Abstract
The abnormal inflammatory responses due to the lung tissue damage and ineffective repair/resolution in response to the inhaled toxicants result in the pathological changes associated with chronic respiratory diseases. Investigation of such pathophysiological mechanisms provides the opportunity to develop the molecular phenotype-specific diagnostic assays and could help in designing the personalized medicine-based therapeutic approaches against these prevalent diseases. As the central hubs of cell metabolism and energetics, mitochondria integrate cellular responses and interorganellar signaling pathways to maintain cellular and extracellular redox status and the cellular senescence that dictate the lung tissue responses. Specifically, as observed in chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis, the mitochondria-endoplasmic reticulum (ER) crosstalk is disrupted by the inhaled toxicants such as the combustible and emerging electronic nicotine-delivery system (ENDS) tobacco products. Thus, the recent research efforts have focused on understanding how the mitochondria-ER dysfunctions and oxidative stress responses can be targeted to improve inflammatory and cellular dysfunctions associated with these pathologic illnesses that are exacerbated by viral infections. The present review assesses the importance of these redox signaling and cellular senescence pathways that describe the role of mitochondria and ER on the development and function of lung epithelial responses, highlighting the cause and effect associations that reflect the disease pathogenesis and possible intervention strategies.
Keywords: Mitochondrial dysfunction, ROS, UPR, DAMPs, Cellular senescence, Cigarette smoke, COPD, Fibrosis
1. Introduction
The environmental toxicants, such as ambient particulate matter, tobacco smoke, biomass fuel smoke, vapors from electronic cigarettes (e-cig) or ozone, are highly oxidizing in nature and cause lung injury by oxidative stress. Mitochondria are the major cellular oxidative stress sensors that regulate metabolism, signaling, and energetics to maintain cellular and tissue homeostasis. In addition, mitochondria are reportedly critical in regulating cellular inflammatory responses, innate immunity, and aging [[1], [2], [3], [4]]. The mitochondrial dysfunction and the dysregulated reactive oxygen species (ROS) production following lung inflammation contributes to various lung diseases and impaired lung functions. At the same time, the protein folding in the endoplasmic reticulum (ER) is susceptible to extracellular stimuli and insults. ER is a highly organized luminal network that plays an important role in protein synthesis, maturation, folding and transport. ER is also the central site for Ca++ storage and homeostasis and regulates cellular redox status and energy stores because this organelle is also the site for lipid, cholesterol, and steroid biosynthesis, and asparagine-linked protein glycosylation [[5], [6], [7], [8], [9]]. The ER couples its quality control machinery to Ca++ and any alterations in intraluminal Ca++ by extra- or intra-cellular factors can cause protein misfolding. Accordingly, when ER homeostasis is disrupted by the inhaled toxicants, the ER develops an unfolded protein response (UPR) in order to maintain cellular homeostasis [[5], [6], [7]]. However, a dysregulated homeostasis following the unresolved stress in mitochondria and ER can initiate cell death pathways. Studies have shown that various signaling pathways, triggered by the UPR and ROS, are crucial to trigger cell death/apoptosis when they fail to restore protein homeostasis and survival [8].
Recent seminal studies have provided in-depth mechanistic insights into how the chronic and acute lung injury disrupts the mitochondrial activity and ER homeostasis that triggers an unresolvable UPR activation, disrupts cellular metabolism/energetics, and causing uncontrolled cell death. Cigarette smoke (CS) and their related products are the major risk factors of respiratory diseases like chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) because CS exposure results in aggregation of oxidative stress-mediated misfolded/unfolded proteins in the lung epithelium [10]. Other than CS exposure, dysregulation in mitophagy, aging, cellular senescence, viral infections, and disruption in cellular circadian rhythms are associated with the development of COPD and fibrosis. Increasing evidence suggests that ER stress and impaired elimination of damaged protein via UPR may play a role in the pathogenesis of COPD [[11], [12], [13]]. In this review, we elaborately discuss the etiological risk factors of COPD as well as the CS-induced oxidative stress, mitochondrial dysfunction, circadian rhythm, aging and cellular senescence mediated ER stress responses. Although the primary focus of this paper is on the cellular stress response in COPD, it is important to acknowledge that there is in fact strong phenotypic similarity between COPD and IPF. Both are characterized by increased and accelerated pulmonary cellular senescence and compromised regenerative potential often induced by fulminating oxidative damage associated with the chronic cigarette smoke exposure [14,15]. As such, when addressing the cellular and redox stress response, some molecular aspects of IPF are also discussed. Additionally, some recent developments that illustrate how targeting the specific ER-mitochondria redox signaling events may be more beneficial in managing chronic lung disease.
1.1. Mitochondrial dysfunction and mitophagy
Damaged or dysfunctional mitochondria are normally cleared from the cell by mitophagy, whereby defective mitochondria are loaded into the autophagosomes, followed by lysosomal degradation. Mitophagy is activated by mitochondrial membrane depolarization followed by stabilization of Pink1 (PTEN-induced putative kinase 1) on mitochondrial outer membranes, which are involved in mitochondrial quality control through fission [16]. Pink1 then recruits an E3 ubiquitin ligase called Parkin (a protein involved in mitochondrial quality control), from the cytosol [17]. Once recruited to mitochondria, Parkin leads to degradation of mitofusin2 (Mfn2, a core protein involved in mitochondrial fusion), which initiates localization of damaged mitochondria with the isolation membranes containing protein microtubule-associated protein light chain 3 (LC3) and formation of autophagosome, where damaged mitochondria are degraded to maintain mitochondrial homeostasis [18]. Pink1 and Parkin knockout (KO) mice exhibit increased ROS levels and dysfunctional mitochondria [19], suggesting that these proteins may play a critical role in cellular senescence [20]. Conversely, Parkin overexpression increases life span in fruit flies, partly due to a reduction in oxidative stress that is mitigated by active mitophagy.
1.2. Mitochondria-ER dysfunctions and ER stress responses in COPD
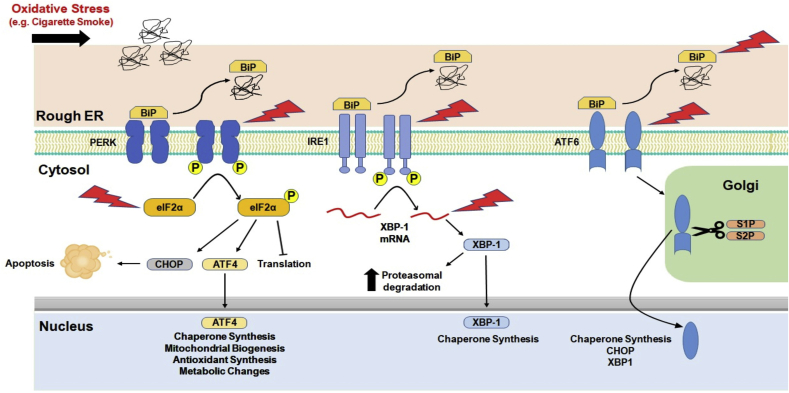
ER stress occurs when the rate at which new protein entering the ER exceeds its folding capacity within the ER and the cells undergo adaptive UPR to defend against ER stress [7]. The UPR is comprised of a series of transcriptional, translational, and post-translational processes that can lower the rate of protein synthesis and increase the protein folding capacity of the ER machinery, enhancing the process of misfolded protein elimination, and escalating the size of the ER compartment thereby reducing the ER stress. Prior studies have shown that various signaling pathways triggered by UPR are crucial within the cells either to trigger cell death/apoptosis when it is unable to restore protein homeostasis and survival [8]. Evidence suggest that UPR is among the key players that could cause lung disease via altered expression of genes and misfolded proteins [9]. UPR restores protein homeostasis via triggering intracellular signal transduction of ER transmembrane proteins such as protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol-requiring enzyme 1 alpha (IRE1α) [7].
ER stress in the lung can occur due to heightened physiological (secretory load) and pathological stress (oxidative stress, CS exposure, hypoxia, starvation, altered calcium levels, and viral infection) that results in increased protein influx, and accumulation of unfolded proteins in the ER lumen. Previous studies have shown that CS-induced ER stress in the lungs of smokers and patients with COPD via oxidant-induced irreversible damage of lung proteins via the ubiquitin-proteasome system or autophagic vacuoles [11,21]. ER in lung fibroblasts from patients with COPD shows more disorganized dots and patchy structure compared to the more reticulated structure observed in never-smokers and ever-smokers [22]. CS-induced increase in poly-ubiquitination of proteins in different lung cell types, in vitro in alveolar and airway epithelial cells, lung fibroblasts and alveolar macrophages, and in vivo in mouse lungs [11,21,[23], [24], [25]]. This supports that CS-induced increase in misfolded proteins in the ER and impairs the ER folding capacity. Chronic CS upregulated protein abundance of the chaperones, glucose-regulated protein of 78 kDa (GRP78), calnexin, and calreticulin, protein disulfide isomerase (PDI) and other signaling molecules that play a vital role in the PERK pathway (phosphorylated-eIF2a, ATF4, and CHOP) in the human lung [10,[25], [26], [27]]. Additional evidence from a mouse model of CS exposure demonstrates increased expression of phospho-eIF2α, CHOP, and p50 ATF6N protein abundance and mRNA levels in mouse lung [11]. Acute vs. chronic CS exposure in mouse showed differences in mRNA levels of ATF4 and CHOP in mouse lungs and alveolar macrophages, suggesting that UPR target genes and proteins at the transcriptional and translational levels are dynamic and cell type-dependent in vivo [23,28,29]. GRP78 is an ER chaperone known to be involved in alveolar epithelial cell survival and gene expression during lung development via modulation of ER stress [[30], [31], [32]]. Previous reports have shown increased GRP78 protein levels in the lungs of smokers and patients with COPD. Furthermore, GRP78 serum levels were significantly increased in COPD that correlated with decline in lung function and severity of disease (emphysema) [33]. In another report, BALF levels of GRP78 were significantly greater in smokers compared to non-smokers and human airway epithelial cells treated with CS extract showed augmented secretion of GRP78 [26]. Overall, these findings on elevated ER stress protein GRP78 levels could be a potential biomarker for CS-induced lung injury and chronic lung diseases.
Surprisingly, COPD patients that were stable along with varying range of disease severity and diaphragm muscle weakness, when analyzed for markers of ER stress/UPR signal mediators (markers of protein misfolding, proteolysis, apoptosis including ATF6, PERK and IRE1 pathways) showed no significant alterations in main respiratory muscle both at the mRNA and protein levels [34]. These findings from stable COPD patients with diaphragm muscle dysfunction and associated severe airflow limitation did not correlate with the status of ER stress and UPR [34]. Hence, therapeutic strategies using ER stress inhibitors or UPR modulators to treat diaphragm muscle dysfunction in COPD patients with advanced disease may not be advisable. A recent report by Aggarwal et al. (2018) reported augmented heme and ER stress marker, GRP78/BIP, in the plasma of very severe (GOLD 4) COPD patients [35]. These findings corroborated with the increase in plasma heme levels of ferrets exposed to chronic CS (6 months) and in mice exposed to bromine (Br2) gas at 400 ppm for 30 min/day for 14–21 days [35]. Although all branches of the UPR in some way regulate CHOP [36], the ATF4 was established as a major CHOP inducer in the ATF4 haplodeficient (ATF4+/−) mice model of Br2 exposure [35]. The mice exposed to Br2 show elevated plasma heme levels resulting in increased ER stress markers such as GRP78, phospho-PERK, pIRE1α, ATF4, ATF6 and CHOP associated with other lung pathological manifestations such as airway enlargement and increased lung compliance that resembles pulmonary fibrosis and emphysema observed in humans [35]. Additionally, attenuation of ER stress using salubrinal (ER stress inhibitor; 1 mg/kg BW), a heme scavenger, abrogates levels/activity of elastase, reduces the inflammatory response (neutrophils and macrophages) and lung fibrotic and emphysematous pathophysiological phenotypes caused by Br2 exposure wild-type and ATF4+/− mice [35].
A previous report showed chlorine gas exposure in mice enhanced the expression of UPR marker p-PERK levels in the lungs and skin (as early as 1–6 h) [37]. Other evidence from literature demonstrates human neutrophil elastase induces UPR by activating PERK-CHOP signaling mediated apoptosis in endothelial cells [38]. Gaseous exposure induced inhalation injury can result in heme-dependent airway damage that might contribute to lung fibrotic and emphysematous changes. These findings support that heme could be a potential culprit associated with silica, quartz and cadmium induced lung injury caused due to environmental exposures. Therapeutic approaches to treat such inhalation injury induced heme levels using heme-scavenging protein (Hx), albumin and haptoglobin along with pharmacological ER stress blockers may be beneficial. Overall, these findings suggest that ER-stress related UPR pathways are essential for normal cellular functions (Table 1) and any disruption in their function can cause pathological changes leading to disease conditions. Thus, these molecular pathways could be potential therapeutic targets against CS-induced lung injury and chronic lung diseases, as outlined in Fig. 1. However, one must be wary when considering UPR components as potential therapeutic targets, as there is often intra- and inter-pathway cross linking, and certain UPR components may interact with others, as well as with other cellular pathways and responses. Furthermore, although the UPR seems to induce various pathologic changes in chronic pulmonary disease, it also induces increased antioxidant expression. Specifically, the PERK/eIF2α/CHOP branch of the UPR has been shown to drive the expression of nuclear factor (erythroid 2)-related factor 2 or Nrf2 [10,36]. In turn, Nrf2 interacts with ATF4 and together they drive the expression of genes involved in glutathione synthesis, hydrogen peroxide scavenging and more [39]. Furthermore, the PERK/eIF2α branch is significantly involved in cell fate decisions by inducing, or preventing apoptosis, particularly upon chronic ER stress. Specifically, Kasai et al. (2019) have shown that when Nrf2 interacts with ATF4, they repress the expression of CHOP, a proapoptotic gene [40,41]. The Ire1 branch has also been implicated in proapoptotic induction via the regulated Ire1-dependent decay pathway (RIDD) and all three branches (ATF6, ATF4 and XBP1) can potentially bind CHOP [42,43]. As such, excessive inhibition of the UPR, specifically of the PERK branch may have deleterious effects and as such further research is required. Whilst, Nrf3 has a crucial role in smooth muscle cells and was involved in the ER-stress induced differentiation [44].
