Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating lung disease of unknown etiology. It is characterized by deposition of extracellular matrix proteins, like collagen and fibronectin in the lung interstitium leading to respiratory failure. Our understanding of the pathobiology underlying IPF is still incomplete; however, it is accepted that aging is a major risk factor in the disease while growing evidence suggests that the mitochondria plays an important role in the initiation and progression of pulmonary fibrosis. Mitochondria dysfunction and metabolic reprogramming had been identified in different IPF lung cells (alveolar epithelial cells, fibroblasts, and macrophages) promoting low resilience and increasing susceptibility to activation of profibrotic responses. Here we summarize changes in mitochondrial numbers, biogenesis, turnover and associated metabolic adaptations that promote disrepair and fibrosis in the lung. Finally, we highlight new possible therapeutic approaches focused on ameliorate mitochondrial dysfunction.
Keywords: Fibrosis, Aging, Mitochondrial dysfunction, Epithelial cells, Fibroblast, Macrophage
Graphical abstract
1. Introduction
Idiopathic Pulmonary Fibrosis (IPF), one of the most common ILDs (interstitial lung diseases), is a chronic, irreversible and fatal lung disease characterized by scarring and thickening of the interstitial tissue in the lung leading to dyspnea and, finally, respiratory failure. Notably, IPF is characterized by changed in the lung alveolar epithelium, fibroblast foci accumulation, and exaggerated extracellular matrix proteins deposition [1]. IPF histology is characterized by an usual interstitial pneumonia pattern, including honeycombing in lower lobes and subpleural regions [2] accompanied by myofibroblast foci located in the more dense fibrotic areas, and the accumulation of hyperplastic type II alveolar epithelial cells (AECII) and the reduction of type I alveolar epithelial cells [3]. Diagnosis of IPF is still a challenge (probably leading to under diagnosis) and it is based on a combination of factors like the presence of dyspnea, cough and impaired gas exchange accompanied with the occurrence of abnormalities in chest imaging. IPF presents a mean survival of 3–5 years after diagnosis. Two approved drugs, pirfenidone and nintedanib show a modest benefit at reducing the lung function decline in one year [4]. New meta-analysis and extended 3-year survival studies show that pirfenidone has a survival benefit in an IPF cohort [5]. Nevertheless, the complete mechanism of action of these drugs is still unknown, maintaining a significant interest to generate new and more effective therapies [6,7].
The most prominent risk factor for IPF is aging. IPF increases its prevalence and incidence with age and typically is diagnosed in the sixth and seventh decades of life. Two-thirds of IPF patients are over 60 years at the time of diagnosis [8]. Incidence increased with age, from 1.1 new cases of IPF per 100,000 person-years in individuals among 18-34 year-old to up to 19.3 in individuals who are >55 year-old [9]. The risk of IPF is up to 7 times higher in the population 70 years old and older [10]. Raghu et al., in 2006 estimated that the prevalence of IPF increased from 4 to 227 per 100,000 persons in the population 75 years or older compared to people younger than 35 years old [8]. After a decade, the revised epidemiology data showed an increased as high as 400 cases per 100,000 people in patients over 65 years old [11]. Several studies have confirmed the increasing trend of IPF prevalence with age, labelling IPF as an age-related lung disease [12].
Aging can be defined as a degenerative process due to accumulation of extrinsic and intrinsic damages that results in cellular dysfunction, altered tissue response and finally death [13]. In order to simplify the study and understanding of the complex mechanisms behind the aging process, nine hallmarks of aging were proposed [14,15]. Those common biochemical processes include genomic instability, telomere shortening, epigenetic alterations, loss in the homeostasis of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, senescence, stem cell exhaustion, and altered intercellular communication. All of these pathways are found, and even exacerbated, in IPF [1,[16], [17], [18], [19], [20], [21], [22]].
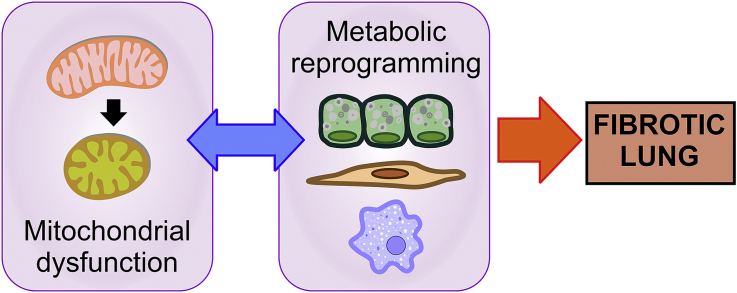
Mitochondria act as a central hub in the mammalian cell; its homeostasis and precise function is critical to ensure cell fitness and assure cell survival. In this organelle, multiple signaling pathways converge and interact to regulate the linked processes of mitochondrial energetics, biogenesis, production of reactive oxygen species (ROS), mitochondrial DNA (mtDNA) preservation and repair, mitophagy and mitochondria-nucleus communication [17]. As the cell age, its mitochondria accumulate abnormalities including morphological alterations (rounded appearance, loss of cristae and inner membranes), reduction in biogenesis, less mtDNA copy numbers but increased mtDNA mutations, leading to functional failure of the respiratory chain capacity and ATP production [16]. In addition, mitochondrial dysfunction is present in aged-related lung diseases [23]. In IPF, many of these regulatory mechanisms that control mitochondrial function are dysregulated in the lung epithelial cells, fibroblasts, and macrophages [16,17] resulting in a maladaptation to cellular stress, creating a vulnerable environment to injury that results in the development of pulmonary fibrosis [[24], [25], [26], [27], [28]]. In this review, we will discuss how mitochondrial dysfunction affects different lung cell populations in the context of IPF, with a focus on epithelial cells, fibroblasts and alveolar macrophages (Fig. 1).
Fig. 1.
Mitochondrial dysfunction in the IPF lung: origins and consequences. The loss of mitochondria homeostasis is a common aspect of the fibrotic lung and can appear at different stages of the mitochondria life cycle. In the IPF lung, the specific features of mitochondrial dysfunction (in red) exhibited are cell-type dependent, triggering an array of diverse metabolic changes and alteration (in blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
2. Aspects of mitochondrial dysfunction in pulmonary fibrosis
In the last decades, the knowledge of the role of mitochondria in normal physiology as well as the onset and progression of diseases has increased dramatically. While generating the vast majority of ATP, mitochondria consume oxygen and produce reactive oxygen species (ROS). However, they also play an important role in other cellular processes, such as signal transduction, cell cycle regulation, oxidative stress, thermogenesis and apoptosis.
2.1. Defective mitochondrial bioenergetics
Mitochondria's most prominent role is the energy transduction to generate ATP. The production of reducing equivalents (NADH and FADH2) is coupled to the oxidation of acetyl-CoA (derived from the citric acid via the TCA –tricarboxylic acid cycle, from pyruvate by glycolysis or from the beta-oxidation of fatty acids). The redox energy stored in those reducing equivalents can be transferred to oxygen (O2), in a process known as oxidative phosphorylation or OXPHOS, to generate a proton gradient that will power a molecular motor (ATP synthase) linked to ATP production. Natural byproducts of the mitochondrial respiration are superoxide anion (O2−·), hydroxyl radical (OH•) and hydrogen peroxide (H2O2) [29]. These mitochondrial reactive oxygen species (mtROS) have a fundamental signaling role and are able to increase the antioxidant capacity of the cell through a process call mitohormesis [30]. This is a tightly regulated signaling mechanisms that is key to orchestrate cell responses to injury. However, in higher concentrations (due to sustained production -usually related with mitochondria injury and dysfunction), mtROS will cause oxidative damage to mitochondrial DNA (mtDNA), lipids and proteins leading to uncoupling of the electron transport chain (ETC) and/or calcium imbalance. As mtROS increase to non-physiological levels (when the established robust antioxidant defense mechanisms of the cell cannot cope with this ROS production), mtROS can leak to the cytosol and activate a plethora of inflammatory mediators such as nuclear factor-kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome, and activator protein-1 (AP-1) [31]. Low levels of superoxide free radicals stimulate the proliferation of fibroblast [32] that can be inhibited by free radical scavengers. In addition, TGF-β1 stimulated normal fibroblast an IPF derived myofibroblast can secrete H2O2 and, in a paracrine way, induce apoptosis in the alveolar epithelial cells [33]. Lastly, these oxidative species are closely involved in the expression and regulation of the matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs, leading to changes in the deposition and degradation of the extracellular matrix [34].
