Abstract
OBJECTIVE:
The frequency of Cushing’s disease (CD), ACTH-independent Cushing’s syndrome (CS) and autonomous cortisol secretion (ACS) in patients with obesity is not well known. Therefore, in the present study, we aimed to assess the frequency of CD, CS and ACS among the patients with obesity.
METHODS:
This study included 813 patients (683 female, mean age 46.47±14.23 yr; mean body mass index (BMI) 37.31±6.50 kg/m2). Patients with obesity were classified further to stages 1, 2, and 3, according to BMI. All patients underwent a low dose dexamethasone suppression test (LDDST). The patients with CD, CS, and ACS and patients with simple obesity were compared concerning gender, age, type-2 diabetes, hypertension (HT) and hyperlipidemia (HL).
RESULTS:
Forty-four (5.4%) out of 813 patients were diagnosed as CD, CS or ACS. CD, CS, and ACS were diagnosed in four (0.4%), two (0.2%), and 33 (4%) patients, respectively. When patients with CD, CS and ACS were compared to the patients with simple obesity, older age at diagnosis, the presence of stage-1 obesity, the presence of HT, and uncontrolled type-2 diabetes were more frequent in patients with CD, CS and ACS (p=0.001, p=0.007, p=0.004, and p=0.0026, respectively).
CONCLUSION:
The frequency of CD, CS, and ACS is high among patients with obesity. Screening for CD, CS, and ACS in patients with stage-I obesity who are older than 50 years of age with uncontrolled type-2 diabetes and HT may be a reasonable approach.
Keywords: Autonomous cortisol secretion, Cushing’s syndrome, obesity
Obesity is one of the most important health problems in the world and its prevalence has been increasing over the last several decades [1]. The prevalence of obesity worldwide has increased more than twice since 1980. Obesity may develop due to many different etiological factors, such as malnutrition, sedentary lifestyle, neuroendocrine causes, genetics and drugs. If remain untreated, it may lead to cardiovascular system (CVS) diseases and hypertension (HT), insulin resistance, and type-2 diabetes, hepatosteatosis, sleep apnea syndrome, gastrointestinal, joint and malignant diseases [2–5].
Cushing’s syndrome (CS) is a rare disease that arises from the body’s prolonged exposure to high endogenous or exogenous glucocorticoids [6]. The incidence of CS is 2–3 per million per year [7]. CS is a multisystem disorder affecting many organs and systems and causes various complications, including CVS diseases and HT, type-2 diabetes and impaired glucose tolerance, obesity, hyperlipidemia (HL), osteoporosis, coagulopathy and high susceptibility to infections [8–11]. Recently, the term ‘’autonomous cortisol secretion (ACS)’’, a condition diagnosed during the multimodal assessment of incidentally discovered adrenal messes or adrenal incidentalomas (AI), has been introduced [12]. ACS is also known as preclinical CS, subclinical CS or subclinical hypercortisolism [12]. ACS is present in up to 20% of AI and is the most common hormonal alteration in patients with AI [13]. Patients with ACS have no classical morphological phenotype of CS but have autonomous cortisol secretion, independent of ACTH secretion [13]. ACS is associated with increased frequency of obesity, metabolic syndrome, HT, prediabetes, type-2 diabetes, osteoporosis, thromboembolic disease, CVS disease, and psychiatric disease compared to non-functional AI [13–15].
Weight gain and obesity is the most frequent comorbidity accompanying endogenous hypercortisolemia and is present in 70–85% of patients with Cushing’s disease (CD), ACTH-independent CS and ACS [16–19]. Although the frequency of obesity in patients with CS and ACS has been investigated in a few studies, the number of studies investigating the frequency of CS and ACS in patients with obesity is quite low, and a wide range of results (between 0.6–9.4%) have been found [16–19]. These controversial results are probably related to the different patient populations included in the studies, the use of different tests for the diagnosis of CS and ACS, and the use of different cut-off values for cortisol after low dose dexamethasone suppression test (LDDST). Therefore, in this study, we aimed to assess the frequency of CS and ACS in patients with obesity, using the diagnostic criteria suggested by the recently published Endocrine Society and the European Endocrinology Society’s clinical practice guidelines [7, 12].
