism by which the other two mechano-gated K2P channels are activated by MTs.
The alkaline-sensitive K2P5.1 and K2P17, but not K2P16.1, were also found to be activated by MTs. While K2P17.1 was only mildly activated (up to a fourfold increase in current, Figure 3A ), K2P5.1 currents increased by up to 17-fold (0.3 mM, Figure 2A ). Even at 60 µM carvacrol, K2P5.1 currents increased by 5.5-fold ( Figure 2C ). The selectivity of the MTs towards these channels was not the same as for mechano-gated channels. While carvacrol activated, to a degree, all channels, K2P5.1 was uniquely activated by cinnamaldehyde, but not by thymol, and K2P17.1 was uniquely activated by β-citronellol and 4MC ( Figure 3A ). K2P18.1 channels were activated by the same MTs, up to 5.3-fold ( Figure 3B ). Our findings indicate a certain degree of selectivity in the sensitivity of different K2P channels to MTS, as some channels are activated by MTs that are inactive against other channels. The origin for this apparent selectivity is unclear since whether membrane-adhered hydrophobic molecules directly bind to channels or if they change membrane properties, causing each channel to react differently to those changes is an ongoing debate (Cristani et al., 2007; Ogawa et al., 2009; Lee, 2011; Nury et al., 2011; Epand et al., 2015; Sacchi et al., 2015). This dilemma applies not only to monoterpenes but also to other lipophilic molecules such as general anesthetics and alcohols (Howard et al., 2014), as well as for endocannabinoids (Oz, 2006) and steroids (Hill et al., 2015), all are allosteric modulators of several structurally different ion channels (Sanchez-Borzone et al., 2014; Oz et al., 2015; Ton et al., 2015). By changing the physicochemical properties of the surrounding membrane environment (Sanchez et al., 2004; Turina et al., 2006; Zunino et al., 2011; Reiner et al., 2013), and energetic requirements for gating-related conformational changes monoterpenes could affect ligand-gated ion channels (LGICs) (Fantini and Barrantes, 2009; Barrantes et al., 2010), and voltage-gated ion channel (VGICs) as reviewed by Oz at al. 2015 (Oz et al., 2015). On the other hand, evidence for direct binding of lipophilic monoterpenes, such as carvacrol and thymol, to specific amino acid residues in the transmembrane domain of Human 5-Hydroxytryptamine Type 3 (5-HT3Rs) were found (Lansdell et al., 2015). Similarly, the different potency of menthol stereoisomers (Walstab et al., 2014) on 5-HT3Rs or GABA(A) receptor (Corvalan et al., 2009) suggest also a degree of selectivity in monoterpenes action on ion channels.
For K2P2.1, it was shown that channel opening would result in a reduction in its voltage dependency (Bockenhauer et al., 2001; Lopes et al., 2005; Cohen et al., 2008). Schewe et al. displayed that, for all mechano-gated K2P channels, activation causes a gating mode shift within the selectivity filter and that these channels can be converted into a “classical” leak mode when stimulated by arachidonic acid or PIP2 (Schewe et al., 2016). This phenomenon was observed in our experiments with K2P2.1, but not with K2P4.1 or K2P10.1 channels. Reduction in the percent of voltage-dependent current was observed with K2P5.1, but not with K2P17.1, while with K2P18.1, an increase in the voltage-dependent current was recorded ( Figure 3C ). The voltage-dependency of the K2P channels’ current is directly related to their open probability. Since in our experiments, only a relative estimation of the open probability of the channel is measured, we believe that it is plausible that “leak-like” behavior of the channel is achieved only at high open probabilities and that under our experimental conditions, this was not always achieved.
The acid-sensitive TASK channels, K2P3.1 and K2P9.1, were affected differently by MTs: a. most tested MTs caused a decrease in TASK channel currents ( Figure 4A ); b. the current decrease rate was high (a few seconds), while the activation rate of other channels was at least an order of magnitude slower ( Figures 4B, C and not shown); and c. unlike other tested K2P channels, TASK channels were affected mostly by linear MTs (β-citronellol and geraniol, Figure 4A ). These three observations suggest that TASK channels are affected by MTs by a different mechanism than the other tested channels. Thus, we further studied the characteristics of the current inhibition of TASK channels by MTs using K2P3.1 and carvone as a model.
