Abstract
OBJECTIVE:
To study the clinical, laboratory, and radiological characteristics of the pediatric patients infected with the new emerging 2019 coronavirus virus (SARS-CoV-2) in Hamadan and Sanandaj, west of Iran.
METHODS:
A descriptive study was conducted in Hamadan and Kurdistan province between March 1 to April 15, 2020. Medical records of the children diagnosed as probable or confirmed cases of COVID-19 disease were extracted and analyzed in this study. We followed the WHO Guideline for the case definition of the patients.
RESULTS:
Thirty patients admitted to the wards specified for COVID-19 diseases. Nineteen (63%) patients categorized as confirmed by Real-Time Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) and 11 (37%) patients as probable according to Computed Tomography (CT) findings of the chest. Sixteen (53.3%) cases were female, the youngest patient was one day old, and the oldest patient was 15 years old. 11 (36.7%) cases had a definite history of close contact. The most common symptoms were fever, cough, and dyspnea, and the most common sign was tachypnea. None of our patients presented with a runny nose. Lymphopenia and marked elevation of the C-reactive Protein observed in four (13.3%) and 12 (40%) cases, respectively. There were 10 (33.3%) cases with normal chest X-rays. Ground-Glass Opacities (GGOs) were the most common CT findings (19, 73.1%). All but one of the patients discharged without sequala. An 11-yrs-old girl expired with a fulminant pneumonia.
CONCLUSION:
COVID-19 is not uncommon in children and could have different presentations. Concomitant use of RT-PCR and chest CT scans in symptomatic cases recommended as a modality of choice to diagnose the disease. Routine laboratory tests, like many other viral infections, may not show significant or specific changes. The superimposed bacterial infection seems not the determinant of clinical outcomes as most patients had a negative evaluation by specific laboratory tests for bacterial infections; got improved dramatically with a short or no antibiotic therapy.
Keywords: Coronavirus, COVID-19 disease, child, diagnosis, imaging, Iran
A new infectious agent belonging to the Coronaviruses group has emerged recently in Wuhan, Hubei Province, China. Its ability to spread out across the country and worldwide is surprising.[1] By the name SARS-COVID-19, World Health Organization (WHO) declared the disease as a pandemic on March 11, 2020, and a new public health emergency.[2] To date (May 4, 2020), WHO has reported more than 3,435,894 confirmed cases and 239,604 deaths, which 82,763 and 8657 belong to the last 24 hours, respectively.[3] More than 190 countries involved.
The first Iranian case of COVID-19 infection disclosed in January 2020. However, the country started reporting cases to the WHO on February 20, 2020.[4] The diseases spread all over the country very fast. According to the last report from the Iranian Ministry of Health, a total of 97,424 confirmed cases, and 6203 deaths occurred to date.[3, 5] According to WHO, Iran has the first in the Eastern Mediterranean Region and the ninth-highest prevalence rate in the world.[3]
A growing number of studies have focused on the epidemiology of COVID-19 diseases in adults. However, there is only a limited number of studies investigated the epidemiology of COVID-19 in children. Early reports from China denoted no involvement of children below 15 years old. Most cases (64.8%) occurred in peoples older than 45 years.[1, 6] However, in the succeeding reports, the number of involved children increased.[7] In a report from China among a total of 44 672 confirmed cases, 416 (0.9%) and 542 (1.2%) of the patients belonged to 0–9- and 10–19-years age groups, respectively.[8] The mortality rates in these groups were 0 and 0.2%, respectively, compared to 3.2% of the total.
