Abstract
Mechanisms underlying the pathogenesis of pulmonary fibrosis remain incompletely understood. Emerging evidence suggests changes in mitochondrial quality control are a critical determinant in many lung diseases, including chronic obstructive pulmonary disease, asthma, pulmonary hypertension, acute lung injury, lung cancer, and in the susceptibility to pulmonary fibrosis. Once thought of as the kidney-bean shaped powerhouses of the cell, mitochondria are now known to form interconnected networks that rapidly and continuously change their size to meet cellular metabolic demands. Mitochondrial quality control modulates cell fate and homeostasis, and diminished mitochondrial quality control results in mitochondrial dysfunction, increased reactive oxygen species (ROS) production, reduced ATP production, and often induces intrinsic apoptosis. Here, we review the role of the mitochondria in alveolar epithelial cells, lung macrophages, and fibroblasts within the context of pulmonary fibrosis.
1. Introduction
Mitochondrial quality control is maintained generally through three different mechanisms: (1) mitochondrial biogenesis; (2) mitochondrial dynamics (fusion and fission); and (3) mitophagy. Broadly, mitochondrial quality control also includes mitochondrial intracellular trafficking/migration and mitochondrial intracellular communication with the nucleus and other organelles, such as endoplasmic reticulum and Golgi apparatus. Mitochondrial dysfunction has been proposed to be a key player in pathogenesis of many pulmonary diseases, such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), pulmonary hypertension, asthma, acute lung injury, and lung cancer [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. Many of these disease conditions, including IPF, are thought to be related to aging, and accumulation of dysfunctional mitochondria is considered a marker for the pathological conditions but is also the key factor that drives disease progression.
Evolutionally, mitochondria originated from the integration of an endosymbiotic alphaproteobacterium in host cells to facilitate a more efficient way of generating ATP through aerobic respiration [10,11]. The eventual transition to an intracellular organelle signifies the importance of mitochondrial quality control in maintaining cellular homeostasis. In the past years, in addition to their function as “the powerhouse of the cell,” mitochondria have been shown to contribute profoundly to the regulation of signaling, metabolism, and cell death [12].
The complexity of mitochondrial quality control in pulmonary fibrosis is also related to various effector lung cells as the etiology of pulmonary fibrosis remains unknown and many hypotheses involving different cell types exist. While most of the research has been conducted on the three main cell types, namely alveolar epithelial cells (AEC), lung macrophages and fibroblasts, there are possible contributions from other cells such as vascular endothelial cells, smooth muscle cells, and fibrocytes. In this review, we concentrate on recent advances in mitochondrial quality control in AECs, lung macrophages, and fibroblasts; however, there have been studies suggesting that mitochondrial biogenesis is upregulated in vascular smooth muscle cells in both asbestos- and bleomycin-injured mice [13].
Given the evolution of the mitochondrion, it is not surprising that it is the only organelle, in addition to the nucleus, that contains its own DNA and transcription system. New mitochondria are not synthesized de novo but are generated through division from an existing mitochondrion. Mitochondrial biogenesis is a highly coordinated process utilizing both mitochondrial and nuclear encoded genes to increase mitochondrial size/mass. It requires synergetic efforts from mitochondria, nucleus, ER, and other organelles in the cell. The best documented regulator in mitochondrial biogenesis is PGC-1α, but other transcriptional factors, such as 5' adenosine monophosphate-activated protein kinase (AMPK) and nuclear factor erythroid 2-related factor 1/2 (Nrf1/2) can also be involved [14]. Biogenesis is not only important during homeostasis and proliferation, but stress signals known to induce mitophagy can also promote biogenesis [15], suggesting biogenesis can serve as a possible rescue mechanism. However, dysregulated biogenesis can also lead to increased mitochondrial ROS (mtROS) production and drive disease progression in pulmonary fibrosis [16,17].
Fusion (mitochondrial elongation) and fission (mitochondrial fragmentation) are not two separate processes but rather are interdependent. It has been hypothesized that mitochondrial dynamics are regulated in response to cellular stress. In mild to moderate stress conditions, cells mainly utilize fusion to combine damaged mitochondria with healthy mitochondria to offset the injuries. This will generate elongated mitochondria that can be spared from mitophagy. During severe stress conditions, normal or elongated mitochondria will undergo fission in which mitochondria will be separated into smaller compartments so the diseased part will be split from the healthy part, limiting further damage. The fragmented and damaged mitochondria will eventually be removed by mitophagy. Regulatory proteins involved in fusion are optic atrophy 1 (OPA1) and mitofusin (MFN1 and MFN2). Proteins involved in fission are dynamin-related protein (Drp1) and its mitochondrial receptors, mitochondrial fission 1 (FIS1) and mitochondrial fission factors [18]. Increased numbers of mitochondria coordinate process involving both mitochondrial biogenesis and fission.
Mitophagy is a highly specialized form of autophagy called macroautophagy. The best-known regulators in mitophagy are PINK1 and PARK2. The canonical PINK1-PARK2-mediated mitophagy includes three steps: (1) PINK1 binds to mitochondrial outer membrane and recruits the E3 ligase PARK2; (2) PARK2 ubiquitinates mitochondrial proteins; and (3) SQSTM1/p62 binds ubiquitinated substrates to LC-3 ligands on autophagosomes. While this three-step process is the main pathway of mitophagy, PINK1 and PARK2 have additional function in maintaining cellular homeostasis. PINK1/PARK2 have been shown to induce ubiquitination of fusion-related proteins, such as MFN1 and MFN2, as a mechanism to diminish the opposing process of mitophagy in neuroblastoma cells [19]. Interestingly, PARK2 binds to MFN2, but not MFN1, in HEK cells [20]. This binding is important for PINK1-mediated PARK2 mitochondrial recruitment, and PINK1/MFN2/PARK2 interaction is critical for the development of dilated cardiomyopathy.
All three mitochondrial quality control processes are considered catabolic and energy demanding. Several of the key proteins in mitochondrial dynamics are GTPases, such as MFN1/2 and Drp1, and ubiquitination of mitophagy-related proteins requires ATP. Mitochondria supply energy for these processes through the highly efficient oxidative phosphorylation (OXPHOS). However, emerging evidence suggests that cellular bioenergetics also change during mitochondrial quality control. It is generally accepted that glycolysis, particularly aerobic glycolysis, is elevated in fibroblasts during pulmonary fibrosis [[21], [22], [23]]. Changes in fatty acid oxidation also have been observed in pulmonary fibrosis, particularly in lung macrophages [17]. Lung macrophages have augmented fatty acid oxidation that facilitates their profibrotic polarization.
Mitochondria contain DNA and their own transcriptional mechanism. Traditionally considered as a surrogate of oxidative injuries during pulmonary fibrosis, mitochondrial DNA (mtDNA) is emerging as an important topic in understanding the pathogenesis of IPF, predicting prognosis, and determining effectiveness of anti-fibrotic drugs. Due to its proximity to the origin of ROS production and lack of the sophisticated mechanisms of protection and repair, mtDNA are prone to sustained injuries [24]. Many studies have evaluated mtDNA, particularly in its oxidized form, as an indicator of oxidative stress in AECs. Animal models with defects in mtDNA repair have exacerbated pulmonary fibrosis [25]. Recent studies have highlighted the biological relevance of mtDNA more than a mere indicator of excessive ROS. Release of mtDNA can serve as a Damage-Associated Molecular Pattern (DAMP) and bind to toll-like receptor 9 (TLR9), which leads to a cascade of intracellular responses involving TGF-β1 production, release and activation [26,27]. Moreover, extracellular mtDNA may be generated in an active production/secretary fashion related to mitochondrial biogenesis or mitophagy and have an autocrine or paracrine effect on fibroblasts to promote their transition into myofibroblasts. Translationally, mtDNA is increased in BAL fluid and serum of various fibrotic lung diseases, including IPF, and is correlated with increased mortality. Moreover, elevated expression of TLR9, the proposed mtDNA receptor, is correlated with rapid progression in IPF [28]. In this review, we will focus on recent advances in mitochondrial quality control in AECs, lung macrophages, and fibroblasts to distinguish their differences during pulmonary fibrosis, a devastating chronic lung disease.