It was clearly evident that carvone almost completely eliminated the voltage-gated portion of K2P3.1 currents ( Figures 5D–F ). Since voltage-dependent gating was shown to originate from the movement of three to four potassium ions into the high electric field of an inactive selectivity filter (Schewe et al., 2016) and since the stability of the selectivity filter was shown to be affected by the concentration of external potassium ions in potassium channels in general (Hille, 2001) and in K2P channels in particular (Zilberberg et al., 2001), we looked at the influence of external potassium concentrations on K2P3.1 activity and sensitivity to carvone. External potassium ion levels had a clear effect on channel gating, as currents decreased dramatically at low external levels ( Figure 6A ). At all potassium levels, carvone reduced K2P3.1 current, while specifically targeting the voltage-dependent portion ( Figures 6C–G ). While the voltage-independent portion of the current behaved, as expected, like a potassium GHK-leak, the voltage-dependent portion displayed an outward rectification behavior that was partly dependent on potassium concentration ( Figure 6F ). We suggest that as external potassium ions stabilize the selectivity filter at its conductive state, they minimize the destabilizing structural changes caused by carvone. For this reason, we suggest that MTs might serve as a useful tool in studying the voltage-dependency of TASK channels as they specifically target the voltage-dependent portion of the current. Recently, it was reported that bupivacaine blocks TASK channels in a voltage-dependent manner by disrupting the K+-flux gating mechanism (Rinne et al., 2019), and that it is located laterally in the side fenestrations of K2P3.1 channels and interacts with residues of the pore helix, and the M2, M3, and M4 segments. It is conceivable that both bupivacaine and MTs bind to a similar binding site within the membrane and, thus, affect channels through a similar mechanism.
K2P channels play a role in various physiological processes such as pain signaling (Li and Toyoda, 2015) heart function (Hancox et al., 2016), and more (Lesage and Barhanin, 2011; Bandulik et al., 2015; Renigunta et al., 2015; Riegelhaupt et al., 2018). For example, activation of mechano-gated K2P, as well as K2P18.1 channels, is expected to result in reduced pain sensation and neuroprotection. Terpenes have been proposed as analgesic agents (Khalilzadeh et al., 2016), as remedies for the treatment of pain and cardiovascular diseases (Magyar et al., 2004; Aydin et al., 2007; Menezes et al., 2010; Peixoto-Neves et al., 2010; Santos et al., 2011; Quintans-Junior et al., 2013; Quintans Jde et al., 2013; Guimaraes et al., 2014) and were shown to possess antitumor, local anesthetic, and anti-ischemic abilities (Koziol et al., 2014). Any of these activities of terpenes that stem from their activity on K2P channels remains to be determined. Even though MTs are regularly consumed by people as food additives, due to their low concentration in food, they are unlikely to have any pharmacological effect. However, the extensive use of MTs in traditional medicine might raise the possibility of their beneficial pharmacological use when given in high concentrations.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee, Ben Gurion University. The project approval number is IL-61-09-2015.
Author Contributions
Conception and design of the study: EA and NZ. Acquisition of data: EA and GB. Analysis and interpretation of data: EA. Writing the manuscript: EA and NZ.
Funding
This work was supported by a grant from the Israel Science Foundation (1877/15) to NZ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Prof. Dierk Thomas for his generous gift of the K2P4.1, K2P10.1, K2P16.1, and K2P17.1 clones.
Abbreviations
MTs, monoterpenes; K2P, two pore-domain potassium channels.