Table 1.
ER and mitochondrial therapeutic targets in chronic pulmonary diseases associated with CS exposure.
| Target | Function | Pathologic Response | Treatment | Reference |
|---|---|---|---|---|
| ER (UPR) | ||||
| GRP78 or BiP (Binding Immunoglobulin Protein) | UPR master regulator: binds unfolded proteins, releases downstream effectors | Binds unfolded proteins, UPR effectors dimerization and activation | Upregulate | [[30], [31], [32], [35]] |
| PERK (Protein Kinase R-like ER Kinase) | UPR effector, Inactivates eIF2 | Activated | De-dimerize | [[25], [26], [27], [34], [37]] |
| eIF2α | Translation initiation factor. Activates ATF4 & CHOP, Attenuates translation | Activated | / | [[10], [34]] |
| ATF4 (Activating Transcription Factor 4) | Transcription factor, Cytoprotective | Activated | Degrade | [[37], [38]] |
| CHOP (C/EBP homologous protein) | Apoptosis initiator | Activated | Degrade | [[38], [40], [41], [42], [43]] |
| IRE1 (Inositol-Requiring Enzyme 1) | UPR effector. Splices XBP-1, allowing translation and activation | Activated | De-dimerize | [[34], [35], [42], [43]] |
| XBP-1 | Transcription factor, Stimulates ER chaperone production and protein degradation | Activated | Degrade (siRNA) | [[42], [43]] |
| ATF6 | UPR effector. Activated by BiP, stimulates ER chaperone production | Activated | Degrade | [[42], [43]] |
| Calnexin | Crucial ER chaperone | / | Upregulate | [[10], [25], [26], [27]] |
| Calreticulin | Crucial ER chaperone | / | Upregulate | [10,25–27] |
| PDI (Protein Disulfide Isomerase) | Crucial ER chaperone | / | Upregulate | [[11], [75]] |
| Golph3 | Golgi-mitochondria transport during biogenesis | Upregulated | Downregulate/degrade | [[84], [85]] |
| Antioxidant Regulators | ||||
| Nrf2 (Nuclear factor erythroid 2-related factor 2) | Transcription factor, Stimulates antioxidant production | / | Upregulate | [[10], [36], [39], [40], [41], [42], [43]] |
| Nrf3 (Nuclear factor (erythroid 2)-like factor 3) | Transcription factor, Reduces antioxidants | Upregulated | Downregulate/degrade | [44] |
| Mitochondria | ||||
| PINK (PTEN-Induced Kinase 1) | Mitochondrial kinase. Recruits Parkin to depolarized mitochondria | / | Upregulates mitophagy, diminish mtDAMPs | [[119], [227], [228], [229], [230]] |
| Parkin | E3 ubiquitin ligase, mediates mitophagy | / | Upregulates mitophagy, diminish mtDAMPs | [119,227–230] |
| Drp1 | Cytosolic, mitochondrial fission effector | / | / | [[20], [140], [222]] |
| Fis1/Mff/MiD49/MiD51 | Mitochondrial fission proteins involved in Drp-1 recruitment | / | / | [20,140,222] |
| Mfn1/Mfn2/Opa1 | Mitochondrial fusion proteins | Opa1 upregulated with CS exposure | / | [20,140,222] |
| Nox4 | Inner mitochondrial membrane enzyme. ROS sensor, immune activator | Upregulated | Downregulate/degrade | [[220], [221]] |
Fig. 1.
Potential therapeutic targets within the unfolded protein response pathways that are involved in chronic airway diseases.
1.3. Circadian clock and ER stress crosstalk in relevance with ER and mitochondria stress in lung diseases
The circadian clock is a self-sustained system of biological oscillations with a period near 24 h that are driven by the circadian timing in mammals [45]. Circadian clock at the cellular level consist of transcription-translation feedback loop of transcriptional regulators called clock genes. The circadian clock molecule BMAL1:CLOCK drives the expression of the repressors PER (period) and CRY (cryptochrome) genes. PER and CRY genes following a series of post-translational modifications repress their own expression by blocking the activity of BMAL1:CLOCK [45]. The detailed molecular circadian clock mechanisms involved in lung pathophysiology have been extensively reviewed previously [46]. Based on the recent reports on circadian clock and UPR in cancer biology and fibrosis [47,48], it will be important to turn our attention towards the role of the circadian clock-ER stress axis in chronic inflammatory lung diseases. Studies that directly support the involvement of circadian clock and ER stress interactions are sparse.
Clock disruption has been associated with lung immune-inflammatory disorders such as COPD asthma, lung cancers, metabolic diseases, and aging [46,[49], [50], [51], [52]]. Understanding the novel interactions and molecular connections that occur between the circadian clock and cellular processes such as the UPR in the context of normal vs. diseased pathophysiology will provide better drug targets for chronotherapeutics of ER-stress induced chronic lung diseases. The ER is one of the other intracellular organelles that display diurnal rhythmicity in their structural proteins, functions, and response to specific stressors [53]. A previous report has demonstrated that secretory pathways that include ribosomes, ER chaperones, Golgi vesicles display rhythmic oscillations similar to those observed in liver secretions [54]. Binding immunoglobulin protein (BiP) is known for its control of URP activation during ER stress [55]. Recently, the link between ER stress and circadian rhythm in fibroblasts and mouse that actively secreting collagen (low during the day and high during the night) was extensively investigated [48,56,57]. A previous study showed levels of extractable collagen-I oscillates with a 24 h period but not the transcript levels of collagen-I polypeptide chains. Hence, the circadian rhythmicity of extracellular collagen observed could be generated post-transcriptionally [57]. Additional evidence suggests that BiP overexpression or treatment with chemical chaperons strengthens circadian rhythm (amplitude of oscillation) in immortalized tail tendon fibroblasts [48]. ER stress is one among the active players involved in the pathogenesis of pulmonary fibrosis [58]. Based on the abovementioned evidence that ER stress activation could dampen circadian rhythms and collagen synthesis, understanding the mechanism by which lung fibroblasts circumvent this physiological control can provide novel drug targets to treat fibrotic lung disease.
Previous reports have evidently shown that ATF4 is a transcriptional regulator of CLOCK and XBP1 splicing by IRE1α follows a 12 h rhythm coordinated by the clock machinery [59,60]. In a recent report, XBP1 regulated the cell-autonomous mammalian 12 h clock independent of the circadian clock to coordinate metabolic and stress rhythms in the ER and mitochondria across species such as nematodes, crustacean, and mammals [61]. Increased XBP1 mRNA splicing via IRE1α-dependent mechanism show reduced Per1 expression in glioblastoma cells [62]. Another report showed ATF4-mediated rhythmic expression of Per2 gene sustains circadian oscillators via cAMP-dependent signaling using in vitro (mouse embryonic fibroblasts) and in vivo (ATF4-null mice) models [60]. Hence, understanding the novel interactions between the ER stress-mediated UPR and circadian clock molecules in normal vs. diseased state will be essential to reduce time-of-day dependent proteotoxicity.
Recently, miR-211 coordination of UPR and circadian clock (CLOCK:BMAL1) during cell survival and tumor progression was demonstrated [47]. An earlier report showed that miR-211 is induced as a result of ER stress possibly via the PERK-eIF2α-ATF4 axis dependent mechanism [63]. Compelling evidence on the UPR-miR-211-clock axis comes from oncogene-induced ER stress and associated PERK activation in tumor cells (c-Myc+) with increased miR-211 and decreased BMAL1 expression [47]. Blocking PERK or miR-211 in cancer cells restored BMAL1 expression and its downstream signaling targets [47]. Melatonin is an indoleamine hormone secreted by the pineal gland that has been shown to regulate circadian rhythms [64]. Additional evidence suggest that melatonin exhibits protective effects against a variety of inflammatory disorders that affect the cardiovascular and pulmonary systems. Previously, melatonin levels were significantly lower in patients with acute COPD exacerbation compared to stable COPD [65]. Melatonin administration in patients with COPD show reduced oxidative stress and improved dyspnea despite no significant change in lung function and their exercise capacity [66]. Melatonin treatment in mice exposed to CS and lipopolysaccharide (LPS) showed protection against fibrotic responses by downregulating TGF-β1, collagen I, and SMAD3. In a recent report, male Wistar rats challenged with CS and LPS to induce COPD when treated with melatonin shows attenuation of COPD development by activating sirtuin-1 (SIRT-1) expression thereby reducing apoptosis and ER stress in the lung [67,68]. The circadian clock has also been investigated in IPF. Recently, Cunningham et al. have presented the core clock protein Rev-Erbα as an important player in epithelial-mesenchymal transition. They found that Rev-Erbα leads to dysregulation of the integrinβ1 adhesion formation and increased myofibroblast activation, suggesting it as a viable therapeutic target for IPF [69]. Melatonin may have a therapeutic role in IPF as well as Sanchez et al. (2018) have shown that mice treated with melatonin have a reduced amount of both Rev-Erbα as well as GRP78 [70]. Research on the permissive or causative role of molecular clock dysfunction-ER cross-talks that constantly occur in chronic inflammatory lung diseases is required. Collectively, these findings support the fact that circadian clock and ER stress/UPR are tightly regulated molecular signaling pathways that can be significantly altered contributing to chronic lung diseases.
1.4. Combustible and electronic cigarettes in mitochondrial and ER stress response
The chemical composition and complexity predominantly depend on the heating conditions, such as the temperature and the oxygen flow. During the combustion process of a cigarette, burning zone temperatures can reach 900 °C. The area behind marginal oxygen levels reaches approximately 700 °C in the distillation zone, where pyrolytic reactions occur. Inhaled smoke drawn through the cigarette is named mainstream smoke. The smoldering end of the cigarette, which reaches approximately 800 °C by diffusion of oxygen has combustion conditions significantly differ from mainstream conditions [71].
Mainstream smoke consists of particle and gas phases, including volatile organic compounds (VOC), and semi-volatile organic compounds (SVOC). Chemical classes that constitute tobacco smoke include aldehydes, ketones, carboxylic acids, phenols, nitriles, alkaloids, and various saturated and unsaturated hydrocarbons. Smoke constituents can act as electrophiles, free radicals, and reactive anions. For example, benzo[a]pyrene converts to quinone, which then produces ROS as byproducts. Hydroxyl ions, which is another crucial ROS, is also formed by hydrogen peroxide via Fenton's reaction in the tar phase or by its compounds such as catechol. Redox cycling mechanisms also form semiquinones and superoxide ions, which are highly reactive. Gas-phase components such as nitric dioxide can form carbon-centered radicals by reacting with other smoke constituents. Superoxide ions react with nitric oxide to form peroxynitrite with high oxidizing capacity. Peroxynitrite can also deplete intracellular antioxidants such as glutathione levels. Reactive oxygen and nitrogen species (ROS and RNS, respectively) generated in mainstream smoke perpetually form reactive intermediates such as acrolein, which are cytotoxic [72].
Vastly produced polycyclic aromatic hydrocarbon (PAH) components in tobacco smoke, such as 1-Nitropyrene, was shown to augment mitochondrial genes and induce deregulation of mitochondrial genes via C/EBPα [73]. Copious amounts of exogenous reactive oxygen and nitrogen species produced in tobacco smoke offset the redox homeostasis in cells inducing oxidative stress. ER stress can result in unfolded protein response (UPR) in cells, including lung cells such as epithelial cells. UPR can cause dissociation of ER signaling molecules such as ATF6, Ire1, and PERK from GRP78/BiP. An increase in BiP is a hallmark of UPR. Upregulation of these molecules induces chaperone assisted folding and degradation of misfolded proteins. PERK activated eIF-2α reduces protein synthesis and therefore preventing protein misfolding. Nrf2, a bZIP protein that regulates antioxidant proteins, is often activated to counteract CS-induced oxidative stress and pathogenesis of emphysema [74].
Nicotine is present in tobacco smoke, e-liquids, and heat-not-burn products. Nicotine has shown to increase the phosphorylation of PERK and eIF-2α. Nicotine was also shown to alter Hsd11b1 (11β-Hydroxysteroid dehydrogenase type 1), placental cell differentiation and function marker. These effects show the potential risks involved with nicotine intake during pregnancy [75]. Another study has also demonstrated an upregulation of ER stress markers (eI2α, Grp78, Arf4, and CHOP) in rat placenta. Additional ER stress-inducing factor, impaired disulfide bond formation, was also observed in nicotine exposed rat placenta, with decreased mRNA levels of protein-disulfide-isomerase (PDI) and oxidoreductase, QSOX1 [76]. Contrary to these findings, Srinivasan et al. showed neuroprotective effects of low-dose nicotine by looking at nAChRs in dopaminergic neurons. They showed nicotine reducing UPR and CHOP activation following ER stress-inducing, tunicamycin (Tu) treatment [77].
Zahedi et al. demonstrated that e-liquid aerosols cause mitochondrial stress in stem cells. Exposure of stem cells to e-cig aerosols showed an increase in the presence of superoxide by MitoSOX. Moreover, protein oxidation was observed along with the aggregation of mitochondrial nucleoids and mitochondrial DNA damage. Furthermore, this study attributed these mitochondrial stress responses to the nicotine present in e-liquids [78].
Tobacco smoke is well known to cause lung epithelial ciliary impairment. Smoke reduces the ciliary beat frequency, motility, and even ciliary loss. Similarly, tobacco smoke has been shown to significantly alter the structure of mitochondria structure significantly by altering the microtubular network and reducing the matrix. Disruption of the internal structure of the mitochondria is associated primarily with the particulate phase of the tobacco smoke, although the gas phase (e.g., volatile phenolic fractions) induces mitochondrial swelling. These effects interfere with mitochondrial energy production, thus ciliary dysfunction [79].