2. Alveolar epithelial cells
The current and long-standing dogma is that recurrent epithelial cell injury and subsequent apoptosis are required for the development of lung fibrosis [[29], [30], [31]]. In response to normal injury, type II AECs differentiate into type I AECs to promote re-epithelialization; however, in pulmonary fibrosis, AECs undergo apoptosis. The injury impairs the ability of the lung to replace type I cells, and type II cells undergo hyperplasia inducing ineffective re-epithelialization. Additionally, the loss of alveolar epithelium basement membrane integrity in the fibrotic lung promotes alveolar collapse. Data supporting this shows that type II AECs from IPF subjects stain positive for pro-apoptotic markers and show a reduction in anti-apoptotic markers compared with control subjects [32]. Using experimental mouse models, the induction of type II AEC apoptosis promotes a fibrotic phenotype [33] and inhibition of apoptosis attenuates pulmonary fibrosis [[34], [35], [36]].
Emerging evidence indicates that mitochondrial damage occurs in IPF type II AECs. These cells from IPF subjects are increased in number and have enlarged and swollen mitochondria with disrupted cristae [5,37]. Excessive mtROS production [38,39] and reduced ETC complex I and IV activity [5] in fibrotic type II AECs contribute to mitochondrial dysfunction (Fig. 1A). Implicating type II AEC mtROS in fibrosis, transgenic mice expressing mitochondrial-targeted catalase are protected from bleomycin- and asbestos-induced pulmonary fibrosis, and type II AECs from these mice show reduced mtROS levels compared to wild type mice [39]. Nrf2 is an oxidant-sensitive transcription factor promoting antioxidant and detoxification genes with a fundamental role in regulating mitochondrial function [40,41]. AECs isolated from Nrf2-deficient mice exhibit sensitivity to oxidant-induced cell death, dysregulated type II AEC proliferation [42], and exaggerated fibrosis is response to bleomycin [43].
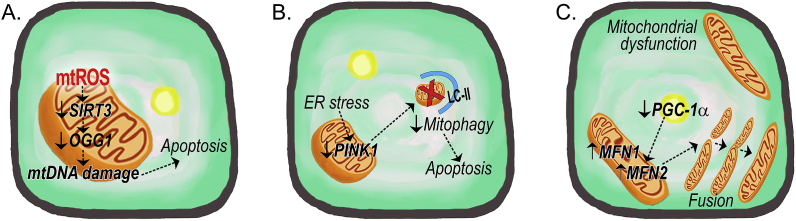
Fig. 1.
Schematic of mitochondrial quality control in fibrotic type II alveolar epithelial cells. (A) The production of mtROS promotes mtDNA damage by reducing the mitochondrial expression of SIRT3 and OGG1 to mediate AEC apoptosis. (B) Fibrotic type II AECs show increased ER stress that inhibits PINK1-mediated mitophagy to promote apoptosis. (C) Reduced PGC-1α expression leads to increased mitochondrial fusion (MFN1 and 2) and the accumulation of swollen and elongated mitochondria fibrotic type II AECs. Abbreviations: LC-II = Microtubule-associated protein 1A/1B-light chain 3; MFN = mitofusion; mtDNA = mitochondrial DNA; mtROS = mitochondrial reactive oxygen species; PINK1 = PTEN-induced putative kinase 1; PGC-1α = peroxisome proliferator-activated receptor-ɣ coactivator 1-α; OGG1 = 8-oxoguanine DNA glycosylase 1; SIRT3 = sirtuin 3.
Mitochondrial DNA (mtDNA) damage is linked to oxidative injury and pulmonary fibrosis. The DNA base excision repair enzyme, 8-oxoguanine DNA glycosylase 1 (OGG1), is critical for repair of type II AEC mtDNA oxidative damage [44]. Type II AECs from IPF subjects show increased levels of DNA oxidation [45], and Ogg1−/−- mice are more susceptible to type II AEC mtDNA damage, oxidant-induced apoptosis, and asbestos-mediated lung fibrosis [25]. Mitochondrial sirtuin 3 (SIRT3), a member of the sirtuin family of NAD-dependent deacetylaces, has been implicated in regulating lung fibrosis. SIRT3 function is decreased in type II AECs from IPF patients [46], and Sirt3−/− mice spontaneously develop lung fibrosis [47] (Fig. 1A). Moreover, SIRT3 modulates mtDNA damage by regulating OGG1 and manganese superoxide dismutase (MnSOD) acetylation [46], suggesting that SIRT3 deficiency leads to the acetylation and inactivation of mitochondrial proteins that augment oxidant-induced type II AEC mtDNA damage and apoptosis, thereby promoting pulmonary fibrosis.
The accumulation of dysfunctional mitochondria that is seen in IPF type II AECs is related to the downregulation of mitophagy [48]. In type II AECs from the IPF lung, PINK1 expression is decreased [5]. Impaired mitophagy is associated with enhanced AEC apoptosis and bleomycin-induced lung fibrosis in Pink1−/− mice [5,37] (Fig. 1B). Moreover, the pro-fibrotic cytokine TGF-β1 may protect lung epithelial cells by inducing PINK1 expression and attenuating AEC apoptosis, while silencing PINK1 in bronchial epithelial cells promotes TGF-β1-mediated apoptosis and mitochondrial depolarization [5,37]. Although Kobayashi et al. observed enhanced bleomycin-induced lung fibrosis in Park2−/− mice, no difference in type II AEC apoptosis was detected in these mice [49]. The ER stress protein, ATF3 (activating transcription factor 3), is a negative regulator of PINK1 gene expression (Fig. 1B). Mice with a conditional deletion of Atf3 in type II AECs were protected against bleomycin-induced fibrosis [50]. Moreover, induction of ER stress reduced PINK1 expression in type II AECs, altered mitochondrial bioenergetics, and increased apoptosis [5], implicating a functional link between endoplasmic reticulum stress, mitochondrial dysfunction, and fibrotic remodeling.
The generation of new and the removal of damaged or dysfunctional mitochondria are highly regulated processes that must be precisely coordinated for the maintenance of mitochondrial and cellular homeostasis. Mitophagy and mitochondrial biogenesis are typically co-dependent. In accordance with reduced mitophagy, PGC-1α expression is also reduced in type II AEC from bleomycin-exposed mice [51] (Fig. 1C). A recent report demonstrated that thyroid hormone (T3) can attenuate bleomycin-induced lung fibrosis by promoting mitochondrial function and morphology, restoring mitochondrial membrane potential (ΔψM), and increasing oxygen consumption rates in type II AECs [51]. The beneficial effects of T3 were associated with inhibition of mitochondria-regulated apoptosis dependent on intact expression of PGC-1α and PINK1 expression in type II AECs.
IPF type II AECs display enhanced mitochondrial fusion with increased MFN2 mRNA expression compared to healthy controls [52]. Type II AECs isolated from bleomycin-exposed mice similarly show increased MFN1, MFN2, and Drp1 expression [53]. Mice with a conditional deletion of Mfn1 or Mfn2 in type II AECs show enhanced fibrosis with Mfn1 deletion leading to mitochondrial fragmentation, and Mfn2 deletion results in swollen mitochondria after bleomycin exposure [53]. Moreover, mice lacking both Mfn1 and Mfn2 in type II AECs develop spontaneous lung fibrosis with excessive mitochondrial dysfunction (Fig. 1C). Since mitochondrial function is closely associated with lipid metabolism, Chung et al. determined that type II AECs require mitofusins for surfactant lipid production providing a mechanistic link between mitochondrial dysfunction, impaired surfactant lipid synthesis in type II cells, and lung fibrosis development [53]. This data supports the altered lipid profiles seen in IPF BAL fluid [54], IPF lung [55], and IPF type II AECs [52].
Mitochondrial dysfunction in fibrotic type II AECs is characterized by excessive mtROS that promotes cell injury or apoptosis. These dysfunctional mitochondria show reduced mitophagy and the diminished expression of PGC-1α promotes the accumulation of dysmorphic mitochondria that become swollen, elongated, and have damaged cristae. These dysfunctional mitochondria are associated with increased ER stress and the down regulation of redox-regulated transcription factors. Because type II AECs are responsible for lipid metabolism and surfactant production, the alteration in metabolism of lipids in pulmonary fibrosis is secondary, in part, to the absence of proper mitochondrial function and mitochondrial-ER interactions.