References
- Alloui A., Zimmermann K., Mamet J., Duprat F., Noel J., Chemin J., et al. (2006). TREK-1, a K+ channel involved in polymodal pain perception. EMBO EMBO J. 25, 2368–2376. 10.1038/sj.emboj.7601116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi E., Blecher G., Zilberberg N. (2020). A regulatory domain in the K2P2.1 (TREK-1) carboxyl-terminal allows for channel activation by monoterpenes. Mol. Cell. Neurosci. In press. 10.1016/j.mcn.2020.103496. [DOI] [PubMed] [Google Scholar]
- Aydin Y., Kutlay O., Ari S., Duman S., Uzuner K., Aydin S. (2007). Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 73, 1365–1371. 10.1055/s-2007-990236 [DOI] [PubMed] [Google Scholar]
- Bandulik S., Tauber P., Lalli E., Barhanin J., Warth R. (2015). Two-pore domain potassium channels in the adrenal cortex. Pflugers Arch. 467, 1027–1042. 10.1007/s00424-014-1628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel O., Shalev S. A., Ofir R., Cohen A., Zlotogora J., Shorer Z., et al. (2008). Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am. J. Hum. Genet. 83, 193–199. 10.1016/j.ajhg.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J., Bermudez V., Borroni M. V., Antollini S. S., Pediconi M. F., Baier J. C., et al. (2010). Boundary lipids in the nicotinic acetylcholine receptor microenvironment. J. Mol. Neurosci. 40, 87–90. 10.1007/s12031-009-9262-z [DOI] [PubMed] [Google Scholar]
- Bavi O., Cox C. D., Vossoughi M., Naghdabadi R., Jamali Y., Martinac B. (2016). Influence of global and local membrane curvature on mechanosensitive ion channels: A finite element approach. Membranes 6, 14. 10.3390/membranes6010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhauer D., Zilberberg N., Goldstein S. A. (2001). KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat. Neurosci. 4, 486–491. 10.1038/87434 [DOI] [PubMed] [Google Scholar]
- Brickley S. G., Aller M. I., Sandu C., Veale E. L., Alder F. G., Sambi H., et al. (2007). TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J. Neurosci. 27, 9329–9340. 10.1523/JNEUROSCI.1427-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S. G., Su Z., Mackinnon R. (2014). Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. U. S. A 111, 3614–3619. 10.1073/pnas.1320768111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanos C., Wang M., Han X., Hansen S. B. (2017). A Soluble Fluorescent Binding Assay Reveals PIP2 Antagonism of TREK-1 Channels. Cell Rep. 20, 1287–1294. 10.1016/j.celrep.2017.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S. (2002). Potassium channel structures. Nat. Rev. Neurosci. 3, 115–121. 10.1038/nrn727 [DOI] [PubMed] [Google Scholar]
- Cohen A., Ben-Abu Y., Hen S., Zilberberg N. (2008). A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 283, 19448–19455. 10.1074/jbc.M801273200 [DOI] [PubMed] [Google Scholar]
- Corvalan N. A., Zygadlo J. A., Garcia D. A. (2009). Stereo-selective activity of menthol on GABA(A) receptor. Chirality 21, 525–530. 10.1002/chir.20631 [DOI] [PubMed] [Google Scholar]
- Cristani M., D’arrigo M., Mandalari G., Castelli F., Sarpietro M. G., Micieli D., et al. (2007). Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J. Agric. Food Chem. 55, 6300–6308. 10.1021/jf070094x [DOI] [PubMed] [Google Scholar]
- Czyzewska M. M., Mozrzymas J. W. (2013). Monoterpene alpha-thujone exerts a differential inhibitory action on GABA(A) receptors implicated in phasic and tonic GABAergic inhibition. Eur. J. Pharmacol. 702, 38–43. 10.1016/j.ejphar.2013.01.032 [DOI] [PubMed] [Google Scholar]
- Decher N., Maier M., Dittrich W., Gassenhuber J., Bruggemann A., Busch A. E., et al. (2001). Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett. 492, 84–89. 10.1016/s0014-5793(01)02222-0 [DOI] [PubMed] [Google Scholar]