E-liquids are available in a plethora of flavors. These flavors are imparted by flavoring chemicals. Cinnamaldehyde, used in cinnamon-flavored e-liquids, have been shown to dysregulate mitochondria in epithelial cells, thereby affecting the ciliary beat function and cell motility. Mitochondrial respiration, glycolysis, and ATP production were significantly affected by cinnamaldehyde vapor. These adverse effects were caused by cinnamaldehyde and the nicotine content did not contribute to these effects. As cilia are an important defense mechanism in blocking foreign material into the respiratory system, cinnamaldehyde inhalation may increase the susceptibility to infections [80]. Lerner et al. showed elevated mitochondrial ROS levels by exposure to short e-cig exposures, including CuO, Cu40, and Cu60 treatments, in human fetal lung fibroblast (HFL-1) cells. This was also supported by decreased COX-II levels demonstrating the vulnerability of complex IV [81]. These studies show that exposure to e-cigarette and e-liquid constituents causes mitochondrial stress in cells.
Aryl hydrocarbon receptor (AhR) agonists such as PAHs, i.e., benzo[a]pyrene, can be detected in the serum of smokers. These ROS producing compounds induced the overexpression of autophagy proteins BECN1 and LC3 and coupled with increased PARK2 pro-fission protein for autophagosomal degradation and reduced mitofusin proteins, MFN1 and MFN2 [82]. These changes in protein expression suggest that CS exposure gives rise to mitochondrial dysregulation and mitophagy. Downregulation of mitofusin proteins has been debatable as some studies observed increased expression of MFN2 by CS exposure [83]. Regulation of expression of these proteins may be attributed to the adaptive response to mitochondrial stress. Moreover, nontoxic doses of cigarette smoke extract have shown unchanged HSP60 (mitoUPR) levels in alveolar epithelial cells [83].
Tobacco smoke also disrupts the trans-Golgi network, where Golgi phosphoprotein 3 (GOLPH3) plays a vital role in protein trafficking and glycosylation. GOLPH3 has been linked with ER in relation to phosphotidylinositol-4-phosphate signal termination mediated membrane docking mechanism [84]. During mitochondrial biogenesis, GOLPH3 transits between Golgi and mitochondria, and the upregulation of GOLPH3 causes mitochondrial dysfunction. Thus, nicotine derived nitrosamine ketones (NNKs) in tobacco smoke can dysregulate ER-Golgi network [85]. Tobacco smoke induces mitochondrial DNA mutations, i.e., point mutations, insertions, deletions. Secondhand smoke is considered to be more toxic than mainstream smoke. Undiluted sidestream smoke yields more harmful constituents compared to the mainstream per pound of smoke [86]. Sidestream smoke at 2 μg/mL is considered toxic for the airway epithelium. Moreover, aged secondhand smoke has been demonstrated to have increased total particulate matter, CO, and nicotine concentrations and toxicity by several folds compared to the mainstream smoke [87]. Neonatal secondhand smoke exposure has shown to significantly increase the mitochondrial superoxide dismutase activity with increased oxidative stress, altered mitochondrial DNA copy number, and deletion levels [88].
Exposure to thirdhand smoke extract has shown to cause cytotoxicity as well as altered mitochondrial morphology and function. Increased mitochondrial hyperfusion, i.e., increased mitochondrial membrane potential, increased superoxide production, and oxidation of proteins. Moreover, decrease mitochondrial fission gene expressions (e.g., Fis1), and uncoupling genes contribute to reduced oxidative phosphorylation (OXPHOS), Ucp2, Ucp3, and Ucp5, have also been observed. These adverse effects are from semivolatile and nonvolatile organic compounds. Tobacco specific nitrosamines, nicotine, and related alkaloids are responsible constituents for mitochondrial stress responses [72,89]. While these responses may be induced as a survival mechanism, chronic thirdhand smoke may cause severe cytotoxicity and apoptosis leading to pathogenesis as seen with stem cell exposures in previous studies [90].
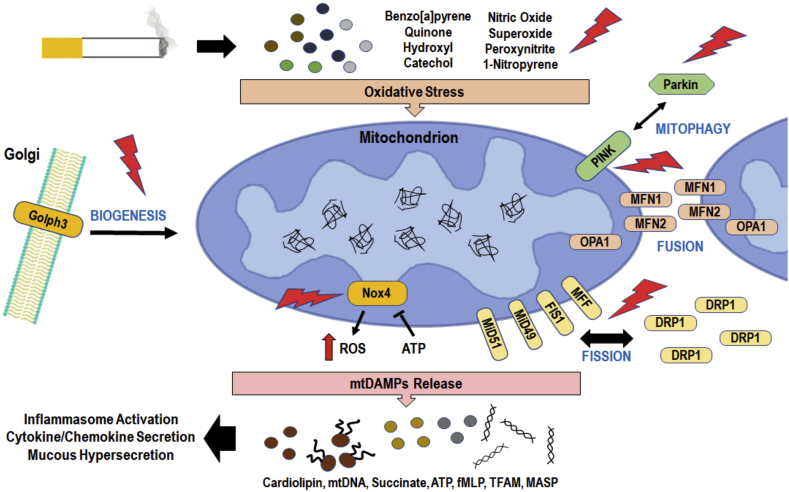
Tobacco smoke, including secondhand, thirdhand, e-cig aerosols, and heated not burned or modified tobacco products release chemical compounds that form ROS and RNS species. These products adversely affect cellular organelles, including mitochondria, endoplasmic reticulum, and Golgi apparatus. Exposure to cigarette smoke compounds alters mitochondrial oxidative phosphorylation and metabolic activity, which are symptoms of debilitating diseases such as COPD and asthma. Molecular signatures of CS-induced stress include altered mitochondrial biogenesis and mitophagy, increased mtDNA mutations, and damage-associated molecular patterns (DAMPs) are closely associated with lung diseases, as discussed later. Overall, these findings on elevated mitochondrial dynamics and the quality control, essential for normal physiological functions are disrupted by the CS-induced oxidative stress (Fig. 2) and these pathways could be potential therapeutic targets against smoking-related chronic lung diseases.
Fig. 2.
Potential therapeutic targets within the mitochondrial oxidative stress response pathways of the airway epithelium.
1.5. Mitochondrial dysfunction in HIV and CS-induced lung diseases
Lung diseases such as COPD, pulmonary hypertension, lung cancer, and pneumonia are emerging as significant comorbidities in the HIV-infected population. Combination antiretroviral therapy (cART) suppresses viral replication but is unable to eradicate HIV due to reactivation of the HIV from latently infected anatomical reservoirs [91,92] upon cessation of cART [[93], [94], [95], [96]]. There is convincing evidence that the lungs are anatomical reservoirs of HIV [[97], [98], [99]]. HIV is found in cell-free bronchoalveolar lavage fluid [97], alveolar macrophages and intrapulmonary lymphocytes [98,99]. HIV infection is an independent risk factor for the development of COPD irrespective of the smoking status [100]. The mechanism by which HIV promotes chronic inflammation predisposing to COPD is not known. HIV patients exhibit increased oxidative stress [101,102]. HIV induces oxidative stress and this has been attributed to several HIV proteins including HIV Tat, Nef, Vpr and gp120 in different cell types [[103], [104], [105]]. HIV infected cells are known to express HIV proteins even in the presence of cART. Proteins like, Tat and Nef are immediate early proteins of HIV and are not suppressed by cART [[106], [107], [108], [109], [110]]. The protein transduction domain of Tat allows its secretion by infected cells and uptake by bystander cells where it mediates pleotropic effects [[111], [112], [113], [114]]. Likewise, Nef has been secreted from cells via extracellular vesicles and exosomes [115,116]. HIV Nef has been shown be expressed in aviremic lungs [110]. We have shown that primary bronchial epithelium cultured ex vivo and bronchial brushings from human subjects express canonical HIV receptors CD4, CCR5, and CXCR4 receptors and can be infected with HIV [117]. We have also demonstrated that cigarette smoke (via TGF-β signaling) increases levels of HIV receptors and facilitates HIV infection of bronchial epithelial cells, increasing the viral burden in the airway possibly explaining worse outcomes in HIV smokers [118].
HIV infection of bronchial epithelial cells and secretion of viral proteins can affect mitochondrial homeostasis. Santerre et al., recently demonstrated that HIV Nef induces the hyperpolarization of mitochondrial membrane as well as mitochondrial morphology causing them to appear hyperfused when compared to Carbonyl cyanide 3-chlorophenylhydrazone treated controls [119]. HIV Vpr has been shown to decrease the mitochondrial membrane potential of CD4 T-cells and bystander cells leading to apoptosis [105]. While HIV Tat has been shown to promote inflammation in cell lines as well as mouse models with secretion of proinflammatory cytokines [120,121], the mechanism by which it promotes inflammation has not been determined. However, recent reports have demonstrated that HIV Tat impairs mitochondrial homeostasis in different cell types [[122], [123], [124]]. Tat expression in the airway epithelial cells or exposure to secreted Tat by other immune cells in the airway can similarly affect mitochondrial homeostasis and promote epithelial cell senescence. In our laboratory we have observed that HIV Tat alters the epithelial microRNAome to suppress some of the key proteins in mitophagy and general macroautophagy with consequent impaired mitophagy and increased cellular senescence in airway epithelial cells (unpublished data). An indirect effect of HIV infection on mitochondrial homeostasis is the use of anti-retrovirals in controlling HIV. Many early reports have implicated cART with mitochondrial dysfunction in pediatric patients as well as adults [125], specifically with use of nucleoside reverse transcriptase inhibitors (NRTI). These have been attributed to a lack of specificity of these inhibitors where they also target mitochondrial DNA leading to decreased levels of mtDNA. Patients on twelve months of NRTI therapy demonstrate decrease mitochondrial DNA [126].
Almost 60% of people living with HIV smoke tobacco [127]. Given that cigarette smoke increases HIV gene expression [128] while also upregulating HIV receptors on primary bronchial epithelial cells [118], it is expected that mitochondrial dysfunction will be exacerbated in HIV smokers compared to HIV non-smokers, consequently exacerbating senescence, inflammation, and lung aging. A number of reports, including ours, have reported that cigarette smoke promotes mitochondrial dysfunction leading to epithelial cell senescence [14,18,20]. Cigarette smoke inhibits Parkin translocation to damaged mitochondria resulting in impaired mitophagy and senescence in mouse models of COPD as well as in lungs of chronic smokers and COPD patients [18]. CS-impaired mitophagy was shown to accelerate lung aging in mouse models of COPD [14]. In addition, IPF lung evaluation has shown that TGF-β1 induces mitochondrial fragmentation, ROS production and in fact increased PINK expression [129]. CS-impaired mitophagy could further strengthen the debilitating effects of IPF. Mitochondrial integrity itself is affected in COPD lung tissues and CS extract (CSE) was shown to promote mitochondrial fragmentation in a ROS dependent manner, consequently promoting senescence in primary human bronchial epithelial cells [20]. In this study, CSE-induced ROS was shown to post-translationally modify DRP-1 resulting in its translocation to mitochondria followed by mitochondrial fragmentation.
1.6. Mitochondrial DAMPs, noncoding RNAs and mucociliary clearance mechanisms
Lung injury and other cellular stress described earlier could trigger the release of mitochondrial damage-associated molecular patterns (mt-DAMPs) into the cytosol or extracellular space [2,130] and activate the innate lung responses via pattern recognition receptors (PRRs) [131]. To prevent the release of these proinflammatory molecules cells have developed various defensive mechanisms including autophagosomal degradation of the damaged mitochondria via mitophagy [2,3,17,132]. However, overwhelming injury or cellular stress releases these mt-DAMPs or mitochondrial alarmins including mitochondrial DNA fragments (mtDNA), N-formyl peptides (fMLP), cardiolipin, succinate, ATP, mitochondrial transcriptional factor A (TFAM) and the mitochondrial antiviral signaling protein (MASP) [2,130]. These mtDAMPs bind to the PRRs resulting in nuclear factor (NF)-κB- and MAPK-dependent proinflammatory response [133]. Accordingly, these pathways are intricately regulated by several genetic and epigenetic factors. In addition, several noncoding RNAs (ncRNAs) generated from the intergenic and intronic regions have now been shown to regulate all stages of gene expression and several cellular functions including the lung innate immune responses. Mucociliary clearance mechanisms are one of the primary innate defense mechanisms that physically clear the airways via mucociliary escalator [134,135]. In the respiratory tract, 9 genes have been found to encode for mucins of which MUC2, MUC5AC, and MUC5B are primarily found in airway secretions [136,137]. The heterogeneity in airway mucins allows entrapment of many inhaled pathogens and toxicants to help clear the airways by mucociliary clearance mechanisms, therefore, a lot of attention has been geared towards understanding the expression and the regulation of these mucins in the context of lung pathologies.
The newly identified ncRNAs along with the well-characterized proteins mediate an intricate crosstalk between mitochondria and the nucleus to maintain the airway epithelial homeostasis. Among ncRNAs, the small microRNAs (miRNAs or miRs) are implicated mainly in post-transcriptional gene regulation and a variety of innate immune processes but how these miRNAs are associated with the mitochondrial homeostasis and mtDAMPs is still unknown. For example, in lung tissues, mtDAMPs induce the miR-223 expression that inhibits NLRP3 inflammasome and IL-1β release and suppressing miR-223 leads to the activation of NLRP3-IL-1β and acute lung injury [138]. NLRP3 activators induce the release of the mitochondrial membrane lipid cardiolipin, another mtDAMP [139]. The miR-30a inhibited AECs-II apoptosis by repressing the mitochondrial fission dependent on Drp-1 leading to lung fibrosis [140]. The miR-34/449 deficiency in ciliated cells results in reduced cilia length and numbers, which is mediated, at least in part, by the post-transcriptional repression of Cp110, a centriolar protein suppressing cilia assembly [141], making these miRs indispensable for mucociliary differentiation. Similarly, miR-183 was shown to be essential for hypercapnia responses by regulating isocitrate dehydrogenase 2 (IDH2) that impairs mitochondrial function and cell as observed in patients with chronic lung diseases [142]. Strikingly, miR-449a is upregulated by more than 1000-fold when epithelial cells from human airways are lifted from a liquid environment to air, allowing them to undergo mucociliary differentiation [143]. Among the mucin expression regulation, miR-134-5p, miR-146a-5p, and let-7 family of miRs have been shown to regulate chronic mucus hypersecretion associated with COPD [144]. Earlier, Pandit et al. (2010) reported that the let-7d miR was associated with IPF, suggesting a potential therapeutic target for controlling IPF associated pathologies [145]. Similarly, miR-21 levels had also been found significantly upregulated in IPF and increased miR-21 levels promoted the TGF-β1 activty [146]. Furthermore, miR-145-5p was recently implicated in modulating TGF-β1 levels and could be useful therapeutic target in restoring the airway epithelial homeostasis [147].