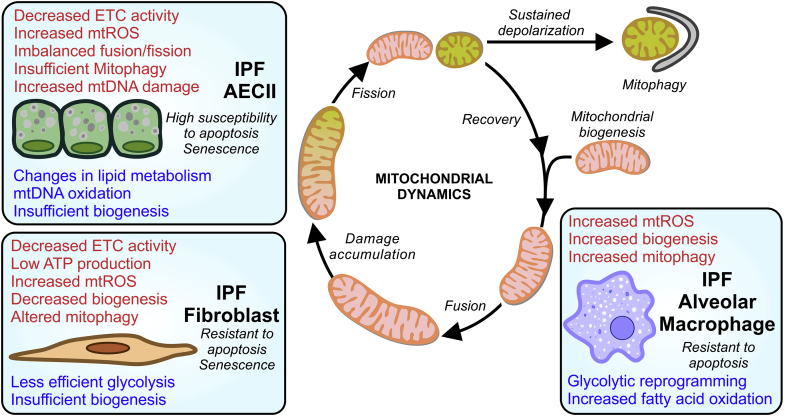
Reduced mitochondrial respiration and oxygen consumption; in tandem with decreased ATP production and increased mitochondrial ROS production have been identified in lung tissue from IPF patients [24,35] (Table 1). In primary cells, type II alveolar epithelial cells (AECII) from IPF lungs show reduced ETC complex I and IV activity [24]. Primary IPF fibroblasts show reduced ATP production linked to rate of oxygen consumption [36,37] and increased mitochondrial ROS [38]. While in IPF primary alveolar macrophages the expression of mitochondria-encoded OXPHOS genes is decreased and the production of mtROS is significantly increased [26,39,40].
Table 1.
Mitochondrial dysfunction in lung fibrosis.
| Feature | Change | Model | References |
|---|---|---|---|
| Mitochondrial respiration | Decreased ETC complex activity, lower OCR | IPF total lung | [24,35] |
| IPF lung fibroblasts | [36,37] | ||
| IPF AECII | [24] | ||
| Murine macrophages | [141] | ||
| Murine AECII | [24,25,43] | ||
| MHV68 mouse model | [24] | ||
| ATP production | Decreased | IPF lung fibroblasts | [36] |
| Mitochondrial reactive oxygen species | Increased | Bleomycin mouse model | [41] |
| Asbestosis mouse model | [41] | ||
| IPF alveolar macrophages | [26,39,40] | ||
| IPF lung fibroblasts | [38] | ||
| Asbestosis alveolar macrophages | [52] | ||
| IPF total lung | [35] | ||
| Mitochondrial biogenesis | Decrease | IPF fibroblast | [50] |
| Bleomycin mouse model | [25,50] | ||
| Increased | Asbestosis alveolar macrophages | [52] | |
| Asbestosis mouse model | [52] | ||
| Mitochondrial dynamics | Imbalanced | MHV68 mouse model | [24] |
| Murine AECII | [56] | ||
| Bleomycin mouse model | [56,117] | ||
| Mitophagy alterations | Reduced levels of mediators of mitochondrial quality control in epithelial cells and fibroblasts | IPF lung | [24,28,44] |
| IPF AECII | [24] | ||
| IPF lung fibroblasts | [28] | ||
| Human bronchial epithelial cells | [44] | ||
| Bleomycin mouse model | [28,58,59] | ||
| MHV68 mouse model | [24] | ||
| Increased mitophagy in macrophages | IPF alveolar macrophages | [26] | |
| Bleomycin mouse model | [26] | ||
| Silicosis mouse model | [60] | ||
| mtDNA | Increased oxidative damage, insufficient mtDNA repair | IPF total lung | [24,46] |
| Human AECII | [24,45] | ||
| Murine AECII | [41] | ||
| Bleomycin mouse model | [41,77] | ||
| Asbestosis mouse model | [41,77,78] | ||
| increased presence of the common mtDNA deletion | IPF total lung | 30940853 [35] |
ETC: electron transport chain. OCR: oxygen consumption rate. AECII: alveolar epithelial cell. MHV68: murine gamma-herpesvirus 68.
This bioenergetics dysfunction has been also found in different experimental models of lung fibrosis [24,41]. High mtROS was detected in mice lungs following exposure to asbestos or bleomycin [27,41]. Carter et al. showed mitochondrial H2O2 production in alveolar macrophages from asbestosis patients and from murine models through Ras-related C3 botulinum toxin substrate (Rac1) protein [42]. In addition, Akt1 (RAC-alpha serine and/or threonine-protein kinase) enhances the production of mitochondrial ROS. Mice harboring a conditional deletion of Akt1 in macrophages decreased TGF-β1 expression and fibroblast differentiation. Furthermore, these macrophage-Akt1 deficient mice were protected from pulmonary fibrosis triggered by asbestos through an increased activation of mitophagy [26]. On the other hand, mice that overexpress mitochondria-targeted human catalase (acting as a ROS scavenger) show protective effects in these models of lung fibrosis [41] cementing the role of mtROS as a powerful mediator in the pathological fibrotic response.
Finally, a profibrotic milieu can lead to mitochondrial dysfunction. In vitro, TGF-β1 treatment of epithelial cells reduces mitochondrial ETC activity, particularly in complex IV, resulting in loss of mitochondrial transmembrane potential and increased production of mtROS [43]. In addition, increased ROS in epithelial cells can oxidize and activate latent TGF-β1 fueling the recruitment of fibroblast [44] while primary murine and human AECII housing dysfunctional mitochondria also increase TGF-β1 transcript levels [24,45,46].
2.2. Changes in mitochondrial life cycle: biogenesis and fusion-fission dynamics
Cells depend on the energy generated in their mitochondria. It is not only the number of mitochondria key in the health status of the cell, but their functional state, which greatly depends on biogenesis and dynamics (including fusion, fission and mitophagy). Mitochondrial biogenesis is controlled by PPARγ coactivator-1α (PGC-1α) and PGC-1β signaling pathways. These are tightly regulated signaling cascades implicating the nuclear respiration transcription factors 1 and 2 (NRF1 and NRF2). NRFs, when co-activated by PGC-1α/β, upregulate expression of the electron transfer chain (ETC) subunits encoded by the nuclear genome and bind to the promoters of genes involved in mtDNA transcription [47]. As the organism ages, the capacity for mitochondrial biogenesis declines by the reduction in activity of upstream activators of PGC-1α/β such as AMP-activated protein kinase (AMPK) as well as SIRT1 [48,49].
Expression of PGC-1α is reduced in primary lung fibroblast isolated from IPF patients and in fibrotic mouse lungs [50]. Reinforcing the role of aging in the susceptibility of lung fibrosis, young mice treated with bleomycin show an initial reduction of PGC-1α that later recovers to allow fibrosis resolution (Table 1). In contrast, after the same bleomycin treatment, old mice are not able to upregulate PGC-1α in the resolution phase, showing low levels of PGC-1α leading to persistent fibrosis [50]. Finally, mice deficient in PGC-1α are more susceptible to bleomycin-induced lung fibrosis [25]. Additionally, the ROS-producing enzyme NADPH oxidase-4 (NOX4), which is upregulated in IPF lung myofibroblasts, reduces mitochondrial biogenesis through direct effect on NRF2 expression independently of PGC-1α [51].
On the other hand, NOX4 (through an upregulation of mitochondrial ROS production) induces mitochondrial biogenesis in macrophages [52]. NOX4 is highly expressed in alveolar macrophages of subjects with asbestosis promoting a profibrotic phenotype. In addition, mice depleted of NOX4 in lung macrophages were protected from asbestos-induced fibrosis. In the case of macrophages, PGC-1α is required for this NOX4-dependent upregulation of mitochondrial biogenesis [52].
Mitochondrial networks are continuously remodeling, undergoing fusion and fission, to allow rapid diffusion of matrix proteins (also spreading metabolites and mtDNA through the network) while the location of the membrane bound respiratory complexes is controlled [53,54]. As the mitochondria age, it reduces its mitochondrial membrane potential (also known as mitochondria depolarization) resulting in a slowly reducing respiratory capacity and gradual deterioration. Beyond its role in morphology, these cycles of fusion/fission work as a very efficient mitochondrial quality control mechanism. They allow for the isolation of damaged (depolarized) mitochondria that need to be disconnected from the functional mitochondrial network. As isolated entities, they can be evaluated to be rescued or to be eliminated by mitophagy (the selective degradation of mitochondria by autophagy) [55]. In IPF lungs and in murine models of lung fibrosis, mitochondrial dynamics are severely imbalance in alveolar epithelial cells (Table 1). An upregulation of mitochondrial fusion markers was found in virus-induced lung fibrosis models; while in bleomycin treated mice, altered mitochondrial dynamics lead to mitochondrial fragmentation [24,56].
2.3. Mitophagy alterations in the IPF lung
Mitophagy can be divided in two phases: the induction of canonical autophagy and the recruitment of mitochondria. The autophagy induction phase relies in the expression of Atg (autophagy-related protein) proteins and the upregulation and recruitment of LC3 (microtubule-associated protein 1A/1B-light chain 3) to autophagosomal membranes with the adaptor molecule p62. The recruitment of mitochondria can be started by multiple mechanisms that could be Parkin dependent or independent. In the Parkin dependent pathway, loss of mitochondrial potential leads to impaired PARL (presenilins-associated rhomboid-like protein)-mediated PINK1 (PTEN-induced putative kinase 1) cleavage, leading to PINK1 stabilization and recruitment and ubiquitination of Parkin. Damaged mitochondria (particularly upon hypoxic damage) may increase the expression of FUNDC1 (FUN14 domain containing 1) and Nix (BNIP3L) in the outer mitochondrial membrane, which directly recruit autophagosomes by interaction with LC3 [57].