- Decher N., Ortiz-Bonnin B., Friedrich C., Schewe M., Kiper A. K., Rinne S., et al. (2017). Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 9, 403–414. 10.15252/emmm.201606690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeure O., Lecerf F., Duby C., Desert C., Ducheix S., Guillou H., et al. (2011). Regulation of LPCAT3 by LXR. Gene 470, 7–11. 10.1016/j.gene.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Dobler T., Springauf A., Tovornik S., Weber M., Schmitt A., Sedlmeier R., et al. (2007). TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J. Physiol. 585, 867–879. 10.1113/jphysiol.2007.145649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. (1997). TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 16, 5464–5471. 10.1093/emboj/16.17.5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus P., Scheubel R. J., Dobrev D., Ravens U., Holtz J., Huetter J., et al. (2005). Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J. Thorac. Cardiovasc. Surg. 129, 1383–1390. 10.1016/j.jtcvs.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Epand R. M., D’souza K., Berno B., Schlame M. (2015). Membrane curvature modulation of protein activity determined by NMR. Biochim. Biophys. Acta 1848, 220–228. 10.1016/j.bbamem.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Fantini J., Barrantes F. J. (2009). Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim. Biophys. Acta 1788, 2345–2361. 10.1016/j.bbamem.2009.08.016 [DOI] [PubMed] [Google Scholar]
- Feliciangeli S., Chatelain F. C., Bichet D., Lesage F. (2015). The family of K2P channels: salient structural and functional properties. J. Physiol. 593, 2587–2603. 10.1113/jphysiol.2014.287268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Honore E. (2004). The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol. Sci. 25, 601–608. 10.1016/j.tips.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Friedrich C., Rinne S., Zumhagen S., Kiper A. K., Silbernagel N., Netter M. F., et al. (2014). Gain-of-function mutation in TASK-4 channels and severe cardiac conduction disorder. EMBO Mol. Med. 6, 937–951. 10.15252/emmm.201303783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R., Alves E. S., Santos M. P., Aquije G. M., Fernandes A. A., Dos Santos R. B., et al. (2008). Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. Braz. J. Microbiol. 39, 163–168. 10.1590/S1517-838220080001000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C., Duprat F., Terrenoire C., Tinel N., Fosset M., Romey G., et al. (2001). Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels. Biochem. Biophys. Res. Commun. 282, 249–256. 10.1006/bbrc.2001.4562 [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Bockenhauer D., O’kelly I., Zilberberg N. (2001). Potassium leak channels and the KCNK family of two-P-domain subunits. Nat. Rev. Neurosci. 2, 175–184. 10.1038/35058574 [DOI] [PubMed] [Google Scholar]
- Guimaraes A. G., Serafini M. R., Quintans-Junior L. J. (2014). Terpenes and derivatives as a new perspective for pain treatment: a patent review. Expert Opin. Ther. Pat. 24, 243–265. 10.1517/13543776.2014.870154 [DOI] [PubMed] [Google Scholar]
- Hancox J. C., James A. F., Marrion N. V., Zhang H., Thomas D. (2016). Novel ion channel targets in atrial fibrillation. Expert Opin. Ther. Targets 20, 947–958. 10.1517/14728222.2016.1159300 [DOI] [PubMed] [Google Scholar]
- Hill M., Duskova M., Starka L. (2015). Dehydroepiandrosterone, its metabolites and ion channels. J. Steroid Biochem. Mol. Biol. 145, 293–314. 10.1016/j.jsbmb.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Hille B. (2001). Ion channels of excitable membranes (Sunderland, Mass: Sinauer; ). . [Google Scholar]
- Howard R. J., Trudell J. R., Harris R. A. (2014). Seeking structural specificity: direct modulation of pentameric ligand-gated ion channels by alcohols and general anesthetics. Pharmacol. Rev. 66, 396–412. 10.1124/pr.113.007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca H. C., Cruz-Mendes Y., Oliveira-Abreu K., Maia-Joca R. P., Barbosa R., Lemos T. L., et al. (2012). Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J. Nat. Prod. 75, 1511–1517. 10.1021/np300050g [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Erickson J. W., Cerione R. A. (2012). C-terminal di-arginine motif of Cdc42 protein is essential for binding to phosphatidylinositol 4,5-bisphosphate-containing membranes and inducing cellular transformation. J. Biol. Chem. 287, 5764–5774. 