The results of an epidemiologic study of COVID-19 diseases in 2143 Chinese pediatric patients have published recently in the Pediatrics journal.[7] Most pediatric cases reported to be asymptomatic or at most have mild symptoms.[9] The prevalence of asymptomatic, mild, and moderate symptoms has reported as 4.4%, 50.9%, and 38.8%, respectively. These are in contrast to adult cases that reported 17.4% as asymptomatic.[10] Moreover, 5% of the cases classified as severe due to an oxygen saturation below 92%; and 0.6% as critical with ARDS, respiratory, other organ failures, including one death in a 4-year-old patient. Although the clinical symptoms of COVID-19 disease are generally mild in children, most cases of severe or critical illness in children are less than one year old.[7] Much remain to know about epidemiology and clinical presentation of the COVID-19 infection in children despite abundant publications. Some suppose a probable role of children as asymptomatic carriers to maintain the circulation of the virus in the community.[11] The differences in immunologic maturation may be an explanation for differences in the prevalence and clinical presentation of COVID infection among adults and children.[12]
Herein, we try to present a series of pediatric patients evaluated and treated in two tertiary pediatric hospitals. We hope the presentation of atypical cases could help unveil some diversity of the clinical behavior of the COVID-19 virus. Patients hospitalized in the referral centers of Hamadan and Kurdistan provinces in the west of Iran.
MATERIALS AND METHODS
In this prospective descriptive study, the clinical and laboratory pattern of children with novel Coronavirus infection was investigated. Medical records of children aged younger than 18 years who diagnosed as confirmed or probable cases of COVID-19 disease reviewed. This study conducted in two university-affiliated hospitals in Hamadan and Sanandaj, west of Iran, from March 1 to April 15, 2020.
Informed consent was taken from all legal guardians of the patients. To inform parents or legal guardians of the child, we used a World Health Organization booklet that was written in Persian and with simple terms (http://nritld.sbmu.ac.ir/uploads/WHO-corona.pdf). This study approved by the Ethics Committee of Kurdistan University of Medical Sciences on behalf of the National Iranian Committee for Ethics in Biomedical Research with registration No. IR.MUK.REC.1399.007 (http://ethics.research.ac.ir/form/92cmt6q8v6ak7fli.pdf).
We followed the WHO Guideline for the case definition of our patients.[13] The following rules were applied.
“Suspect case
A. A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath), AND a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days before symptom onset;
OR
B. A patient with an acute respiratory illness AND having been in contact with a confirmed or probable COVID-19 case (see definition of contact) in the last 14 days before symptom onset;
OR
C. A patient with severe acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath, AND requiring hospitalization) AND in the absence of an alternative diagnosis that thoroughly explains the clinical presentation.
Probable case
A. A suspect case for whom testing for the COVID-19 virus is inconclusive.
OR
B. A suspect case for whom testing could not perform for any reason.
Confirmed case
A person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms”.[13]
All the chest images, including Computed Tomography (CT) scans and X-rays images of the patients, were reviewed by an experienced radiologist. In the case of any disagreement, the images were reviewed again by another radiologist separately. Interpretation and classification of the patients’ radiological findings were performed using the instructions of the Radiological Society of North America (RSNA) consensus statement, which were also approved by the Society of Thoracic Radiology.[14] According to this guideline, patients’ chest CT Scan findings were divided into four categories, comprising of typical, indeterminate, atypical, and negative findings for COVID-19 pneumonia. The following CT features used for classification: Ground-Glass Opacities and their locations as more specific subpleural vs. central, intralobular lines (crazy-paving), consolidations with or without adjacent halo sign, and also the presences of nodules, fine interstitial infiltrations, pleural effusion, or lymphadenopathy.[15] Specific attention was paid for the distribution of the infiltrations as unilateral or bilateral and involvement of multiple lobes. Regarding the importance of chest CT scan and radiography in the diagnosis of COVID-19, an extra definition for probable COVID-19 diseases included based on the compatible chest CT scan results according to the recommendations of Iranian Expert’s Consensus Statement.[16]
According to the Iranian national guideline of pediatrics COVID-19, the absolute lymphocytes count (ALC) considered as lymphopenia in the following conditions: ALC<3000/µL in infants (1 month to 12 months), <2000/µL in Children 1–5 years of age, and <1100/µL in children older than five years.[16] The laboratory results were interpreted based on the patient’s age and the manufacturer’s test kits. We also matched cut-offs of laboratory reference values to valid online databases.[17–20]
All data were analyzed using Stata software, version 14.2 (StataCorp, TX, USA) with a significance level of 0.05.