Ultrastructural micrography of IPF lungs showed an increased number of mitochondria in AECIIs when compared with aged–matched lungs (Table 1). These accumulated organelles presented a swollen phenotype with aberrant cristae morphology and where co-localized in areas of the lung with increased expression of endoplasmic reticulum stress (ER stress) markers. Our studies demonstrated that primary AECII from IPF lungs are deficient in PINK1 resulting in the accumulation dysfunctional mitochondria with reduced electron transport chain activity [24]. In addition, the same AECIIs upregulated the expression of ATF3 (activating transcription factor 3), a negative transcriptional regulator if PINK1, suggesting that this factor (intimately link with the chronic induction of ER stress) may play a crucial role in age‐related susceptibility to mitochondrial dysfunction and lung fibrosis [45]. Finally, IPF lungs show a reduced expression of Parkin that can modulate the differentiation of primary IPF fibroblast into myofibroblast through the regulation of the platelet-derived growth factor receptor phosphorylation [28]. In animal models, both PINK1 −/− and Parkin −/− mice were more susceptible to experimental lung fibrosis [24,28,44]. In contrast, conditional AECII ATF3 knockout mice were protected against bleomycin-induced pulmonary fibrosis [45]. Recent reports [58] highlight the role of mitochondrial homeostasis and quality control in the context of the origin of the mitochondrial damage. Epithelial cells derived from PINK1 deficient mice showed increased production of reactive oxygen species and cell death in response to TGF-β1, which reinforced PINK1's protective role against TGF-β1 fibrotic responses [44]. In addition, epithelial cells with decreased PINK1 expression show low mitochondrial membrane potential and upregulated expression of profibrotic factors [24,46]. In culture fibroblast, Parkin is involved in the ROS-mediated myofibroblast differentiation [28] while PINK1 appeared to regulate the TGF-β-induced myofibroblast differentiation [59].
In contrast, isolated IPF alveolar macrophages exhibit a profibrotic phenotype with resistance to apoptosis [26] and have been established as the main source of TGF-β1. Increased activation of protein kinase B (Akt1) with a concomitant upregulation of mitochondrial ROS production induce mitophagy as a protective measure. Mitophagy modulates macrophage apoptosis that stabilizes macrophages to release TGF-β1 and stimulate local fibroblast activation. Blocking mitophagy in alveolar macrophages is protective against bleomycin-induced fibrosis [26]. In agreement with these results, new studies have shown that autophagy activation reduced alveolar macrophage apoptosis in models of silicosis [60]. This finding exemplifies the role of mitophagy as an adaptive survival response that is cell-type specific and, in this case, serves to preserve and promote macrophage signaling necessary for repair and resolution.
2.4. Mitochondrial DNA damage and repair
Human mitochondrial DNA is a multi-copied, super-coiled, double-stranded, closed circular genome of approximately 16.5 kilobases. It encodes 37 intron-free genes that translate into 2 ribosomal RNAs (12S and 16S rRNAs), 22 transfer RNAs (tRNAs) and 13 proteins [61]. Mitochondrial DNA (mtDNA) replication is cell cycle-independent; however, its replication and transcription machinery is not self-supported depending heavily on imported nuclear gene products as the DNA polymerase γ (pol γ), the mtDNA helicase (like Twinkle), the mitochondrial RNA polymerase (mtRPOL) or the mitochondrial transcription factor A (Tfam) [62,63]. The fact that all the proteins in the oxidative phosphorylation (OXPHOS) system are encoded in the mtDNA (except the nuclear DNA-encoded complex II), makes the maintenance of its integrity critical. So to accomplish it, mitochondria have deployed different mtDNA repair pathways to cope with replication errors or other types of damage [64]. One of the most common lesions in the mtDNA is the oxidation of guanidine to 8-oxoguanine, which can be repaired by a base excision repair mechanism [65]. There is also evidence of other pathways of DNA repair in the mitochondria such as double-strand break repair, direct reversal and mismatch repair [66].
Aging has been reported to affect mtDNA transcription activity and replication control. Age-related differences in the amount of mtDNA transcript levels correlate with lower synthesis rates and oxidative enzyme activities [67] and it is concomitant with an increase in the mtDNA mutation accumulation [68]. In addition, mtDNA copy numbers can change during the course of life. In the case of the lung, mtDNA copies seemed to be statistically different with age [69] and also to change greatly in cases of age-related pulmonary diseases [24,46,69,70]. Michikawa et al. reported a higher accumulation of point mutations at the control replication region (D-loop) with age, with some mutation only appearing in the elderly population [71]. Nevertheless, other sites of the mtDNA sequence can undergo an age-related accumulation of point mutations or specific deletions [72]. For example, the appearance and accumulation of a common deletion (4977 bp deletion between nucleotides 8470 and 13447) is more frequent in the erderly population [73] and, though it can has a tissue-specific distribution [74], it has been detected in the aged lung [75]. When comparing aged-matched controls and IPF patients, the common mtDNA deletion was present at low levels in control while it was significantly more frequent in the disease lung [35].
There is early evidence that mitochondria DNA oxidative damage could probably have a role in lung fibrosis (Table 1). In a proteomics analysis of IPF lung, the expression of DNA damage protein was upregulated [76]. As previously mentioned, the most common ROS-induced mtDNA lesion is the adduct 8-hydroxyguanine (8-oxo-G), that can lead to a transversion mutation by mispairing during replication. Mitochondrial DNA isolated from IPF lungs show differences in copy numbers [24] and a higher rate of DNA lesions by long extension PCR when compared to control lungs [46]. In addition, increased 8-hydroxy-2′-deoxyguanosine (8-OH-dG) content per μg of isolated mtDNA was found in lungs from IPF patients and age-matched donor control when compared with young donor lungs [46]. The enzyme best characterized in mtDNA repair is 8-oxoguanine DNA glycosylase 1 (OGG1). OGG1 corrects the 8-hydroxyguanine lesion through the base excision repair mechanism. In animal models, OGG1 deficient mice show increased susceptibility to asbestos-induced pulmonary fibrosis by increasing AECII mtDNA damage and apoptosis [27]. What is becoming clear is the involvement of mitochondrial ROS in pulmonary fibrosis, since in both bleomycin and asbestos-induced model of lung fibrosis, a transgenic mouse over-expressing catalase in the mitochondria show reduced fibrosis, AECII mtROS, mtDNA damage and apoptosis [41]. In addition, the aforementioned effects seem to be amplified in the Sirtuin 3 deficient mice opening the possibility that the acetylation/deacetylation state of mitochondrial ROS detoxifying enzymes plays also an important role in the preservation of mtDNA [77]. Also, the benefits of the mtDNA preservation in AECII has been shown in the presence of the antiaging molecular Klotho [78]. Finally, Jeager at al investigated the role of large-scale somatically acquired mutations in mitochondrial DNA [35] and its relationship with respiratory chain dysfunction. They found that ILD lungs (from IPF and other connective-tissue ILD) contained more malondialdehyde, a marker of ROS-production, and mtDNA deletions [35]. Altogether, these data strengthen the role of mtDNA oxidation, damage and mutation with concomitant respiratory chain dysfunction in the perpetuation of the pathological production of mtROS in the fibrotic lung.
Damaged mitochondria are also the source of damage-associated molecular patterns (DAMPs) that play a key role in the propagation of inflammatory responses. DAMPs include peptides (such as TFAM and formyl-peptides), lipids (like cardiolipin), metabolites (i.e. succinate and/or ATP) and mitochondrial DNA [79]. On entering the cytoplasm, mtDNA can trigger responses by activating any of this four innate immune receptors: cGAS (cytosolic cyclic GMP-AMP synthase) [80], endosomal TLR9 [46,81], and the two inflammasomes related receptors AIM2 (Absent In Melanoma 2) and NLRP3 (NOD, LRR and Pyrin domain-containing protein 3) [82,83]. On the extracellular space, mtDNA (due to its similarities with bacteria DNA) can mediate a pro-inflammatory response through the TLR9 signaling pathway. In the lung, mtDNA has demonstrated its pro-inflammatory potential since the intravenous injection of mtDNA (accompanied with formyl-peptides) caused a severe inflammation in rats [84]. However, extracellular mtDNA can also be sensed by other non-immune cells types [85] and cause upregulation of pro-inflammatory a pro-fibrotic markers. Human primary fibroblast increase the expression of pro-fibrotic markers (collagen and fibronectin) and augments the expression marker of activation such as α-smooth muscle actin (αSMA) in the presence of mtDNA [46,70]. In contrast, IPF-derived primary fibroblast are not sensitive to the mtDNA stimulus [46]. Primary human lung epithelial cells can upregulated the release of TGF-β1 after stimulations with extracellular mtDNA, which is mediated by the activation of TLR9 [46].
