Abstract
Oxidative stress is a major driving mechanism in the pathogenesis of COPD. There is increased oxidative stress in the lungs of COPD patients due to exogenous oxidants in cigarette smoke and air pollution and due to endogenous generation of reactive oxygen species by inflammatory and structural cells in the lung. Mitochondrial oxidative stress may be particularly important in COPD. There is also a reduction in antioxidant defences, with inactivation of several antioxidant enzymes and the transcription factors Nrf2 and FOXO that regulate multiple antioxidant genes. Increased systemic oxidative stress may exacerbate comorbidities and contribute to skeletal muscle weakness. Oxidative stress amplifies chronic inflammation, stimulates fibrosis and emphysema, causes corticosteroid resistance, accelerates lung aging, causes DNA damage and stimulates formation of autoantibodies. This suggests that treating oxidative stress by antioxidants or enhancing endogenous antioxidants should be an effective strategy to treat the underlying pathogenetic mechanisms of COPD. Most clinical studies in COPD have been conducted using glutathione-generating antioxidants such as N-acetylcysteine, carbocysteine and erdosteine, which reduce exacerbations in COPD patients, but it is not certain whether this is due to their antioxidant or mucolytic properties. Dietary antioxidants have so far not shown to be clinically effective in COPD. There is a search for more effective antioxidants, which include superoxide dismutase mimetics, NADPH oxidase inhibitors, mitochondria-targeted antioxidants and Nrf2 activators.
Keywords: Reactive oxygen species, Inflammation, NADPH oxidase Inhibitor, Mitochondria-targeted antioxidants, Nrf2 activator
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major and increasing global health problem, which is now the third leading cause of death worldwide and a major cause of morbidity [1,2]. It currently affects around 10% of the population over 45 years of age but this rises to 50% in heavy smokers and it has been estimated that the cumulative life-time risk of developing COPD is now over 25% [3]. The increase in COPD globally is greatest in low income countries, where indoor air pollution, such as exposure to biomass smoke, is as common as cigarette smoking as a risk factor [4,5]. There is increasing evidence that increased oxidative stress in the lungs is a major driving mechanism of the disease through multiple and interacting molecular mechanisms [6,7]. Oxidative stress arises as a result of endogenous anti-oxidant defences being impaired and/or overwhelmed by the presence of reactive oxygen species (ROS) [8]. COPD is characterized by chronic inflammation and fibrosis of the small airways and destruction of the lung parenchyma (emphysema) [9,10]. A striking feature of COPD is its failure to resolve when exposure to cigarette smoke has stopped which has led to the suggestion that other endogenous factors, such as autoimmunity or persistent infection may also be driving the disease [10]. Oxidative stress appears to drive may of the pathogenetic mechanisms involved in COPD and its progression (Fig. 1). This suggests that reducing oxidative stress by antioxidants or by enhancing endogenous antioxidants may be a useful therapeutic approach. However, it has proved difficult to discover safe and effective antioxidants for COPD, possible because the levels of oxidative stress in the lungs is very high. The aim of therapy is to restore normal redox balance in the lungs without reducing the benefits of oxidant signaling. There is a lack of useful biomarkers to identify which patients will benefit most from antioxidant therapy or to indicate what doses are needed to restore redox balance in COPD patients.
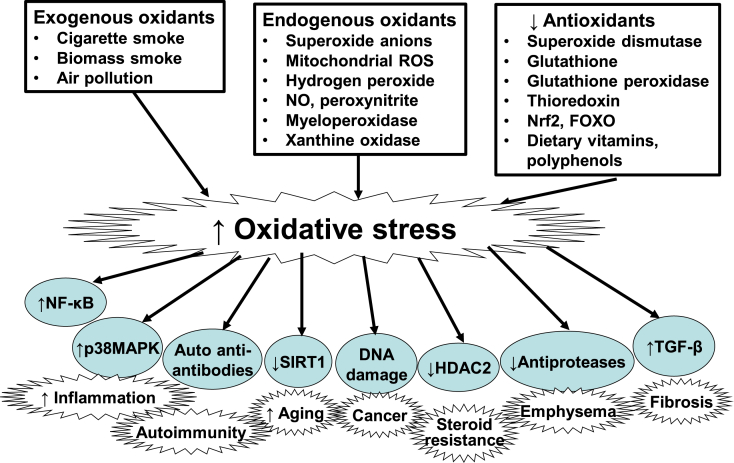
Fig. 1.
Increased oxidative stress in COPD and its consequences. Increased lung oxidative stress in COPD may be from exogenous oxidants (mainly cigarette smoke, biomass smoke, air pollution), endogenous oxidants (superoxide anions, hydrogen peroxide, mitochondrial oxidants, peroxynitrite, myeloperoxidase, xanthine oxidase) and by reduced antioxidants (superoxide dismutase, glutathione, thioredoxin, Nrf2, FOXO, and dietary vitamins and polyphenols). Oxidative stress drives COPD through activation of several mechanisms, including the proinflammatory transcription factor nuclear factor-KB (NF-κB), p38 mitogen-activate protein kinase (MAPK), generation of autoantibodies to carbonylated proteins, reduced expression of sirtuin-1, DNA damage, reduced histone deacetylase (HDAC)-2 expression, reduced activity of antiproteases and increased release of transforming growth factor(TGF)-β.
2. Lung and systemic oxidative stress in COPD
Oxidative stress is increased in COPD patients, particularly during acute exacerbations. Cigarette smoke, air pollution and biomass smoke are major exogenous sources of oxidative stress in the lungs, but oxidative stress persists even in ex-smokers, indicating that oxidative stress also arises endogenously. Alveolar macrophage numbers are enormously increased in the lungs of COPD patients and are more activated compared to control subjects, releasing increased amounts of ROS in the form of superoxide anions and hydrogen peroxide (H2O2) [11]. Activated neutrophils are also increased in the lungs of COPD patients and activated peripheral blood neutrophils from COPD patients release increased amounts of ROS, particularly during exacerbations [12]. Lung tissue from COPD patients shows increased lipid peroxidation, as measured by 4-hydroxy-2-nonenal (4HNE), which reflects an effect of ROS on endogenous lipids [13].
Increased lung oxidative stress has been demonstrated in COPD patients by measuring various markers of oxidative stress in the breath. Ethane, a volatile product of lipid peroxidation, is increased in exhaled breath of COPD patients and this is correlated with disease severity [14]. COPD patients have increased concentrations of H2O2, malondialdehyde, 4HNE and 8-isoprostane in exhaled breath condensate [[15], [16], [17], [18]] and these are further increased during exacerbations [19,20]. The increased markers of oxidative stress remain elevated in ex-smokers, indicating that they are derived from endogenous oxidative stress, presumably reflecting persistent lung inflammation [18]. Increased oxidative (superoxide anions) and nitrative stress (nitric oxide [NO]) result in the formation of peroxynitrite, which is increased in exhaled breath condensate of patients with COPD [21]. This may also be reflected by an increase in tyrosine nitration, as a result of peroxynitrite, in induced sputum and lungs of patients with COPD [22,23]. Oxidative stress is also increased in skeletal muscle of patients with COPD and may contribute to muscle weakness [24].