RESULTS
In this prospective descriptive study, a total number of 30 cases with a confirmed or probable diagnosis of COVID-19 were evaluated. Of the total cases, 19 (63%) patients were classified as confirmed cases of COVID19 and 11 (37%) patients were classified as probable cases COVID-19 according to the positive Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) result and CT-scan findings, respectively. 16 (53.3%) cases were female, the youngest patient was one day old, and the oldest case was 15 years old. The mean (±SD) and median ages of patients were six (±5) and 5.5 yrs., respectively.
Among all the cases, 23 (76.6%) cases did not have any underlying predisposing conditions, and only 11 (36.7%) cases had a definite history of close contact with the confirmed or probable COVID-19 cases.
The most common symptoms were fever, cough, and dyspnea, and the most common sign was tachypnea. None of our patients presented with a runny nose (Table 1).
TABLE 1.
Epidemiologic and clinical characteristics of the pediatric COVID-19 patients
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Girl | 16 | 53.3 |
| Boy | 14 | 46.7 |
| Age/years | ||
| 0–<1 mos. | 5 | 16.7 |
| 1–<12 mos. | 1 | 3.3 |
| 1–<3 yrs. | 5 | 16.7 |
| 3–<6 yrs. | 6 | 20 |
| 6–<12 yrs. | 4 | 13.3 |
| 12–18 yrs. | 9 | 30 |
| History of contact | ||
| With Confirmed cases | 7 | 23.3 |
| With Suspicious cases | 4 | 13.3 |
| Symptoms | ||
| Fever | 23 | 76.7 |
| Cough | 16 | 53.3 |
| Diarrhea | 3 | 10 |
| Nausea | 8 | 26.7 |
| Vomiting | 7 | 23.3 |
| Dyspnea | 20 | 66.7 |
| Sore throat | 6 | 20 |
| Rhinorrhea | 0 | 0 |
| Signs | ||
| Tachypnea | 23 | 76.7 |
| Lung crackles | 6 | 20 |
| Decrease consciousness | 4 | 13.3 |
| SpO2 | ||
| 90–93% | 5 | 16.7 |
| <90% | 12 | 40 |
| Underlying conditions | ||
| No specific underlying disease | 23 | 76.6 |
| Chronic kidney disease | 1 | 3.3 |
| Immunocompromised condition | 4 | 13.3 |
| Cerebral palsy | 2 | 6.7 |
Abnormal laboratory results include leukocytosis, lymphopenia, thrombocytopenia, thrombocytosis, and elevated ESR observed in 12 (40), four (13.3%), four (13.3%), one (3.3%) and 16 (53.3%) cases, respectively. Other laboratory findings are presented in Table 2.
TABLE 2.