MATERIALS and METHODS
Patients
In this study, 813 consecutive subjects who admitted for obesity to our Endocrinology and Metabolism Diseases outpatient clinic between January 01, 2010, and December 31, 2015, were included. The study protocol was approved by the local ethics committee (approval no: 2015.91.08.04 approval date: 27.08.2015) and this study was conducted in accordance with the Declaration of Helsinki. In our center, the LDDST test is routinely performed in all patients admitted for obesity.
Patients with a previous diagnosis of CD, CS or ACS, patients <18 years of age, pregnant women, patients with liver and kidney failure (GFR <60 ml/min), patients using oral, parenteral or topical glucocorticoids, oral contraceptives or drugs that could induce CYP 3A4 enzymes, patients with type-1 DM, and those with a history of psychiatric illnesses, including major depression and obsessive-compulsive disorder, were not included in this study.
Obesity was diagnosed according to BMI [(BMI=body weight (kg)/height (m2)]. The patients with BMI between 30–34.9 kg/m2, 35–39.9 kg/m2 and >40 kg/m2 were classified as stage 1, 2, and 3, respectively [20].
Type-2 diabetes in this study was defined as a fasting plasma glucose (FPG) ≥126 mg/dl and an HbA1c ≥6.5% or 2. hour plasma glucose ≥200 mg/dl after a 75 gr Oral Glucose Tolerance Test (OGTT). Patients under current treatment for Type-2 diabetes, including oral antidiabetic agents, insulin or GLP-1 analogs, were also included. Patients using insulin for at least three months, either alone or in combination with oral antidiabetics who had HbA1c >7% were defined as uncontrolled type-2 diabetes [21].
Hypertension was defined as a systolic blood pressure ≥140 and/or diastolic blood pressure ≥90 mmHg, or current use of any antihypertensive drug by the patients [21].
Hyperlipidemia was defined as an LDL cholesterol level >130 mg/dl and/or a triglyceride level >150 mg/dl, or current use of statins or fibrates [22].
All patients underwent LDDST. One mg dexamethasone was administered by oral route between 23:00–24:00 pm and serum cortisol levels were assessed the following day between 08:00 and 09:00 am. Plasma ACTH levels were measured in all patients with a serum cortisol level >1.8 µg/dl after LDDST. A serum cortisol level of >1.8 µg/dl after LDDST accompanied by a plasma ACTH level of <10 pg/ml, assessed for at least twice, was defined as ACTH-independent hypercortisolism. Among patients with a plasma ACTH <10 pg/ml, patients with a clinical manifestation of CS, including central obesity, thin extremities, buffalo hump, round face, purple stria on abdominal skin, and easy bruising underwent two days 2 mg DST. A serum cortisol level of >1.8 µg after two days, 2 mg DST accompanied by a plasma ACTH level of <10 pg/mL in a patient with clinical manifestations was accepted as CS. 24-hour urine free cortisol, midnight salivary cortisol, and serum DHEASO4 levels were also assessed in patients diagnosed as CS. However, patients with a serum cortisol level of >1.8 µg/dl without clinical manifestations of CS accompanied by a plasma ACTH levels of <10 pg/mL, performed for at least twice were diagnosed as ACS. Patients with CS and those with ACS underwent upper abdominal magnetic resonance imaging (MRI) or computed tomography (CT) to detect the presence of an adrenal tumor or hyperplasia. Patients with plasma ACTH levels >10 pg/ml, assessed for at least twice, underwent two days 2 mg DST as well. A serum cortisol level >1.8 µg/dl was accepted as a positive result. Midnight salivary cortisol and 24-hour urine free cortisol levels were assessed on two separate days in these patients, and CD was diagnosed if the two of the aforementioned tests were higher than the predetermined cut-off values. These patients underwent 8 mg DST and pituitary MRI. Although patients with a history of psychiatric illnesses were not included in this study, a CRH-DST was performed to exclude the presence of pseudo-CS in patients who were newly diagnosed with a psychiatric disease such as depression, after LDDST was performed [7].