Increased oxidative stress in COPD also reflects a reduction in endogenous antioxidant defences in COPD patients. Concentrations of glutathione are lower in bronchoalveolar lavage fluid from COPD patients with frequent exacerbations compared to those with stable COPD [25]. Extracellular superoxide dismutase (SOD3) polymorphisms are more frequent in COPD and its expression is increased in sputum of COPD patients, although there is reduced expression around small airways [26,27]. The transcription factors Nrf2 (nuclear factor erythroid 2-related factor 2) and FOXO3a (Forkhead box O3a) regulate multiple antioxidant gens and both are reduced in COPD lungs [28,29].
3. Sources of endogenous ROS
The lung is particularly vulnerable to injury from environmental oxidative stress due in part to its anatomical structure. But lungs are also constantly exposed to sources of endogenous ROS generated by mitochondrial respiration and inflammatory responses to bacterial and viral infections within the lung. The continued presence of oxidative stress in COPD arises from activated neutrophils and macrophages, as well as lung epithelial cells. Indeed, lung epithelial cells of COPD patients produce oxidative stress derived from mitochondrial respiration [30]. Other sources of intracellular ROS include the cytoplasmic ROS generating enzymes, such as membrane-bound NADPH oxidases (NOX) and the xanthine/xanthine oxidase system, as well as neutrophil derived myeloperoxidase (MPO) [6].
Superoxide anions are produced endogenously mainly by NOX and are relatively weak oxidizing agents, but are rapidly converted to more damaging ROS species, such as the hydroxyl radical and H2O2, or the very powerful and damaging peroxynitrite radical formed when in the presence of nitric oxide [21]. Similarly MPO, released from activated neutrophils, which are recruited into the lungs of COPD patients, produces very destructive hypochlorous acid, which chlorinates tyrosine residues in proteins, with the formation of 3-chlorotyrosine, which is increased in sputum of COPD patients [31]. However, in healthy cells intracellular antioxidant defences are able to efficiently mop up these damaging ROS species, thus limiting their cellular effects, whereas in COPD these antioxidant defences are overwhelmed.
ROS generation may also result in the formation of reactive carbonyls through lipid peroxidation and glycoxidation of sugars, resulting in the formation of several aldehydes that result in protein carbonylation [32]. This accumulation of reactive carbonyls and subsequent protein carbonylation has been referred to as ‘carbonyl stress’ and is associated with chronic disease and ageing. Unlike other post-translational modifications, protein carbonylation is non-enzymatic and targets specific peptide residues, such as lysine, arginine, cysteine and histidine. Protein carbonylation is present in both smokers and COPD patients and is correlated with disease severity [33]. Protein carbonylation may modify protein function, disrupting normal cell function and physiological mechanisms.
4. Effects of increased oxidative stress
Increased oxidative stress is a major mechanism driving the pathophysiology of COPD and has several effects on the lungs (Fig. 1).
4.1. Increased inflammation
Over 50 cytokines and chemokines are released in the lungs of patients with COPD [34]. Many of the intracellular signalling pathways triggered and/or driving the release of these inflammatory mediators are sensitive to oxidative stress as they incorporate redox-sensitive molecular targets, such as the transcription factor nuclear factor-κB (NF-κB) and signalling molecules such as Ras/Rac, Jun-N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK) and protein tyrosine phosphatases. Oxidative stress activates NF-κB pathways and NF-κB expression and activation are increased in COPD, particularly in airway epithelial cells and macrophages. Oxidative stress also activates transforming growth factor(TGF)-β signalling pathways, which themselves induce oxidative stress [35] and are involved in small airway fibrosis. This may be enhanced by the inhibitory effect of TGF-β on Nrf2, resulting in reduced expression of endogenous antioxidants [36]. Oxidative stress increases the expression of MMP9, a key elastolytic enzyme involved in emphysema, and further enhances elastolysis through oxidative inactivation of α1-antitrypsin and secretory leukoprotease inhibitor, resulting in enhanced neutrophil elastase activity [37].
4.2. Corticosteroid resistance
The ability of corticosteroids to repress pro-inflammatory gene expression is also impaired in COPD as a result of oxidative stress and mediated though reduced activity and expression of histone deacetylase-2 (HDAC2), which is required for inflammatory gene suppression [38]. Oxidative stress impairs HDAC2 function by activation of phosphoinositide-3-kinase(PI3K)-δ, which leads to phosphorylation and ubiquitination of HDAC2 [39] and through the formation of peroxynitrite, which inactivates HDAC2 by tyrosine nitration and ubiquitination [40]. This prevents the corticosteroid from switching off activated inflammatory genes, resulting in amplified inflammation and corticosteroid resistance.
4.3. Accelerated aging and cellular senescence
Patients with COPD appear to have acceleration of the normal lung aging process and the accumulation of senescent cells [28]. This appears to be due to activation of cellular senescence pathways and a loss of endogenous anti-aging molecules, such as sirtuin-1. Oxidative stress may accelerate telomere shortening leading to the activation of the DNA damage response that leads to cell cycle arrest through activation of p53 and p21CIP1, but may also activate the p16INK4 stress-induced senescence pathway [41]. Oxidative stress reduces sirtuin-1 enzyme activity and expression, and this is associated with increased acetylation of NF-κB and increased expression of MMP-9 [42]. ROS activate the PI3K-mTOR (mammalian target of rapamycin) pathway that results in increased microRNA-34a, which suppresses sirtuin-1 [43].
4.4. Autoimmunity
There is increasing evidence for autoimmunity in COPD lungs, especially in severe disease, with autoantibodies against epithelial and endothelial cells [44]. Oxidative stress may cause carbonylation of proteins (“carbonyl stress”), which creates neoantigens against which autoantibodies may develop. In COPD patients there is evidence for autoantibodies against carbonyl-modified proteins and since these may be complement-fixing this could contribute to lung parenchymal damage [33].