3. Lung macrophages
Macrophages are innate immune cells comprised of tissue-resident or recruited/infiltrating monocytes that are critical for maintaining homeostasis and repair of damaged tissue. Tissue-resident alveolar macrophages are derived from the yolk sac or fetal liver [[56], [57], [58]]. They are long-living cells, capable of self-renewing, and are maintained independently of circulating monocytes [59,60]. It is well established that recruited monocytes contribute to lung fibrosis as deletion of chemokine receptor 2 (CCR2), the receptor utilized for monocyte recruitment, or systemic administration of clodronate liposomes, which depletes circulating monocytes, protects mice from pulmonary fibrosis [[61], [62], [63], [64]]. However, the deletion of tissue-resident and monocyte-derived macrophages late in fibrosis delays resolution [64]. Although type II AEC dysfunction may initiate the development of IPF [[29], [30], [31]], recent findings suggest that repeated AEC injury is not required for fibrosis progression. Our group demonstrated that increased activation and mitochondrial localization of the small GTPase, Rac1, in monocyte-derived macrophages mediates lung fibrosis in the absence of lung injury and exacerbated fibrosis in the presence of injury [65]. The augmented Rac1 activity does not induce type II AEC injury or apoptosis; however, macrophage-derived TGF-β1 production and activation are increased to mediate fibrosis by promoting monocyte-derived macrophage/fibroblast interaction.
The generation of mtROS plays a critical role in lung injury and subsequent fibrosis [66,67]. Lung macrophages from IPF subjects generate increased mtROS [6,68], similar to the increase seen in lung macrophages from asbestosis subjects [69,70]. Levels of mtROS can be reduced in fibrotic macrophages by silencing the iron-sulfur protein, Rieske, in complex III of the mitochondrial ETC [67,71]. The increased ROS levels in IPF lung macrophages is due to the increased recruitment of monocyte-derived macrophages [72]. The increase in ROS levels is lung specific as this increase was not detected in blood monocytes isolated from IPF subjects.
Among the three superoxide dismutase (SOD) enzymes, Cu,Zn-SOD (SOD1), which is located in the cytosol and mitochondrial intermembrane space, is increased in asbestosis lung macrophages and contributes to the increased mtROS production by the dismutation of superoxide to hydrogen peroxide [66] (Fig. 2A). In addition, mitochondrial SOD1 accelerates the development of pulmonary fibrosis by augmenting the profibrotic polarization of lung macrophages via redox regulation of the Jumonji domain-containing protein 3 [70,73].
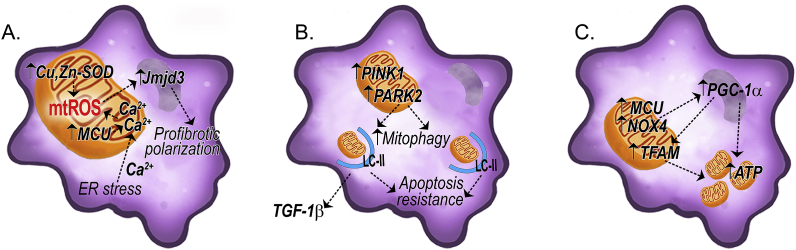
Fig. 2.
Schematic of mitochondrial quality control in fibrotic macrophages. (A) Fibrotic lung macrophages show increased mtROS mediated by Cu,Zn-SOD and MCU. Enhanced mtROS polarized macrophages to a profibrotic phenotype via Jmjd3 in a redox-dependent manner. (B) The increased mitophagy seen in fibrotic lung macrophages increases TGF-β1 secretion and leads to apoptosis resistance. (C) Increased mitochondrial biogenesis in fibrotic lung macrophages is induced via upregulation of PGC-1α and increased ATP production. Abbreviations: Jmjd3 = Jumonji domain containing 3; MCU = mitochondrial calcium uniporter; PARK2 = E3 ubiquitin-protein ligase parkin; TFAM = mitochondrial transcription factor A; TGF-β1 = transforming growth factor β1; SOD = superoxide dismutase.
Mitochondrial quality control in lung macrophages, including processes such as mitophagy and mitochondrial biogenesis, are critical determinants in pulmonary fibrosis. Diminished mitochondrial quality control results in augmented mitochondrial dysfunction and increased mtROS that leads to decrease ATP production and promotes intrinsic apoptosis [[74], [75], [76]]. Lung macrophages polarize to a profibrotic phenotype and are resistant to apoptosis in IPF subjects secondary to enhanced mitophagy [6] (Fig. 2B). Furthermore lung macrophage mitochondria must undergo mitophagy to produce TGF-β1, as Park2−/− mice fail to secrete TGF-β1 and are protected from bleomycin-induced fibrosis [6]. Translational support of the importance of mitophagy in fibrosis progression showed that IPF subjects taking the rapamycin analog, everolimus, which increases mitophagy in macrophages, had disease progression [77]. Moreover, rapamycin-treated mice showed increased active TGF-β1 in BAL fluid and exacerbation of fibrosis [6]. These studies suggest that mitophagy contributes to macrophage apoptosis resistance, which is an essential feature in the pathogenesis of the disease.
The removal of dysfunctional mitochondria by mitophagy in fibrotic lung macrophages is associated with increased mitochondrial biogenesis in these cells. Levels of PGC-1α are increased in lung macrophages from IPF and asbestosis subjects [16,17] (Fig. 2C). NOX4, which is also present in mitochondria [78], has been shown to regulate mitochondrial biogenesis in fibrotic lung macrophages via mtROS production [16]. Moreover, NOX4 regulated PGC-1α expression, and PGC-1α is required for NOX4-mediated mitochondrial biogenesis in fibrotic macrophages (Fig. 2C). The observed increase in mitochondrial biogenesis appears to be critical for sustaining the profibrotic phenotype of macrophages. Nox4−/− macrophages fail to polarize to the profibrotic phenotype and show reduced mtROS production [16], suggesting that mtROS and biogenesis are critical for the maintenance of the profibrotic macrophage phenotype.
Metabolic reprogramming that entails fatty acid oxidation (FAO) and OXPHOS is a key feature of profibrotic macrophages [79,80]. Recent evidence indicates that metabolic reprogramming shifts glycolysis to FAO in macrophages from bleomycin- or asbestos-injured mice [16,17]. The metabolic reprogramming in profibrotic macrophages is necessary to support long-term cellular activities (i.e., lung remodeling), as well as to promote the apoptotic resistance that is seen in these cells [6,[81], [82], [83]]. Studies indicate that ATP levels are increased in BAL fluid and within lung macrophages from subjects with lung fibrosis [84,85], and PGC-1α is required for ATP production in lung macrophage [85] (Fig. 2C). Moreover, extracellular ATP has been shown in promote mitochondrial dysfunction and mtDNA oxidation in mouse macrophages [86].
Calcium homeostasis plays a critical role in mitochondrial quality control. IPF and asbestosis subjects have higher mitochondrial calcium levels in lung macrophages compared with normal subjects [17,85]. The mitochondrial calcium uniporter (MCU), a highly selective ion channel transporting calcium into the mitochondria, is also increased in the lung macrophages from fibrosis subjects (Fig. 2A). An increase in mitochondrial calcium levels is associated with a loss of mitochondrial membrane potential (ΔψM). Lung macrophages isolated from asbestos-exposed WT mice showed a loss in ΔψM, which was not seen in asbestos-exposed MCU ± mice [85]. During ER stress, calcium is released from the ER and enters mitochondria. ER stress has been reported in macrophages isolated from IPF and asbestosis subjects and in bleomycin and asbestosis-exposed mice [87,88] (Fig. 2A). Calcium influx into the mitochondria is suggested to regulate OXPHOS and may promote an adaptive response to acute ER stress [85,89]. Moreover, MCU regulates PGC-1α expression (Fig. 2C) to mediate metabolic reprogramming in fibrotic lung macrophages [17].
Mitochondrial-derived ROS have been linked to inflammasome activation [90], and asbestos or silica, which can cause pulmonary fibrosis, are known to activate the NLRP3 inflammasome [91,92]. Likewise, lung macrophages from IPF subjects show increased NLRP3 inflammasome expression [93]. Vimentin intermediate filaments are known to modulate mitochondrial motility [94], and lack of vimentin results in increased ROS production [95]. Furthermore, mtROS is required for inflammasome priming [96], and vimentin is critical for NLRP3 inflammasome assembly and activation in pulmonary fibrosis [97].