Laboratory Analysis
Blood samples of all patients were drawn through the antecubital vein after overnight fasting and were collected into a dry tube. Serum cortisol and plasma ACTH levels were measured using electrochemiluminescence immunoassay (ECLIA) method (Elecsys and Cobas Modular analytics E 710, Cobas e 601, Mannheim, Germany). Salivary cortisol was measured by the ECLIA method (Roche Cobas 8000, Tokyo, Japan). A cotton tube (Salivette, Sarstedt, Nümbrecht, Germany) was chewed for 2–3 minutes by the patient and was delivered to the laboratory in the following day. The urine free-cortisol levels were analyzed by chromatography-mass spectrometry method (Thermo Scientific TSQ Endura Triple-Stage Quadrupole Mass Spectrometer). Serum cholesterol and triglyceride levels were measured by enzymatic, colorimetric methods (Cobas c 501, Mannheim, Germany). Serum HDL cholesterol level was measured by a homogeneous colorimetric enzyme assay (Cobas c 501, Mannheim, Germany). Serum LDL cholesterol level was calculated using the formula (LDL=Total Cholesterol - Triglyceride/5 - HDL). Serum glucose levels were measured by enzymatic reference method with hexokinase (Cobas c 501, Mannheim, Germany), and Serum HbA1c level was measured by high-performance liquid chromatography and mass spectroscopy method (Adams A1C HA-8160, Koka, Japan).
Imaging Studies
The pituitary and upper abdominal MRI imaging were performed using a GE healthcare device (Optima MR360 1.5 Tesla, Milwaukee, Wisconsin, USA). Multiphasic upper abdominal CT scans were performed by an 80-line detector CT (160 slices) scanner (Aquilion Prime, Toshiba Medical Systems, Nasu, Japan).
Statistical Analysis
The statistical analysis was performed using PASW Statistics 18 for Windows statistical software package (IBM Corp., Armonk, NY). Data were expressed as mean±standard deviation, frequency, and percentages as appropriate. A chi-square test was used to compare categorical data, and an independent-sample t-test was used to compare non-categorical variables. A p-value of <0.05 was considered statistically significant.
RESULTS
A total of 813 patients [683 (84%) female, 130 (16%) male] who were diagnosed with obesity, according to the BMI, were included in this study. The characteristics of patients are shown in Table 1.
TABLE 1.
The characteristics of the 813 patients with obesity included in this study
| Mean±SD | |
|---|---|
| Age (year) | 46.47±14.63 |
| Weight (kg) | 98.44±19.03 |
| Height (cm) | 161.29±8.32 |
| BMI (kg/m2) | 37.31±6.50 |
SD: Standard deviation; BMI: Body mass index.
In this study, serum cortisol levels failed to suppress in 44 (5.4%) patients with obesity. Among these patients, four (0.4%), four (4%) and 31 (3.8%) were diagnosed as CS, CD, and ACS, respectively, while five (0.6%) patients lost to follow-up. The flowchart of the study is presented in Figure 1. CRH-DST was performed in three patients with a newly diagnosed depression, of whom, one patient was diagnosed as CD, one as CS and one as ACS. The characteristics and baseline biochemical results of the 44 patients with CD, CS, and ACS are shown in Table 2.
FIGURE 1.
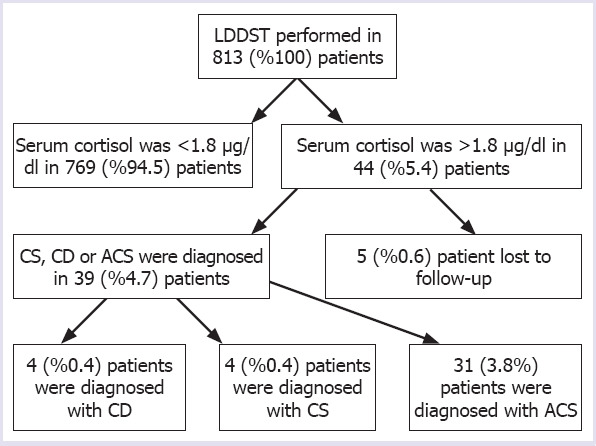
Flowchart of this study.
CD: Cushings’ disease; CS: ACTH independent Cushings’ syndrome; ACS: Autonomous cortisol secretion; LDDST: Low dose dexamethasone supression test.
TABLE 2.