4.5. DNA damage
Oxidative stress causes direct damage to DNA. There is increased in the expression of 8-hydroxy-2-deoxyguanosine, a biomarker of oxidative damage of DNA, in peripheral lung of normal smokers and patients with COPD, presumably reflecting the oxidative stress of cigarette smoking [45]. There are normally efficient molecular mechanisms for DNA repair; apurinic/apyradymic (AP) sites are common lesions in DNA in the repair of oxidized bases. In lung of normal smokers an increase in AP sites reflects active DNA repair, whereas this is reduced in lungs of COPD patients, indicating a defective DNA repair in COPD. Nuclear expression of the DNA repair protein Ku86 is significantly reduced in COPD compared to normal smoker lungs, indicating a defect on double-stranded DNA repair in COPD [45]. Reduced Ku86 is also seen in mice exposed to the oxidative stress of cigarette smoke and in human small airway epithelial cells exposed to H2O2. The defect in COPD repair as a result of oxidative stress may account for the increased prevalence of lung cancer in patients with COPD compared to smokers without airway obstruction [46].
5. Strategies for reducing oxidative stress
As discussed above, oxidative stress is a major driving mechanism for the pathophysiology of COPD so reducing oxidative stress is an important therapeutic strategy [6,47,48] (Table 1).
Table 1.
Antioxidants for COPD therapy.
| Antioxidant type | Examples | Studies in COPD |
|---|---|---|
| Thiol antioxidants | N-acetylcycsteine | Reduced exacerbations |
| Carbocisteine | “ | |
| Erdosteine | “ | |
| Inhaled glutathione | Not tested | |
| Dietary antioxidants | Vitamin C (ascorbic acid) | No clinical trials |
| Vitamin E (α-tocopherol) | “ | |
| Resveratrol | Anti-inflammatory in vitro | |
| (-)-Epigallocatechol | Not tested | |
| SOD Mimetics | AEOL 10150 | Effective in animal models |
| GPx Mimetics | Ebselen | Effective in animal models |
| NOX inhibitors | Apocynin | Reduces inflammation |
| Setanaxib (GKT137831) | No studies | |
| Spin-trap antioxidants | Disulfenton sodium (NXY-059) | No studies |
| Thioredoxan reductase inhibitors | Ethasalen | No studies |
| Xanthine oxidase inhibitors | Allopurinol | Anti-inflammatory |
| Myeloperoxidate inhibitors | AZD 5904 | Effective in animal models |
| iNOS inhibitors | L-NIL | Effective in animal studies |
| Mitochondria-targeted antioxidants | mitoQ, mitoTEMPO | Effective in vitro |
| SkQ1 | No clinical studies | |
| Nrf2 activators | Sulforaphane | Clinical trial negative |
| Bardoxelone methyl | Effective in animal models | |
| Dimethylfumarate (BG-12) | Not tested |
Abbreviations: SOD: superoxide dismutase; GPx: glutathione peroxidase; NOX: NADPH oxidase; iNOS: inducible nitric oxide synthase; L-NIL: L-N6-(1-iminoethyl)lysin; Nrf2: nuclear erythroid-2 related factor 2.
5.1. Thiol-based antioxidants
N-acetylcysteine (NAC) is a thiol compound that was developed as is a mucolytic agent as it breaks down mucin disulfide cross-links to reduce mucus viscosity. It also has antioxidant properties through increasing glutathione concentrations, which are reduced in COPD [49]. Several small studies indicated that NAC reduces exacerbations of COPD [50], but a large clinical trial of low dose NAC (BRONCUS: Bronchitis Randomised on NAC Study, n = 523, giving 600 mg daily) showed no exacerbation reduction or reduction in disease progression, apart from a possible effect in patients not treated with inhaled corticosteroids (ICS) [51]. In a large study (over 1000 Chinese COPD patients) using a higher dose of NAC (600 mg twice daily) there was a reduction in acute exacerbations of about 20% [52]. Further analysis of these data showed that this reduction was greatest in those patients who were current smokers and those not treated with ICS [53].
Carbocisteine, another thiol mucolytic drug with antioxidant effects, gave a small reduction in exacerbations in COPD patients who were not treated with ICS [54], and this has been confirmed in a meta-analysis of 4 placebo-controlled studies [55]. A related thiol antioxidant erdosteine also has some evidence for clinical benefit in COPD patients, with reduction in the number of mild (but not moderate or severe) exacerbations when added to other treatments [56] and confirmed in a meta-analysis [57,58]. Overall, these thiol-basedmucolytic drugs have a modest effect in reducing exacerbations, even when added to ICS and long-acting bronchodilators, but there is little effect on lung function or quality of life [59,60]. However, the drugs are relatively well tolerated so may be useful in clinical management of COPD. It is not certain whether the clinical effects of these drugs are through their mucolytic properties through an improvement in mucociliary clearance, or due to their antioxidant effects in the lungs, which have not been documented in these studies. NAC and has also been delivered by nebulization and a lysine derivative nacystelyn by dry powder inhaler, but there is no convincing evidence for antioxidant or anti-inflammatory effects in COPD. Nebulized glutathione has been investigated in other lung diseases, but appears to be ineffective and induces bronchoconstriction in asthmatic patients [61]. One of the problems with thiol-based antioxidants that because of their thiol structure, they may be rapidly inactivated by the high level of oxidative stress in COPD lungs. This has prompted a search for more effective antioxidant molecules.
5.2. Dietary antioxidants
Diets poor in antioxidants have been associated with worse lung function and a risk factor for the development of COPD, but it has been difficult to show that antioxidant vitamins specifically improve established COPD [62]. Dietary antioxidants include vitamin C (ascorbic acid), vitamin E (α-tocopherol), resveratrol, and flavonoids, such as quercetin, but so far improving dietary antioxidant intake has not been shown to improve lung function or clinical features of COPD [47,63]. The Mediterranean diet is rich in dietary antioxidants and there is some retrospective evidence that it may mitigate against the development of COPD, although confounding factors such as poverty are difficult to separate out [64]. The dietary polyphenol resveratrol, found in red-skinned fruits and red wine has antioxidant and anti-inflammatory effects in COPD cells in vitro and reduces pulmonary neutrophilic inflammation induced by lipopolysaccharide in rats in vivo [65,66]. However, despite its availability in health food stores, it has poor oral bioavailability, leading to the development of more potent and orally bioavailable analogs. Inhaled resveratrol reduces accelerated lung aging in a telomerase-deficient mouse model [67]. (-)-Epigallocatechin is a polyphenol found in green tea that is reported to activate FOXO3a, a transcription factor that regulates antioxidant genes such as SOD and catalase [68].