Lung macrophages contribute to the pathogenesis of pulmonary fibrosis by initiating an immune response and by generating mtROS. The increase in mtROS promotes mitophagy to clear the dysfunctional mitochondria. Mitochondria from fibrotic lung macrophages maintain functionality through increased biogenesis and fission. The impact of the increased mitochondrial turnover leads to enhanced mitochondrial ATP content. The increased mtROS that is seen in these cells also promotes ER stress and an influx of calcium into the mitochondria. Lung macrophages in pulmonary fibrosis exhibit apoptosis resistance, and their prolonged survival is associated with disease progression. Fibrotic lung macrophages also undergo metabolic reprogramming to FAO, which is regulated by mtROS, to support the long-term cellular activities necessary for lung remodeling.
4. Lung fibroblasts
IPF lung fibroblasts have reduced mitochondrial mass, disrupted membranes, and altered cristae compared with lung fibroblasts from normal subjects, suggesting changes in mitochondrial homeostasis [98]. Studies concentrating on the three different aspects of mitochondrial homeostasis (biogenesis, dynamics, and mitophagy) provide many potential mechanisms of how fibroblast mitochondrial quality control actively participate in the pathogenesis of pulmonary fibrosis.
Much of the research on mitochondrial biogenesis focuses on PGC-1α, although contradicting data exist. TGF-β1 has been shown to activate PGC-1α; and induce mitochondrial biogenesis and bioenergetics in fibroblasts in vitro [99,100]. This augmented mitochondrial biogenesis is required to maintain the differentiated status of myofibroblasts. Interestingly, increasing mitochondrial biogenesis does not increase fibronectin and collagen IA1 expression, suggesting a multi-latitude of regulatory mechanisms for matrix protein production or degradation. In contrast, another study showed that PGC-1α level is suppressed in primary IPF fibroblasts, and overexpression PGC-1α can reverse the profibrotic phenotype of IPF fibroblasts with reduction of collagen production [101]. Inhibiting PGC-1α in normal primary lung fibroblasts and IMR90 lung fibroblasts leads to augmented collagen protein production. Activation of PGC-1α with a PPARɣ activator, rosiglitazone, can induce PGC-1α expression and reduce collagen gene expression. A PGC-1α-independent mechanism of mitochondrial biogenesis was also reported [41]. TGF-β1 induces NOX4 expression in fibroblasts and subsequent mtROS production [102] (Fig. 3A). NOX4, which is increased in IPF fibroblasts and promotes pulmonary fibrosis, suppresses mitochondrial biogenesis, and reduces OXPHOS. NOX4 modulates mitochondrial biogenesis by regulating Nrf2 expression and its nuclear translocation (Fig. 3B). Pharmacological inhibition or genetic silencing of NOX4 promotes mitochondrial biogenesis in fibroblasts.
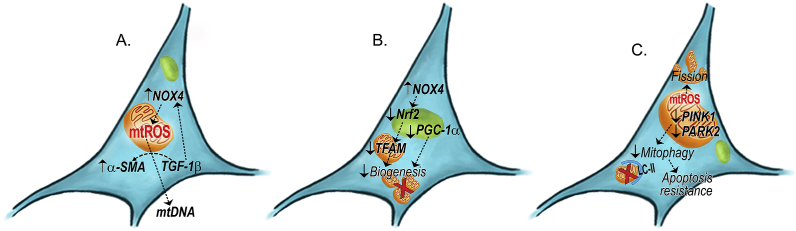
Fig. 3.
Schematic of mitochondrial quality control in fibrotic fibroblasts. (A) Increased mtROS promotes the release of mtDNA in fibrotic lung fibroblasts through TGF-β1-mediated NOX4 signaling. (B) The reduced biogenesis seen in fibrotic fibroblasts is through a PGC-1α-independent mechanism and a NOX4-mediated downregulation of Nrf2. (C) Reduced mitophagy in fibrotic lung fibroblasts promotes apoptosis resistance, and the increased fission is mediated by mtROS production. Abbreviations: α-SMA = α-smooth muscle actin; NOX4 = NADPH-oxidase 4; Nrf2 = Nuclear factor erythroid-derived 2-like 2.
The AMPK pathway plays a critical role in myofibroblasts differentiation and autophagy. Early research has shown that despite AMPK pathway activation in IPF lungs, there is no change of autophagy [103]. To note, these were measured in whole lung homogenates that lack specificity to cell types. TGF-β1 inhibits autophagy in human lung fibroblasts. Moreover, inhibition of autophagy by silencing LC3 increases α-SMA expression in fibroblasts. A recent study addressed AMPK activities in different cells types in pulmonary fibrosis. While AMPK activities remain unchanged in airway epithelial cells between normal and IPF lungs, AMPK activities are significantly reduced in the fibrotic foci where active myofibroblasts are located [104]. IPF fibroblasts have reduced AMPK activity and reduced autophagy, which is linked to mitochondrial dysfunction and metabolic reprogramming. IPF myofibroblasts have more fragmented mitochondria secondary to a defective mechanism involving mitophagy (Fig. 3C). Activation of AMPK pathway with a pharmacological activator, metformin, reversed established pulmonary fibrosis in an animal model of pulmonary fibrosis [104]. AMPK activation also transposed the apoptosis-resistant phenotype observed in IPF fibroblasts. One of the proposed mechanisms of fibroblast reversal from apoptotic resistance is through increasing AMPK-activated mitochondrial biogenesis.
Several recent studies have specifically evaluated mitophagy in pulmonary fibrosis by focusing on mitophagy-specific proteins, such as PINK1 and PARK2. TGF-β1 treatment inhibits PINK1 and PARK2 expression in lung fibroblast cell lines [105] (Fig. 3C). Targeting PINK1 and PARK2, Kobayashi et al. showed that silencing either gene is able to induce α-SMA expression in lung fibroblasts with a greater extent seen in PARK2−/− fibroblasts [49]. Although no significant mitochondrial damage was observed by electron microscopy, increased mtROS production was detected biochemically, which can be attenuated by either N-acetylcysteine (NAC) or Mito-TEMPO. Surprisingly, no changes in both fusion-related (OPA1 and MFN1/2) and fission-related (Drp1 and FIS1) proteins were observed in PARK2 null fibroblasts compared with wildtype fibroblasts. Additionally, no differences were observed in ATP production between wildtype and PARK2−/− fibroblasts. Silencing PARK2 resulted in increased phosphorylation of Akt1 and PDGF, and the Akt1/2 inhibitor blocked α-SMA expression and PDGFR phosphorylation in PARK2−/− fibroblasts, suggesting an important role of Akt in mitochondrial dynamics and fibrosis progression [106]. Using a PDGF inhibitor, AG1296, the authors demonstrate that mice are protected from developing pulmonary fibrosis and conclude that in fibroblasts, PARK2-mediated mitophagy mainly regulates fibroblast differentiation through PDGFR/Akt signaling pathway. The same authors further demonstrate that pirfenidone, one of the two FDA-approved anti-fibrotic medications, induce PARK2 but not PINK1 expression and promote mitophagy in fibroblasts. Mechanistically, pirfenidone inhibits ROS production and abrogates Akt activation in PARK2 null fibroblasts. Further studies are needed to address the knowledge gap of mitophagy-specific genes in different types of fibrosis-promoting cells during fibrogenesis.
One proposed mechanism of regulating PARK2-mediated mitophagy in pulmonary fibrosis is through miR-1224–5p [105]. TGF-β1 treatment inhibits PARK2 mitochondrial translocation, which is rescued by a miR-1224–5p inhibitor in vitro. miR-1224–5p binds to the promoter region of becn1 gene and inhibits its expression. Immunoprecipitation experiments further verified that beclin-1, which can also induce autophagy/mitophagy, binds to PARK2. In an experimental silicosis model, miR-1224–5p levels are increased, and the expression of beclin-1 is reduced in mice exposed to silica [107]. Similarly, these authors showed that silencing PARK2 leads to activation of Akt/PDGFR pathways and increased α-SMA expression in fibroblasts.