Characteristics and baseline biochemical results of the 44 patients with CD, CS and ACS
| Mean±SD | |
|---|---|
| Age (year) | 60.06±11.61 |
| Weight (kg) | 87.26±14.02 |
| Height (cm) | 158.01±6.37 |
| BMI (kg/m2) | 34.40±4.22 |
| Basal cortisol (µg/dL) | 17.97±7.50 |
| LDDST (µg/dL) | 5.66±5.76 |
| 2 days 2 mg DST (µg/dL)* | 6.30±6.26 |
| ACTH (pg/ml)* | 19.16±23.24 |
| UFC (µg/24 h)* | 357.48±539.62 |
SD: Standard deviation; BMI: Body mass index; LDDST: Low dose dexamethasone supression test; DST: Dexamethasone supression test; UFC: Urinary free cortisol; CS: ACTH-independent Cushing’s syndrome; CD: Cushing’s disease; ACS: Autonomous cortisol secretion;
Performed in 39 patients.
In the present study, no significant relationship was found between patients with CD, CS or ACS and simple obesity in terms of gender and HL (p>0.05 for both comparisons). However, age, particularly >50 years, HT, and stage-1 obesity were more frequent in patients with CD, CS or ACS, as compared to patients with simple obesity (p=0.001, p=0.007, and p=0.004, respectively) (Tables 3, 4). However, the frequency of type-2 diabetes was slightly higher in patients with CD, CS or ACS compared to patients with simple obesity (p=0.038). On the other hand, the frequency of uncontrolled type-2 diabetes was significantly more common in patients with CD, CS or ACS, as compared to patients with simple obesity (p=0.026) (Tables 3, 4).
TABLE 3.
Distribution of the CD, CS and ACS in 44 patients according to age, gender and BMI
| Diagnosis | p | ||
|---|---|---|---|
| Simple obesity % | CD, CS, and ACS % | ||
| Gender | |||
| Male | 15.7 | 20.5 | 0.406 |
| Female | 84.3 | 79.5 | |
| BMI | |||
| Stage 1 | 41.1 | 61.4 | |
| Stage 2 | 26.3 | 27.3 | 0.007 |
| Stage 3 | 32.6 | 11.4 | |
| Age | |||
| 18–20 | 3.6 | 0 | |
| 21–30 | 14.4 | 0 | |
| 31–40 | 17.2 | 6.8 | |
| 41–50 | 24.7 | 9.1 | 0.001 |
| 51–60 | 23.9 | 27.3 | |
| 61–70 | 12.7 | 38.6 | |
| 71–80 | 2.9 | 13.6 | |
| >80 | 0.5 | 4.5 | |
BMI: Body mass index; CD: Cushings’ disease; CS: ACTH-independent Cushings’ syndrome; ACS: Autonomous cortisol secretion.
TABLE 4.
The frequency of comorbidities among patients with CD, CS, and ACS and simple obesity
| Comorbidity | Diagnosis | p | |
|---|---|---|---|
| Obesity % | CD, CS, ACS % | ||
| Hiperlipidemia | |||
| No | 28.4 | 26.8 | 0.831 |
| Yes | 71.6 | 73.2 | |
| Type-2 DM | |||
| No | 73.4 | 59.1 | 0.038 |
| Yes | 26.6 | 40.9 | |
| Hypertension | |||
| No | 68.6 | 47.7 | 0.004 |
| Yes | 31.4 | 52.3 | |
| Uncontrolled Type-2 DM | |||
| No | 79.3 | 62.5 | 0.026 |
| Yes | 20.7 | 37.5 | |
CD: Cushings’ disease; CS: ACTH-independent Cushings’ syndrome; ACS: Autonomous cortisol secretion.
In our study, an adrenal mass was detected by upper abdominal MRI or CT in 35 patients (4.3%) with suppressed ACTH levels. Among these patients, 12 (34.2%) patients, 12 (34.2%) patients and 11 (31.4%) patients had masses located on the right, left and bilateral adrenal glands, respectively. All patients with CD underwent endoscopic endonasal transsphenoidal surgery, while three patients with adrenal masses underwent adrenalectomy, based on the recommendations of the current guidelines [7, 12].
DISCUSSION
In this study, we assessed the frequency of CD, CS, and ACS among patients with obesity. According to results obtained from the present study, CD, CS, and ACS were diagnosed in four (0.4%), two (0.2%), and 33 (4%) patients, respectively. However, CD, CS or ACS were more frequent in patients more than 50 years old with stage 1 obesity, HT, and uncontrolled type-2 diabetes, as compared with younger obese patients without HT and those with well-controlled type-2 diabetes.