10.1074/jbc.M111.336487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D., Kim D. (2006). TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 291, C138–C146. 10.1152/ajpcell.00629.2005 [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Mizuta K., Fujita T., Kumamoto E. (2013). Inhibition by menthol and its related chemicals of compound action potentials in frog sciatic nerves. Life Sci. 92, 359–367. 10.1016/j.lfs.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Khalilzadeh E., Hazrati R., Saiah G. V. (2016). Effects of topical and systemic administration of Eugenia caryophyllata buds essential oil on corneal anesthesia and analgesia. Res. Pharmaceut. Sci. 11, 293. 10.4103/1735-5362.189297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Bang H., Kim D. (2000). TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 275, 9340–9347. 10.1074/jbc.275.13.9340 [DOI] [PubMed] [Google Scholar]
- Kim S., Thiessen P. A., Bolton E. E., Chen J., Fu G., Gindulyte A., et al. (2016). PubChem Substance and Compound databases. Nucleic Acids Res. 44, D1202–D1213. 10.1093/nar/gkv951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol A., Stryjewska A., Librowski T., Salat K., Gawel M., Moniczewski A., et al. (2014). An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 14, 1156–1168. 10.2174/1389557514666141127145820 [DOI] [PubMed] [Google Scholar]
- Lafreniere R. G., Cader M. Z., Poulin J. F., Andres-Enguix I., Simoneau M., Gupta N., et al. (2010). A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 16, 1157–1160. 10.1038/nm.2216 [DOI] [PubMed] [Google Scholar]
- Lansdell S. J., Sathyaprakash C., Doward A., Millar N. S. (2015). Activation of human 5-hydroxytryptamine type 3 receptors via an allosteric transmembrane site. Mol. Pharmacol. 87, 87–95. 10.1124/mol.114.094540 [DOI] [PubMed] [Google Scholar]
- Lauritzen I., Chemin J., Honore E., Jodar M., Guy N., Lazdunski M., et al. (2005). Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 6, 642–648. 10.1038/sj.embor.7400449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. (2011). Biological membranes: the importance of molecular detail. Trends Biochem. Sci. 36, 493–500. 10.1016/j.tibs.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Lesage F., Barhanin J. (2011). Molecular physiology of pH-sensitive background K(2P) channels. Physiol. (Bethesda) 26, 424–437. 10.1152/physiol.00029.2011 [DOI] [PubMed] [Google Scholar]
- Li X. Y., Toyoda H. (2015). Role of leak potassium channels in pain signaling. Brain Res. Bull. 119, 73–79. 10.1016/j.brainresbull.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Li Fraine S., Patel A., Duprat F., Sharif-Naeini R. (2017). Dynamic regulation of TREK1 gating by Polycystin 2 via a Filamin A-mediated cytoskeletal Mechanism. Sci. Rep. 7, 17403. 10.1038/s41598-017-16540-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C. M. B., Rohács T., Czirják G., Balla T., Enyedi P., Logothetis D. E. (2005). PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J. Physiol. 564, 117–129. 10.1113/jphysiol.2004.081935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon R. (2003). Potassium channels. FEBS Lett. 555, 62–65. 10.1016/S0014-5793(03)01104-9 [DOI] [PubMed] [Google Scholar]
- Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., et al. (2007). Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. 10.1038/nature05544 [DOI] [PubMed] [Google Scholar]
- Magyar J., Szentandrassy N., Banyasz T., Fulop L., Varro A., Nanasi P. P. (2004). Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Eur. J. Pharmacol. 487, 29–36. 10.1016/j.ejphar.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Marei G. I. K., Abdel Rasoul M. A., Abdelgaleil S. (2012). Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pesticide Biochem. Physiol. 103 (2012), 56–61. 10.1016/j.pestbp.2012.03.004 [DOI] [Google Scholar]
- Menezes I. A., Barreto C. M., Antoniolli A. R., Santos M. R., De Sousa D. P. (2010). Hypotensive activity of terpenes found in essential oils. Z. Naturforsch. C. 65, 562–566. 10.1515/znc-2010-9-1005 [DOI] [PubMed] [Google Scholar]