5.3. Antioxidant mimetics
Antioxidant mimetics are designed to restore depleted endogenous antioxidants such as SOD, catalase and glutathione peroxidase (GPx) [69]. SOD mimetics include metalloporphyrins, such as AEOL 10113 and AEOL 10150 and manganese-containing molecules, such as M40419. These drugs have been shown to be effective in various in vivo animal models of oxidative stress, including tobacco smoke-exposed mice who show a reduced inflammatory response [70]. AEOL 10150 is used for the treatment of radiation pneumonitis and derivatives are currently being developed for COPD patients. GPx include selenium and non-selenium containing antioxidant enzymes that catalyse the breakdown of H2O2. GPx-1 is reduced in COPD lungs and plasma, suggesting that GPx-1 mimetics may be useful therapeutically [71]. GPx transgenic mice are protected against the development of inflammation and emphysema after cigarette smoke exposure, whereas GPx gene knockout increases the lung response to smoke [72]. Ebselen is a GPx mimetic that is effective in reducing airway inflammation induced by ozone in rats [73] and inflammatory cytokines in the lungs of cigarette smoke exposed mice [74], but no clinical studies in COPD have been reported.
5.4. NOX inhibitors
NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (NOX) is a membrane bound complex that is the major source of ROS in COPD through the generation of superoxide anions. Several isoforms of the catalytic component of NOX exist, including NOX1-5 and the dual oxidases DUOX1 and 2 [8]. Several NOX inhibitors have been developed to counteract oxidative stress [75,76]. Apocynin is a non-selective NOX inhibitor and given systemically to cigarette-smoke exposed mice reduces inflammatory cytokines and chemokines in bronchoalveolar lavage fluid [74]. When given by nebulization to COPD patients apocynin reduces exhaled H2O2 and nitrite concentrations in exhaled breath condensate, but no clinical parameters were reported [77]. Several polyphenols, including quercetin and resveratrol, have been shown to inhibit NOX activity, although they also have other actions. It has proved difficult to develop more selective NOX inhibitors. Setanaxib (GKT137831) is a dual Nox1/4 inhibitor which attenuates acute lung injury induced by ischemia-reperfusion injury, but has not been studied in models of COPD [78]. This drug is now in clinical trials and a trial of setanaxib is currently underway in patients with idiopathic pulmonary fibrosis (NCT03865927).
5.5. Other small molecule antioxidants
Several synthetic small molecule antioxidants have been developed, although few have proceeded to clinical trials. Nitrone spin-trap antioxidants, such as disufentan sodium (NXY-059), were developed as neuroprotective agents but failed in clinical trials of acute stroke. Edaravone is another free radical scavenger molecule, which has been approved for the treatment of amyotrophic lateral sclerosis, but has not been tested in COPD [79]. Thioredoxin is an endogenous redox balance regulator that counteracts ROS and may be reduced in COPD [80]. Systemic administration of thioredoxin-1 is effective in murine models of COPD, including exacerbations, with a reduction in neutrophilic inflammation [81].
Xanthine oxidase may also generate superoxide anions and is increased in lungs of mice exposed to cigarette smoke [82]. Xanthine oxidase shows increased expression in bronchial mucosal lining fluid of COPD patients and is correlated with the increase expression of proinflammatory cytokines [83]. The xanthine oxidase inhibitor allopurinol reduces 3-nitrotyrosine expression in sputum cells of COPD patients and increases fractional exhaled NO (FeNO), presumably by blocking the superoxide generation so that peroxynitrite is not formed [84].
5.6. Peroxidase inhibitors
Neutrophil derived myeloperoxidase (MPO) is markedly increased in COPD, reflecting neutrophil activation in the lungs [85]. Several MPO inhibitors have been developed [86]. A selective irreversible MPO inhibitor, the 2-thioxanthine AZD5904, reduces oxidative stress and the development of emphysema in guinea pigs exposed to cigarette smoke [87]. However, although well tolerated in human volunteers, this drug was discontinued for unknown reasons.
5.7. Inhibiting nitrative stress
Superoxide anions combine rapidly with NO to form the highly reactive peroxynitrite ions, which results in the formation of 3-nitrotyrosine adducts of amino acids in proteins that may affect their function as enzymes, ion channels or structural proteins. Peroxynitrite is increased in exhaled breath condensate of COPD patients [21] and 3-nitrotyrosine is expressed in sputum cells and airways of COPD patients [23,88]. The formation of peroxynitrite in the airways of COPD patients may explain why FeNO is low in COPD patients, as all of the free NO is avidly bound by superoxide anions. NO may be generated in COPD lungs by type 1 NOS (also known as neuronal NOS), which is expressed in alveolar epithelial cells, in response to oxidative stress [89]. Mice exposed to cigarette smoke show increased expression of inducible NOS (iNOS, type 2 NOS) and are protected from the development of emphysema by knockout of the iNOS gene and by selective iNOS inhibitors [90]. Nebulized aminoguanidine, a relatively selective inhibitor of iNOS, partially reduces central and peripheral exhaled NO in COPD patients, but fails to eliminate exhaled NO, indicating that neuronal NOS is the likely source and that selective iNOS inhibitors may not be useful in reducing peroxynitrite in COPD patients [91]. Several highly selective iNOS inhibitors have been developed for clinical administration. An oral selective iNOS inhibitor L-NIL (L-N6-(1-iminoethyl)lysine) is very effective in reducing FeNO in patients with asthma, but has not been tested in COPD patients [92].
5.8. Mitochondria-targeted antioxidants
There is increasing evidence that mitochondria are dysfunctional in COPD [41]. There is an increase in mitochondrial mass, fusion and disruption of mitochondria with mitochondrial membrane leakiness [93]. This is explained by impairment of autophagy mechanisms that remove damaged mitochondria (mitophagy). Dysfunctional leaky mitochondria may be a major source of ROS in COPD [94,95]. Drugs are now able to selectively target mitochondria drugs that selectively target mitochondria [[96], [97], [98]]. Mitochondria-targeted (mt) antioxidants based on the structure of ubiquinone are concentrated 50-100-fold in mitochondria. and have been shown to be more effective than conventional antioxidants in several animal models of aging [98,99] Mt-antioxidants include mitoQ, mito-TEMPO, pyrroloquinoline quinone and SkQ1, which are now in clinical trials for several age-related diseases. In human airway epithelial cells in vitro cigarette smoke extract causes mitochondrial dysfunction and release of mROS, which is inhibited by mito-TEMPO [100]. In a murine model of chronic oxidative stress that involves long-term ozone exposure, mitoQ treatment reduces airway hyperresponsiveness, neutrophilic inflammation and lung inflammatory mediators [94]. No clinical studies in COPD patients have been reported.