3. Alterations on cell metabolism in the fibrotic lung
Metabolic changes are increasingly recognized as a hallmark of fibrosis across many organ types. In the field of pulmonary fibrosis, studies suggest that metabolic dysregulation acts as a pivotal contributor to the pathogenesis of the disease. Cell-type-specific metabolic changes have been described in the IPF lung. Fibrotic lung tissue in IPF has an augmented metabolic activity measured by increased fluorodeoxyglucose uptake on PET scan [86]. These increased uptake correlates with disease progression and mortality [86] and can be developed as a tool to stratify IPF patients [87]. However, the complexity of the IPF lung is also reflected in these metabolic changes upon fibrosis, where epithelial cells shift towards a change in lipid metabolism and the fibroblast changes tend to benefit the upregulation of glycolytic pathways.
AECII display downregulation of genes involved in lipid synthesis, elongation, and metabolism identified through single-cell RNA sequencing [88]. Similarly, Romero et al. recently reported that levels of stearoyl CoA desaturase (SCD1), an enzyme involved in the desaturation of fatty acids were reduced in IPF lung tissues and that pharmacological inhibition of this enzyme caused ER stress and induced fibrotic remodeling in the mouse lung [89]. Additionally, alveolar macrophages in fibrotic lung tissue increase fatty acid oxidation in response to the glycolytic shift [90]. Emerging evidence has demonstrated that metabolically targeted therapy might be an essential strategy for fibrosis reduction. Rangarajan et al. recently showed that metformin, an activator of AMPKα, and also an inhibitor of lipid synthesis, reverses established fibrosis in mice [91]. Moreover, new reports show that fenofibrate and ciprofibrate, FDA approved drugs for lowering circulating lipids, can effectively reduce lung fibrosis in mice and decrease collagen production and myofibroblast differentiation in IPF fibroblasts [92]. Thus, there is a considerable need to learn how metabolic adaptations affect repair responses to identify novel targets for new lung fibrosis therapies.
3.1. Metabolic changes and reprogramming
Now that the “omics” approaches are mastered [[93], [94], [95]] and we are venturing into the new realm of single-cell RNA sequencing in complex tissue [88,[96], [97], [98]], we can start to develop a comprehensive picture of the transcription and translation changes and its implication in the modification of metabolic profile in the IPF lung [95] (Table 2). Combining mass spectrometry metabolomics analysis and microarray-derived gene expression, De Perrot's group found that IPF lung samples showed altered metabolites production and downregulated expression of key enzymes involved in several metabolic pathways, including glycolysis and key mitochondrial-related pathways such as mitochondrial beta-oxidation and tricarboxylic acid cycle [95]. Recently, the potential role of the AECII damaged mitochondria was studied in detail using murine models. Mitofusin is a family of proteins fundamental to the balance of mitochondrial fusion and fission dynamics that has been shown to be upregulated in the AECII of IPF lungs [88]. Mice which AECII are defective of mitofusin 1 (Mfn1) or mitofusin 2 (Mfn2) show higher susceptibility to bleomycin-induced lung fibrosis; while deletion of both, in the same cell type, induced spontaneous lung fibrosis [56]. Transcriptomic analysis of those murine lungs showed upregulated purine metabolism and downregulated lipid metabolism after bleomycin [56], hinting at changes in the production of lipids in AECII that can modify the surfactant synthesis with detrimental consequences in the maintenance of epithelial barrier. However, the mechanistic details of the changes in lipid metabolism (and its consequences) in the IPF lung remain elusive.
Table 2.
Metabolic consequences of mitochondrial dysfunction in the fibrotic lung.
| Metabolic pathway | Change | Model | Reference |
|---|---|---|---|
| Lipid metabolism | Changes in lipid metabolism | IPF lung | [95] |
| Murine AECII | [56] | ||
| Bleomycin mouse model | [56] | ||
| role of dietary fats | Bleomycin mouse model | [101] | |
| IPF risk factor | [100] | ||
| role of fatty acid receptors and free fatty acids | Bleomycin mouse model | [99] | |
| IPF clinical trial | [142] | ||
| Glycolysis | Alterations in fibroblast | IPF total lung | [37] |
| IPF lung myofibroblasts | [37,109] | ||
| Bleomycin mouse model | [37,109,143] | ||
| Role of Fructose-1,6-Bisphosphate | IPF lung | [95] | |
| Murine fibroblast | [110] | ||
| Bleomycin mouse model | [110,144] | ||
| Other | low AMPK activity | IPF total lung | [91] |
| Bleomycin mouse model | [91] | ||
| role of Thyroid hormone in epithelial cells | IPF total lung | [25] | |
| Bleomycin mouse model | [25] | ||
| Metabolic reprograming in macrophages | IPF alveolar macrophages | [40,113] | |
| Murine alveolar macrophages | [90,113] | ||
| Bleomycin mouse model | [90,113] |
Free fatty acids (FFA) are essential nutrients. FFA can contain medium-chain FFAs (as the decanoic acid) or long-chain FFAs, as palmitate (PA). During fasting, the FFA can be hydrolyzed from adipose triglycerides (TA) stores or from liver-derivate TA to provide energy for tissues. However, recent evidence shows that besides FFA role in the metabolic change, FFA can modulate fibrosis by the activity regulation of GPR40 and GPR84, G protein–coupled receptors with free fatty acid ligands, using the GPR84 antagonist compound PBI-4050. PBI-4050 significantly attenuated fibrosis in a variety of injury contexts in several tissue fibrosis models, including lung fibrosis [99]. In addition, the fatty acid metabolism has found altered in IPF lung. For instance, there is an increment of FFA in the parenchyma, principally seen by steric acid and palmitate accumulation (a saturated FFA). In a multicenter case-control study in Japan, they authors found that a high risk of IPF was associated with the intake of mono unsaturated and n-6 polyunsaturated fatty acids [100]. Chu et al. used these results to study the role of fatty acid intake (using a high-fat diet chow) in animal models of lung fibrosis. In accordance with the reported risk, a high-fatty-acid diet rich in PA results in both an increase in mortality and a higher susceptibility of developing pulmonary fibrosis (in the bleomycin mouse model) by enhancing ER stress and apoptosis of the epithelial cells [101].
Cells need to maintain ATP levels to keep homeostasis and to prevent diseases [102]. One of the main sources for ATP production is glucose. Glucose is the final product of the carbohydrate metabolism and it can be converted to energy by the activation of glycolysis under anaerobic conditions, by complete oxidation under aerobic conditions, and by the pentose phosphate pathway [[103], [104], [105]]. Perturbation in the glycolytic pathway has been describe in the fibrotic diseases. Changes in glycolysis and fatty acid oxidation have reciprocal effect in the upregulation and downregulation of extracellular matrix deposition [106]. In the lung, IPF myofibroblast not also prefers aerobic glycolysis as a first source of glucose-derived energy but glycolysis is required to initiate its differentiation, and promoting fibrosis progression [37]. Myofibroblasts profibrotic phenotype including contractibility have been shown to be affected by alterations in the metabolic reprograming [107] meanwhile TGF-β collagen production depends on glucose uptake Selvarajah [108]. Supporting the importance of glucose metabolism in lung fibrosis, in vivo, attenuation of HIF-1α/PDK1–mediated glycolytic reprogramming it is enough to reduce lung fibrotic injury in the bleomycin model [109].
Fructose-1,6-Bisphosphate (FBP) is an intermediate metabolite in the breakdown of glucose by glycolysis. Zhao et al. found significant less amount of FBP and phosphoenolpyruvate, another glycolytic metabolite, in IPF lungs compared with healthy counterparts. According with the author, glycolysis energy metabolism (among other metabolic pathways) are key in the pathogenesis of IPF [95]. However, the fibrotic biological mechanism affected by low levels of fructose 1,6-bisphosphate remains unclear. Recently, FBP has been shown to regulate fibrosis by both directly reducing collagen expression and other extracellular matrix components and preventing their degradation in the mouse model [110]. Together, these results underline the need for a better understanding of the metabolic regulation of cellular key process in the fibrosis lung. One example is the promising therapeutic option of thyroid hormone delivery in the resolution of murine fibrosis [25].
Furthermore, Free Fatty Acid (FFA) metabolism can be regulated by the AMP-activated protein kinase (AMPK), a “metabolic master switch”, that senses the charge of cellular energy. AMPK can both switches on FFA oxidation or switches off FFA synthesis during cellular stress [111]. In the fibrotic context, AMPK activators seems to protect from lung injury that could result in fibrosis [112]. In both aging, a risk fibrotic factor, as well as in IPF, AMPK activity has been found reduced which results in metabolic changes accompanied by the reduction of the autophagy activity in lung fibroblasts [91]. These findings emphasize the importance of metabolic reprograming in IPF, principally in the fibroblast population. However, there is an increased interest in understand how perturbations in the metabolism derived from mitochondrial dysfunction affect other lung cell populations including macrophages.