Limited studies have investigated the role of mitochondrial fusion and fission in fibroblasts during pulmonary fibrosis. One study showed that astaxanthin, a carotenoid usually used as dietary supplement, can induce Drp1 expression and mitochondrial translocation that leads to enhanced fission and apoptosis in human fibroblasts and bleomycin-injured mice. However, TGF-β1 treatment does not alter mitochondrial fission [108]. The authors proposed that the protective mechanism of astaxanthin in bleomycin-injured mice is secondary to fission and apoptosis of myofibroblasts; however, additional experiments utilizing mice harboring a selective deletion of fission-associated genes, such as Drp1, are required to validate these observations.
Mitochondrial DNA is an emerging research area due to its translational significance as a biomarker of mitochondrial homeostasis and cellular injuries in various disease conditions, such as ARDS, COPD and various fibrotic lung diseases, including IPF. Studies have shown that IPF fibroblasts produce increased amount of mtDNA in vitro [23]. Similarly, normal lung fibroblasts have increased mtDNA production upon stimulation of TGF-β1. Furthermore, mtDNA is elevated in bronchoalveolar lavage fluid and plasma of IPF subjects (Fig. 3A). From a biomarker standpoint, elevated serum mtDNA is correlated with increased all-cause mortality in IPF, and mtDNA is reduced in IPF subjects that respond to pirfenidone treatment [23]. Although the authors did not specifically address whether increasing mtDNA is a direct result of either mitochondrial biogenesis or mitophagy, they showed that TGF-β1-stimulated fibroblasts have reduced mitochondrial mass, elongation and enlargement, and maintenance of ΔψM. The authors postulate that elevated extracellular mtDNA is not related to cell viability (i.e., not secondary to increasing apoptosis or necrosis), but rather is the result of a potential active releasing/production that may enhance fibroblasts differentiation into myofibroblasts (Fig. 3A). In contrast, Bueno et al., showed that there are no differences in mtDNA production by IPF fibroblasts compared with age-matched normal fibroblasts [109]. Given mtDNA is found to be elevated in various fibrotic lung diseases, including IPF, sarcoidosis, hypersensitivity pneumonitis, and ILD caused by connective tissue diseases, it is critical to further delineate the role(s) and mechanism(s) that lead to increased extracellular mtDNA given its translational importance in determining drug efficacy and predicting prognosis, at least, in IPF.
During fibrosis, fibroblasts have augmented mitochondrial dysfunction and generate increased mtROS while having suppressed mitophagy and mitochondrial biogenesis that promotes differentiation into myofibroblasts. The suppression of mitophagy and biogenesis also contributes to apoptosis resistance. The evidence supporting the role of mtDNA as a biomarker and prognostic indicator has translational and clinical importance. Thus, attenuation of the production and release of mtDNA from fibroblasts during differentiation may be a therapeutic target.
5. Conclusion
The most common form of pulmonary fibrosis, idiopathic pulmonary fibrosis is a devastating disease affecting approximately 3 million people worldwide and has a high mortality rate [110]. The currently approved therapies, pirfenidone and nintedanib, have limited efficacy in that neither drug is associated with improvements in quality of life or mortality and only show modest improvements in disease symptoms [111,112]. Emerging evidence suggest that the contribution of mitochondrial dysfunction is a key player in the pathophysiology of lung diseases, such as IPF.
Mitochondrial quality control is an important determinant for bioenergetic changes that occur in response to metabolic stress. Disrupted mitochondrial quality control is associated with changes in mitochondrial morphology, OXPHOS, excessive mtROS production, decline in mitochondrial membrane potential, altered energy production, modulated intracellular calcium flux, and activation of cell death mechanisms/apoptosis (Table 1). Changes in mitochondrial quality control including mitophagy, mitochondrial biogenesis, and mitochondrial dynamics (fission and fusion) are critical to maintain cell homeostasis. A recent study highlighted a core set of genes that changed in the IPF lung before fibrosis was histologically evident and continued to change with disease progression [113]. Systems biology analysis revealed that genes related to mitochondrial transport, mitochondrial trafficking, and mitochondrial organization were found to be altered with advanced fibrosis, potentially explaining the loss of mitochondrial quality control that occurs during fibrogenesis.
Table 1.
Changes of key factors in mitochondrial quality control during pulmonary fibrosis.
| mtROS | Biogenesis | Dynamics |
Mitophagy | Bioenergetics | ATP | Apoptosis | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Fusion | Fission | ||||||||
| AEC II | ++ | ─ | + | ─ | ─ | Glycolysis | ─ | + | [5,[37], [38], [39],43,45,48,50,51,53,54] |
| Macrophages | +++ | ++ | + | + | ++ | β-oxidation | ++ | ─ | [6,16,17,[65], [66], [67],[69], [70], [71],83,85] |
| Fibroblasts | ++ | +/─ | ─ | ++ | ─ | Glycolysis | ─ | ─ | [41,49,[98], [99], [100], [101],[104], [105], [106], [107]] |
Table 1: Comparison of mitochondrial quality control and its key modulator/effectors between AECII, macrophages, and fibroblasts during pulmonary fibrosis. AECII have attenuated biogenesis and mitophagy with predominantly glycolysis-driven metabolism. This leads to reduced ATP and increased mtROS production, which leads to apoptosis. Macrophages have augmented biogenesis and mitophagy with augmented fatty acids β-oxidation and ATP production. As the result, they become resistant to apoptosis. Fibroblasts have increased fission but reduced mitophagy, collectively causing increased mtROS and reduced ATP production. Metabolically, fibroblasts also have increased glycolysis and shown to be apoptosis resistance.
The mitochondrial quality control system plays various roles in different cell types within the context of pulmonary fibrosis. While type II AECs, lung macrophages, and fibroblasts all show increased mtROS production in fibrotic lungs, their response to the excessive ROS differs. Type II AECs undergo apoptosis in response to increased mtROS; however, lung macrophages and fibroblasts display apoptosis resistance. Mitochondria from fibrotic type II AECs become swollen and enlarged, but mitophagy and biogenesis are reduced. In contrast, dysfunctional mitochondria from fibrotic lung macrophages are removed via mitophagy, subsequently biogenesis increases and mitochondria undergo fission. Fibrotic fibroblasts show reduced mitophagy and mitochondrial biogenesis, but enhanced fission/fragmentation is seen, leading to a pool of dysfunctional mitochondria. In response to increasing oxidative stress, all three cell types undergo a dramatic metabolic reprogramming (Table 1). A loss in lipid synthesis in type II AECs leads to impaired production of cholesterol and phospholipids required for surfactant synthesis. Fatty acid oxidation and OXPHOS in fibrotic lung macrophages promote the sustained profibrotic phenotype that potentiates dysregulated repair. Many rate-limiting glycolytic enzymes are upregulated in fibrotic lung fibroblasts promoting an increase in glycolytic flux. These metabolic reprogramming events are critical for driving the profibrotic behaviors and contribute to the development and progression of lung fibrosis.
A randomized clinical trial using NAC failed to improve clinical outcomes (changes of lung function, rate of death, or acute exacerbation) in patients with IPF [114]. Although attempts to alter ROS with general antioxidants have been largely unsuccessful, the failure of the NAC trial may have resulted from an incomplete understanding of role of mtROS and IPF development. Strategies to target mitochondria with organelle specific drugs are being developed. MitoQ, a mitochondrial targeted antioxidant, has been shown to attenuate expression of TGF-β1 and NOX4 in IPF lung fibroblasts [115]. In addition, transgenic mice expressing mitochondrial catalase are protected from asbestos- and bleomycin-induced lung fibrosis and had lower levels of mtDNA damage [39]. Furthermore, using lung tissue and/or cells from patients undergoing biopsy or lung transplantation to develop 3D lung organoids (pulmospheres) may aide in the ability to target cell-specific mitochondria for a personalized medicine approach [116]. The growing interest in mitochondrial biology may provide new avenues to identify novel therapeutics.
Declaration of competing interest
The authors have declared that no conflict of interest exists.