5.9. Nrf2 activators
The transcription factor Nrf2 regulates multiple antioxidant genes and appears to be defective in COPD cells as it does not increase its activity in response to ROS as in normal cells [101]. This may reflect the fact that Nrf2 is acetylated in COPD cells, as a result of reduced HDAC2 and this acetylated form, while translocating to the nucleus after dissociation from Keap1 (Kelch-like ECH-associated protein 1), does not effectively transactivate antioxidant genes, such as heme oxygenase-1 which has a protective role in the lungs [102]. This suggests that Nrf2 activators might be effective against oxidative stress in COPD. Several electrophilic modifiers of Keap1 and drugs that interfere with the protein-protein interaction between Nrf2 and Keap1 have been developed and some of these have been into clinical trials [103]. Sulforaphane is a compound that is found in cruciferous vegetables, such as broccoli and wasabi, that reacts with Cys residues of Keap1 in the cytoplasm to form thioacyl adducts, so that Nrf2 translocates to the nucleus to switch on multiple antioxidant genes. However, sulforaphane has poor specificity and toxicity [104]. A clinical trial of sulforaphane in COPD patients over 4 weeks failed to increase antioxidant gene targets of Nrf2 or reduce oxidative stress and inflammation [105]. A synthetic triterpenoid bardoxolone methyl is effective in a cigarette smoke exposed mouse model of COPD [106], but a Phase 3 clinical trial in renal disease was terminated due to adverse effects and increased mortality [107]. Dimethyl fumarate (BG-12) is also an Nrf2 activator and has been approved for use in multiple sclerosis, although side effect such as flushing, nausea and diarrhoea are reported [108]. A microparticulate formulation of BG-12 has been developed for aerosol administration, but no results in animal models of COPD have been reported [109]. All of these Nrf2 activators lack specificity and there is a search for drugs that interfere with the protein-protein interaction between Nrf2 and Keap1 [110].
6. Conclusions
Increased oxidative stress in the lungs of COPD patients is a major driving mechanism for chronic inflammation, disease progression and exacerbations, whereas systemic oxidative stress is linked to the worsening of comorbidities. Targeting oxidative stress with antioxidants is therefore a logical approach in a common disease where there are no effective disease modifying therapies. Several different approaches to reducing oxidative stress in COPD have been explored in animal models of the disease, but few have been tested clinically. Thiol-based antioxidants, which were developed as mucolytic therapies, have been the most widely studied and appear to reduce exacerbations even on top of long-acting bronchodilators and ICS, but have little effect on symptoms and quality of life and their effect on disease progression is unknown. Although these drugs are well tolerated, their limited clinical efficacy may be explained by their inactivation by oxidative stress, prompting a search for more effective antioxidants. Perhaps the most promising drugs are mt-antioxidants as mitochondrial dysfunction may be a major source of ROS in COPD. Several mt-antioxidants have been developed and some are in clinical trials for other chronic diseases, so there is a good case for their study in COPD patients. SOD mimetics and the GPx mimetic ebselen are effective in animal models of COPD and have already been studied in other clinical diseases. Selective NOX inhibitors are now in development and, since NOX is a major source of ROS in COPD, these inhibitors may prove to be useful in COPD. Nrf2 activators are also an attractive approach, as Nrf2-regulated antioxidant genes are not activated approximately in COPD, but it has proved difficult to develop potent and specific Nrf2 activators. There is no doubt that targeting oxidative stress in COPD is likely to be effective if more effective drugs can be discovered, so it is an important area for drug development in the future.
Declaration of competing interest
No conflict of interest.
References
- 1.Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators G.C.R.D. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Resp Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon A.S., Warner L., Cascagnette P., Victor J.C., To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. doi: 10.1016/S0140-6736(11)60990-2. [DOI] [PubMed] [Google Scholar]
- 4.Salvi S.S., Barnes P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 5.Sood A., Assad N.A., Barnes P.J. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur. Respir. J. 2018;51:1700698. doi: 10.1183/13993003.00698-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 7.McGuinness A.J., Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J. Clin. Med. 2017;6 doi: 10.3390/jcm6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 9.Hogg J.C., Timens W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 10.Barnes P.J., Burney P.G.J., Silverman E.K., Celli B.R., Vestbo J., Wedzicha J.A., Wouters E.F.M. Chronic obstructive pulmonary disease. Nature Rev Primers. 2015;1:1–21. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 11.Schaberg T., Klein U., Rau M., Eller J., Lode H. Subpopulations of alveolar macrophages in smokers and nonsmokers: relation to the expression of CD11/CD18 molecules and superoxide anion production. Am. J. Respir. Crit. Care Med. 1995;151:1551–1558. doi: 10.1164/ajrccm.151.5.7735614. [DOI] [PubMed] [Google Scholar]
- 12.Noguera A., Batle S., Miralles C., Iglesias J., Busquets X., Macnee W., Agusti A.G. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax. 2001;56:432–437. doi: 10.1136/thorax.56.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman I., van Schadewijk A.A., Crowther A.J., Hiemstra P.S., Stolk J., Macnee W., de Boer W.I. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 14.Paredi P., Kharitonov S.A., Leak D., Ward S., Cramer D., Barnes P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000;162:369–373. doi: 10.1164/ajrccm.162.2.9909025. [DOI] [PubMed] [Google Scholar]
- 15.Bartoli M.L., Novelli F., Costa F., Malagrino L., Melosini L., Bacci E., Cianchetti S., Dente F.L., Di Franco A., Vagaggini B., Paggiaro P.L. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediat. Inflamm. 2011:891752. doi: 10.1155/2011/891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corradi M., Pignatti P., Manini P., Andreoli R., Goldoni M., Poppa M., Moscato G., Balbi B., Mutti A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur. Respir. J. 2004;24:1011–1017. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekhuijzen P.N.R., Aben K.H.H., Dekker I., Aarts L.P.H.J., Wielders P.L.M., van Herwarden C.L.A., Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;154:813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 18.Montuschi P., Collins J.V., Ciabattoni G., Lazzeri N., Corradi M., Kharitonov S.A., Barnes P.J. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am. J. Respir. Crit. Care Med. 2000;162:1175–1177. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- 19.Biernacki W.A., Kharitonov S.A., Barnes P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax. 2003;58:294–298. doi: 10.1136/thorax.58.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paredi P., Kharitonov S.A., Barnes P.J. Analysis of expired air for oxidation products. Am. J. Respir. Crit. Care Med. 2002;166:S31–S37. doi: 10.1164/rccm.2206012. [DOI] [PubMed] [Google Scholar]