Obesity is the most common metabolic problem in developed countries. In 2013, 57.6% of people living in the United States were found to be obese or overweight. It is estimated that this rate will increase to 75% in 2020 [23]. According to 2016’s WHO data, 66.8% of the people in Turkey were overweight (64% male and 69.3% female), of whom 31.2% had obesity (24.4% of the males and 39.2% of the females) [23].
Chronic hypercortisolism is characterized by central obesity with peripheral fat tissue accumulation in the abdominal region [24]. The 11β-HSD-1 enzyme can convert the inactive glucocorticoid cortisone to the active glucocorticoid cortisol within the fat tissue. Obesity has not been observed in 11β-HSD1 inhibited mice, while 11β-HSD1 overexpression in mice has been associated with metabolic syndrome and central obesity [24]. The 11β-HSD1 expression is thought to cause differentiation in peripheral and visceral fat tissue ratio, but there are few studies in humans. However, the structure and function of visceral adipose tissue have been suggested to be different in healthy females as compared to female patients with CS [24]. In female patients with CS, an increase in adipogenesis and a decrease in lipolytic capacity have also been shown in abdominal fat cells [24]. Thus, hypercortisolism due to any cause may lead to fat accumulation and subsequent obesity.
Although obesity has been reported in 70–85% in patients with CD, CS, and ACS, the routine screening of patients with obesity for CD, CS or ACs is not recommended in the current guidelines and screening for CS has only been recommended only in patients with frank manifestations, patients with the poorly controlled type-2 diabetes and HT, progressive osteoporosis, and hypokalemia [7]. In this case, ACS, which is not associated with clinical manifestations of CS, but is associated with metabolic complications of CS is generally missed. ACS has many complications due to excessive cortisol release, as well as obesity [25]. For instance, the risk of mortality, particularly due to cardiovascular diseases, is four times higher in ACS compared to healthy people. Furthermore, the prevalence of HT, type-2 diabetes, metabolic syndrome, dyslipidemia, coagulopathy, infectious diseases, osteoporosis, and psychiatric disorders also increased in patients with ACS [25].
The frequency of CD, CS or ACS in patients with obesity has been reported between 0.6%–9.4% in different studies [16–19]. Generally, the main reason for this discordant results is the different cut-off values for cortisol after LDDST. In our study, we determined the cut-off value of cortisol after LDDST as <1.8 µg/dl, as recommended by the recent Endocrine Society and the European Society for Endocrinology’s clinical practice guidelines [7, 12]. A cut-off value of 1.8 µg/dl (50 nmol/L) for cortisol after LDDST significantly increases the sensitivity of the LDDST [12, 26]. However, this situation may lead to an increase in the rate of false-positive results and a decrease in the specificity as well [7, 12, 26]. However, the basic rule for a screening strategy is to achieve the maximum sensitivity, and more importantly, the diagnosis ACS may be missed when a cut-off value of 5 µg/dl (138 nmol/L) for cortisol after LDDST is used. Therefore, In our study, the higher frequency of CD, CS, and ACS in patients with obesity is due to the cut-off value used for cortisol levels after LDDST. In a study conducted by Tiryakioglu et al. [16], the frequency of CD and ACS in patients with obesity was 9.3%. However, in their study, 9, 3 and one patients had CD, ACS, and CS due to an adrenocortical carcinoma, respectively. However, in our study, only four of the cases were diagnosed as CD, while the majority of the cases had ACS. We believe that the reason for the high frequency of CD in the study of Tiryakioglu et al. [16] is most probably due to a more specific group of patients included in their study.
In our study, we found a significant relationship between BMI stage with CD, CS, and ACS as well. CD, CS, and ACS were more common in patients with stage 1 obesity, while stage 2 and 3 obesity were not found to be associated with CD, CS or ACS, a result that has not been found in previous studies. In contrast, some studies suggest an absence of a relationship between ACS and obesity [27]. In a study performed by Fierabracci et al. [27], the frequency of ACS was found to be 0.8% among 783 patients with obesity undergoing bariatric surgery. However, in our study, CD, CS and ACS were diagnosed in five patients (1.4%) falling into stage-3 obesity, which is almost close to the results reported by Fierabracci et al. [27]. Although weight gain and central obesity are typical for CD, CS, and ACS, many CS patients have no morbid obesity. Therefore, we believe that the lower frequency of ACS found in the study of Fierabracci et al. is due to the higher frequency of stage-3 obesity in patients included in their study. Our study results are also in line with the study conducted by the European Registry on Cushings Syndrome (ERCUSYN) [28], in which the mean BMI of the patients diagnosed with all subtypes of CS in 23 European countries was 31±7 kg/m2 [28]. The lower frequency of morbid obesity in patients CD, CS, and ACS is may be related to a four-fold increased risk of CVS diseases as well as congestive heart failure, which may lead to premature mortality [29].