The other major species of ncRNAs are long ncRNAs (lncRNAs) that are >200 bases long with characteristic 3D structures and are involved in the regulation of gene expression and several disease conditions. Nevertheless, not much is known about the role of lncRNAs in mtDAMPs and MCC mechanisms associated with lung pathophysiologies. Most lncRNAs that are transcribed in the nucleus also reside in mitochondria and play a key role in regulating mitochondrial responses. For example, lncRNA RMRP or mitochondrial RNA-processing endoribonuclease is required for mitochondrial DNA replication and RNA processing, and the lncRNA SRA or steroid receptor RNA activator is a key modulator of hormone signaling found in both mitochondria and nucleus. The mitochondrial DNA also encodes for a set of lncRNAs like lncND5, lncND6, and lncCyt b RNA encoded on the complementary strands of ND5, ND6 and Cyt b genes as reviewed recently [148]. These mitochondrial DNA-encoded lncRNAs also appear to function in the nucleus and the molecular mechanisms underlying trafficking of the mitochondrial-encoded lncRNAs to the nucleus are now beginning to emerge [148]. There is also growing evidence towards mitochondria importing the cytosolic ncRNAs via yet-to-be discovered mechanisms [149].
The cellular DAMPs play a pivotal role in COPD pathogenesis with several of them significantly elevated in the lung tissues and blood circulation of COPD patients compared to both smoking and nonsmoking controls [150,151]. Moreover, during COPD exacerbations, the RAGE ligands like HMGB1, S100A8, and LL-37 are reportedly elevated [152]. These pathological findings have been successfully recapitulated in mouse models with most of the cellular DAMPs like galectin-3, S100A9, and dsDNA secreted into the inflamed airway tissues following CS exposure in susceptible mice [151,153]. However, studies are needed to ascertain whether the airway tissue of COPD patients is predisposed to release mtDAMPs upon lung injury and whether elevated mtDAMPs contribute to the development of COPD. It is noteworthy that mitochondria-targeted inhibitors suppressed the DAMP-mediated radiation-induced lung tissue damage in a mouse model [154]. Thus, substantial research efforts are needed to develop an understanding of the role of ncRNA in the lung pathophysiologies specifically with regards to the regulation of mtDAMPs and mucociliary clearance mechanisms.
1.7. Mitochondria, stem cells, and aging lung diseases
Human lungs comprise of 40 different types of cells or even more, share to form the structure and physiological function [155]. Among them, the lung stem cells are responsible for regenerating new tissues and repair injury. Mitochondria are energy storehouses that produce ATP through oxidative phosphorylation (OXPHOS). There are extensive studies that demonstrated the important role of mitochondria in the regulation of stem cell function. It also plays an important role in cell signaling and any mitochondrial stress can generate ROS in stem cells. Alteration in mitochondrial function of stem cells from various organ-specific cells may produce age-related changes in self-renewal and differentiation. The stem cells originating from different organs have diverse metabolic properties, the majority of stem cells use the glycolytic pathway of metabolism such as human and murine hematopoietic stem cells, murine neural stem cells, human embryonic stem cells, and mesenchymal stem cells [[156], [157], [158], [159], [160], [161]]. The glycolytic pathway of respiration helps in maintenance of self-renewal and differentiation of stem cells. These stem cells have low rate of mitochondrial oxidative respiration. Though the stem cells require glycolytic pathway for self-renewal, the oxidative pathway of respiration is required for differentiation [162,163]. The decline in function of mitochondria in stem cells leads to alteration in stem cell functions and self-renewal and aging [[164], [165], [166]].
The stem cells are required to differentiate and self-renew for the maintenance of homeostasis of the various organs in the body. Exposure to these agents or conditions leads to exhaustion of the stem cells and unable to cope up with the requirement of various cell types in the organs and develop age-related diseases [[167], [168], [169]]. Recently, several types of stem cells have been reported in the lungs with the self-renewal and differentiation properties that included tracheal club cells (clara cells) and alveolar type II epithelial cells. The alveolar type II cells differentiate to alveolar type I cells [[170], [171], [172]]. The altered function of basal cells, endothelial progenitor cells, and hematopoietic stem cells have been reported in smokers and COPD patients [[173], [174], [175]]. Additional studies have been reported showing altered function of mesenchymal stem cells in experimental mouse models [176,177]. Studies have shown that stem cell exhaustion, alveolar epithelial cell injury and hyperplasia are associated with pulmonary fibrosis [[178], [179], [180], [181], [182]]. A decline in mitochondrial functions has been associated with age-dependent abnormal function of organs in experimental mouse models [183,184].
Aging causes many structural and functional changes in the lungs, such as enlarged alveoli, reduced surface area of gas exchange, loss of alveolar attachment, reduced elastic recoil, and increased gas trapping [185,186]. Aging is also associated with increased incidence of COPD and IPF [187,188]. However, the cellular and molecular mechanisms of occurrence of these diseases are still unknown. There are several mechanisms have been postulated by many researchers in the last several years. The age-associated changes in oxidative stress, telomerase length, immune functions, extracellular matrix, and anti-aging molecules, have been reported [189]. There is increased oxidative stress observed in lungs and oxidative damage to mtDNA in skeletal muscle cells of COPD patients [190,191]. Changes in telomere length reported in former smokers, COPD and IPF patients [192,193]. Additionally, increased incidence of infections and autoimmune diseases reported in aging populations [194]. The alveolar structure is composed of collagen and elastin proteins in extracellular matrix (ECM). Aging causes changes in ECM, which leads to decline in lung function [186,195]. Further, changes in several anti-aging molecules reported in aging conditions such as klotho gene, senescence marker protein-30, and SIRT-1. The SIRT-1 expression was suppressed in the lungs of cigarette smoke-exposed rats and in the lung cells from COPD patients [[196], [197], [198], [199]].
Recently, stem cell therapy initiated in human and experimental models of acute lung injury and chronic diseases. There was some success achieved in acute lung injury models. However, human trials done recently were mostly unsuccessful in COPD patients. We have recently shown that exosomes-derived from MSCs restore cigarette smoke-induced lung abnormalities via attenuating mitochondrial function [200].
The regenerative therapy attempted recently using two approaches. The extrinsic approach by infusion of exogenous stem cells to repair the damaged structures of the lungs using embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells, and human lung stem cells. The second approach intrinsic therapy was done by administering the small molecule to stimulate the endogenous lung stem/progenitor cells to repair the damaged tissues using retinoid compounds [[201], [202], [203]].
1.8. Mitochondria, telomeres, and telosome/shelterin complex crosstalk
Perinuclear mitochondrial accumulation or clustering transmits a nuclear signal via mtROS. Oxidative and genotoxic stress are well-known initiators of telomere dysfunction. We have shown that CS treatment causes perinuclear mitochondria clustering along with nuclear ROS accumulation and DNA damage foci formation, as well as injurious lung responses [204].
Telomeres have specialized nucleoprotein structures that protect or “cap” the linear ends of eukaryotic chromosomes from DNA repair, preventing aberrant repair and end-to-end chromosome fusions. Telomeres constitute TTAGGG DNA tandem repeats that are bound to a multiprotein complex called shelterin/telosome that contains six vital proteins TRF1, TRF2, POT1 (POT1a and POT1b in mouse), TPP1 (ACD/TPP1 i.e. shelterin component TPP1), TIN2 and RAP1. Telomere loop formation by TRF2 protects its ends from recognition by DNA damage pathways. Telomere shortening is associated with aging, and current studies suggest an important role of telomere structure/organization, telomeric DNA damage, and telomere end-capping proteins during aging and in various age-associated disorders. Mitochondrial dysfunction and telomere attrition may be involved in cellular senescence. This may be due to TIN2 (shelterin protein) localization into mitochondria apart from its nuclear compartment, leading to mitochondrial dysfunction [2].
CS treatment causes translocation and mitochondrial localization of TIN2 in human lung cells [2]. A close correlation between perinuclear mitochondria and telomeric DNA damage foci is observed in the presence of CS treatment. Furthermore, CS treatment causes telomere shortening and shelterin TPP1 reduction in lung epithelial cells and fibroblasts. The precise mechanisms on how mitochondria coordinate with shelterin telomeres to regulate CS-induced lung injurious responses are not known.
1.9. Dysregulated mitochondrial DNA and proteins in chronic lung diseases
As discussed before, mitochondria play a vital role in cell fate, differentiation, and disease pathogenesis [[205], [206], [207]], thus, mitochondrial dysfunction affects almost all the organs, such as liver, heart, kidney, brain, and lungs [[208], [209], [210], [211], [212]]. Because mitochondria are major producers of ROS, the mtDNA are at a higher risk of injury than nuclear DNA (nDNA) because the histone-shield and the self-repair ability are absent in mitochondria [[213], [214], [215]]. In this section, we focus on the mitochondrial targets (nucleic acids or protein markers) specifically associated with chronic lung diseases. Recently, Carpagnano et al. reported that the ratio of mtDNA and nDNA, could serve as an effective biomarker to identify COPD, asthma, and asthma-COPD overlap syndrome (ACOS) [216]. In addition, they report that higher content of mtDNA is closely associated with COPD than asthma in ACOS patients [217]. Similarly, Liu et al. reported that lower mtDNA copy number in leukocyte was found associated with COPD patient compared to healthy control [218]. Bindu et al. demonstrated SIRT3 serves as a protector of mtDNA damage and prevents the pathogenesis of pulmonary fibrosis and myofibroblast differentiation but not in COPD and asthma [219]. These studies nonetheless suggest that mtDNA can be a marker of chronic lung disease or can be a therapeutic target for dysregulated mitochondrial associated diseases.
Mitochondrial ROS is generated as a by-product of biosynthesis and is probably responsible for the upregulated ROS level in patients with chronic lung diseases. For instance, Hecker et al. reported that ROS related markers: NADPH oxidase-4 (Nox4) and Nrf2 were protective against IPF in a mouse model [220]. Nox4 acts as a sensor and checkpoint for metabolic/energetic process in mitochondria, and is, therefore, proposed as a novel intervention strategy to minimize the drug-resistance in cancer [221]. CS exposure is associated with fragmented mitochondria and mtROS generation, and COPD patients had smaller size mitochondria compared to the healthy controls [20]. The mtROS induced dysregulation of mitochondrial fission and fusion proteins (Fis1 and Drp1) affect the mitochondrial dynamics in human bronchial epithelial cells [20]. Interestingly, long-term CS exposed BEAS-2B cells presented only increased optic atrophy 1/mitochondrial dynamin-like GTPase (OPA1) at a relatively higher concentration, while other mitochondrial fission/fusion proteins (Mfn1, Mfn2, Fis1, and Drp1) and TFAM remained unchanged [222]. These studies indicate that in addition to causing a direct lung injury, CS exposure could induce mtROS production and induce mitochondrial dysregulation.
Besides mitochondrial fission/fusion proteins, there are several proteins involved in mitochondrial dysfunction associated chronic lung diseases, such as PINK-Parkin, which are required for mitochondrial degradation via mitophagy; or PPARγ coactivator-1 (PGC-1α/β) which are responsible for mitochondrial biogenesis to maintain cellular energetics [16]. Recently, Yu et al. reported that resistance to IPF in a mouse model is dependent on the intact PGC-1α, and PINK1 pathways, because knocking down either of these pathways resulted in the development of IPF-like conditions [19]. In the pulmonary arterial smooth muscle cells lacking sirtuin-1 and -3 (SIRT-1 and -3) or PGC-1α, the mitochondrial biogenesis and dynamics were suppressed resulting in reduced mtDNA content [223]. SIRT family of proteins, as discussed before, are well-known in aging-related chronic lung diseases, such as COPD and IPF [224]. Interestingly, although the preventing mitochondrial biogenesis by regulating PGC-1α/β is beneficial in IPF a similar mechanism induces cellular aging in COPD [16].
In addition, damaged mitochondrial accumulation was found in COPD lungs, which might be due to the ineffective mitophagy [20]. The appropriate removal of the damaged mitochondria is vital because mitophagy can help to protect organs from injury caused by dysregulated mtROS and mtDNA release, which could cause inflammation and apoptosis [225,226]. Previous reports have been discussed that damaged mitochondria were found in COPD lung tissues along with the lower level of PARK2, which indicated that ineffective mitophagy was associated with COPD pathogenesis [16,227,228]. The deficiency of PINK1-PARK2 induced myofibroblast differentiation and proliferation, which are associated with IPF pathogenesis [229]. Moreover, TGF-β treatment inhibited the expression of PINK1 and consequently promoted IPF [230].
1.10. Therapeutic potential of mitochondria transfer: role of Miro1
Recent studies have highlighted a strong translational and therapeutic potential of mitochondrial transfer, particularly in diseases associated with mitochondrial abnormalities/dysfunction. Particularly relevant are the data suggesting that mitochondrial transfer can rejuvenate cellular respiration. It has been shown that mitochondrial transport machinery including mitochondrial Rho-GTPase 1 (Miro1) regulates intracellular mitochondria movement and transfer of mitochondria between cells that occur via tunneling nanotubes and enhances MSC rescue efficacy. A recent study found that the defects in mitochondrial motility and distribution due to Miro1 deficiency are enough to cause neurological disease, also implicating in CS-induced lung inflammation [231]. This strategy provides a promising translational therapeutic tool for mitochondria transfer in conditions including the response to CS exposure, where mitochondria are dysfunctional. We have shown that bone marrow-MSCs form intercellular tunneling nanotubes and transfer their healthy mitochondria into lung epithelial cells treated with CSE. We reveal the mechanisms by which the mitochondria are transferred into lung epithelial cells containing dysfunctional mitochondria in COPD/emphysema, and whether Miro1-mediated mitochondrial transfer protects against emphysema in a mouse model reverses the injuries.