- 21.Osoata G.O., Hanazawa T., Brindicci C., Ito M., Barnes P.J., Kharitonov S., Ito K. Peroxynitrite elevation in exhaled breath condensate of COPD and its inhibition by fudosteine. Chest. 2009;135:1513–1520. doi: 10.1378/chest.08-2105. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M., Sugiura H., Yamagata S., Koarai A., Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am. J. Respir. Crit. Care Med. 2000;160:701–706. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- 23.Ricciardolo F.L., Caramori G., Ito K., Capelli A., Brun P., Abatangelo G., Papi A., Chung K.F., Adcock I., Barnes P.J., Donner C.F., Rossi A., Di Stefano A. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005;116:1028–1035. doi: 10.1016/j.jaci.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Barreiro E., Peinado V.I., Galdiz J.B., Ferrer E., Marin-Corral J., Sanchez F., Gea J., Barbera J.A. Cigarette smoke-induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am. J. Respir. Crit. Care Med. 2010;182:477–488. doi: 10.1164/rccm.200908-1220OC. [DOI] [PubMed] [Google Scholar]
- 25.Drost E.M., Skwarski K.M., Sauleda J., Soler N., Roca J., Agusti A., Macnee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regan E.A., Mazur W., Meoni E., Toljamo T., Millar J., Vuopala K., Bowler R.P., Rahman I., Nicks M.E., Crapo J.D., Kinnula V.L. Smoking and COPD increase sputum levels of extracellular superoxide dismutase. Free Radic. Biol. Med. 2011;51:726–732. doi: 10.1016/j.freeradbiomed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Yao H., Arunachalam G., Hwang J.W., Chung S., Sundar I.K., Kinnula V.L., Crapo J.D., Rahman I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes P.J., Baker J., Donnelly L.E. Cellular senescence as a mechanism and target in chronic lung diseases. Am. J. Respir. Crit. Care Med. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 29.Hwang J.W., Rajendrasozhan S., Yao H., Chung S., Sundar I.K., Huyck H.L., Pryhuber G.S., Kinnula V.L., Rahman I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 2011;187:987–998. doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Toorn M., Rezayat D., Kauffman H.F., Bakker S.J., Gans R.O., Koeter G.H., Choi A.M., van Oosterhout A.J., Slebos D.J. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L109–114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell C., Newbold P., White P., Thong B., Stone H., Stockley R.A. 3-Chlorotyrosine in sputum of COPD patients: relationship with airway inflammation. COPD. 2010;7:411–417. doi: 10.3109/15412555.2010.528086. [DOI] [PubMed] [Google Scholar]
- 32.Negre-Salvayre A., Coatrieux C., Ingueneau C., Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkham P.A., Caramori G., Casolari P., Papi A., Edwards M., Shamji B., Triantaphyllopoulos K., Hussain F., Pinart M., Khan Y., Heinemann L., Stevens L., Yeadon M., Barnes P.J., Chung K.F., Adcock I.M. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of COPD. Am. J. Respir. Crit. Care Med. 2011;184:796–802. doi: 10.1164/rccm.201010-1605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes P.J. The cytokine network in COPD. Am. J. Respir. Cell Mol. Biol. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 35.Gorowiec M.R., Borthwick L.A., Parker S.M., Kirby J.A., Saretzki G.C., Fisher A.J. Free radical generation induces epithelial-to-mesenchymal transition in lung epithelium via a TGF-beta1-dependent mechanism. Free Radic. Biol. Med. 2012;52:1024–1032. doi: 10.1016/j.freeradbiomed.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Michaeloudes C., Sukkar M.B., Khorasani N.M., Bhavsar P.K., Chung K.F. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taggart C., Cervantes-Laurean D., Kim G., McElvaney N.G., Wehr N., Moss J., Levine R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 38.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 39.To Y., Ito K., Kizawa Y., Failla M., Ito M., Kusama T., Elliot M., Hogg J.C., Adcock I.M., Barnes P.J. Targeting phosphoinositide-3-kinase-δ with theophylline reverses corticosteroid insensitivity in COPD. Am. J. Respir. Crit. Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osoata G., Yamamura S., Ito M., Vuppusetty C., Adcock I.M., Barnes P.J., Ito K. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem. Biophys. Res. Commun. 2009;384:366–371. doi: 10.1016/j.bbrc.2009.04.128. [DOI] [PubMed] [Google Scholar]
- 41.Birch J., Barnes P.J., Passos J.F. Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol. Ther. 2018;183:34–49. doi: 10.1016/j.pharmthera.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Nakamaru Y., Vuppusetty C., Wada H., Milne J.C., Ito M., Rossios C., Elliot M., Hogg J., Kharitonov S., Goto H., Bemis J.E., Elliott P., Barnes P.J., Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. Faseb. J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 43.Baker J., Vuppusetty C., Colley T., Papaioannou A., Fenwick P., Donnelly L., Ito K., Barnes P.J. Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci. Rep. 2016;6:35871. doi: 10.1038/srep35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feghali-Bostwick C.A., Gadgil A.S., Otterbein L.E., Pilewski J.M., Stoner M.W., Csizmadia E., Zhang Y., Sciurba F.C., Duncan S.R. Autoantibodies in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177:156–163. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caramori G., Adcock I.M., Casolari P., Ito K., Jazrawi E., Tsaprouni L., Villetti G., Civelli M., Carnini C., Chung K.F., Barnes P.J., Papi A. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 46.Adcock I.M., Caramori G., Barnes P.J. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011;81:265–284. doi: 10.1159/000324601. [DOI] [PubMed] [Google Scholar]
- 47.Biswas S., Hwang J.W., Kirkham P.A., Rahman I. Pharmacological and dietary antioxidant therapies for chronic obstructive pulmonary disease. Curr. Med. Chem. 2013;20:1496–1530. doi: 10.2174/0929867311320120004. [DOI] [PubMed] [Google Scholar]