In this study, the frequency of uncontrolled type-2 diabetes was significantly more common in patients with CD, CS, and ACS, as compared to patients with simple obesity, while a marginally increased frequency of type-2 diabetes was found in patients with CD, CS, and ACS compared to patients with simple obesity (Table 4). Studies indicate an increased frequency of type-2 diabetes in patients with CD, CS, and ACS [30–33]. The frequency of diabetes is high in the presence of CD, CS, and ACS, and when cases with prediabetes (impaired fasting glucose and impaired glucose tolerance) were included, the frequency may even approach to 70% [30]. Rossi et al. [31] found a significantly increased frequency of type-2 diabetes among patients with ACS. In their study, 12 out of 50 patients with adrenal incidentaloma were diagnosed with ACS, and type-2 diabetes was present in five of them (41.8%) [31]. In another study in which a cortisol cut-off value of <1.8 µg/dl after LDDST was used, the ACS frequency was more common in patients with type-2 diabetes compared to the control group (9.4% vs 2.1%, respectively) [32]. The results of our study are close to what reported in prior studies. Among the patients with CD, CS, and ACS in our study, 41 and 37.5% had type-2 diabetes and uncontrolled type-2 diabetes, respectively (Table 4). However, in a study conducted by Terzolo et al. [33], the frequency of ACS among patients with type-2 diabetes was found as 0.6%. However, they used a cut-off value of 5.0 µg/dl for cortisol after LDDST, a fact that can explain the lower frequency of ACS among patients with type-2 diabetes in their study [33]. Thus, it should be emphasized that the cut-off value for cortisol after LDDST is the main determinant of outcome during screening for any kind of endogenous hypercortisolism.
Systolic blood pressure and weight are positively correlated with each other. For each 4.5 kg gain in weight, systolic blood pressure increased by 4.4 mmHg in men and 4.2 mmHg in women in our country, a linear relationship was found between blood pressure and obesity in the TEKHARF study [34]. Framingham study data also indicate that 70% of the male and more than 60% of the female patients with HT have obesity [35–37]. Higher blood pressure values in patients with adrenal incidentaloma associated ACS compared to the control group were also reported in a study carried-out by Tauchmanova et al. [9]. In another study performed by Akaza et al. [38], the prevalence of HT was 56% among patients with ACS. Our study results also confirm the results of the previous studies. Although in our study, any degree of HT was present in 31.4% of the patients with simple obesity, HT was present in 52.3% of the patients with CD, CS, and ACS, as compared to patients with simple obesity (Table 4).
Although CD, CS, and ACS are rare in the general population, recent studies have found a higher frequency of these diseases among high-risk populations. For example, ACS frequency was found to be 8% in 423 patients with resistant HT [39]. Therefore, some authors recommend screening for CS in patients with uncontrolled type-2 DM and HT, metabolic syndrome, PCOS, osteoporosis, depression, and patients with adrenal incidentaloma [13].
In our study, no significant relationship was found between HL with CD, CS and ACS. Although some studies have found an increased prevalence of HL among patients with ACS compared to healthy subjects [9], our study included only patients with obesity. Considering the high frequency of HL in patients with obesity, the lack of a relationship is not a surprising outcome.
This study has some limitations. The most important limitation of our study was the retrospective nature of this study. We believe that this restriction is not important because all patients admitting to our center for obesity are routinely evaluated for CS by LDDST. However, it is possible that a few patients with obesity may not be assessed by LDDST. However, the strength of our study is derived from the inclusion of a large number of patients in this study with available all the aforementioned laboratory and imaging results.