1.11. Iron deficiency as a comorbidity to chronic pulmonary disease
Chronic pulmonary diseases and other chronic inflammatory conditions are associated with the iron deficiency and related comorbidities. In fact, Nickol et al. (2015) found that 18% of COPD patients had a non-anemic functional iron deficiency versus only 5% of healthy adults [232]. This comorbidity is due to cytokine stimulation of hepcidin expression in monocytes, macrophages, hepatocytes and various other cells leading to iron trapping within those cells and a functional iron deficiency [233,234]. However, Cloonan et al. (2016) have shown that intracellular iron metabolism may in fact be an important factor in COPD pathogenesis [235]. They have identified a pathway in which the iron-responsive element-binding protein 2 (IRP2) increases the expression of mitochondrial cytochrome c oxidase 1 (COX-1), a terminal electron acceptor in the process of oxidative phosphorylation, which has a proposed role as a regulator of mitochondrial iron metabolism [236]. IRP2 therefore leads to an increased iron load in mitochondria, which seems to have a highly detrimental effect, for as Cloonan et al. (2016) have shown, IRP2−/− mice are significantly less susceptible to chronic CS-induced pulmonary injury, changes in mucociliary clearance, and most importantly, mitochondrial aberrations such as cytochrome c release, decrease in mitochondrial membrane potential and mitochondrial ROS generation. Although the extent of the role that mitochondrial iron loading may have on COPD pathogenesis is not clearly established, further studies on the iron-induced mitochondrial stress responses and potential therapeutic targeting may be highly beneficial.
2. Conclusions
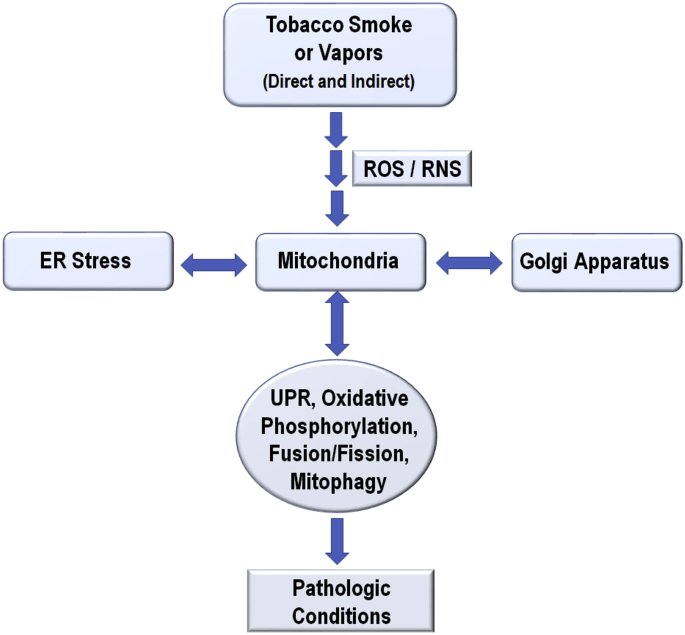
Chronic pulmonary diseases, including COPD and IPF, present with similar cellular pathophysiology. There is a significant increase in ROS/RNS and cellular redox conditions involved in the pathology of mitochondrial dysfunction and senescence. The subcellular interaction between the rough ER, mitochondria, and Golgi apparatus is disrupted leading to dysregulated UPR and oxidative stress responses leading to chronic pathologic conditions (Fig. 3). CS coupled with viral or aging insults induces protein misfolding and protein influx into the ER, overburdening the primary chaperones (GRP78, PDI, calnexin, and calreticulin), thus initiating the UPR. Ample evidence supports the activation of the PERK, IRE1, and ATF6 pathways downstream of GRP78 delocalization. Increased induction of chaperones or GRP78 or deactivation/degradation of UPR downstream components would significantly alleviate pulmonary injury. Additionally, the delivery of ROS scavengers to the appropriate location or any reduction of the CS-induced ROS may profoundly improve chronic pulmonary disease outcomes.
Fig. 3.
Chronic exposures to direct or indirect tobacco smoke or vapors from electronic cigarettes induce molecular dysregulation in mitochondria, ER, and Golgi apparatus that leads to pathologic conditions observed in COPD and IPF.
CS and e-liquid aerosols induce a significant mitochondrial superoxide increase, respiration reduction, and affect ciliary function. Evidence suggests an increase in autophagy (increased BECN1 and LC3) however a significant decrease in mitophagy as heavily fragmented and damaged mitochondria with low membrane potentials are observed. Few significant changes in fusion/fission proteins have been reported. In COPD, increased mitochondrial biogenesis through Golph3 seems to have a detrimental effect. Oxidative stress and reduced mitophagy lead to the release of mtDAMPs and initiation of both intra and extracellular inflammatory pathways. In all discussed chronic pulmonary conditions, an increase in mitophagy via the PINK/Parkin pathway would remove damaged mitochondria and alleviate the condition. Additional factors, such as non-coding RNAs play an increasingly pivotal role and provide a unique therapeutic opportunity; however, extensive further research is required. Modulation of miR-223, 30, 449a, 34/449 and 183 can regulate inflammatory response, mucociliary differentiation, and chronic mucous secretion.
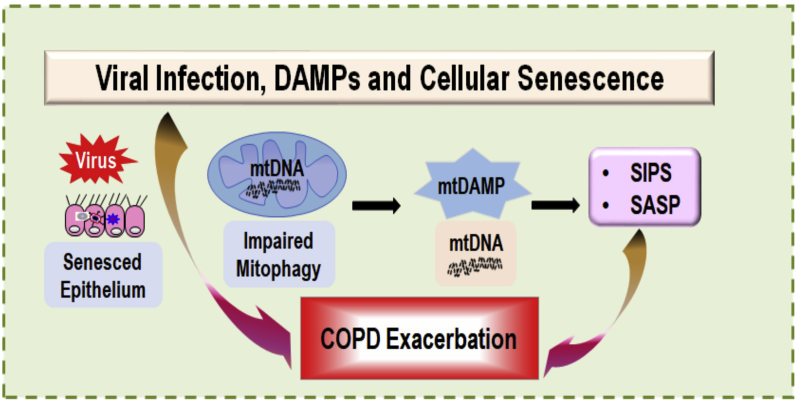
There is evidence that concomitant viral infection (e.g., HIV) and CS exposure further augment oxidative stress leading to release of cellular DAMPs and induce cellular senescence. Thus, the airway mucosal viral infection, DAMPs accumulation, and cellular senescence aggravate the ongoing inflammatory and lung pathologic responses (Fig. 4). In case of HIV infection, the HIV Vpr has been shown to decrease mitochondrial membrane potential and induce apoptosis. Concomitant exposure additionally reduces the level of mitophagy, and CS increases HIV receptor levels and facilitates HIV infection.
Fig. 4.
Viral infection, DAMPs, and cellular senescence of airway epithelium aggravate the ongoing inflammatory and pathologic responses.
Chronic pulmonary pathology is commonly age-related, and loss of stem cells plays a pivotal role. The conversion from glycolysis to OXPHOS is crucial for stem cell differentiation, and as such, loss of mitochondrial quality control reduces tissue regenerative and repair capability and leads to faster pulmonary aging. Mitochondrial DNA damage and telomere reduction have also been reported in COPD patients, along with reduced anti-aging molecules such as the klotho gene, senescence marker 30 and SIRT1. Therapeutic induction of these genes would significantly improve care, especially since recent stem cell or exosome treatments have been unsuccessful in COPD patients.
Finally, increasing evidence has shown that the molecular clock plays a quintessential role. ATF4 was shown to be a transcription factor for CLOCK and oncogenic PERK expression was shown to reduce BMAL1 expression. Both ATF4 and PERK induce miR-211 and induced GRP78 overexpression increases the amplitude of circadian protein oscillations. The molecular clock genes require investigation for therapeutic potential, like melatonin, which was found reduced in COPD patients, reduced oxidative stress and increased SIRT1 expression. Overall, CS-induced oxidative stress, mitochondrial dysfunction, circadian rhythm, aging and cellular senescence mediated ER stress are involved in the pathogenesis of chornic lung diseases. Targeting the specific ER-mitochondria redox signaling events may provide novel therapeutic strategies which would be more beneficial in managing chronic lung diseases.
Authors’ contributions
All authors wrote and revised/edited the manuscript.
Declaration of competing interest
Authors declare no conflict of interest with work described in this manuscript.
Acknowledgements
This study was supported by the NIH R01 HL135613, R21 ES028006, R01 HL133404, and R01 HL137738 (to I.R.) and NIH R01 HL142543 (to I.K.S). FIU investigators acknowledge the support by NIH R01 HL149898, R21 AI144374, R21 AI117560, Herbert Wertheim College of Medicine and Office of Research & Economic Development Start-Up Funds. Thanks to Krishna P. Maremanda, PhD for coordinating and collecting initial manuscript drafts.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101443.
List of abbreviations
- ATF
activating transcription factor
- ATP
Adenosine triphosphate
- BALF
Bronchoalveolar lavage fluid
- BiP
Binding immunoglobulin protein
- BMAL1
Brain and Muscle ARNT-Like 1
- C/EBPα
CCAAT-enhancer-binding proteins
- cART
Combination antiretroviral therapy
- CLOCK
Circadian Locomotor Output Cycles Kaput
- COPD
chronic obstructive pulmonary disease
- CRY
cryptochrome
- CS/CSE
Cigarette smoke/Cigarette smoke extract
- DRP-1
dynamin-related protein 1
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- GOLPH3
Golgi phosphoprotein 3
- GRP78
glucose regulated protein of 78 kDa
- HIV
human immunodeficiency virus
- IPF
idiopathic pulmonary fibrosis
- IRE1α
inositol-requiring enzyme 1 alpha
- LC3
MAP kinase light chain 3
- lncRNA
Long noncoding RNA
- LPS
lipopolysaccharide
- Mfn1
mitofusin1
- Mfn2
mitofusin2
- miR
microRNA
- mt DAMPs
mitochondrial damage-associated molecular patterns
- mtDNA
mitochondrial DNA
- MUC
Mucin
- nAChRs
Nicotinic acetylcholine receptors
- Nef
Negative Regulatory Factor
- NLRP3
nucleotide-binding domain, leucine-rich repeat Pyrin Domain Containing 3
- NRT1
nucleoside reverse transcriptase inhibitors
- OPA1
optic atrophy 1/mitochondrial dynamin-like GTPase
- OXPHOS
oxidative phosphorylation
- PAH
polycyclic aromatic hydrocarbons
- PDI
protein disulfide isomerase
- PER
period
- PERK
protein kinase RNA (PKR)-like ER kinase
- Pink1
PTEN-induced putative kinase 1
- POT1
Protection Of Telomeres 1
- QSOX1
quiescin sulfhydryl oxidase 1
- RAP1
Repressor/Activator Protein 1
- RNS
Reactive nitrogen species
- ROS
reactive oxygen species
- SVOC
semi-volatile organic compounds
- Tat
Trans-Activator of Transcription
- TGF
β1 transforming growth factor beta-1
- TIN2
TRF1- and 2-Interacting Nuclear Protein 2
- TRFs
Telomeric Repeat Factors
- UPR
unfolded protein response
- VOC
Volatile organic compounds
- Vpr
Viral Protein R
- XBP1
X-box binding protein 1
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dela Cruz C.S., Kang M.J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion. 2018;41:37–44. doi: 10.1016/j.mito.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner C.A., Sundar I.K., Rahman I. Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int. J. Biochem. Cell Biol. 2016;81(Pt B):294–306. doi: 10.1016/j.biocel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J. Clin. Invest. 2016;126(3):809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filadi R., Theurey P., Pizzo P. The endoplasmic reticulum-mitochondria coupling in health and disease: molecules, functions and significance. Cell Calcium. 2017;62:1–15. doi: 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Marchi S., Bittremieux M., Missiroli S., Morganti C., Patergnani S., Sbano L. Endoplasmic reticulum-mitochondria communication through Ca(2+) signaling: the importance of mitochondria-associated membranes (MAMs) Adv. Exp. Med. Biol. 2017;997:49–67. doi: 10.1007/978-981-10-4567-7_4. [DOI] [PubMed] [Google Scholar]
- 7.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufey E., Sepulveda D., Rojas-Rivera D., Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am. J. Physiol. Cell Physiol. 2014;307(7):C582–C594. doi: 10.1152/ajpcell.00258.2014. [DOI] [PubMed] [Google Scholar]
- 9.Chen A.C.H., Burr L., McGuckin M.A. Oxidative and endoplasmic reticulum stress in respiratory disease. Clin. Transl. Immunol. 2018;7(6) doi: 10.1002/cti2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelsen S.G. The unfolded protein response in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2016;13(Suppl 2):S138–S145. doi: 10.1513/AnnalsATS.201506-320KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenche H., Baty C.J., Vedagiri K., Shapiro S.D., Blumental-Perry A. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. Faseb. J. 2013;27(3):965–977. doi: 10.1096/fj.12-216234. [DOI] [PubMed] [Google Scholar]
- 12.Hassan T., Carroll T.P., Buckley P.G., Cummins R., O'Neill S.J., McElvaney N.G. miR-199a-5p silencing regulates the unfolded protein response in chronic obstructive pulmonary disease and alpha1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2014;189(3):263–273. doi: 10.1164/rccm.201306-1151OC. [DOI] [PubMed] [Google Scholar]
- 13.Tran I., Ji C., Ni I., Min T., Tang D., Vij N. Role of cigarette smoke-induced aggresome formation in chronic obstructive pulmonary disease-emphysema pathogenesis. Am. J. Respir. Cell Mol. Biol. 2015;53(2):159–173. doi: 10.1165/rcmb.2014-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashid K., Sundar I.K., Gerloff J., Li D., Rahman I. Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci. Rep. 2018;8(1):9023. doi: 10.1038/s41598-018-27209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American thoracic S, and European respiratory S. American thoracic society/European respiratory society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American thoracic society (ATS), and the European respiratory society (ERS) was adopted by the ATS board of directors, june 2001 and by the ERS executive committee, june 2001. Am. J. Respir. Crit. Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 16.Tsubouchi K., Araya J., Kuwano K. PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm. Regen. 2018;38(1):18. doi: 10.1186/s41232-018-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piantadosi C.A., Suliman H.B. Mitochondrial dysfunction in lung pathogenesis. Annu. Rev. Physiol. 2017;79:495–515. doi: 10.1146/annurev-physiol-022516-034322. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad T., Sundar I.K., Lerner C.A., Gerloff J., Tormos A.M., Yao H. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29(7):2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G., Tzouvelekis A., Wang R., Herazo-Maya J.D., Ibarra G.H., Srivastava A. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24(1):39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara H., Araya J., Ito S., Kobayashi K., Takasaka N., Yoshii Y. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;305(10):L737–L746. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 21.Somborac-Bacura A., van der Toorn M., Franciosi L., Slebos D.J., Zanic-Grubisic T., Bischoff R. Cigarette smoke induces endoplasmic reticulum stress response and proteasomal dysfunction in human alveolar epithelial cells. Exp. Physiol. 2013;98(1):316–325. doi: 10.1113/expphysiol.2012.067249. [DOI] [PubMed] [Google Scholar]