- Muruganathan U., Srinivasan S., Vinothkumar V. (2017). Antidiabetogenic efficiency of menthol, improves glucose homeostasis and attenuates pancreatic beta-cell apoptosis in streptozotocin-nicotinamide induced experimental rats through ameliorating glucose metabolic enzymes. BioMed. Pharmacother. 92, 229–239. 10.1016/j.biopha.2017.05.068 [DOI] [PubMed] [Google Scholar]
- Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., et al. (2011). X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431. 10.1038/nature09647 [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shinoda T., Cornelius F., Toyoshima C. (2009). Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc. Natl. Acad. Sci. U. S. A 106, 13742–13747. 10.1073/pnas.0907054106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortar G., Schiano Moriello A., Morera E., Nalli M., Di Marzo V., De Petrocellis L. (2014). Effect of acyclic monoterpene alcohols and their derivatives on TRP channels. Bioorg. Med. Chem. Lett. 24, 5507–5511. 10.1016/j.bmcl.2014.10.012 [DOI] [PubMed] [Google Scholar]
- Oz M., Lozon Y., Sultan A., Yang K. H., Galadari S. (2015). Effects of monoterpenes on ion channels of excitable cells. Pharmacol. Ther. 152, 83–97. 10.1016/j.pharmthera.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Oz M. (2006). Receptor-independent effects of endocannabinoids on ion channels. Curr. Pharm. Des. 12, 227–239. 10.2174/138161206775193073 [DOI] [PubMed] [Google Scholar]
- Parnas M., Peters M., Dadon D., Lev S., Vertkin I., Slutsky I., et al. (2009). Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 45, 300–309. 10.1016/j.ceca.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto-Neves D., Silva-Alves K. S., Gomes M. D., Lima F. C., Lahlou S., Magalhaes P. J., et al. (2010). Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin. Pharmacol. 24, 341–350. 10.1111/j.1472-8206.2009.00768.x [DOI] [PubMed] [Google Scholar]
- Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., et al. (2004). UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Pham Q. D., Topgaard D., Sparr E. (2015). Cyclic and Linear Monoterpenes in Phospholipid Membranes: Phase Behavior, Bilayer Structure, and Molecular Dynamics. Langmuir 31, 11067–11077. 10.1021/acs.langmuir.5b00856 [DOI] [PubMed] [Google Scholar]
- Quintans Jde S., Menezes P. P., Santos M. R., Bonjardim L. R., Almeida J. R., Gelain D. P., et al. (2013). Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in beta-cyclodextrin. Phytomedicine 20, 436–440. 10.1016/j.phymed.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Quintans-Junior L. J., Barreto R. S., Menezes P. P., Almeida J. R., Viana A. F., Oliveira R. C., et al. (2013). beta-Cyclodextrin-complexed (-)-linalool produces antinociceptive effect superior to that of (-)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 113, 167–172. 10.1111/bcpt.12087 [DOI] [PubMed] [Google Scholar]
- Reed A. P., Bucci G., Abd-Wahab F., Tucker S. J. (2016). Dominant-Negative Effect of a Missense Variant in the TASK-2 (KCNK5) K+ Channel Associated with Balkan Endemic Nephropathy. PloS One 11, e0156456. 10.1371/journal.pone.0156456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner G. N., Delgado-Marín L., Olguín N., Sánchez-Redondo S., Sánchez-Borzone M., Rodríguez-Farré E., et al. (2013). Gabaergic Pharmacological Activity of Propofol Related Compounds as Possible Enhancers of General Anesthetics and Interaction with Membranes. Cell Biochem. Biophysics 67, 515–525. 10.1007/s12013-013-9537-4 [DOI] [PubMed] [Google Scholar]
- Renigunta V., Schlichthörl G., Daut J. (2015). Much more than a leak: structure and function of K2P-channels. Pflügers Archiv. Eur. J. Physiol. 467, 867–894. 10.1007/s00424-015-1703-7 [DOI] [PubMed] [Google Scholar]