Acknowledgements
JLC is supported by a Parker B. Francis fellowship and American Lung Association grant RG-507440. CH is supported by the National Institute of Health grant 5T32HL105346. ABC is supported by the National Institute of Health grants 2R01ES015981-12, P01HL114470-7, and 1 I01 CX001715-01 to the Department of Veteran Affairs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101426.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mizumura K., Cloonan S.M., Nakahira K., Bhashyam A.R., Cervo M., Kitada T., Glass K., Owen C.A., Mahmood A., Washko G.R., Hashimoto S., Ryter S.W., Choi A.M. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu W., Koeck T., Lara A.R., Neumann D., DiFilippo F.P., Koo M., Janocha A.J., Masri F.A., Arroliga A.C., Jennings C., Dweik R.A., Tuder R.M., Stuehr D.J., Erzurum S.C. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mabalirajan U., Dinda A.K., Kumar S., Roshan R., Gupta P., Sharma S.K., Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J. Immunol. 2008;181:3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 4.Trian T., Benard G., Begueret H., Rossignol R., Girodet P.O., Ghosh D., Ousova O., Vernejoux J.M., Marthan R., Tunon-de-Lara J.M., Berger P. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueno M., Lai Y.C., Romero Y., Brands J., St Croix C.M., Kamga C., Corey C., Herazo-Maya J.D., Sembrat J., Lee J.S., Duncan S.R., Rojas M., Shiva S., Chu C.T., Mora A.L. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Investig. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson-Casey J.L., Deshane J.S., Ryan A.J., Thannickal V.J., Carter A.B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. 2016;44:582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B.B., Coon T.A., Glasser J.R., Zou C., Ellis B., Das T., McKelvey A.C., Rajbhandari S., Lear T., Kamga C., Shiva S., Li C., Pilewski J.M., Callio J., Chu C.T., Ray A., Ray P., Tyurina Y.Y., Kagan V.E., Mallampalli R.K. E3 ligase subunit Fbxo15 and PINK1 kinase regulate cardiolipin synthase 1 stability and mitochondrial function in pneumonia. Cell Rep. 2014;7:476–487. doi: 10.1016/j.celrep.2014.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan J.J., Marsboom G., Fang Y.H., Toth P.T., Morrow E., Luo N., Piao L., Hong Z., Ericson K., Zhang H.J., Han M., Haney C.R., Chen C.T., Sharp W.W., Archer S.L. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2013;187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehman J., Zhang H.J., Toth P.T., Zhang Y., Marsboom G., Hong Z., Salgia R., Husain A.N., Wietholt C., Archer S.L. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz R.M., Dayhoff M.O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978;199:395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- 11.Yang D., Oyaizu Y., Oyaizu H., Olsen G.J., Woese C.R. Mitochondrial origins. Proc. Natl. Acad. Sci. U. S. A. 1985;82:4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roger A.J., Munoz-Gomez S.A., Kamikawa R. The origin and diversification of mitochondria. Curr. Biol. 2017;27:R1177–R1192. doi: 10.1016/j.cub.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Carraway M.S., Suliman H.B., Kliment C., Welty-Wolf K.E., Oury T.D., Piantadosi C.A. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxidants Redox Signal. 2008;10:269–275. doi: 10.1089/ars.2007.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J. Clin. Investig. 2016;126:809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suliman H.B., Welty-Wolf K.E., Carraway M.S., Schwartz D.A., Hollingsworth J.W., Piantadosi C.A. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J. 2005;19:1531–1533. doi: 10.1096/fj.04-3500fje. [DOI] [PubMed] [Google Scholar]
- 16.He C., Larson-Casey J.L., Davis D., Hanumanthu V.S., Longhini A.L.F., Thannickal V.J., Gu L., Carter A.B. NOX4 modulates macrophage phenotype and mitochondrial biogenesis in asbestosis. JCI Insight. 2019;4:e126551. doi: 10.1172/jci.insight.126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu L., Larson-Casey J.L., Andrabi S.A., Lee J.H., Meza-Perez S., Randall T.D., Carter A.B. Mitochondrial calcium uniporter regulates PGC-1alpha expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol. 2019;26:101307. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gegg M.E., Cooper J.M., Chau K.Y., Rojo M., Schapira A.H., Taanman J.W. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Dorn G.W., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottmann R.M., Kulkarni A.A., Smolnycki K.A., Lyda E., Dahanayake T., Salibi R., Honnons S., Jones C., Isern N.G., Hu J.Z., Nathan S.D., Grant G., Phipps R.P., Sime P.J. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am. J. Respir. Crit. Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie N., Tan Z., Banerjee S., Cui H., Ge J., Liu R.M., Bernard K., Thannickal V.J., Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu C., Sun H., Gulati M., Herazo-Maya J.D., Chen Y., Osafo-Addo A., Brandsdorfer C., Winkler J., Blaul C., Faunce J., Pan H., Woolard T., Tzouvelekis A., Antin-Ozerkis D.E., Puchalski J.T., Slade M., Gonzalez A.L., Bogenhagen D.F., Kirillov V., Feghali-Bostwick C., Gibson K., Lindell K., Herzog R.I., Dela Cruz C.S., Mehal W., Kaminski N., Herzog E.L., Trujillo G. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2017;196:1571–1581. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yakes F.M., Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheresh P., Morales-Nebreda L., Kim S.J., Yeldandi A., Williams D.B., Cheng Y., Mutlu G.M., Budinger G.R., Ridge K., Schumacker P.T., Bohr V.A., Kamp D.W. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am. J. Respir. Cell Mol. Biol. 2015;52:25–36. doi: 10.1165/rcmb.2014-0038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang F., Marangoni R.G., Zhou X., Yang Y., Ye B., Shangguang A., Qin W., Wang W., Bhattacharyya S., Wei J., Tourtellotte W.G., Varga J. Toll-like receptor 9 signaling is augmented in systemic sclerosis and elicits transforming growth factor beta-dependent fibroblast activation. Arthritis Rheum. 2016;68:1989–2002. doi: 10.1002/art.39655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meneghin A., Choi E.S., Evanoff H.L., Kunkel S.L., Martinez F.J., Flaherty K.R., Toews G.B., Hogaboam C.M. TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem. Cell Biol. 2008;130:979–992. doi: 10.1007/s00418-008-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trujillo G., Meneghin A., Flaherty K.R., Sholl L.M., Myers J.L., Kazerooni E.A., Gross B.H., Oak S.R., Coelho A.L., Evanoff H., Day E., Toews G.B., Joshi A.D., Schaller M.A., Waters B., Jarai G., Westwick J., Kunkel S.L., Martinez F.J., Hogaboam C.M. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001510. 57ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., Chapman H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korfei M., Ruppert C., Mahavadi P., Henneke I., Markart P., Koch M., Lang G., Fink L., Bohle R.M., Seeger W., Weaver T.E., Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson W.E., Cheng D.S., Degryse A.L., Tanjore H., Polosukhin V.V., Xu X.C., Newcomb D.C., Jones B.R., Roldan J., Lane K.B., Morrisey E.E., Beers M.F., Yull F.E., Blackwell T.S. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plataki M., Koutsopoulos A.V., Darivianaki K., Delides G., Siafakas N.M., Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 33.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J.F., Liu X., White E.S., Thannickal V.J., Moore B.B., Christensen P.J., Simon R.H. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwano K., Kunitake R., Maeyama T., Hagimoto N., Kawasaki M., Matsuba T., Yoshimi M., Inoshima I., Yoshida K., Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 35.Lee C.G., Cho S.J., Kang M.J., Chapoval S.P., Lee P.J., Noble P.W., Yehualaeshet T., Lu B., Flavell R.A., Milbrandt J., Homer R.J., Elias J.A. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang H.R., Cho S.J., Lee C.G., Homer R.J., Elias J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 37.Patel A.S., Song J.W., Chu S.G., Mizumura K., Osorio J.C., Shi Y., El-Chemaly S., Lee C.G., Rosas I.O., Elias J.A., Choi A.M., Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor- Beta1 in pulmonary fibrosis. PLoS One. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panduri V., Weitzman S.A., Chandel N.S., Kamp D.W. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L1220–L1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.J., Cheresh P., Jablonski R.P., Morales-Nebreda L., Cheng Y., Hogan E., Yeldandi A., Chi M., Piseaux R., Ridge K., Michael Hart C., Chandel N., Scott Budinger G.R., Kamp D.W. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic. Biol. Med. 2016;101:482–490. doi: 10.1016/j.freeradbiomed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard K., Logsdon N.J., Miguel V., Benavides G.A., Zhang J., Carter A.B., Darley-Usmar V.M., Thannickal V.J. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J. Biol. Chem. 2017;292:3029–3038. doi: 10.1074/jbc.M116.752261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy N.M., Kleeberger S.R., Cho H.Y., Yamamoto M., Kensler T.W., Biswal S., Reddy S.P. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am. J. Respir. Cell Mol. Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H.Y., Reddy S.P., Yamamoto M., Kleeberger S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.J., Cheresh P., Williams D., Cheng Y., Ridge K., Schumacker P.T., Weitzman S., Bohr V.A., Kamp D.W. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J. Biol. Chem. 2014;289:6165–6176. doi: 10.1074/jbc.M113.515130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwano K., Nakashima N., Inoshima I., Hagimoto N., Fujita M., Yoshimi M., Maeyama T., Hamada N., Watanabe K., Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur. Respir. J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 46.Jablonski R.P., Kim S.J., Cheresh P., Williams D.B., Morales-Nebreda L., Cheng Y., Yeldandi A., Bhorade S., Pardo A., Selman M., Ridge K., Gius D., Budinger G.R.S., Kamp D.W. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017;31:2520–2532. doi: 10.1096/fj.201601077R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundaresan N.R., Bindu S., Pillai V.B., Samant S., Pan Y., Huang J.Y., Gupta M., Nagalingam R.S., Wolfgeher D., Verdin E., Gupta M.P. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3beta. Mol. Cell. Biol. 2015;36:678–692. doi: 10.1128/MCB.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi K., Araya J., Minagawa S., Hara H., Saito N., Kadota T., Sato N., Yoshida M., Tsubouchi K., Kurita Y., Ito S., Fujita Y., Takasaka N., Utsumi H., Yanagisawa H., Hashimoto M., Wakui H., Kojima J., Shimizu K., Numata T., Kawaishi M., Kaneko Y., Asano H., Yamashita M., Odaka M., Morikawa T., Nakayama K., Kuwano K. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 2016;197:504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 50.Bueno M., Brands J., Voltz L., Fiedler K., Mays B. St Croix C, Sembrat J, Mallampalli RK, Rojas M, Mora AL. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell. 2018:17. doi: 10.1111/acel.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu G., Tzouvelekis A., Wang R., Herazo-Maya J.D., Ibarra G.H., Srivastava A., de Castro J.P.W., DeIuliis G., Ahangari F., Woolard T., Aurelien N., Arrojo E.D.R., Gan Y., Graham M., Liu X., Homer R.J., Scanlan T.S., Mannam P., Lee P.J., Herzog E.L., Bianco A.C., Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y., Mizuno T., Sridharan A., Du Y., Guo M., Tang J., Wikenheiser-Brokamp K.A., Perl A.T., Funari V.A., Gokey J.J., Stripp B.R., Whitsett J.A. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung K.P., Hsu C.L., Fan L.C., Huang Z., Bhatia D., Chen Y.J., Hisata S., Cho S.J., Nakahira K., Imamura M., Choi M.E., Yu C.J., Cloonan S.M., Choi A.M.K. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat. Commun. 2019;10:3390. doi: 10.1038/s41467-019-11327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson P.C., Watters L.C., King T.E., Mason R.J. Idiopathic pulmonary fibrosis. Abnormalities in bronchoalveolar lavage fluid phospholipids. Am. Rev. Respir. Dis. 1988;137:585–591. doi: 10.1164/ajrccm/137.3.585. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y.D., Yin L., Archer S., Lu C., Zhao G., Yao Y., Wu L., Hsin M., Waddell T.K., Keshavjee S., Granton J., de Perrot M. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir. Res. 2017;4:e000183. doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D.A., Perlman H., Malissen B., Zelzer E., Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S.W., Forsberg E.C., Tanaka M., van Rooijen N., Garcia-Sastre A., Stanley E.R., Ginhoux F., Frenette P.S., Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssen W.J., Barthel L., Muldrow A., Oberley-Deegan R.E., Kearns M.T., Jakubzick C., Henson P.M. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore B.B., Paine R., 3rd, Christensen P.J., Moore T.A., Sitterding S., Ngan R., Wilke C.A., Kuziel W.A., Toews G.B. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 62.Osterholzer J.J., Olszewski M.A., Murdock B.J., Chen G.H., Erb-Downward J.R., Subbotina N., Browning K., Lin Y., Morey R.E., Dayrit J.K., Horowitz J.C., Simon R.H., Sisson T.H. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J. Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K.J.N., Abdala-Valencia H., Yacoub T.J., Chi M., Chiu S., Gonzalez-Gonzalez F.J., Gates K., Lam A.P., Nicholson T.T., Homan P.J., Soberanes S., Dominguez S., Morgan V.K., Saber R., Shaffer A., Hinchcliff M., Marshall S.A., Bharat A., Berdnikovs S., Bhorade S.M., Bartom E.T., Morimoto R.I., Balch W.E., Sznajder J.I., Chandel N.S., Mutlu G.M., Jain M., Gottardi C.J., Singer B.D., Ridge K.M., Bagheri N., Shilatifard A., Budinger G.R.S., Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibbons M.A., MacKinnon A.C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A.T., van Rooijen N., Haslett C., Howie S.E., Simpson A.J., Hirani N., Gauldie J., Iredale J.P., Sethi T., Forbes S.J. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 65.Larson-Casey J.L., Vaid M., Gu L., He C., Cai G.Q., Ding Q., Davis D., Berryhill T.F., Wilson L.S., Barnes S., Neighbors J.D., Hohl R.J., Zimmerman K.A., Yoder B.K., Longhini A.L.F., Hanumanthu V.S., Surolia R., Antony V.B., Carter A.B. Increased flux through the mevalonate pathway mediates fibrotic repair without injury. J. Clin. Investig. 2019;129:4962–4978. doi: 10.1172/JCI127959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He C., Murthy S., McCormick M.L., Spitz D.R., Ryan A.J., Carter A.B. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J. Biol. Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murthy S., Ryan A., He C., Mallampalli R.K., Carter A.B. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 2010;285:25062–25073. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strausz J., Muller-Quernheim J., Steppling H., Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 69.Murthy S., Larson-Casey J.L., Ryan A.J., He C., Kobzik L., Carter A.B. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 2015;29:3527–3536. doi: 10.1096/fj.15-271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He C., Ryan A.J., Murthy S., Carter A.B. Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J. Biol. Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osborn-Heaford H.L., Ryan A.J., Murthy S., Racila A.M., He C., Sieren J.C., Spitz D.R., Carter A.B. Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J. Biol. Chem. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiemle-Kallee J., Kreipe H., Radzun H.J., Parwaresch M.R., Auerswald U., Magnussen H., Barth J. Alveolar macrophages in idiopathic pulmonary fibrosis display a more monocyte-like immunophenotype and an increased release of free oxygen radicals. Eur. Respir. J. 1991;4:400–406. [PubMed] [Google Scholar]