- 48.Thomson N.C. Targeting oxidant-dependent mechanisms for the treatment of respiratory diseases and their comorbidities. Curr. Opin. Pharmacol. 2018;40:1–8. doi: 10.1016/j.coph.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Biswas S.K., Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol. Aspect. Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grandjean E.M., Berthet P., Ruffmann R., Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Therapeut. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 51.Decramer M., Rutten-van Molken M. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 52.Zheng J.P., Wen F.Q., Bai C.X. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. The Lancet Resp Med. 2014;2:187–194. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 53.Papi A., Zheng J., Criner G.J., Fabbri L.M., Calverley P.M.A. Impact of smoking status and concomitant medications on the effect of high-dose N-acetylcysteine on chronic obstructive pulmonary disease exacerbations: a post-hoc analysis of the PANTHEON study. Respir. Med. 2019;147:37–43. doi: 10.1016/j.rmed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Zheng J.P., Kang J., Huang S.G., Chen P., Yao W.Z., Yang L., Bai C.X., Wang C.Z., Wang C., Chen B.Y., Shi Y., Liu C.T., Chen P., Li Q., Wang Z.S., Huang Y.J., Luo Z.Y., Chen F.P., Yuan J.Z., Yuan B.T., Qian H.P., Zhi R.C., Zhong N.S. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008;371:2013–2018. doi: 10.1016/S0140-6736(08)60869-7. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Z., Yang D., Huang X., Xiao Z. Effect of carbocisteine on patients with COPD: a systematic review and meta-analysis. Int J COPD. 2017;12:2277–2283. doi: 10.2147/COPD.S140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calverley P.M., Page C., Dal Negro R.W., Fontana G., Cazzola M., Cicero A.F., Pozzi E., Wedzicha J.A. Effect of erdosteine on COPD exacerbations in COPD patients with moderate airflow limitation. Int J COPD. 2019;14:2733–2744. doi: 10.2147/COPD.S221852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dal Negro R.W., Wedzicha J.A., Iversen M., Fontana G., Page C., Cicero A.F., Pozzi E., Calverley P.M.A. Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study. Eur. Respir. J. 2017:50. doi: 10.1183/13993003.00711-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cazzola M., Calzetta L., Page C., Rogliani P., Matera M.G. Impact of erdosteine on chronic bronchitis and COPD: a meta-analysis. Pulm. Pharmacol. Therapeut. 2018;48:185–194. doi: 10.1016/j.pupt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Poole P., Sathananthan K., Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019;5 doi: 10.1002/14651858.CD001287.pub6. Cd001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogliani P., Matera M.G., Page C., Puxeddu E., Cazzola M., Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir. Res. 2019;20:104. doi: 10.1186/s12931-019-1078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marrades R.M., Roca J., Barbera J.A., de Jover L., MacNee W., Rodriguez-Roisin R. Nebulized glutathione induces bronchoconstriction in patients with mild asthma. Am. J. Respir. Crit. Care Med. 1997;156:425–430. doi: 10.1164/ajrccm.156.2.9611001. [DOI] [PubMed] [Google Scholar]
- 62.Scoditti E., Massaro M., Garbarino S., Toraldo D.M. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. 2019;11(6) doi: 10.3390/nu11061357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsiligianni I.G., van der M.T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir. Res. 2010;11:171. doi: 10.1186/1465-9921-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer A., Johansson I., Blomberg A., Sundstrom B. Adherence to a mediterranean-like diet as a protective factor Against COPD: a nested case-control study. COPD. 2019;16:272–277. [Google Scholar]
- 65.Culpitt S.V., Rogers D.F., Fenwick P.S., Shah P., de Matos C., Russell R.E., Barnes P.J., Donnelly L.E. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birrell M.A., McCluskie K., Wong S., Donnelly L.E., Barnes P.J., Belvisi M.G. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-κB-independent mechanism. Faseb. J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- 67.Navarro S., Reddy R., Lee J., Warburton D., Driscoll B. Inhaled resveratrol treatments slow ageing-related degenerative changes in mouse lung. Thorax. 2017;72:451–459. doi: 10.1136/thoraxjnl-2016-208964. [DOI] [PubMed] [Google Scholar]
- 68.Bartholome A., Kampkotter A., Tanner S., Sies H., Klotz L.O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys. 2010;501:58–64. doi: 10.1016/j.abb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 69.Day B.J. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov. Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 70.Smith K.R., Uyeminami D.L., Up Kodavanti, Crapo J.D., Chang L.Y., Pinkerton K.E. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic. Biol. Med. 2002;33:1106–1114. doi: 10.1016/s0891-5849(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 71.Vlahos R., Bozinovski S. Glutathione peroxidase-1 as a novel therapeutic target for COPD. Redox Rep. 2013;18:142–149. doi: 10.1179/1351000213Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geraghty P., Hardigan A.A., Wallace A.M., Mirochnitchenko O., Thankachen J., Arellanos L., Thompson V., D'Armiento J.M., Foronjy R.F. The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis. A key determinant of airway inflammation and alveolar destruction. Am. J. Respir. Cell Mol. Biol. 2013;49:721–730. doi: 10.1165/rcmb.2013-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishii Y., Hashimoto K., Hirano K., Morishima Y., Mochizuki M., Masuyama K., Nomura A., Sakamoto T., Uchida Y., Sagai M., Sekizawa K. Ebselen decreases ozone-induced pulmonary inflammation in rats. Lung. 2000;178:225–234. doi: 10.1007/s004080000026. [DOI] [PubMed] [Google Scholar]
- 74.Oostwoud L.C., Gunasinghe P., Seow H.J., Ye J.M., Selemidis S., Bozinovski S., Vlahos R. Apocynin and ebselen reduce influenza A virus-induced lung inflammation in cigarette smoke-exposed mice. Sci. Rep. 2016;6:20983. doi: 10.1038/srep20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altenhofer S., Radermacher K.A., Kleikers P.W., Wingler K., Schmidt H.H. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxidants Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cifuentes-Pagano E., Csanyi G., Pagano P.J. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell. Mol. Life Sci. 2012;69:2315–2325. doi: 10.1007/s00018-012-1009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefanska J., Sarniak A., Wlodarczyk A., Sokolowska M., Doniec Z., Bialasiewicz P., Nowak D., Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Therapeut. 2012;25:343–348. doi: 10.1016/j.pupt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Cui Y., Wang Y., Li G., Ma W., Zhou X.S., Wang J., Liu B. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice. PloS One. 2018;13 doi: 10.1371/journal.pone.0209444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhandari R., Kuhad A., Kuhad A. Edaravone: a new hope for deadly amyotrophic lateral sclerosis. Drugs Today. 2018;54:349–360. doi: 10.1358/dot.2018.54.6.2828189. [DOI] [PubMed] [Google Scholar]
- 80.Zhou J., Wang C., Wu J., Fukunaga A., Cheng Z., Wang J., Yamauchi A., Yodoi J., Tian H. Anti-allergic and anti-inflammatory effects and molecular mechanisms of thioredoxin on respiratory system diseases. Antioxidants Redox Signal. 2020;60:304–321. doi: 10.1089/ars.2019.7807. [DOI] [PubMed] [Google Scholar]