In IPF, macrophages can play a different phagocytic and immune role depending on their subtype (31351434). Interstitial macrophages (IM), for example, act as local immune defense of the lung upon injury; while the role of the alveolar macrophages (AM) in the fibrotic pathogenesis have been recently studied. Interestingly, in murine models, the profibrotic phenotype of the AM is heavily dependent on glycolysis but not dependent on fatty acid oxidation [90]. Finally, the mitochondrial calcium uniporter (MCU) regulates the mitochondrial calcium influx, which is central to sense the cellular energy status. IPF lung macrophages have increased MCU expression as well as increased calcium in the mitochondria [113]. In addition, mice expressing a dominant-negative MCU in macrophages show protection in a fibrotic model due to metabolic reprograming [113].
4. Mitochondrial dysfunction role in epigenetic changes and senescence
Epigenetics play a pivotal role during the aging process and have a role in various age-related diseases. Epigenetic mechanisms modulate gene activity in the absence of DNA sequence changes, including DNA methylation, histone modification, and expression of noncoding RNAs.
4.1. Modifications to the genetic material: epigenetic changes and cell-free DNA
Some of the epigenetic changes found in IPF can be connected with mitochondria dysfunction and/or pathological levels of mtROS. Using a new developed imaging technique, Qian et al. were able to study the nuclear effects of mitochondrial dysfunction [114]. One single event of mitochondrial singlet oxygen production is able to persist as a wave of other mtROS intermediates that can be detected in the nucleus. They were able to identify nuclear oxidative stress but no nuclear DNA strand breaks. However, DNA double-strand breaks occurred exclusively in telomeres as a direct consequence of mitochondrial dysfunction [114]. These results could be reveling the missing link between mitochondria dysfunction and telomere dysfunction, both of them hallmarks of the IPF lung [16].
In IPF, the expression patterns of microRNAs are dysregulated. MicroRNAs expression can both promotes and attenuates fibrosis in a target-depended manner [115]. Lately, it has been shown that miR-30a may function as a novel therapeutic target for lung fibrosis by blocking mitochondrial fission mediated by Drp-1 [116]. Zhang et al. found that one possible mechanism of this Drp-1 regulation could be the decreased expression level of the key enzyme TET1, which is a miR-30a target that regulates Drp-1 promoter hydroxymethylation [117].
As previously mentioned, due to its ancestral bacterial origin, the hypomethylated CpG-rich mitochondrial DNA is an innate immune agonist. Exposure to extracellular circulating levels of mtDNA can promote inflammation in several manners, including the induction of inflammatory cells infiltration producing proinflammatory cytokines [118] and by the promotion of extracellular neutrophil traps formations [119]. New reports show that extracellular mtDNA can also activate other cells types [46,70]. In IPF patients, cell-free mtDNA levels in bronchoalveolar lavage fluid (BALF) and plasma are increased compared to healthy controls [46,70], and those plasma mtDNA levels can be associated with disease progression [46,70] and higher mortality in patients with IPF [70]. Nonetheless, still we do not have a complete picture regarding the origin of that extracellular mtDNA in the lung. It has been proposed that fibroblast could be the original source of that cell-free mtDNA [70]. However, other reports show that only lung primary fibroblast derived from young donor lung differentially release mtDNA compared to old lung donor or IPF fibroblast where the extracellular mtDNA detected correlate with the leak of nuclear DNA [46]. Another possible source of mtDNA could be the IPF lung epithelial cells bearing low PINK1 levels and showing dysregulation of the mitochondria homeostasis and accumulation of damaged mitochondria [46]. In an extended ILD cohort, including patients diagnosed with IPF, hypersensitivity pneumonitis (HP) or autoimmune-related ILD, all showed higher levels of extracellular mtDNA in their BALF and plasma samples compared with aged-matched control. In BALF there is a striking inverse correlation between in lung expression of PINK1 and the number of mtDNA copies found in the fluid [46].
4.2. Mitochondrial dysfunction is associated with senescence
Senescence is a hypo-replicative state characterized in part by the expression of certain cell cycle cyclin inhibitors and the acquisition of a senescence-associated secretory phenotype (SASP). Senescence can be induced as a response to different stimuli including oxidative stress, DNA damage, and mitochondrial dysfunction. Growing evidence suggest that mitochondrial dysfunction due to mitophagy defects (including PINK1 deficiency [45]), or altered NAD+/NADH ratio and increased AMPK can trigger cellular senescence [120]. There are also new reports linking senescence with some of the metabolic changes discussed above and with mitochondrial ROS production (mtROS production can drive senescence through a sustained expression of p21 [121]).
In lung fibroblast, defects in PGC1-α expression [50]as well as low levels of ATP (because of a diminished glycolytic capacity and abnormalities in oxidative phosphorylation in primary IPF fibroblast) [36] correlates with a senescence phenotype characterized by overexpression of cell cycle inhibitors. The regulation of cell cycle inhibitory proteins (such as p16INK4a overexpression) [122,123] and the accumulation of DNA damage [124] correlates with loss of the regenerative potential and promotion of senescence. In human primary IPF-derived AECII present a reduced regenerative potential [125] while primary mouse AECII cells overexpressed senescence markers in a fibrotic model [126,127]. In accordance with these findings, use of senolytics to deplete senescence cells has resulted in the attenuation of experimental lung fibrosis [126,128].
Senescent cells secret a cell a specific repertoire of factors known as the senescence‐associated secretory phenotype (SASP) [36]; however, epithelial cells, fibroblasts, or myofibroblasts secret a characteristic subtype of SASP, which it is composed of a defined cell-dependent subset of pro-fibrotic and pro-inflammatory cytokines [120,126,129]. Recently, new insights in the mitochondria to nucleus axis show that melatonin, a pineal gland produced hormone, can regulate SASP production [130] meanwhile prevents lung injury by improving mitochondrial dysfunction [127].
5. Targeting mitochondria as a new therapeutic approach
After decades of research on the mechanism involved in idiopathic pulmonary fibrosis, only two therapeutic have been approved. However, few studies have shown any consistent improvement on survival. There are new targets being evaluated and new drugs in the pipeline (Fig. 2) [3,131]. As we have been discussing, loss of mitochondrial homeostasis (and the metabolic changes that arise as its consequence) plays a key role in the pathophysiology of IPF. Therapies targeting mitochondria had been mainly focused on improving mitochondrial biogenesis, as well as to restore mitophagy and to prevent mitochondrial apoptosis. Therapies like urolithin A, a promoter of mitophagy, has shown to promote mitochondrial gene expression and modulate lipid metabolism in healthy elderly individuals [132,133]. Furthermore, urolithin B, a metabolite of ellagitannin-rich foods, controlled inflammation and fibrosis in a preclinical model of renal fibrosis [134]. In alveolar epithelial cells, it have been shown that the combination use of senolytics and antioxidants can ameliorate fibrosis by cleaning senescent cells from injured areas as well as preventing SASP signaling activation [3]. Pathways that regulate SASP indirectly are also been explored as therapeutic targets. Rapamycin, for instance, limits SASP production by direct inhibition of the mTOR pathway, when other drugs inhibit other important SASP pathways [135]. In the case of rupatadine, it had been found that its potent action includes preventing the activation of the p53–p21 axis and attenuating the expression of CCAAT/enhancer-binding protein-β (C/EBPβ), a positive modulator of SASP production [136]. However, there is growing evidence that senolytic drugs have very strict cell-type effects suggesting that further studies are needed to advance this field [136].
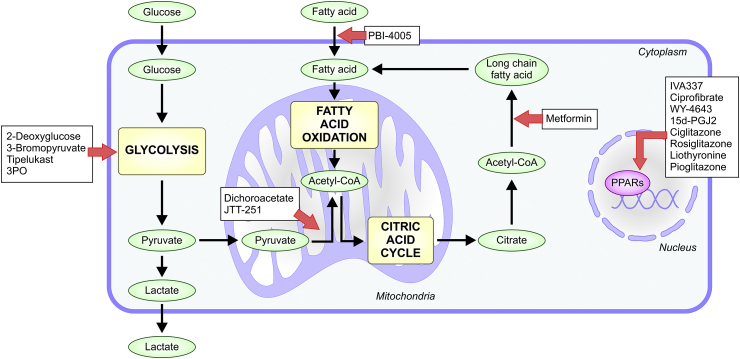
Fig. 2.
Metabolic pathways targeted by new antifibrotic drugs. In order to maintain cellular homeostasis and prevent profibrotic events, modulation of glycolysis, fatty acid oxidation and synthesis are key. New potential therapies are in development targeting these main metabolic pathways; with some of them already at different stages in clinical trials. Drugs are highlighted in white boxes, main metabolic pathways in yellow boxes, metabolites in green and transcription factors in pink. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
Alterations in the metabolic pathways, in fibrotic conditions, have been found in metabolomics studies. Zhao et al. recently summarized the importance of targeting metabolic dysregulation as a fibrotic therapeutic option. Metabolic dysregulated pathways indirectly affected mitochondrial homeostasis [134]. Targeting SIRT3 could by one part control myofibroblast transformation by inhibiting TGFβ1 signaling [137] and also regulates the acetylation and function of the mitochondrial DNA repair enzyme 8-oxoguanine-DNA glycosylase-1 (OGG1), which hydrolyses oxidized guanine residues (8-Oxo-dG), reducing mtDNA damage. These results suggested a protective role of SIRT3 in injury and fibrosis [138].