- 22.Weidner J., Jarenback L., Aberg I., Westergren-Thorsson G., Ankerst J., Bjermer L. Endoplasmic reticulum, Golgi, and lysosomes are disorganized in lung fibroblasts from chronic obstructive pulmonary disease patients. Phys. Rep. 2018;6(5) doi: 10.14814/phy2.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geraghty P., Wallace A., D'Armiento J.M. Induction of the unfolded protein response by cigarette smoke is primarily an activating transcription factor 4-C/EBP homologous protein mediated process. Int. J. Chronic Obstr. Pulm. Dis. 2011;6:309–319. doi: 10.2147/COPD.S19599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraghty P., Baumlin N., Salathe M.A., Foronjy R.F., D'Armiento J.M. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediat. Inflamm. 2016;2016:9461289. doi: 10.1155/2016/9461289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelsen S.G., Duan X., Ji R., Perez O., Liu C., Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am. J. Respir. Cell Mol. Biol. 2008;38(5):541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 26.Aksoy M.O., Kim V., Cornwell W.D., Rogers T.J., Kosmider B., Bahmed K. Secretion of the endoplasmic reticulum stress protein, GRP78, into the BALF is increased in cigarette smokers. Respir. Res. 2017;18(1):78. doi: 10.1186/s12931-017-0561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenche H., Ye Z.W., Vedagiri K., Richards D.M., Gao X.H., Tew K.D. Adverse outcomes associated with cigarette smoke radicals related to damage to protein-disulfide isomerase. J. Biol. Chem. 2016;291(9):4763–4778. doi: 10.1074/jbc.M115.712331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijt S.H., Keller I.E., John G., Kohse K., Yildirim A.O., Eickelberg O. Acute cigarette smoke exposure impairs proteasome function in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303(9):L814–L823. doi: 10.1152/ajplung.00128.2012. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Wu Z.Z., Wang W. Inhibition of endoplasmic reticulum stress alleviates cigarette smoke-induced airway inflammation and emphysema. Oncotarget. 2017;8(44):77685–77695. doi: 10.18632/oncotarget.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pobre K.F.R., Poet G.J., Hendershot L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J. Biol. Chem. 2019;294(6):2098–2108. doi: 10.1074/jbc.REV118.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flodby P., Li C., Liu Y., Wang H., Marconett C.N., Laird-Offringa I.A. The 78-kD glucose-regulated protein regulates endoplasmic reticulum homeostasis and distal epithelial cell survival during lung development. Am. J. Respir. Cell Mol. Biol. 2016;55(1):135–149. doi: 10.1165/rcmb.2015-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxidants Redox Signal. 2009;11(9):2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merali S., Barrero C.A., Bowler R.P., Chen D.E., Criner G., Braverman A. Analysis of the plasma proteome in COPD: novel low abundance proteins reflect the severity of lung remodeling. COPD. 2014;11(2):177–189. doi: 10.3109/15412555.2013.831063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreiro E., Salazar-Degracia A., Sancho-Munoz A., Aguilo R., Rodriguez-Fuster A., Gea J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J. Appl. Physiol. 1985;126(6):1572–1586. doi: 10.1152/japplphysiol.00670.2018. 2019. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S., Ahmad I., Lam A., Carlisle M.A., Li C., Wells J.M. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight. 2018;3(21) doi: 10.1172/jci.insight.120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayaub E.A., Kolb P.S., Mohammed-Ali Z., Tat V., Murphy J., Bellaye P.S. GRP78 and CHOP modulate macrophage apoptosis and the development of bleomycin-induced pulmonary fibrosis. J. Pathol. 2016;239(4):411–425. doi: 10.1002/path.4738. [DOI] [PubMed] [Google Scholar]
- 37.Li C., Weng Z., Doran S.F., Srivastava R.K., Afaq F., Matalon S. Chlorine induces the unfolded protein response in murine lungs and skin. Am. J. Respir. Cell Mol. Biol. 2013;49(2):197–203. doi: 10.1165/rcmb.2012-0488RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grechowa I., Horke S., Wallrath A., Vahl C.F., Dorweiler B. Human neutrophil elastase induces endothelial cell apoptosis by activating the PERK-CHOP branch of the unfolded protein response. FASEB J. 2017;31(9):3868–3881. doi: 10.1096/fj.201700012R. [DOI] [PubMed] [Google Scholar]
- 39.He C.H., Gong P., Hu B., Stewart D., Choi M.E., Choi A.M. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 40.Kasai S., Yamazaki H., Tanji K., Engler M.J., Matsumiya T., Itoh K. Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control. J. Clin. Biochem. Nutr. 2019;64(1):1–12. doi: 10.3164/jcbn.18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milisav I., Suput D., Ribaric S. Unfolded protein response and macroautophagy in alzheimer's, Parkinson's and prion diseases. Molecules. 2015;20(12):22718–22756. doi: 10.3390/molecules201219865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepe A.E., Xiao Q., Zampetaki A., Zhang Z., Kobayashi A., Hu Y. Crucial role of nrf3 in smooth muscle cell differentiation from stem cells. Circ. Res. 2010;106(5):870–879. doi: 10.1161/CIRCRESAHA.109.211417. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18(3):164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundar I.K., Yao H., Sellix M.T., Rahman I. Circadian molecular clock in lung pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309(10):L1056–L1075. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bu Y., Yoshida A., Chitnis N., Altman B.J., Tameire F., Oran A. A PERK-miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol. 2018;20(1):104–115. doi: 10.1038/s41556-017-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickard A., Chang J., Alachkar N., Calverley B., Garva R., Arvan P. Preservation of circadian rhythms by the protein folding chaperone, BiP. FASEB J. 2019;33(6):7479–7489. doi: 10.1096/fj.201802366RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao H., Sundar I.K., Huang Y., Gerloff J., Sellix M.T., Sime P.J. Disruption of sirtuin 1-mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2015;53(6):782–792. doi: 10.1165/rcmb.2014-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papagiannakopoulos T., Bauer M.R., Davidson S.M., Heimann M., Subbaraj L., Bhutkar A. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metabol. 2016;24(2):324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durrington H.J., Gioan-Tavernier G.O., Maidstone R.J., Krakowiak K., Loudon A.S.I., Blaikley J.F. Time of day affects eosinophil biomarkers in asthma: implications for diagnosis and treatment. Am. J. Respir. Crit. Care Med. 2018 doi: 10.1164/rccm.201807-1289LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hood S., Amir S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 2017;127(2):437–446. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chedid A., Nair V. Diurnal rhythm in endoplasmic reticulum of rat liver: electron microscopic study. Science. 1972;175(4018):176–179. doi: 10.1126/science.175.4018.176. [DOI] [PubMed] [Google Scholar]
- 54.Robles M.S., Cox J., Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10(1) doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 56.Garva R., Yeung C.Y.C., Pickard A., Claverley B., Adamson A., Lu Y.H. Circadian clock regulation of the secretory pathway in fibroblasts. Int. J. Exp. Pathol. 2017;98(6) A7-A. [Google Scholar]
- 57.Yeung C.-Y.C., Garva R., Pickard A., Chang J., Holmes D.F., Lu Y. Circadian clock regulation of the secretory pathway. bioRxiv. 2018:304014. [Google Scholar]
- 58.Kropski J.A., Blackwell T.S. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Invest. 2018;128(1):64–73. doi: 10.1172/JCI93560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cretenet G., Le Clech M., Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metabol. 2010;11(1):47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Koyanagi S., Hamdan A.M., Horiguchi M., Kusunose N., Okamoto A., Matsunaga N. cAMP-response element (CRE)-mediated transcription by activating transcription factor-4 (ATF4) is essential for circadian expression of the Period2 gene. J. Biol. Chem. 2011;286(37):32416–32423. doi: 10.1074/jbc.M111.258970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu B., Zhang Q., Pan Y., Mace E.M., York B., Antoulas A.C. A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metabol. 2017;25(6):1305–13019 e9. doi: 10.1016/j.cmet.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pluquet O., Dejeans N., Bouchecareilh M., Lhomond S., Pineau R., Higa A. Posttranscriptional regulation of PER1 underlies the oncogenic function of IREalpha. Can. Res. 2013;73(15):4732–4743. doi: 10.1158/0008-5472.CAN-12-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chitnis N.S., Pytel D., Bobrovnikova-Marjon E., Pant D., Zheng H., Maas N.L. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol. Cell. 2012;48(3):353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018;175(16):3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gumral N., Naziroglu M., Ongel K., Beydilli E.D., Ozguner F., Sutcu R. Antioxidant enzymes and melatonin levels in patients with bronchial asthma and chronic obstructive pulmonary disease during stable and exacerbation periods. Cell Biochem. Funct. 2009;27(5):276–283. doi: 10.1002/cbf.1569. [DOI] [PubMed] [Google Scholar]
- 66.de Matos Cavalcante A.G., de Bruin P.F., de Bruin V.M., Nunes D.M., Pereira E.D., Cavalcante M.M. Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled study. J. Pineal Res. 2012;53(3):238–244. doi: 10.1111/j.1600-079X.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 67.Shin N.R., Park J.W., Lee I.C., Ko J.W., Park S.H., Kim J.S. Melatonin suppresses fibrotic responses induced by cigarette smoke via downregulation of TGF-beta1. Oncotarget. 2017;8(56):95692–95703. doi: 10.18632/oncotarget.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He B., Zhang W., Qiao J., Peng Z., Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can. J. Physiol. Pharmacol. 2019;97(5):386–391. doi: 10.1139/cjpp-2018-0529. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham P.S., Meijer P., Nazgiewicz A., Anderson S.G., Borthwick L.A., Bagnall J. The circadian clock protein REVERBalpha inhibits pulmonary fibrosis development. Proc. Natl. Acad. Sci. U. S. A. 2019 doi: 10.1073/pnas.1912109117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez D.I., Gonzalez-Fernandez B., Crespo I., San-Miguel B., Alvarez M., Gonzalez-Gallego J. Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. J. Pineal Res. 2018;65(3) doi: 10.1111/jpi.12506. [DOI] [PubMed] [Google Scholar]
- 71.Biondi-Zoccai G., Sciarretta S., Bullen C., Nocella C., Violi F., Loffredo L. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the sapienza university of rome-vascular assessment of proatherosclerotic effects of smoking (SUR - VAPES) 2 randomized trial. J. Am. Heart Assoc. 2019;8(6) doi: 10.1161/JAHA.118.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bahl V., Weng N.J., Schick S.F., Sleiman M., Whitehead J., Ibarra A. Cytotoxicity of thirdhand smoke and identification of acrolein as a volatile thirdhand smoke chemical that inhibits cell proliferation. Toxicol. Sci. 2016;150(1):234–246. doi: 10.1093/toxsci/kfv327. [DOI] [PubMed] [Google Scholar]
- 73.Li X., Yang H., Sun H., Lu R., Zhang C., Gao N. Taurine ameliorates particulate matter-induced emphysema by switching on mitochondrial NADH dehydrogenase genes. Proc. Natl. Acad. Sci. U. S. A. 2017;114(45):E9655–E9664. doi: 10.1073/pnas.1712465114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pickworth W.B., Rosenberry Z.R., Yi D., Pitts E.N., Lord-Adem W., Koszowski B. Cigarillo and little cigar mainstream smoke constituents from replicated human smoking. Chem. Res. Toxicol. 2018;31(4):251–258. doi: 10.1021/acs.chemrestox.7b00312. [DOI] [PubMed] [Google Scholar]
- 75.Wong M.K., Holloway A.C., Hardy D.B. Nicotine directly induces endoplasmic reticulum stress response in rat placental trophoblast giant cells. Toxicol. Sci. 2016;151(1):23–34. doi: 10.1093/toxsci/kfw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong M.K., Nicholson C.J., Holloway A.C., Hardy D.B. Maternal nicotine exposure leads to impaired disulfide bond formation and augmented endoplasmic reticulum stress in the rat placenta. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srinivasan R., Henley B.M., Henderson B.J., Indersmitten T., Cohen B.N., Kim C.H. Smoking-relevant nicotine concentration attenuates the unfolded protein response in dopaminergic neurons. J. Neurosci. 2016;36(1):65–79. doi: 10.1523/JNEUROSCI.2126-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zahedi A., Phandthong R., Chaili A., Leung S., Omaiye E., Talbot P. Mitochondrial stress response in neural stem cells exposed to electronic cigarettes. iScience. 2019;16:250–269. doi: 10.1016/j.isci.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennedy J.R., Elliott A.M. Cigarette smoke: the effect of residue on mitochondrial structure. Science. 1970;168(3935):1097–1098. doi: 10.1126/science.168.3935.1097. [DOI] [PubMed] [Google Scholar]
- 80.Clapp P.W., Lavrich K.S., van Heusden C.A., Lazarowski E.R., Carson J.L., Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316(3):L470–L486. doi: 10.1152/ajplung.00304.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lerner C.A., Rutagarama P., Ahmad T., Sundar I.K., Elder A., Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016;477(4):620–625. doi: 10.1016/j.bbrc.2016.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gannon A.M., Stampfli M.R., Foster W.G. Cigarette smoke exposure elicits increased autophagy and dysregulation of mitochondrial dynamics in murine granulosa cells. Biol. Reprod. 2013;88(3):63. doi: 10.1095/biolreprod.112.106617. [DOI] [PubMed] [Google Scholar]
- 83.Ballweg K., Mutze K., Konigshoff M., Eickelberg O., Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307(11):L895–L907. doi: 10.1152/ajplung.00180.2014. [DOI] [PubMed] [Google Scholar]
- 84.Cai Y., Deng Y., Horenkamp F., Reinisch K.M., Burd C.G. Sac1-Vps74 structure reveals a mechanism to terminate phosphoinositide signaling in the Golgi apparatus. J. Cell Biol. 2014;206(4):485–491. doi: 10.1083/jcb.201404041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu K.N., Kim H.J., Kim S., Dawaadamdin O., Lee A.Y., Hong S.H. Cigarette smoking condensate disrupts endoplasmic reticulum-golgi network homeostasis through GOLPH3 expression in normal lung epithelial cells. Nicotine Tob. Res. 2016;18(9):1877–1885. doi: 10.1093/ntr/ntw079. [DOI] [PubMed] [Google Scholar]
- 86.Schick S., Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tobac. Contr. 2005;14(6):396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schick S.F., Glantz S.A. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tobac. Contr. 2006;15(6):424–429. doi: 10.1136/tc.2006.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fetterman J.L., Pompilius M., Westbrook D.G., Uyeminami D., Brown J., Pinkerton K.E. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE(-/-) mice. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0066835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bahl V., Shim H.J., Jacob P., 3rd, Dias K., Schick S.F., Talbot P. Thirdhand smoke: chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol. Vitro. 2016;32:220–231. doi: 10.1016/j.tiv.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bahl V., Johnson K., Phandthong R., Zahedi A., Schick S.F., Talbot P. From the cover: thirdhand cigarette smoke causes stress-induced mitochondrial hyperfusion and alters the transcriptional profile of stem cells. Toxicol. Sci. 2016;153(1):55–69. doi: 10.1093/toxsci/kfw102. [DOI] [PubMed] [Google Scholar]