- 81.Tanabe N., Hoshino Y., Marumo S., Kiyokawa H., Sato S., Kinose D., Uno K., Muro S., Hirai T., Yodoi J., Mishima M. Thioredoxin-1 protects against neutrophilic inflammation and emphysema progression in a mouse model of chronic obstructive pulmonary disease exacerbation. PloS One. 2013;8 doi: 10.1371/journal.pone.0079016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kayyali U.S., Budhiraja R., Pennella C.M., Cooray S., Lanzillo J.J., Chalkley R., Hassoun P.M. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol. Appl. Pharmacol. 2003;188:59–68. doi: 10.1016/s0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 83.Komaki Y., Sugiura H., Koarai A., Tomaki M., Ogawa H., Akita T., Hattori T., Ichinose M. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease's airways. Pulm. Pharmacol. Therapeut. 2005;18:297–302. doi: 10.1016/j.pupt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Ichinose M., Sugiura H., Yamagata S., Koarai A., Tomaki M., Ogawa H., Komaki Y., Barnes P.J., Shirato K., Hattori T. Xanthine oxidase inhibition reduces reactive nitrogen species production in COPD airways. Eur. Respir. J. 2003;22:457–461. doi: 10.1183/09031936.03.00052002. [DOI] [PubMed] [Google Scholar]
- 85.Zhu A., Ge D., Zhang J., Teng Y., Yuan C., Huang M., Adcock I.M., Barnes P.J., Yao X. Sputum myeloperoxidase in chronic obstructive pulmonary disease. Eur. J. Med. Res. 2014;19:12. doi: 10.1186/2047-783X-19-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lazarevic-Pasti T., Leskovac A., Vasic V. Myeloperoxidase inhibitors as potential drugs. Curr. Drug Metabol. 2015;16:168–190. doi: 10.2174/138920021603150812120640. [DOI] [PubMed] [Google Scholar]
- 87.Churg A., Marshall C.V., Sin D.D., Bolton S., Zhou S., Thain K., Cadogan E.B., Maltby J., Soars M.G., Mallinder P.R., Wright J.L. Late intervention with a myeloperoxidase inhibitor stops progression of experimental COPD. Am. J. Respir. Crit. Care Med. 2012;185:34–43. doi: 10.1164/rccm.201103-0468OC. [DOI] [PubMed] [Google Scholar]
- 88.Sugiura H., Ichinose M., Tomaki M., Ogawa H., Koarai A., Kitamuro T., Komaki Y., Akita T., Nishino H., Okamoto S., Akaike T., Hattori T. Quantitative assessment of protein-bound tyrosine nitration in airway secretions from patients with inflammatory airway disease. Free Radic. Res. 2004;38:49–57. doi: 10.1080/10715760310001633817. [DOI] [PubMed] [Google Scholar]
- 89.Brindicci C., Kharitonov S.A., Ito M., Elliott M.W., Hogg J.C., Barnes P.J., Ito K. Nitric oxide synthase isoenzyme expression and activity in peripheral lungs of COPD patients. Am. J. Respir. Crit. Care Med. 2010;181:21–30. doi: 10.1164/rccm.200904-0493OC. [DOI] [PubMed] [Google Scholar]
- 90.Seimetz M., Parajuli N., Pichl A. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 91.Brindicci C., Ito K., Torre O., Barnes P.J., Kharitonov S.A. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy controls, healthy smokers and COPD patients. Chest. 2009;135:353–367. doi: 10.1378/chest.08-0964. [DOI] [PubMed] [Google Scholar]
- 92.Hansel T.T., Kharitonov S.A., Donnelly L.E., Erin E.M., Currie M.G., Moore W.M., Manning P.T., Recker D.P., Barnes P.J. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. Faseb. J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 93.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J. Clin. Invest. 2016;126:809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wiegman C.H., Michaeloudes C., Haji G., Narang P., Clarke C.J., Russell K.E., Bao W., Pavlidis S., Barnes P.J., Kanerva J., Bittner A., Rao N., Murphy M.P., Kirkham P.A., Chung K.F., Adcock I.M. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015;136:769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Belchamber K.B.R., Singh R., Batista C.M., Whyte M.K., Dockrell D.H., Kilty I., Robinson M.J., Wedzicha J.A., Barnes P.J., Donnelly L.E. Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur. Respir. J. 2019;54:1802244. doi: 10.1183/13993003.02244-2018. [DOI] [PubMed] [Google Scholar]
- 96.Zinovkin R.A., Zamyatnin A.A. Mitochondria-targeted drugs. Curr. Mol. Pharmacol. 2019;12:202–214. doi: 10.2174/1874467212666181127151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sureshbabu A., Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front. Physiol. 2013;4:384. doi: 10.3389/fphys.2013.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy M.P. Antioxidants as therapies: can we improve on nature? Free Radic. Biol. Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 99.Kolosova N.G., Stefanova N.A., Muraleva N.A., Skulachev V.P. The mitochondria-targeted antioxidant SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in rats. Aging. 2012;4:686–694. doi: 10.18632/aging.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hara H., Araya J., Ito S., Kobayashi K., Takasaka N., Yoshii Y., Wakui H., Kojima J., Shimizu K., Numata T., Kawaishi M., Kamiya N., Odaka M., Morikawa T., Kaneko Y., Nakayama K., Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;305:L737–746. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 101.Zhao H., Eguchi S., Alam A., Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312:L155–l162. doi: 10.1152/ajplung.00449.2016. [DOI] [PubMed] [Google Scholar]
- 102.Mercado N., Thimmulappa R., Thomas C.M., Fenwick P.S., Chana K.K., Donnelly L.E., Biswal S., Ito K., Barnes P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011;406:292–298. doi: 10.1016/j.bbrc.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W., Dinkova-Kostova A.T. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 104.Morimitsu Y., Nakagawa Y., Hayashi K., Fujii H., Kumagai T., Nakamura Y., Osawa T., Horio F., Itoh K., Iida K., Yamamoto M., Uchida K. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J. Biol. Chem. 2002;277:3456–3463. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 105.Wise R.A., Holbrook J.T., Criner G. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PloS One. 2016;11 doi: 10.1371/journal.pone.0163716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sussan T.E., Rangasamy T., Blake D.J. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U.S.A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossing P. Diabetic nephropathy: could problems with bardoxolone methyl have been predicted? Nat. Rev. Nephrol. 2013;9:128–130. doi: 10.1038/nrneph.2013.13. [DOI] [PubMed] [Google Scholar]
- 108.Gold R., Kappos L., Arnold D.L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M.T., Yang M., Sheikh S.I., Dawson K.T. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;20(367):1098–1107. doi: 10.1056/NEJMoa1114287. %. [DOI] [PubMed] [Google Scholar]
- 109.Muralidharan P., Hayes D., Jr., Black S.M., Mansour H.M. Microparticulate/nanoparticulate powders of a novel Nrf2 activator and an aerosol performance enhancer for pulmonary delivery targeting the lung Nrf2/Keap-1 pathway. Mol Syst Des Eng. 2016;1:48–65. doi: 10.1039/C5ME00004A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang Z.Y., Lu M.C., Xu L.L., Yang T.T., Xi M.Y., Xu X.L., Guo X.K., Zhang X.J., You Q.D., Sun H.P. Discovery of potent Keap1-Nrf2 protein-protein interaction inhibitor based on molecular binding determinants analysis. J. Med. Chem. 2014;57:2736–2745. doi: 10.1021/jm5000529. [DOI] [PubMed] [Google Scholar]