- 73.He C., Larson-Casey J.L., Gu L., Ryan A.J., Murthy S., Carter A.B. Cu,Zn-Superoxide dismutase-mediated redox regulation of Jumonji domain containing 3 modulates macrophage polarization and pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2016;55:58–71. doi: 10.1165/rcmb.2015-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim N.C., Tresse E., Kolaitis R.M., Molliex A., Thomas R.E., Alami N.H., Wang B., Joshi A., Smith R.B., Ritson G.P., Winborn B.J., Moore J., Lee J.Y., Yao T.P., Pallanck L., Kundu M., Taylor J.P. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron. 2013;78:65–80. doi: 10.1016/j.neuron.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song M., Chen Y., Gong G., Murphy E., Rabinovitch P.S., Dorn G.W., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ. Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winklhofer K.F. Parkin and mitochondrial quality control: toward assembling the puzzle. Trends Cell Biol. 2014;24:332–341. doi: 10.1016/j.tcb.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Malouf M.A., Hopkins P., Snell G., Glanville A.R. Everolimus in IPFSI. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16:776–783. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- 78.Graham K.A., Kulawiec M., Owens K.M., Li X., Desouki M.M., Chandra D., Singh K.K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang S.C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A.M., Beatty W., Love-Gregory L., Lam W.Y., O'Neill C.M., Yan C., Du H., Abumrad N.A., Urban J.F., Jr., Artyomov M.N., Pearce E.L., Pearce E.J. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez-Prados J.C., Traves P.G., Cuenca J., Rico D., Aragones J., Martin-Sanz P., Cascante M., Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 81.Odegaard J.I., Chawla A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., Pearce E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larson-Casey J.L., Murthy S., Ryan A.J., Carter A.B. Modulation of the mevalonate pathway by Akt regulates macrophage survival and development of pulmonary fibrosis. J. Biol. Chem. 2014;289:36204–36219. doi: 10.1074/jbc.M114.593285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V.F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 85.Gu L., Larson-Casey J.L., Carter A.B. Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization. FASEB J. 2017;31:3072–3083. doi: 10.1096/fj.201601371R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald K.A., Underhill D.M., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryan A.J., Larson-Casey J.L., He C., Murthy S., Carter A.B. Asbestos-induced disruption of calcium homeostasis induces endoplasmic reticulum stress in macrophages. J. Biol. Chem. 2014;289:33391–33403. doi: 10.1074/jbc.M114.579870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao Y., Wang Y., Zhang Z., He L., Zhu J., Zhang M., He X., Cheng Z., Ao Q., Cao Y., Yang P., Su Y., Zhao J., Zhang S., Yu Q., Ning Q., Xiang X., Xiong W., Wang C.Y., Xu Y. Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol. Ther. 2016;24:915–925. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griffiths E.J., Rutter G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 90.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 91.Cassel S.L., Eisenbarth S.C., Iyer S.S., Sadler J.J., Colegio O.R., Tephly L.A., Carter A.B., Rothman P.B., Flavell R.A., Sutterwala F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lasithiotaki I., Giannarakis I., Tsitoura E., Samara K.D., Margaritopoulos G.A., Choulaki C., Vasarmidi E., Tzanakis N., Voloudaki A., Sidiropoulos P., Siafakas N.M., Antoniou K.M. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur. Respir. J. 2016;47:910–918. doi: 10.1183/13993003.00564-2015. [DOI] [PubMed] [Google Scholar]
- 94.Nekrasova O.E., Mendez M.G., Chernoivanenko I.S., Tyurin-Kuzmin P.A., Kuczmarski E.R., Gelfand V.I., Goldman R.D., Minin A.A. Vimentin intermediate filaments modulate the motility of mitochondria. Mol. Biol. Cell. 2011;22:2282–2289. doi: 10.1091/mbc.E10-09-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mor-Vaknin N., Punturieri A., Sitwala K., Markovitz D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 96.Bauernfeind F., Bartok E., Rieger A., Franchi L., Nunez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.dos Santos G., Rogel M.R., Baker M.A., Troken J.R., Urich D., Morales-Nebreda L., Sennello J.A., Kutuzov M.A., Sitikov A., Davis J.M., Lam A.P., Cheresh P., Kamp D., Shumaker D.K., Budinger G.R., Ridge K.M. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015;6:6574. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alvarez D., Cardenes N., Sellares J., Bueno M., Corey C., Hanumanthu V.S., Peng Y., D'Cunha H., Sembrat J., Nouraie M., Shanker S., Caufield C., Shiva S., Armanios M., Mora A.L., Rojas M. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L1164–L1173. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun Q., Fang L., Tang X., Lu S., Tamm M., Stolz D., Roth M. TGF-beta upregulated mitochondria mass through the SMAD2/3-->C/EBPbeta-->PRMT1 signal pathway in primary human lung fibroblasts. J. Immunol. 2019;202:37–47. doi: 10.4049/jimmunol.1800782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bernard K., Logsdon N.J., Ravi S., Xie N., Persons B.P., Rangarajan S., Zmijewski J.W., Mitra K., Liu G., Darley-Usmar V.M., Thannickal V.J. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J. Biol. Chem. 2015;290:25427–25438. doi: 10.1074/jbc.M115.646984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caporarello N., Meridew J.A., Jones D.L., Tan Q., Haak A.J., Choi K.M., Manlove L.J., Prakash Y.S., Tschumperlin D.J., Ligresti G. PGC1alpha repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax. 2019;74:749–760. doi: 10.1136/thoraxjnl-2019-213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patel A.S., Lin L., Geyer A., Haspel J.A., An C.H., Cao J., Rosas I.O., Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rangarajan S., Bone N.B., Zmijewska A.A., Jiang S., Park D.W., Bernard K., Locy M.L., Ravi S., Deshane J., Mannon R.B., Abraham E., Darley-Usmar V., Thannickal V.J., Zmijewski J.W. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Q., Xu T., Liu Y., Li Y., Yuan J., Yao W., Xu Q., Yan W., Ni C. miR-1224-5p mediates mitochondrial damage to affect silica-induced pulmonary fibrosis by targeting BECN1. Int. J. Mol. Sci. 2017:18. doi: 10.3390/ijms18112357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurita Y., Araya J., Minagawa S., Hara H., Ichikawa A., Saito N., Kadota T., Tsubouchi K., Sato N., Yoshida M., Kobayashi K., Ito S., Fujita Y., Utsumi H., Yanagisawa H., Hashimoto M., Wakui H., Yoshii Y., Ishikawa T., Numata T., Kaneko Y., Asano H., Yamashita M., Odaka M., Morikawa T., Nakayama K., Kuwano K. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir. Res. 2017;18:114. doi: 10.1186/s12931-017-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji X., Wu B., Fan J., Han R., Luo C., Wang T., Yang J., Han L., Zhu B., Wei D., Chen J., Ni C. The anti-fibrotic effects and mechanisms of MicroRNA-486-5p in pulmonary fibrosis. Sci. Rep. 2015;5:14131. doi: 10.1038/srep14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang J., Xu P., Wang Y., Wang M., Li H., Lin S., Mao C., Wang B., Song X., Lv C. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission. J. Cell Mol. Med. 2015;19:2215–2231. doi: 10.1111/jcmm.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bueno M., Zank D., Buendia-Roldan I., Fiedler K., Mays B.G., Alvarez D., Sembrat J., Kimball B., Bullock J.K., Martin J.L., Nouraie M., Kaufman B.A., Rojas M., Pardo A., Selman M., Mora A.L. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PLoS One. 2019;14:e0218003. doi: 10.1371/journal.pone.0218003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez F.J., Collard H.R., Pardo A., Raghu G., Richeldi L., Selman M., Swigris J.J., Taniguchi H., Wells A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 111.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., Lederer D.J., Nathan S.D., Pereira C.A., Sahn S.A., Sussman R., Swigris J.J., Noble P.W., Group A.S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 112.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., Kim D.S., Kolb M., Nicholson A.G., Noble P.W., Selman M., Taniguchi H., Brun M., Le Maulf F., Girard M., Stowasser S., Schlenker-Herceg R., Disse B., Collard H.R., nvestigators I.T. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 113.McDonough J.E., Ahangari F., Li Q., Jain S., Verleden S.E., Herazo-Maya J., Vukmirovic M., DeIuliis G., Tzouvelekis A., Tanabe N., Chu F., Yan X., Verschakelen J., Homer R.J., Manatakis D.V., Zhang J., Ding J., Maes K., De Sadeleer L., Vos R., Neyrinck A., Benos P.V., Bar-Joseph Z., Tantin D., Hogg J.C., Vanaudenaerde B.M., Wuyts W.A., Kaminski N. Transcriptional regulatory model of fibrosis progression in the human lung. JCI Insight. 2019:4. doi: 10.1172/jci.insight.131597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Idiopathic Pulmonary Fibrosis Clinical Research N Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr., Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain M., Rivera S., Monclus E.A., Synenki L., Zirk A., Eisenbart J., Feghali-Bostwick C., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J. Biol. Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Surolia R., Li F.J., Wang Z., Li H., Liu G., Zhou Y., Luckhardt T., Bae S., Liu R.M., Rangarajan S., de Andrade J., Thannickal V.J., Antony V.B. 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. JCI Insight. 2017;2:e91377. doi: 10.1172/jci.insight.91377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.