Conclusion
Although the routine screening for CD, CS or ACS is not recommended in patients presenting with obesity in most of the current guidelines, the frequency of CD, CS, and ACS were high among patients with obesity in our study. CD, CS or ACS were more frequent in patients more than 50 years old with stage 1 obesity, HT, and uncontrolled type-2 diabetes, as compared with younger obese patients without HT and those with well-controlled type-2 diabetes. Therefore, screening for CD, CS, and ACS in patients with stage-I obesity who are older than 50 years old with uncontrolled type-2 diabetes and HT may be a reasonable approach.
Footnotes
Ethics Committee Approval: Namik Kemal University Clinical Research Ethics Committee granted approval for this study (date: 27.08.2015, number: 2015.91.08.04).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – SSZ; Design – SSZ; Supervision – SSZ, RVA; Fundings – BT, RVA; Materials – BT, GE; Data collection and/or processing – IY; Analysis and/or interpretation – BT, RVA; Literature review – GE, IY; Writing – RVA; Critical review – SSZ.
REFERENCES
- 1.World Health Organization. Obesity:preventing and managing the global epidemic:report of a WHO Consultation on Obesity, Geneva, 3-5 June 1997. [Accessed Mar 30, 2018]. Available at: https://apps.who.int/iris/handle/10665/63854 . [PubMed]
- 2.Bray GA. Classification and evaluation of the obesities. Med Clin North Am. 1989;73:161–84. doi: 10.1016/s0025-7125(16)30697-6. [DOI] [PubMed] [Google Scholar]
- 3.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10:498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbeck KS, Lister NB, Gow ML, Baur LA. Treatment of adolescent obesity. Nat Rev Endocrinol. 2018;14:331–44. doi: 10.1038/s41574-018-0002-8. [DOI] [PubMed] [Google Scholar]
- 5.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147–62. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 7.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing's syndrome:an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–40. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing's syndrome:state of the art. Lancet Diabetes Endocrinol. 2016;4:611–29. doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 9.Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–8. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 10.Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, et al. Subclinical Cushing's syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am. 2005;34:423–39. doi: 10.1016/j.ecl.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Di Somma C, Pivonello R, Loche S, Faggiano A, Marzullo P, Di Sarno A, et al. Severe impairment of bone mass and turnover in Cushing's disease:comparison between childhood-onset and adulthood-onset disease. Clin Endocrinol (Oxf) 2002;56:153–8. doi: 10.1046/j.0300-0664.2001.01454.doc.x. [DOI] [PubMed] [Google Scholar]
- 12.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas:European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1–34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 13.Araujo-Castro M, Sampedro Núñez MA, Marazuela M. Autonomous cortisol secretion in adrenal incidentalomas. Endocrine. 2019;64:1–13. doi: 10.1007/s12020-019-01888-y. [DOI] [PubMed] [Google Scholar]
- 14.Sbardella E, Minnetti M, D'Aluisio D, Rizza L, Di Giorgio MR, Vinci F, et al. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol. 2018;178:501–11. doi: 10.1530/EJE-17-0986. [DOI] [PubMed] [Google Scholar]
- 15.Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired Glucose Metabolism in Primary Aldosteronism Is Associated With Cortisol Cosecretion. J Clin Endocrinol Metab. 2019;104:3192–202. doi: 10.1210/jc.2019-00299. [DOI] [PubMed] [Google Scholar]
- 16.Tiryakioglu O, Ugurlu S, Yalin S, Yirmibescik S, Caglar E, Yetkin DO, et al. Screening for Cushing's syndrome in obese patients. Clinics (Sao Paulo) 2010;65:9–13. doi: 10.1590/S1807-59322010000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin M, Kebapcilar L, Taslipinar A, Azal O, Ozgurtas T, Corakci A, et al. Comparison of 1 mg and 2 mg overnight dexamethasone suppression tests for the screening of Cushing's syndrome in obese patients. Intern Med. 2009;48:33–9. doi: 10.2169/internalmedicine.48.1234. [DOI] [PubMed] [Google Scholar]
- 18.Janković D, Wolf P, Anderwald CH, Winhofer Y, Promintzer-Schifferl M, Hofer A, et al. Prevalence of endocrine disorders in morbidly obese patients and the effects of bariatric surgery on endocrine and metabolic parameters. Obes Surg. 2012;22:62–9. doi: 10.1007/s11695-011-0545-4. [DOI] [PubMed] [Google Scholar]