Laboratory findings of 30 pediatric COVID-19 patients
| Parameter | n | % |
|---|---|---|
| WBC | ||
| 5000> | 3 | 10 |
| 5000–15000 | 15 | 50 |
| >15000 | 12 | 40 |
| Lymphocytea | ||
| Lymphopenia | ||
| <3000 for <1 yr. | 4 | 13.3 |
| <2000 1–5 yrs. | 0 | 0 |
| <1100 for >5 yrs. | 0 | 0 |
| No lymphopenia | 26 | 86.7 |
| PLTb | ||
| <150000 | 4 | 13.3 |
| 150000–450000 | 25 | 83.3 |
| >450000 | 1 | 3.3 |
| ESRc | ||
| Normal (<25) | 14 | 46.7 |
| Mild elevation (25–59) | 4 | 13.3 |
| Moderate elevation (60–99) | 6 | 20 |
| Marked elevation (>100) | 1 | 3.3 |
| N/Ad | 5 | 16.7 |
| CRPe | ||
| Negative | 7 | 23.3 |
| 1+ | 11 | 36.7 |
| 2+ | 9 | 30 |
| 3+ | 3 | 10 |
| LDHf,g | ||
| 300≥ | 0 | 0 |
| 300< | 18/18 (100) | |
| >450 | 16/18 (89) | |
| Mean (±SD) | 585 (±154) | |
| Min./Max. | 439–976 | |
| CPKh | ||
| High value | 4 | 13.3 |
| >750 | 3 | 10 |
| ALTi | ||
| >60 | 4 | 13.3 |
| ASTj | ||
| >40 | 6 | 20 |
| Real-time rt-PCR | ||
| Positive | 19 | 63 |
a: Lymphopenia was defined as <3000 for <1 yr., <2000 for 1–5 yrs., and <1100 for >5 yrs;[16] b: Platelet count; c: Erythrocyte sedimentation rate (ESR); d: N/A: Not available; e: C-reactive protein; f: LDH data were not available for 12 patients; g: Reference range lactate dehydrogenase (LDH): 0–5 yrs.: 140–304; 5–10 yrs.: 142–290; 10–15 yrs.: 115–257; >15 yrs.: 93–198 IU/L;[17] h: Reference range creatine phosphokinase (CPK): Male: 55–170, Female: 30–135, Newborn: 68–580 units/L;[18] i: Reference range alanaine transaminase (ALT): 20–60 IU/L;[19] j: Reference range aspartate transaminase (AST): Males: 6–34 IU/L, Females: 8–40 IU/L.[20]
The frequencies of various CT features of the chest were as follows: Ground-Glass Opacities (GGOs) 19 (73.1%), multiple consolidations 11 (42.3%), multiple consolidations with surrounding halo sign two (7.6%), single opacity two (7.6%), nodules 4 (15.4%), fine interstitial infiltrations five (19.2%) (Fig. 1). We also found pleural effusion in five (19.2%), and one case (3.3) of hilar lymphadenopathy; features classified as Atypical in RSNA classification. According to RSNA guidelines, the CT scans of the 13 (50%), five (19.2%), seven (26.9%), and one (3.8%) of the patients were classified as typical, indeterminate, atypical, and negative appearances for COVID-19 pneumonia, respectively. Chest CT evaluation did not perform for four newborns due to fear of radiation and as clinically not indicated.
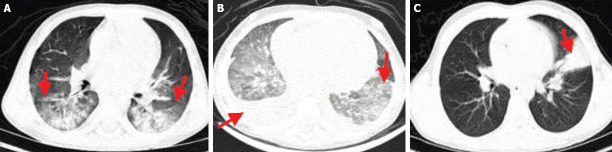
FIGURE 1.
Chest CT imaging features of the confirmed COVID-19 in children. (A) Typical appearance: Bilateral peripheral Ground Glass Opacity (GGO) and consolidation (arrow) in the chest CT scan of a 9-year-old boy. (B) Indeterminate appearance: Bilateral diffuse GGOs and non-rounded morphology associated with bilateral pleural effusion (arrow) in the chest CT scan of a 16-month-old girl. (C) Atypical appearance: Segmental consolidation in left upper lobe (arrow) without GGO or other abnormalities in the chest CT scan of an 11-year-old girl.
There were 10 (33.3%) cases with normal chest X-rays. Further evaluation revealed positive RT-PCR in seven (70%) and GGOs in CT in six (60%) of these patients.
Two newborns were evaluated for contact investigation. They bore from RT-PCR positive mothers and had no symptoms, and were clinically asymptomatic, but both had positive nasopharyngeal RT-PCR. These infants followed for two weeks and had no sequala without any specific treatments. Two more infected newborns were hospitalized with the diagnosis of Sepsis-Like Illness and Transient Tachypnea of Neonate. No specific therapy was administered, and they remained asymptomatic after discharge.