- 91.Cory T.J., Schacker T.W., Stevenson M., Fletcher C.V. Overcoming pharmacologic sanctuaries. Curr. Opin. HIV AIDS. 2013;8(3):190–195. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer S., Josefsson L., Coffin J.M. HIV reservoirs and the possibility of a cure for HIV infection. J. Intern. Med. 2011;270(6):550–560. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 93.Chun T.W., Davey R.T., Jr., Engel D., Lane H.C., Fauci A.S. Re-emergence of HIV after stopping therapy. Nature. 1999;401(6756):874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 94.Davey R.T., Jr., Bhat N., Yoder C., Chun T.W., Metcalf J.A., Dewar R. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brennan T.P., Woods J.O., Sedaghat A.R., Siliciano J.D., Siliciano R.F., Wilke C.O. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J. Virol. 2009;83(17):8470–8481. doi: 10.1128/JVI.02568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joos B., Fischer M., Kuster H., Pillai S.K., Wong J.K., Boni J. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl. Acad. Sci. U. S. A. 2008;105(43):16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Twigg H.L., Soliman D.M., Day R.B., Knox K.S., Anderson R.J., Wilkes D.S. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am. J. Respir. Crit. Care Med. 1999;159(5 Pt 1):1439–1444. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 98.Nakata K., Weiden M., Harkin T., Ho D., Rom W.N. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol. Med. 1995;1(7):744–757. [PMC free article] [PubMed] [Google Scholar]
- 99.Rose R.M., Krivine A., Pinkston P., Gillis J.M., Huang A., Hammer S.M. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am. Rev. Respir. Dis. 1991;143(4 Pt 1):850–854. doi: 10.1164/ajrccm/143.4_Pt_1.850. [DOI] [PubMed] [Google Scholar]
- 100.Crothers K., Butt A.A., Gibert C.L., Rodriguez-Barradas M.C., Crystal S., Justice A.C. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 101.Elbim C., Pillet S., Prevost M.H., Preira A., Girard P.M., Rogine N. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. J. Virol. 1999;73(6):4561–4566. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wanchu A., Rana S.V., Pallikkuth S., Sachdeva R.K. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Res. Hum. Retrovir. 2009;25(12):1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 103.Price T.O., Ercal N., Nakaoke R., Banks W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045(1–2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 104.Olivetta E., Pietraforte D., Schiavoni I., Minetti M., Federico M., Sanchez M. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochem. J. 2005;390(Pt 2):591–602. doi: 10.1042/BJ20042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arunagiri C., Macreadie I., Hewish D., Azad A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2(1):69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- 106.Kelly J., Beddall M.H., Yu D., Iyer S.R., Marsh J.W., Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372(2):300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y., Marsh J.W. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J. Virol. 2003;77(19):10376–10382. doi: 10.1128/JVI.77.19.10376-10382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Y., Marsh J.W. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293(5534):1503–1506. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- 109.Ensoli B., Bellino S., Tripiciano A., Longo O., Francavilla V., Marcotullio S. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PloS One. 2010;5(11) doi: 10.1371/journal.pone.0013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chelvanambi S., Bogatcheva N., Bednorz M., Agarwal S., Maier B., Alves N.J. HIV-nef protein persists in the lungs of aviremic HIV patients and induces endothelial cell death. Am. J. Respir. Cell Mol. Biol. 2018 doi: 10.1165/rcmb.2018-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ensoli B., Barillari G., Salahuddin S.Z., Gallo R.C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 112.Frankel A.D., Pabo C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 113.Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R.A. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993;67(1):277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang H.C., Samaniego F., Nair B.C., Buonaguro L., Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11(12):1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 115.Lenassi M., Cagney G., Liao M., Vaupotic T., Bartholomeeusen K., Cheng Y. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McNamara R.P., Costantini L.M., Myers T.A., Schouest B., Maness N.J., Griffith J.D. Nef secretion into extracellular vesicles or exosomes is conserved across human and simian immunodeficiency viruses. mBio. 2018;9(1) doi: 10.1128/mBio.02344-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cinnapaiyan S., Parira T., Dutta Ra M., Morris A., Nair M., Unwalla H.J. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PloS One. 2017 Jan 6;12(1):e0169161. doi: 10.1371/journal.pone.0169161. eCollection 2017.PMID: 28060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chinnapaiyan S., Dutta R., Bala J., Parira T., Agudelo M., Nair M. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci. Rep. 2018;8(1):7984. doi: 10.1038/s41598-018-26095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santerre M., Chatila W., Wang Y., Mukerjee R., Sawaya B.E. HIV-1 Nef promotes cell proliferation and microRNA dysregulation in lung cells. Cell Cycle. 2019;18(2):130–142. doi: 10.1080/15384101.2018.1557487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geraghty P., Hadas E., Kim B.H., Dabo A.J., Volsky D.J., Foronjy R. HIV infection model of chronic obstructive pulmonary disease in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312(4):L500–L509. doi: 10.1152/ajplung.00431.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cota-Gomez A., Flores A.C., Ling X.F., Varella-Garcia M., Flores S.C. HIV-1 Tat increases oxidant burden in the lungs of transgenic mice. Free Radic. Biol. Med. 2011;51(9):1697–1707. doi: 10.1016/j.freeradbiomed.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rozzi S.J., Avdoshina V., Fields J.A., Mocchetti I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Dis. 2018;4:8. doi: 10.1038/s41420-017-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodriguez-Mora S., Mateos E., Moran M., Martin M.A., Lopez J.A., Calvo E. Intracellular expression of Tat alters mitochondrial functions in T cells: a potential mechanism to understand mitochondrial damage during HIV-1 replication. Retrovirology. 2015;12:78. doi: 10.1186/s12977-015-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thangaraj A., Periyasamy P., Liao K., Bendi V.S., Callen S., Pendyala G. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14(9):1596–1619. doi: 10.1080/15548627.2018.1476810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crain M.J., Chernoff M.C., Oleske J.M., Brogly S.B., Malee K.M., Borum P.R. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J. Infect. Dis. 2010;202(2):291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masyeni S., Sintya E., Megawati D., Sukmawati N.M.H., Budiyasa D.G., Aryastuti S.A. Evaluation of antiretroviral effect on mitochondrial DNA depletion among HIV-infected patients in Bali. HIV AIDS (Auckl) 2018;10:145–150. doi: 10.2147/HIV.S166245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chinnapaiyan S., Unwalla H.J. Mucociliary dysfunction in HIV and smoked substance abuse. Front. Microbiol. 2015;6:1052. doi: 10.3389/fmicb.2015.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ande A., McArthur C., Kumar A., Kumar S. Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expet Opin. Drug Metabol. Toxicol. 2013;9(11):1453–1464. doi: 10.1517/17425255.2013.816285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel A.S., Song J.W., Chu S.G., Mizumura K., Osorio J.C., Shi Y. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grazioli S., Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front. Immunol. 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pouwels S.D., Heijink I.H., van Oosterhout A.J., Nawijn M.C. A specific DAMP profile identifies susceptibility to smoke-induced airway inflammation. Eur. Respir. J. 2014;43(4):1183–1186. doi: 10.1183/09031936.00127813. [DOI] [PubMed] [Google Scholar]
- 132.Mizumura K., Cloonan S.M., Nakahira K., Bhashyam A.R., Cervo M., Kitada T. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hansel T.T., Barnes P.J. New drugs for exacerbations of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):744–755. doi: 10.1016/S0140-6736(09)61342-8. [DOI] [PubMed] [Google Scholar]
- 134.Button B., Anderson W.H., Boucher R.C. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann. Am. Thorac. Soc. 2016;13(Suppl 2):S156–S162. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou-Suckow Z., Duerr J., Hagner M., Agrawal R., Mall M.A. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 136.Ridley C., Thornton D.J. Mucins: the frontline defence of the lung. Biochem. Soc. Trans. 2018;46(5):1099–1106. doi: 10.1042/BST20170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma J., Rubin B.K., Voynow J.A. Mucins, mucus, and goblet cells. Chest. 2018;154(1):169–176. doi: 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 138.Feng Z., Qi S., Zhang Y., Qi Z., Yan L., Zhou J. Ly6G+ neutrophil-derived miR-223 inhibits the NLRP3 inflammasome in mitochondrial DAMP-induced acute lung injury. Cell Death Dis. 2017;8(11) doi: 10.1038/cddis.2017.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iyer S.S., He Q., Janczy J.R., Elliott E.I., Zhong Z., Olivier A.K. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mao C., Zhang J., Lin S., Jing L., Xiang J., Wang M. MiRNA-30a inhibits AECs-II apoptosis by blocking mitochondrial fission dependent on Drp-1. J. Cell Mol. Med. 2014;18(12):2404–2416. doi: 10.1111/jcmm.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song R., Walentek P., Sponer N., Klimke A., Lee J.S., Dixon G. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature. 2014;510(7503):115–120. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vohwinkel C.U., Lecuona E., Sun H., Sommer N., Vadasz I., Chandel N.S. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011;286(43):37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lize M., Herr C., Klimke A., Bals R., Dobbelstein M. MicroRNA-449a levels increase by several orders of magnitude during mucociliary differentiation of airway epithelia. Cell Cycle. 2010;9(22):4579–4583. doi: 10.4161/cc.9.22.13870. [DOI] [PubMed] [Google Scholar]
- 144.Tasena H., Faiz A., Timens W., Noordhoek J., Hylkema M.N., Gosens R. microRNA-mRNA regulatory networks underlying chronic mucus hypersecretion in COPD. Eur. Respir. J. 2018;52(3) doi: 10.1183/13993003.01556-2017. [DOI] [PubMed] [Google Scholar]
- 145.Pandit K.V., Corcoran D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;182(2):220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dutta R.K., Chinnapaiyan S., Rasmussen L., Raju S.V., Unwalla H.J. A neutralizing aptamer to TGFBR2 and miR-145 antagonism rescue cigarette smoke- and TGF-beta-mediated CFTR expression. Mol. Ther. 2019;27(2):442–455. doi: 10.1016/j.ymthe.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong Y., Yoshitomi T., Hu J.F., Cui J. Long noncoding RNAs coordinate functions between mitochondria and the nucleus. Epigenet. Chromatin. 2017;10(1):41. doi: 10.1186/s13072-017-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim K.M., Noh J.H., Abdelmohsen K., Gorospe M. Mitochondrial noncoding RNA transport. BMB Rep. 2017;50(4):164–174. doi: 10.5483/BMBRep.2017.50.4.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Golec M., Reichel C., Mackiewicz B., Skorska C., Curzytek K., Lemieszek M. Cathelicidin LL-37, granzymes, TGF-beta1 and cytokines levels in induced sputum from farmers with and without COPD. Ann. Agric. Environ. Med. 2009;16(2):289–297. [PubMed] [Google Scholar]
- 151.Heijink I.H., Pouwels S.D., Leijendekker C., de Bruin H.G., Zijlstra G.J., van der Vaart H. Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release. Am. J. Respir. Cell Mol. Biol. 2015;52(5):554–562. doi: 10.1165/rcmb.2013-0505OC. [DOI] [PubMed] [Google Scholar]
- 152.Pouwels S.D., Nawijn M.C., Bathoorn E., Riezebos-Brilman A., van Oosterhout A.J., Kerstjens H.A. Increased serum levels of LL37, HMGB1 and S100A9 during exacerbation in COPD patients. Eur. Respir. J. 2015;45(5):1482–1485. doi: 10.1183/09031936.00158414. [DOI] [PubMed] [Google Scholar]
- 153.Matheson M., Rynell A.C., McClean M., Berend N. Cigarette smoking increases neutrophil formyl methionyl leucyl phenylalanine receptor numbers. Chest. 2003;123(5):1642–1646. doi: 10.1378/chest.123.5.1642. [DOI] [PubMed] [Google Scholar]
- 154.Atkinson J., Kapralov A.A., Yanamala N., Tyurina Y.Y., Amoscato A.A., Pearce L. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat. Commun. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Franks T.J., Colby T.V., Travis W.D., Tuder R.M., Reynolds H.Y., Brody A.R. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc. Am. Thorac. Soc. 2008;5(7):763–766. doi: 10.1513/pats.200803-025HR. [DOI] [PubMed] [Google Scholar]