- 19.Ness-Abramof R, Nabriski D, Apovian CM, Niven M, Weiss E, Shapiro MS, et al. Overnight dexamethasone suppression test:a reliable screen for Cushing's syndrome in the obese. Obes Res. 2002;10:1217–21. doi: 10.1038/oby.2002.166. [DOI] [PubMed] [Google Scholar]
- 20.Nuttall FQ. Body Mass Index:Obesity, BMI, and Health:A Critical Review. Nutr Today. 2015;50:117–28. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong ND, Amsterdam EA, Ballantyne C, et al. Spotlight from the American Society for Preventive Cardiology on Key Features of the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guidelines on the Management of Blood Cholesterol. Am J Cardiovasc Drugs. 2020;20:1–9. doi: 10.1007/s40256-019-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Global Health Observatory (GHO) data. [Accessed Mar 30, 2020]. Available at: http://www.who.int/gho/ncd/risk_factors/overweight/en/
- 24.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–81. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, et al. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess:A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171:107–16. doi: 10.7326/M18-3630. [DOI] [PubMed] [Google Scholar]
- 26.Boscaro M, Barzon L, Sonino N. The diagnosis of Cushing's syndrome:atypical presentations and laboratory shortcomings. Arch Intern Med. 2000;160:3045–53. doi: 10.1001/archinte.160.20.3045. [DOI] [PubMed] [Google Scholar]
- 27.Fierabracci P, Pinchera A, Martinelli S, Scartabelli G, Salvetti G, Giannetti M, et al. Prevalence of endocrine diseases in morbidly obese patients scheduled for bariatric surgery:beyond diabetes. Obes Surg. 2011;21:54–60. doi: 10.1007/s11695-010-0297-6. [DOI] [PubMed] [Google Scholar]
- 28.Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. ERCUSYN Study Group. The European Registry on Cushing's syndrome:2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383–92. doi: 10.1530/EJE-11-0272. [DOI] [PubMed] [Google Scholar]
- 29.Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, et al. Incidence and late prognosis of cushing's syndrome:a population-based study. J Clin Endocrinol Metab. 2001;86:117–23. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 30.Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies:the impact of new treatment modalities. Acta Diabetol. 2009;46:85–95. doi: 10.1007/s00592-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 31.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, et al. Subclinical Cushing's syndrome in patients with adrenal incidentaloma:clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–8. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 32.Chiodini I, Torlontano M, Scillitani A, Arosio M, Bacci S, Di Lembo S, et al. Association of subclinical hypercortisolism with type 2 diabetes mellitus:a case-control study in hospitalized patients. Eur J Endocrinol. 2005;153:837–44. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- 33.Terzolo M, Reimondo G, Chiodini I, Castello R, Giordano R, Ciccarelli E, et al. Screening of Cushing's syndrome in outpatients with type 2 diabetes:results of a prospective multicentric study in Italy. J Clin Endocrinol Metab. 2012;97:3467–75. doi: 10.1210/jc.2012-1323. [DOI] [PubMed] [Google Scholar]
- 34.Onat A, Can G, Yüksel H, Ademoglu E, Erginel-Ünaltuna N, Kaya A, et al. TEKHARF 2017 - Tıp Dünyasının Kronik Hastalıklara Yaklaşımına Öncülük. İstanbul: Logos Yayıncılık; 2017. [Google Scholar]
- 35.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension:role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–15S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 36.Narkiewicz K. Obesity-related hypertension:relevance of vascular responses to mental stress. J Hypertens. 2002;20:1277–8. doi: 10.1097/00004872-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Sharma AM, Engeli S. Managing big issues on lean evidence:treating obesity hypertension. Nephrol Dial Transplant. 2002;17:353–5. doi: 10.1093/ndt/17.3.353. [DOI] [PubMed] [Google Scholar]
- 38.Akaza I, Yoshimoto T, Iwashima F, Nakayama C, Doi M, Izumiyama H, et al. Clinical outcome of subclinical Cushing's syndrome after surgical and conservative treatment. Hypertens Res. 2011;34:1111–5. doi: 10.1038/hr.2011.90. [DOI] [PubMed] [Google Scholar]
- 39.Martins LC, Conceição FL, Muxfeldt ES, Salles GF. Prevalence and associated factors of subclinical hypercortisolism in patients with resistant hypertension. J Hypertens. 2012;30:967–73. doi: 10.1097/HJH.0b013e3283521484. [DOI] [PubMed] [Google Scholar]