In the clinical management of the patients, other than respiratory support, the following antiviral drugs prescribed: Hydroxychloroquine 23 (76.7%), Ritonavir/Lopinavir 19 (63.3). Other administered antibiotics were ceftriaxone 17 (56.7), azithromycin two (6.7), meropenem six (20%), clindamycin three (10%), vancomycin six (20%), oseltamivir three (10%) cases.
The outcomes for hospitalized patients were evaluated. The duration of the hospitalization was between 7–10 days in 80% of the patients. Only five patients needed longer staying in the hospital. All children followed for two weeks after discharge. No case of relapse or worsening respiratory symptoms were reported. We had a case of mortality in an 11-yr-child with ALL in remission by chemotherapy. The parents of the child were from a city with high prevalence and mortalities, and both had a history of unexplained cough and fever for ten days. She referred to the hospital with early mild respiratory distress and febrile neutropenia and later diagnosis of pneumonia. No clues found for bacterial infection. The CBC showed severe neutropenia; all cultures, including two Bactec blood cultures, were negative. The child RT-PCR was negative, but she had extensive Ground Glass Opacities in the CT of both lungs. Unfortunately, she expired 48 hours after hospitalization.
DISCUSSION
In this study, we reported 30 pediatric patients diagnosed with COVID-19 infection in Hamadan and Sanandaj cities. The case definitions made according to WHO guidelines and the Iranian national Guideline for COVID-19 disease.[16] We used Real-Time RT-PCR for the diagnosis cases as confirmed and typical or highly compatible findings in CT for probable cases. In this study, 19 (63%) patients have had positive RT-PCR and classified as confirmed, and 11 (37%) cases have been classified as probable according to CT findings and negative RT-PCRs. However, three probable cases had close contacts with confirmed cases in the first-degree family members. WHO Guideline for the case definition limited the close contact with a patient with COVID-19 to the suspicious group of the case definition;[13] this is more acceptable when the reported cases from many countries were sparse. However, currently, in the high prevalence setting of COVID-19 disease, it seems in the case of negative RT-PCR, a compatible or typical CT with a history of close contact with a confirmed case should be regarded as a confirmed case definition. This is especially true when there is no other explanation for respiratory illness or distress. The RT-PCR performed on pharyngeal and nasal swabs in their best situations have yielded a low sensitivity. These figures measured 32% and 63%, respectively, when all requirements for sampling and transferring samples met done.[21]
The most common symptoms of our patients were fever, cough, and dyspnea, and the most common sign was tachypnea. None of our patients presented with a runny nose. The same findings reported by a study from China.[22] The most common presentations for COVID-19 in our pediatric cases were pneumonia. Fourteen (46.7%) cases were hospitalized as early diagnosis of lower respiratory tract infection. These classifications relayed on respiratory symptoms, mainly as dry cough and fever, respiratory distress, and chest X-rays evaluation. The early diagnosis included multilobar pneumonia (7 cases, 23.3%), bronchiolitis (two cases, 6.7%), atypical pneumonia (2 cases-6.7%), lobar pneumonia (3 cases-10%). Other diagnoses included Fever without Focus, Sepsis-Like Illness, Kawasaki disease, Encephalitis, Febrile Neutropenia, Gastroenteritis with severe dehydration, and Urinary Tract Infection. Further evaluation by RT-PCR and chest CT categorized these patients to COVID-19 disease.
Among all cases, 11 (36.7%) cases had a history of close contact with a confirmed or probable COVID-19 case. Xia et al.[22] reported that 13% of their cases had a history of close contact; however, in two other studies, close contact rate was reported as 90%.[23, 24]
The Iranian pediatric guideline for COVID-19 also recommended the administration of antibacterial drugs in patients with various severity.[16] However, our approach to COVID-19 pediatric patients relied on the severity of pneumonia, as recommended by WHO in Integrated Management of childhood illness (IMCI).[25, 26] We also added the criteria from Chest-X rays, CT, RT-PCR for risk assessment, and classifying the severity of the diseases. Accordingly, we did not prescribe antibacterial drugs routinely for all pneumonia cases. In the case of initial antibiotic therapy, the indications were reviewed shortly in 48 hours, and they discontinued to follow de-escalation therapy. We noted no complication or worsening of symptoms in carefully selected patients despite the early discontinuation of the antibiotics.