- 156.Filippi M.D., Ghaffari S. Mitochondria in the maintenance of hematopoietic stem cells: new perspectives and opportunities. Blood. 2019;133(18):1943–1952. doi: 10.1182/blood-2018-10-808873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takubo K., Nagamatsu G., Kobayashi C.I., Nakamura-Ishizu A., Kobayashi H., Ikeda E. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12(1):49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu W.M., Liu X., Shen J., Jovanovic O., Pohl E.E., Gerson S.L. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12(1):62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lange C., Turrero Garcia M., Decimo I., Bifari F., Eelen G., Quaegebeur A. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 2016;35(9):924–941. doi: 10.15252/embj.201592372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang J., Khvorostov I., Hong J.S., Oktay Y., Vergnes L., Nuebel E. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30(24):4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen C.T., Shih Y.R., Kuo T.K., Lee O.K., Wei Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cell. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 162.Zheng X., Boyer L., Jin M., Mertens J., Kim Y., Ma L. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. 2016;5 doi: 10.7554/eLife.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang Y., Luo B., Deng Z., Wang B., Liu F., Li J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ. 2016;4 doi: 10.7717/peerj.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Anso E., Weinberg S.E., Diebold L.P., Thompson B.J., Malinge S., Schumacker P.T. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat. Cell Biol. 2017;19(6):614–625. doi: 10.1038/ncb3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Khacho M., Clark A., Svoboda D.S., MacLaurin J.G., Lagace D.C., Park D.S. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum. Mol. Genet. 2017;26(17):3327–3341. doi: 10.1093/hmg/ddx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 167.Ganuza M., Saiz-Ladera C., Canamero M., Gomez G., Schneider R., Blasco M.A. Genetic inactivation of Cdk7 leads to cell cycle arrest and induces premature aging due to adult stem cell exhaustion. EMBO J. 2012;31(11):2498–2510. doi: 10.1038/emboj.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Richardson R.B. Age-specific bone tumour incidence rates are governed by stem cell exhaustion influencing the supply and demand of progenitor cells. Mech. Ageing Dev. 2014;139:31–40. doi: 10.1016/j.mad.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 169.Boyette L.B., Tuan R.S. Adult stem cells and diseases of aging. J. Clin. Med. 2014;3(1):88–134. doi: 10.3390/jcm3010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kotton D.N., Morrisey E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20(8):822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rawlins E.L., Giangreco A. The best laid schemes of airway repair. Eur. Respir. J. 2014;44(2):299–301. doi: 10.1183/09031936.00088014. [DOI] [PubMed] [Google Scholar]
- 172.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ryan D.M., Vincent T.L., Salit J., Walters M.S., Agosto-Perez F., Shaykhiev R. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paschalaki K.E., Starke R.D., Hu Y., Mercado N., Margariti A., Gorgoulis V.G. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cell. 2013;31(12):2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brittan M., Hoogenboom M.M., Padfield G.J., Tura O., Fujisawa T., Maclay J.D. Endothelial progenitor cells in patients with chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;305(12):L964–L969. doi: 10.1152/ajplung.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Paxson J.A., Gruntman A.M., Davis A.M., Parkin C.M., Ingenito E.P., Hoffman A.M. Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cell. Dev. 2013;22(24):3214–3225. doi: 10.1089/scd.2012.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bustos M.L., Huleihel L., Kapetanaki M.G., Lino-Cardenas C.L., Mroz L., Ellis B.M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am. J. Respir. Crit. Care Med. 2014;189(7):787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rock J., Konigshoff M. Endogenous lung regeneration: potential and limitations. Am. J. Respir. Crit. Care Med. 2012;186(12):1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 180.King T.E., Jr., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 181.Chapman H.A. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 182.Chilosi M., Doglioni C., Murer B., Poletti V. Epithelial stem cell exhaustion in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2010;27(1):7–18. [PubMed] [Google Scholar]
- 183.Rockstein M., Brandt K.F. Enzyme changes in flight muscle correlated with aging and flight ability in the male housefly. Science. 1963;139(3559):1049–1051. doi: 10.1126/science.139.3559.1049. [DOI] [PubMed] [Google Scholar]
- 184.Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D.L. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Song J.W., Hong S.B., Lim C.M., Koh Y., Kim D.S. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur. Respir. J. 2011;37(2):356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 186.Janssens J.P., Pache J.C., Nicod L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999;13(1):197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 187.Miravitlles M., Soriano J.B., Garcia-Rio F., Munoz L., Duran-Tauleria E., Sanchez G. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 188.Navaratnam V., Fleming K.M., West J., Smith C.J., Jenkins R.G., Fogarty A. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66(6):462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 189.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harbor Symp. Quant. Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 190.Puente-Maestu L., Perez-Parra J., Godoy R., Moreno N., Tejedor A., Torres A. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2009;40(6):746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]
- 191.Puente-Maestu L., Perez-Parra J., Godoy R., Moreno N., Tejedor A., Gonzalez-Aragoneses F. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur. Respir. J. 2009;33(5):1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 192.Saretzki G., Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann. N. Y. Acad. Sci. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 193.Chan S.R., Blackburn E.H. Telomeres and telomerase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359(1441):109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dorshkind K., Montecino-Rodriguez E., Signer R.A. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 2009;9(1):57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 195.Kohansal R., Martinez-Camblor P., Agusti A., Buist A.S., Mannino D.M., Soriano J.B. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am. J. Respir. Crit. Care Med. 2009;180(1):3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 196.Suga T., Kurabayashi M., Sando Y., Ohyama Y., Maeno T., Maeno Y. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 2000;22(1):26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- 197.Mori T., Ishigami A., Seyama K., Onai R., Kubo S., Shimizu K. Senescence marker protein-30 knockout mouse as a novel murine model of senile lung. Pathol. Int. 2004;54(3):167–173. doi: 10.1111/j.1440-1827.2003.01603.x. [DOI] [PubMed] [Google Scholar]
- 198.Yang S.R., Wright J., Bauter M., Seweryniak K., Kode A., Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292(2):L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 199.Rajendrasozhan S., Yang S.R., Kinnula V.L., Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177(8):861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maremanda K.P., Sundar I.K., Rahman I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol. Appl. Pharmacol. 2019;385:114788. doi: 10.1016/j.taap.2019.114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Akram K.M., Patel N., Spiteri M.A., Forsyth N.R. Lung regeneration: endogenous and exogenous stem cell mediated therapeutic approaches. Int. J. Mol. Sci. 2016;17(1) doi: 10.3390/ijms17010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun Z., Li F., Zhou X., Chung K.F., Wang W., Wang J. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J. Thorac. Dis. 2018;10(2):1084–1098. doi: 10.21037/jtd.2018.01.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ng-Blichfeldt J.P., Gosens R., Dean C., Griffiths M., Hind M. Regenerative pharmacology for COPD: breathing new life into old lungs. Thorax. 2019;74(9):890–897. doi: 10.1136/thoraxjnl-2018-212630. [DOI] [PubMed] [Google Scholar]
- 204.Nyunoya T., Mebratu Y., Contreras A., Delgado M., Chand H.S., Tesfaigzi Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am. J. Respir. Cell Mol. Biol. 2014;50(3):471–482. doi: 10.1165/rcmb.2013-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Green D.R., Galluzzi L., Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333(6046):1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.DiMauro S., Hirano M. Pathogenesis and treatment of mitochondrial disorders. Adv. Exp. Med. Biol. 2009;652:139–170. doi: 10.1007/978-90-481-2813-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Koh H., Park G.-S., Shin S.-M., Park C.E., Kim S., Han S.J. Mitochondrial mutations in cholestatic liver disease with biliary atresia. 2018;8(1):905. doi: 10.1038/s41598-017-18958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D. vol. 13. 2017. pp. 498–507. (Ripk3 Induces Mitochondrial Apoptosis via Inhibition of FUNDC1 Mitophagy in Cardiac IR Injury). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heidari R., Jafari F., Khodaei F., Shirazi Yeganeh B., Niknahad H.J.N. Mechanism of Valproic acid‐induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. 2018;23(4):351–361. doi: 10.1111/nep.13012. [DOI] [PubMed] [Google Scholar]
- 211.Ren X., Keeney J.T., Miriyala S., Noel T., Powell D.K., Chaiswing L. The triangle of death of neurons: oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-α. 2019;134:1–8. doi: 10.1016/j.freeradbiomed.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lavrich K.S., Corteselli E.M., Wages P.A., Bromberg P.A., Simmons S.O., Gibbs-Flournoy E.A. Investigating mitochondrial dysfunction in human lung cells exposed to redox-active PM components. 2018;342:99–107. doi: 10.1016/j.taap.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Masayesva B.G., Mambo E., Taylor R.J., Goloubeva O.G., Zhou S., Cohen Y. vol. 15. 2006. pp. 19–24. (Mitochondrial DNA Content Increase in Response to Cigarette Smoking). 1. [DOI] [PubMed] [Google Scholar]
- 214.Cannino G., Ferruggia E., Luparello C., Rinaldi A.M.J.M. Cadmium and mitochondria. 2009;9(6):377–384. doi: 10.1016/j.mito.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 215.Pieters N., Koppen G., Smeets K., Napierska D., Plusquin M., De Prins S. Decreased mitochondrial DNA content in association with exposure to polycyclic aromatic hydrocarbons in house dust during wintertime: from a population enquiry to cell culture. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Carpagnano G.E., Lacedonia D., Carone M., Soccio P., Cotugno G., Palmiotti G. Study of mitochondrial DNA alteration in the exhaled breath condensate of patients affected by obstructive lung diseases. 2016;10(2) doi: 10.1088/1752-7155/10/2/026005. [DOI] [PubMed] [Google Scholar]
- 217.Carpagnano G.E., Lacedonia D., Malerba M., Palmiotti G., Cotugno G., Carone M. Analysis of mitochondrial DNA alteration in new phenotype. ACOS. 2016;16(1):31. doi: 10.1186/s12890-016-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu S.-F., Kuo H.-C., Tseng C.-W., Huang H.-T., Chen Y.-C., Tseng C.-C. Leukocyte mitochondrial DNA copy number is associated with chronic obstructive pulmonary disease. 2015;10(9) doi: 10.1371/journal.pone.0138716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bindu S., Pillai V.B., Kanwal A., Samant S., Mutlu G.M., Verdin E. SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial. DNA damage. 2016;312(1):L68–L78. doi: 10.1152/ajplung.00188.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. 2014;6(231) doi: 10.1126/scitranslmed.3008182. 231ra47-ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shanmugasundaram K., Nayak B.K., Friedrichs W.E., Kaushik D., Rodriguez R., Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017;8(1):997. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hoffmann R.F., Zarrintan S., Brandenburg S.M., Kol A., de Bruin H.G., Jafari S. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. 2013;14(1):97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li P., Liu Y., Burns N., Zhao K.S., Song R. SIRT1 is required for mitochondrial biogenesis reprogramming in hypoxic human pulmonary arteriolar smooth muscle cells. Int. J. Mol. Med. 2017;39(5):1127–1136. doi: 10.3892/ijmm.2017.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Faner R., Rojas M., MacNee W., Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012;186(4):306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 225.Kepp O., Galluzzi L., Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 2011;12(3):199. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- 226.Nakamura M., Ohsawa S., Igaki T. Mitochondrial defects trigger proliferation of neighbouring cells via a senescence-associated secretory phenotype in Drosophila. Nat. Commun. 2014;5:5264. doi: 10.1038/ncomms6264. [DOI] [PubMed] [Google Scholar]
- 227.Fujii S., Hara H., Araya J., Takasaka N., Kojima J., Ito S. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. OncoImmunology. 2012;1(5):630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ito S., Araya J., Kurita Y., Kobayashi K., Takasaka N., Yoshida M. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11(3):547–559. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kobayashi K., Araya J., Minagawa S., Hara H., Saito N., Kadota T. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 2016;197(2):504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 230.Sosulski M.L., Gongora R., Danchuk S., Dong C., Luo F., Sanchez C.G. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell. 2015;14(5):774–783. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sundar I.K., Maremanda K.P., Rahman I. Mitochondrial dysfunction is associated with Miro1 reduction in lung epithelial cells by cigarette smoke. Toxicol. Lett. 2019 doi: 10.1016/j.toxlet.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nickol A.H., Frise M.C., Cheng H.Y., McGahey A., McFadyen B.M., Harris-Wright T. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open. 2015;5(7) doi: 10.1136/bmjopen-2015-007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ueda N., Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10(9) doi: 10.3390/nu10091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang X., Rovin B.H. Hepcidin expression by human monocytes in response to adhesion and pro-inflammatory cytokines. Biochim. Biophys. Acta. 2010;1800(12):1262–1267. doi: 10.1016/j.bbagen.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cloonan S.M., Glass K., Laucho-Contreras M.E., Bhashyam A.R., Cervo M., Pabon M.A. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat. Med. 2016;22(2):163–174. doi: 10.1038/nm.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gattermann N., Retzlaff S., Wang Y.L., Hofhaus G., Heinisch J., Aul C. Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood. 1997;90(12):4961–4972. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.