Lipid metabolism have also been shown affected in the fibrotic lung. Targeting regulators of fatty acid oxidation pathways is a promising option. For example, in renal fibrosis (where epithelial cells show defective fatty acid oxidation) the use of the synthetic compound C75, a fatty acid synthase inhibitor, is enough to maintain renal cell viability and to reduce fibrosis in rodent models [139]. Peroxisome proliferator-activated receptors (PPARs) are a set of transcription factors, mainly regulated by fatty acids, which modulate lipid metabolism and inflammation [140]. Dual and pan-PPAR agonist have been seen to have a potential role in attenuating pulmonary fibrosis [134]; however, there is need to have a better understanding of interactions among different isoforms.
Other preclinical studied drug focused on modulate metabolism perturbations (Fig. 2), which effect is indirectly related with the mitochondria, is metformin (and possibly other AMPK activators). Metformin, which target energy sensor AMPK, shows promising therapeutic effects in pulmonary fibrosis. Restoring AMPK activity in IPF myofibroblast reduces their profibrotic phenotype liked to an enhanced mitochondrial biogenesis, and by the other hand, metformin accelerated the fibrotic resolution in bleomycin-injured mice [91]. Finally, there is a growing interest in drugs that improves the glycolytic flux. Some of them, including 2-Deoxyglucose, 3-bromopyruvate, Tipelukast, and 3PO (3-(3-pyridinyl)−1-(4-pyridinyl)−2-propen-1-one) have been seen that reduce fibrosis by reducing myofibroblast collagen production meanwhile restoring glycolysis [134]. However, further studies are required in this field.
Taken together, the definition of abnormalities in mitochondria function and metabolism in the IPF lung cells open a new avenue of therapeutic approaches. We anticipate therapies based in these novel pathogenic insights will improve survival and life quality of IPF patients.
Funding
This work was supported by the National Institutes of Health [R01 HL131789-03, R01 HL149825, 5 R01 HL123766-04 and U01 HL145550-01]; and the Aging Institute at the University of Pittsburgh
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101509.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Selman M., Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir. Res. 2002;3:3. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz D.A. Idiopathic pulmonary fibrosis is a genetic disease involving mucus and the peripheral airways. Ann Am Thorac Soc. 2018;15:S192–S197. doi: 10.1513/AnnalsATS.201802-144AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora A.L., Rojas M., Pardo A., Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017;16:810. doi: 10.1038/nrd.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleetwood K., McCool R., Glanville J., Edwards S.C., Gsteiger S., Daigl M. Systematic review and network meta-analysis of idiopathic pulmonary fibrosis treatments. J Manag Care Spec Pharm. 2017;23:S5–S16. doi: 10.18553/jmcp.2017.23.3-b.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margaritopoulos G.A., Trachalaki A., Wells A.U., Vasarmidi E., Bibaki E., Papastratigakis G. Pirfenidone improves survival in IPF: results from a real-life study. BMC Pulm. Med. 2018;18:177. doi: 10.1186/s12890-018-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher T.M., Molina-Molina M., Russell A.M., Bonella F., Jouneau S., Ripamonti E. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC Pulm. Med. 2017;17:124. doi: 10.1186/s12890-017-0468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamantopoulos A., Wright E., Vlahopoulou K., Cornic L., Schoof N., Maher T.M. The burden of illness of idiopathic pulmonary fibrosis: a comprehensive evidence review. Pharmacoeconomics. 2018;36:779–807. doi: 10.1007/s40273-018-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghu G., Weycker D., Edelsberg J., Bradford W.Z., Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G., Chen S.Y., Hou Q., Yeh W.S., Collard H.R. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur. Respir. J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 10.Choi W.I., Dauti S., Kim H.J., Park S.H., Park J.S., Lee C.W. Risk factors for interstitial lung disease: a 9-year Nationwide population-based study. BMC Pulm. Med. 2018;18:96. doi: 10.1186/s12890-018-0660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G., Chen S.Y., Yeh W.S., Maroni B., Li Q., Lee Y.C. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 12.Martinez F.J., Collard H.R., Pardo A., Raghu G., Richeldi L., Selman M. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 13.Phillip J.M., Aifuwa I., Walston J., Wirtz D. The mechanobiology of aging. Annu. Rev. Biomed. Eng. 2015;17:113–141. doi: 10.1146/annurev-bioeng-071114-040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodig S., Cepelak I., Pavic I. Hallmarks of senescence and aging. Biochem. Med. 2019;29 doi: 10.11613/BM.2019.030501. 030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora A.L., Bueno M., Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J. Clin. Invest. 2017;127:405–414. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zank D.C., Bueno M., Mora A.L., Rojas M. Idiopathic pulmonary fibrosis: aging, mitochondrial dysfunction, and cellular bioenergetics. Front. Med.(Lausanne). 2018;5:10. doi: 10.3389/fmed.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati S., Thannickal V.J. The aging lung and idiopathic pulmonary fibrosis. Am. J. Med. Sci. 2019;357:384–389. doi: 10.1016/j.amjms.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Stanley S.E., Armanios M. Short telomeres: a repeat offender in IPF. Lancet Respir. Med. 2014;2:513–514. doi: 10.1016/S2213-2600(14)70140-7. [DOI] [PubMed] [Google Scholar]
- 20.Romero F., Summer R. Protein folding and the challenges of maintaining endoplasmic reticulum proteostasis in idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2017;14:S410–S413. doi: 10.1513/AnnalsATS.201703-207AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore C., Blumhagen R.Z., Yang I.V., Walts A., Powers J., Walker T. Resequencing study confirms that host defense and cell senescence gene variants contribute to the risk of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;200:199–208. doi: 10.1164/rccm.201810-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora A.L., Rojas M. Adult stem cells for chronic lung diseases. Respirology. 2013;18:1041–1046. doi: 10.1111/resp.12112. [DOI] [PubMed] [Google Scholar]
- 23.Ryter S.W., Rosas I.O., Owen C.A., Martinez F.J., Choi M.E., Lee C.G. Mitochondrial dysfunction as a pathogenic mediator of chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2018;15:S266–S272. doi: 10.1513/AnnalsATS.201808-585MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueno M., Lai Y.C., Romero Y., Brands J., St Croix C.M., Kamga C. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G., Tzouvelekis A., Wang R., Herazo-Maya J.D., Ibarra G.H., Srivastava A. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.l, Deshane J.S., Ryan A.J., Thannickal V.J., Carter A.B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. 2016;44:582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheresh P., Morales-Nebreda L., Kim S.J., Yeldandi A., Williams D.B., Cheng Y. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am. J. Respir. Cell Mol. Biol. 2015;52:25–36. doi: 10.1165/rcmb.2014-0038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K., Araya J., Minagawa S., Hara H., Saito N., Kadota T. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 2016;197:504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 29.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 30.Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piantadosi C.A., Suliman H.B. Mitochondrial dysfunction in lung pathogenesis. Annu. Rev. Physiol. 2017;79:495–515. doi: 10.1146/annurev-physiol-022516-034322. [DOI] [PubMed] [Google Scholar]
- 32.Murrell G.A., Francis M.J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem. J. 1990;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. Faseb. J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 34.Tan R.J., Fattman C.L., Niehouse L.M., Tobolewski J.M., Hanford L.E., Li Q. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2006;35:289–297. doi: 10.1165/rcmb.2005-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeger V.K., Lebrecht D., Nicholson A.G., Wells A., Bhayani H., Gazdhar A. Mitochondrial DNA mutations and respiratory chain dysfunction in idiopathic and connective tissue disease-related lung fibrosis. Sci. Rep. 2019;9:5500. doi: 10.1038/s41598-019-41933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez D., Cardenes N., Sellares J., Bueno M., Corey C., Hanumanthu V.S. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L1164–L1173. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie N., Tan Z., Banerjee S., Cui H., Ge J., Liu R.M. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuliga M., Pechkovsky D.V., Read J., Waters D.W., Blokland K.E.C., Reid A.T. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell Mol. Med. 2018;22:5847–5861. doi: 10.1111/jcmm.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsitoura E., Vasarmidi E., Bibaki E., Trachalaki A., Koutoulaki C., Papastratigakis G. Accumulation of damaged mitochondria in alveolar macrophages with reduced OXPHOS related gene expression in IPF. Respir. Res. 2019;20:264. doi: 10.1186/s12931-019-1196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larson Casey J.L., Vaid M., Gu L., He C., Cai G.Q., Ding Q. Increased flux through the mevalonate pathway mediates fibrotic repair without injury. J. Clin. Invest. 2019;129:4962–4978. doi: 10.1172/JCI127959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S.J., Cheresh P., Jablonski R.P., Morales-Nebreda L., Cheng Y., Hogan E. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic. Biol. Med. 2016;101:482–490. doi: 10.1016/j.freeradbiomed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osborn-Heaford H.L., Ryan A.J., Murthy S., Racila A.M., He C., Sieren J.C. Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J. Biol. Chem. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon Y.S., Lee J.H., Hwang S.C., Choi K.S., Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 44.Patel A.S., Song J.W., Chu S.G., Mizumura K., Osorio J.C., Shi Y. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PloS One. 2015;10 doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bueno M., Brands J., Voltz L., Fiedler K., Mays B., St Croix C. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell. 2018;17 doi: 10.1111/acel.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bueno M., Zank D., Buendia-Roldan I., Fiedler K., Mays B.G., Alvarez D. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PloS One. 2019;14 doi: 10.1371/journal.pone.0218003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarpulla R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 48.Jager S., Handschin C., Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. P Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009;458:1056–U1140. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caporarello N., Meridew J.A., Jones D.L., Tan Q., Haak A.J., Choi K.M. PGC1alpha repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax. 2019;74:749–760. doi: 10.1136/thoraxjnl-2019-213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard K., Logsdon N.J., Miguel V., Benavides G.A., Zhang J., Carter A.B. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J. Biol. Chem. 2017;292:3029–3038. doi: 10.1074/jbc.M116.752261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He C., Larson Casey J.L., Davis D., Hanumanthu V.S., Longhini A.L.F., Thannickal V.J. NOX4 modulates macrophage phenotype and mitochondrial biogenesis in asbestosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisner V., Picard M., Hajnoczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018;20:755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra P., Chan D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Twig G., Hyde B., Shirihai O.S. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Bba-Bioenerg. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung K.P., Hsu C.L., Fan L.C., Huang Z., Bhatia D., Chen Y.J. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat. Commun. 2019;10:3390. doi: 10.1038/s41467-019-11327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding W.X., Yin X.M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganzleben I., He G.W., Gunther C., Prigge E.S., Richter K., Rieker R.J. PGAM5 is a key driver of mitochondrial dysfunction in experimental lung fibrosis. Cell. Mol. Life Sci. 2019;76:4783–4794. doi: 10.1007/s00018-019-03133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sosulski M.L., Gongora R., Danchuk S., Dong C., Luo F., Sanchez C.G. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell. 2015;14:774–783. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du S., Li C., Lu Y., Lei X., Zhang Y., Li S. Dioscin alleviates crystalline silica-induced pulmonary inflammation and fibrosis through promoting alveolar macrophage autophagy. Theranostics. 2019;9:1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montoya J., Lopez-Perez M.J., Ruiz-Pesini E. Mitochondrial DNA transcription and diseases: past, present and future. Biochim. Biophys. Acta. 2006;1757:1179–1189. doi: 10.1016/j.bbabio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Campbell C.T., Kolesar J.E., Kaufman B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 63.McKinney E.A., Oliveira M.T. Replicating animal mitochondrial DNA. Genet. Mol. Biol. 2013;36:308–315. doi: 10.1590/S1415-47572013000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kazak L., Reyes A., Holt I.J. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 65.Ba X., Boldogh I. 8-Oxoguanine DNA glycosylase 1: beyond repair of the oxidatively modified base lesions. Redox Biol. 2018;14:669–678. doi: 10.1016/j.redox.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saki M., Prakash A. DNA damage related crosstalk between the nucleus and mitochondria. Free Radic. Biol. Med. 2017;107:216–227. doi: 10.1016/j.freeradbiomed.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andreu A.L., Arbos M.A., Perez-Martos A., Lopez-Perez M.J., Asin J., Lopez N. Reduced mitochondrial DNA transcription in senescent rat heart. Biochem. Biophys. Res. Commun. 1998;252:577–581. doi: 10.1006/bbrc.1998.9703. [DOI] [PubMed] [Google Scholar]
- 68.Manczak M., Jung Y., Park B.S., Partovi D., Reddy P.H. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J. Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 69.Lee H.C., Lu C.Y., Fahn H.J., Wei Y.H. Aging- and smoking-associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Lett. 1998;441:292–296. doi: 10.1016/s0014-5793(98)01564-6. [DOI] [PubMed] [Google Scholar]
- 70.Ryu C., Sun H., Gulati M., Herazo-Maya J.D., Chen Y., Osafo-Addo A. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2017;196:1571–1581. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michikawa Y., Mazzucchelli F., Bresolin N., Scarlato G., Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 72.Fayet G., Jansson M., Sternberg D., Moslemi A.R., Blondy P., Lombes A. Ageing muscle: clonal expansions of mitochondrial DNA point mutations and deletions cause focal impairment of mitochondrial function. Neuromuscul. Disord. 2002;12:484–493. doi: 10.1016/s0960-8966(01)00332-7. [DOI] [PubMed] [Google Scholar]
- 73.Lee H.C., Pang C.Y., Hsu H.S., Wei Y.H. Differential accumulations of 4,977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim. Biophys. Acta. 1994;1226:37–43. doi: 10.1016/0925-4439(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 74.Zheng Y., Luo X., Zhu J., Zhang X., Zhu Y., Cheng H. Mitochondrial DNA 4977 bp deletion is a common phenomenon in hair and increases with age. Bosn. J. Basic Med. Sci. 2012;12:187–192. doi: 10.17305/bjbms.2012.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fahn H.J., Wang L.S., Hsieh R.H., Chang S.C., Kao S.H., Huang M.H. Age-related 4,977 bp deletion in human lung mitochondrial DNA. Am. J. Respir. Crit. Care Med. 1996;154:1141–1145. doi: 10.1164/ajrccm.154.4.8887618. [DOI] [PubMed] [Google Scholar]
- 76.Korfei M., Schmitt S., Ruppert C., Henneke I., Markart P., Loeh B. Comparative proteomic analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and lung transplant donor lungs. J. Proteome Res. 2011;10:2185–2205. doi: 10.1021/pr1009355. [DOI] [PubMed] [Google Scholar]
- 77.Jablonski R.P., Kim S.J., Cheresh P., Williams D.B., Morales-Nebreda L., Cheng Y. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. Faseb. J. 2017;31:2520–2532. doi: 10.1096/fj.201601077R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S.J., Cheresh P., Eren M., Jablonski R.P., Yeldandi A., Ridge K.M. Klotho, an antiaging molecule, attenuates oxidant-induced alveolar epithelial cell mtDNA damage and apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L16–L26. doi: 10.1152/ajplung.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grazioli S., Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front. Immunol. 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maekawa H., Inoue T., Ouchi H., Jao T.M., Inoue R., Nishi H. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 2019;29:1261–1273. doi: 10.1016/j.celrep.2019.09.050. e1266. [DOI] [PubMed] [Google Scholar]
- 81.Bao W., Xia H., Liang Y., Ye Y., Lu Y., Xu X. Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci. Rep. 2016;6 doi: 10.1038/srep22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Q., Zhang D., Hu D., Zhou X., Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018;103:115–124. doi: 10.1016/j.molimm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X.J. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Nuevo A., Zorzano A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress. 2019;3:195–207. doi: 10.15698/cst2019.06.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Justet A., Laurent-Bellue A., Thabut G., Dieudonne A., Debray M.P., Borie R. [(18)F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir. Res. 2017;18:74. doi: 10.1186/s12931-017-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Win T., Screaton N.J., Porter J.C., Ganeshan B., Maher T.M., Fraioli F. Pulmonary (18)F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF) Eur. J. Nucl. Med. Mol. Imag. 2018;45:806–815. doi: 10.1007/s00259-017-3917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y., Mizuno T., Sridharan A., Du Y., Guo M., Tang J. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1 doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romero F., Hong X., Shah D., Kallen C.B., Rosas I., Guo Z. Lipid synthesis is required to resolve endoplasmic reticulum stress and limit fibrotic responses in the lung. Am. J. Respir. Cell Mol. Biol. 2018;59:225–236. doi: 10.1165/rcmb.2017-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie N., Cui H., Ge J., Banerjee S., Guo S., Dubey S. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L834–L844. doi: 10.1152/ajplung.00235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rangarajan S., Bone N.B., Zmijewska A.A., Jiang S., Park D.W., Bernard K. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Samah M., El-Aidy Ael R., Tawfik M.K., Ewais M.M. Evaluation of the antifibrotic effect of fenofibrate and rosiglitazone on bleomycin-induced pulmonary fibrosis in rats. Eur. J. Pharmacol. 2012;689:186–193. doi: 10.1016/j.ejphar.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 93.Yu G., Ibarra G.H., Kaminski N. Fibrosis: lessons from OMICS analyses of the human lung. Matrix Biol. 2018;68–69:422–434. doi: 10.1016/j.matbio.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vukmirovic M., Kaminski N. Impact of transcriptomics on our understanding of pulmonary fibrosis. Front. Med. 2018;5:87. doi: 10.3389/fmed.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Y.D., Yin L., Archer S., Lu C., Zhao G., Yao Y. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res. 2017;4 doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gokey J.J., Snowball J., Sridharan A., Speth J.P., Black K.E., Hariri L.P. MEG3 is increased in idiopathic pulmonary fibrosis and regulates epithelial cell differentiation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morse C., Tabib T., Sembrat J., Buschur K.L., Bittar H.T., Valenzi E. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peyser R., MacDonnell S., Gao Y., Cheng L., Kim Y., Kaplan T. Defining the activated fibroblast population in lung fibrosis using single-cell sequencing. Am. J. Respir. Cell Mol. Biol. 2019;61:74–85. doi: 10.1165/rcmb.2018-0313OC. [DOI] [PubMed] [Google Scholar]