A valuable finding in the COVID-19 patients might be the association of increased CRP with lymphopenia.[9] Our result revealed lymphopenia in four (13.3%) cases, while Xia et al.[22] reported 35% lymphopenia in pediatric cases. Lymphopenia reported as a risk factor in adult COVID-19 patients; it reported as a dominant picture in non-survivors vs. survivor’s patients.[27] In a systematic review and meta-analysis of published articles, lymphopenia and increased LDH reported in 70 and 40% of the adult patients, respectively.[28] In a retrospective case series from China, lymphopenia was reported for 42% of the adult patients. We compared the association of lymphopenia, LDH, CRP, and WBC counts with clinical indices of COVID-19 disease severity, including duration of fever, tachypnea, hypoxia as measured by SpO2, persistent lungs crackles, the severity of lung involvement according to RSNA classification, duration of hospitalization, and need to respiratory support and invasive ventilation. However, we did not present data due to the low power of our study and the limited number of patients to conclude a statistically defendable inference. Nevertheless, we found no association among laboratory tests and clinical findings to assign them as risk factors for severe COVID-19 diseases. To our knowledge, reports about the prognostic significance of laboratory tests in COVID-19 disease in children are sparse, which remained under-researched. In a case series from Iran, three of nine pediatric COVID-19 patients had lymphopenia. No associations with severe diseases have been reported.[29] In a recent complete review of COVID-19 diseases in children, no prognostic importance has assigned for laboratory findings, such as lymphopenia and LDH.[30] It seems these factors have fewer essential roles in defining the severity of pediatric COVID-19 disease in children; however, more extensive studies with more cases required to assign prognostic significance of laboratory findings and deduce reliable conclusions.
CT scans of the patients with COVID-19 show different characteristics at different stages of the disease.[31] The findings in the progression of the disease generally include local subpleural GGOs, multilobar GGOs, consolidation, and an obvious crazy-paving pattern with interlobular septal thickening and fibrosis lesions.[15, 31] The CT scans of our cases revealed GGOs in 19 (73.1%), multiple consolidations in 11 (42.3%) and consolidations with surrounding halo sign in two (7.6%) of cases that are compatible with other studies and illustrated the importance of this modality for early diagnosis of pediatric COVID-19 disease. Xia et al.[22] reported that CT showed GGOs in 60% of the cases and consolidation with surrounding halo sign in 10% of the cases.
According to RSNA guidelines for COVID-19 pneumonia classification, typical, indeterminate, atypical, and negative appearances found in 13 (50%), 5 (19.2%), 7 (26.9%), and 1 (3.8%) of our patients’ CT scans, respectively. Our findings indicated the importance of CT scans for early diagnosis; however, confirmed cases with RT-PCR showed typical appearance in only 20.7% of the CT scans, which is highly suggestive for COVID-19 pneumonia and 79.7% of the CT scans showed other appearances which may consider as inconsistent with COVID-19 pneumonia.[14] Our findings may indicate high positive predictive value tor typical appearance of CT scan but low negative predictive values for other appearances, including indeterminate, atypical, or even negative appearances. Moreover, the RSNA definitions criteria for interpreting CT scans should be generalized to children with care. It seems in locations reporting community transmission of COVID-19 disease according to WHO classification,[13] and presence of a history of close contact with a COVID-19 patient, physicians should have a high clinical suspicion for COVID-19 disease in the symptomatic child, even in the presence of inconsistent atypical CT scan appearances.