- Riegelhaupt P. M., Tibbs G. R., Goldstein P. A. (2018). HCN and K2P Channels in Anesthetic Mechanisms Research. Methods Enzymol. 602, 391–416. 10.1016/bs.mie.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Rinne S., Kiper A. K., Vowinkel K. S., Ramirez D., Schewe M., Bedoya M., et al. (2019). The molecular basis for an allosteric inhibition of K+-flux gating in K2P channels. eLife 8, e39476. 10.7554/eLife.39476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi M., Balleza D., Vena G., Puia G., Facci P., Alessandrini A. (2015). Effect of neurosteroids on a model lipid bilayer including cholesterol: An Atomic Force Microscopy study. Biochim. Biophys. Acta 1848, 1258–1267. 10.1016/j.bbamem.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Sanchez M. E., Turina A. V., Garcia D. A., Nolan M. V., Perillo M. A. (2004). Surface activity of thymol: implications for an eventual pharmacological activity. Colloids Surf. B. Biointerf. 34, 77–86. 10.1016/j.colsurfb.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Sanchez-Borzone M., Delgado-Marin L., Garcia D. A. (2014). Inhibitory effects of carvone isomers on the GABAA receptor in primary cultures of rat cortical neurons. Chirality 26, 368–372. 10.1002/chir.22328 [DOI] [PubMed] [Google Scholar]
- Sano Y., Inamura K., Miyake A., Mochizuki S., Kitada C., Yokoi H., et al. (2003). A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J. Biol. Chem. 278, 27406–27412. 10.1074/jbc.M206810200 [DOI] [PubMed] [Google Scholar]
- Santos M. R. R. V., Moreira F. V. V., Fraga B. P., Souza D. O. P. D., Bonjardim L. R., Quintans-Junior L. J. (2011). Cardiovascular effects of monoterpenes: a review. Rev. Bras. Farmacogn. 21, 764–771 10.1590/S0102-695X2011005000119 [DOI] [Google Scholar]
- Schewe M., Nematian-Ardestani E., Sun H., Musinszki M., Cordeiro S., Bucci G., et al. (2016). A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K+ Channels. Cell 164, 937–949. 10.1016/j.cell.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Wiedmann F., Schweizer P. A., Katus H. A., Thomas D. (2014). Inhibition of cardiac two-pore-domain K+ (K2P) channels–an emerging antiarrhythmic concept. Eur. J. Pharmacol. 738, 250–255. 10.1016/j.ejphar.2014.05.056 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Wiedmann F., Zhou X. B., Heijman J., Voigt N., Ratte A., et al. (2017). Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy. Eur. Heart J. 38, 1764–1774. 10.1093/eurheartj/ehw559 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Wiedmann F., Gaubatz A. R., Ratte A., Katus H. A., Thomas D. (2018). New Targets for Old Drugs: Cardiac Glycosides Inhibit Atrial-Specific K2P3.1 (TASK-1) Channels. J. Pharmacol. Exp. Ther. 365, 614–623. 10.1124/jpet.118.247692 [DOI] [PubMed] [Google Scholar]
- Ton H. T., Smart A. E., Aguilar B. L., Olson T. T., Kellar K. J., Ahern G. P. (2015). Menthol Enhances the Desensitization of Human alpha3beta4 Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 88, 256–264. 10.1124/mol.115.098285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toncheva D., Mihailova-Hristova M., Vazharova R., Staneva R., Karachanak S., Dimitrov P., et al. (2014). NGS nominated CELA1, HSPG2, and KCNK5 as candidate genes for predisposition to Balkan endemic nephropathy. BioMed. Res. Int. 2014, 920723. 10.1155/2014/920723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina A. V., Nolan M. V., Zygadlo J. A., Perillo M. A. (2006). Natural terpenes: self-assembly and membrane partitioning. Biophys. Chem. 122, 101–113. 10.1016/j.bpc.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Walstab J., Wohlfarth C., Hovius R., Schmitteckert S., Roth R., Lasitschka F., et al. (2014). Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterol. Motil. 26, 810–820. 10.1111/nmo.12334 [DOI] [PubMed] [Google Scholar]
- Xu H., Delling M., Jun J. C., Clapham D. E. (2006). Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 9, 628–635. 10.1038/nn1692 [DOI] [PubMed] [Google Scholar]
- Zilberberg N., Ilan N., Goldstein S. A. (2001). KCNKO: opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron 32, 635–648. 10.1016/S0896-6273(01)00503-7 [DOI] [PubMed] [Google Scholar]
- Zunino M. P., Turina A. V., Zygadlo J. A., Perillo M. A. (2011). Stereoselective effects of monoterpenes on the microviscosity and curvature of model membranes assessed by DPH steady-state fluorescence anisotropy and light scattering analysis. Chirality 23, 867–877. 10.1002/chir.20998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.