- 99.Gagnon L., Leduc M., Thibodeau J.F., Zhang M.Z., Grouix B., Sarra-Bournet F. A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am. J. Pathol. 2018;188:1132–1148. doi: 10.1016/j.ajpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 100.Miyake Y., Sasaki S., Yokoyama T., Chida K., Azuma A., Suda T. Dietary fat and meat intake and idiopathic pulmonary fibrosis: a case-control study in Japan. Int. J. Tubercul. Lung Dis. 2006;10:333–339. [PubMed] [Google Scholar]
- 101.Chu S.G., Villalba J.A., Liang X., Xiong K., Tsoyi K., Ith B. Palmitic acid-rich high-fat diet exacerbates experimental pulmonary fibrosis by modulating endoplasmic reticulum stress. Am. J. Respir. Cell Mol. Biol. 2019;61:737–746. doi: 10.1165/rcmb.2018-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rohas L.M., St-Pierre J., Uldry M., Jager S., Handschin C., Spiegelman B.M. A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7933–7938. doi: 10.1073/pnas.0702683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Streffer C. Glucose-, energy-metabolism and cell proliferation in tumors. Adv. Exp. Med. Biol. 1994;345:327–333. doi: 10.1007/978-1-4615-2468-7_43. [DOI] [PubMed] [Google Scholar]
- 104.Goodpaster B.H., Sparks L.M. Metabolic flexibility in health and disease. Cell Metabol. 2017;25:1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang P., Du W., Wu M. Regulation of the pentose phosphate pathway in cancer. Protein Cell. 2014;5:592–602. doi: 10.1007/s13238-014-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao X., Psarianos P., Ghoraie L.S., Yip K., Goldstein D., Gilbert R. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat Metab. 2019;1:147–157. doi: 10.1038/s42255-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 107.Bernard K., Logsdon N.J., Ravi S., Xie N., Persons B.P., Rangarajan S. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J. Biol. Chem. 2015;290:25427–25438. doi: 10.1074/jbc.M115.646984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Selvarajah B., Azuelos I., Plate M., Guillotin D., Forty E.J., Contento G. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-beta1-induced collagen biosynthesis. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aav3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goodwin J., Choi H., Hsieh M.H., Neugent M.L., Ahn J.M., Hayenga H.N. Targeting hypoxia-inducible factor-1alpha/pyruvate dehydrogenase kinase 1 Axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2018;58:216–231. doi: 10.1165/rcmb.2016-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dias H.B., de Oliveira J.R., Donadio M.V.F., Kimura S. Fructose-1,6-bisphosphate prevents pulmonary fibrosis by regulating extracellular matrix deposition and inducing phenotype reversal of lung myofibroblasts. PloS One. 2019;14 doi: 10.1371/journal.pone.0222202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hardie D.G., Pan D.A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 112.Park C.S., Bang B.R., Kwon H.S., Moon K.A., Kim T.B., Lee K.Y. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012;84:1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 113.Gu L., Larson Casey J.L., Andrabi S.A., Lee J.H., Meza-Perez S., Randall T.D. Mitochondrial calcium uniporter regulates PGC-1alpha expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol. 2019;26:101307. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian W., Kumar N., Roginskaya V., Fouquerel E., Opresko P.L., Shiva S. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2019;116:18435–18444. doi: 10.1073/pnas.1910574116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Selman M., Lopez-Otin C., Pardo A. Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. J. 2016;48:538–552. doi: 10.1183/13993003.00398-2016. [DOI] [PubMed] [Google Scholar]
- 116.Mao C., Zhang J., Lin S., Jing L., Xiang J., Wang M. MiRNA-30a inhibits AECs-II apoptosis by blocking mitochondrial fission dependent on Drp-1. J. Cell Mol. Med. 2014;18:2404–2416. doi: 10.1111/jcmm.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang S., Liu H., Liu Y., Zhang J., Li H., Liu W. miR-30a as potential therapeutics by targeting TET1 through regulation of drp-1 promoter hydroxymethylation in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu X., Wu G., Yao Y., Zeng J., Shi D., Lv T. Intratracheal administration of mitochondrial DNA directly provokes lung inflammation through the TLR9-p38 MAPK pathway. Free Radic. Biol. Med. 2015;83:149–158. doi: 10.1016/j.freeradbiomed.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 119.Chrysanthopoulou A., Mitroulis I., Apostolidou E., Arelaki S., Mikroulis D., Konstantinidis T. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014;233:294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- 120.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janzen V., Forkert R., Fleming H.E., Saito Y., Waring M.T., Dombkowski D.M. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 123.Singh K.P., Bennett J.A., Casado F.L., Walrath J.L., Welle S.L., Gasiewicz T.A. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem Cell. Dev. 2014;23:95–106. doi: 10.1089/scd.2013.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., Weissman I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 125.Kasper M., Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci. Rep. 2017;37 doi: 10.1042/BSR20171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehmann M., Korfei M., Mutze K., Klee S., Skronska-Wasek W., Alsafadi H.N. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L., Li F., Su X., Li Y., Wang Y., Fang R. Melatonin prevents lung injury by regulating apelin 13 to improve mitochondrial dysfunction. Exp. Mol. Med. 2019;51:73. doi: 10.1038/s12276-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Merkt W., Bueno M., Mora A.L., Lagares D. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Semin. Cell Dev. Biol. 2019 Dec 23 doi: 10.1016/j.semcdb.2019.12.008. pii: S1084-9521(18)30264-7, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8 doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu S., Wang X., Geng P., Tang X., Xiang L., Lu X. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J. Pineal Res. 2017;63 doi: 10.1111/jpi.12405. [DOI] [PubMed] [Google Scholar]
- 131.Sontake V., Gajjala P.R., Kasam R.K., Madala S.K. New therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin. Ther. Targets. 2019;23:69–81. doi: 10.1080/14728222.2019.1552262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fivenson E.M., Lautrup S., Sun N., Scheibye-Knudsen M., Stevnsner T., Nilsen H. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017;109:202–209. doi: 10.1016/j.neuint.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Felix A.A. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 134.Zhao X., Kwan J.Y.Y., Yip K., Liu P.P., Liu F.F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2019 doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 135.Malouf M.A., Hopkins P., Snell G., Glanville A.R. Everolimus in IPFSI. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16:776–783. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- 136.Lv X.X., Wang X.X., Li K., Wang Z.Y., Li Z., Lv Q. Rupatadine protects against pulmonary fibrosis by attenuating PAF-mediated senescence in rodents. PloS One. 2013;8 doi: 10.1371/journal.pone.0068631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sosulski M.L., Gongora R., Feghali-Bostwick C., Lasky J.A., Sanchez C.G. Sirtuin 3 deregulation promotes pulmonary fibrosis. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:595–602. doi: 10.1093/gerona/glw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng Y., Ren X., Gowda A.S., Shan Y., Zhang L., Yuan Y.S. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4:e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang H.M., Ahn S.H., Choi P., Ko Y.A., Han S.H., Chinga F. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grunwell J.R., Yeligar S.M., Stephenson S., Ping X.D., Gauthier T.W., Fitzpatrick A.M. TGF-beta1 suppresses the type I IFN response and induces mitochondrial dysfunction in alveolar macrophages. J. Immunol. 2018;200:2115–2128. doi: 10.4049/jimmunol.1701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khalil N., Manganas H., Ryerson C.J., Shapera S., Cantin A.M., Hernandez P. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2019;53 doi: 10.1183/13993003.00663-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Avouac J., Konstantinova I., Guignabert C., Pezet S., Sadoine J., Guilbert T. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Ann. Rheum. Dis. 2017;76:1931–1940. doi: 10.1136/annrheumdis-2016-210821. [DOI] [PubMed] [Google Scholar]
- 144.Jost R.T., Dias H.B., Krause G.C., de Souza R.G., de Souza T.R., Nunez N.K. Fructose-1,6-Bisphosphate prevents bleomycin-induced pulmonary fibrosis in mice and inhibits the proliferation of lung fibroblasts. Inflammation. 2018;41:1987–2001. doi: 10.1007/s10753-018-0842-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.