In a study with 2143 pediatric patients from China, Dong et al.[7] reported only one case mortality. Most reported pediatric cases had mild or no symptoms. However, clinical presentations and courses were not explained. In another study from North of Iran, nine children presented all with mild respiratory symptoms and short clinical courses, were discharged early from hospitals.[29] The authors concluded that COVID-19 diseases in children are self-limited disease, and most do not need multiple antiviral and antibiotic therapy. Similarly, in our study, all patients were discharged without sequala except an 11-year-old girl who expired with fulminant pneumonia.
Recent studies suggest that the number of COVID-19 infections in children may be underestimated and is currently increasing.[32] The children may not be able to comply with infection control measures, and as most infections in children are asymptomatic,[7] they may have an important role in the transmission of the infection to adults. There are rationales for health-policy makers to pay attention more to the epidemiological aspect of COVID-19 in children.
Limitations of This Study
Our study is a prospective observational cross-sectional study with several limitations. First of all, it cannot help to determine a definite cause and effect relationship of proposed variables. The number of cases and the timing of snapshots was limited and may not be representative of the whole. Some information was missing in patients’ medical records. Moreover, the case definition for the probable case may not be able to differentiate between a COVID-19 infection and an active disease. In a limited number of cases, superimposed viral and bacterial infection may mimic the clinical picture of COVID-19 misleading the clinician. However, the only infection that may stimulate the COVID-19 epidemic is seasonal influenza. Seasonal influenza rarely becomes epidemic in the warm Iranian months of March and April.
Conclusion
COVID-19 is not uncommon in children and could have a variety of presentations. Early manifestations of the disease vary and should be differentiated from several common diseases in children. However, the involvement of the lower respiratory tract is a common and dominant picture. Underlying diseases may play a role in aggravating or obscuring the clinical pictures of the disease. Throat sampling is challenging to perform RT-PCR in children, especially in cases where the child is not cooperative, and there are many false negatives. CT scans are essential in diagnosing the disease in symptomatic children. Concomitant use of RT-PCR and chest CT scans in symptomatic cases recommended as a modality of choice to diagnose the disease, and in cases where chest CT is negative, the clinician should consider a carrier state or co-infections.
Differentiation of COVID-19 disease from an asymptomatic infection should be based on the concomitant consideration of clinical symptoms or signs and paraclinical diagnostic findings on PCR and CT scans. Routine laboratory tests, like many other viral infections, may not show significant or specific changes. The superimposed bacterial infection seems not the determinant of clinical outcomes as most patients had a negative evaluation by specific laboratory tests for bacterial infections; got improved dramatically with a short or no antibiotic therapy.
Acknowledgements
The authors would like to acknowledge the Research and Technology Deputies of Kurdistan and Hamadan Universities of Medical Sciences for supporting the current study. We also thank our pediatric residents, especially Dr. Shirin Behzadi, and Dr. Mehrnaz Aligholipour, Dr. Ali Akbar Mortezazadeh, and the nurses of the pediatric wards who devoted their time to the coordination of different parts of the study. The quality of this manuscript greatly enhanced by the gracious assistance of Dr. Neda Pak, who sacrificed her time for critical discussions about radiological issues.
Footnotes
Ethics Committee Approval: This study approved by the Ethics Committee of Kurdistan University of Medical Sciences on behalf of the National Iranian Committee for Ethics in Biomedical Research with registration No. IR.MUK.REC.1399.007 (date: 13.04.2020).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – JS, IS; Design – JS, IS; Supervision – JS, IS, BM, SN, ZS, GS; Fundings – IS, JS; Materials – JS, IS, ZS, GS, BM, SN; Data collection and/or processing – JS, IS, ZS, GS, BM, SN; Analysis and/or interpretation – JS, IS, ZS, GS, BM, SN; Literature review – JS, IS, ZS, GS, BM, SN; Writing – JS, IS, ZS; Critical review – JS, IS.
REFERENCES
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Technical Guidanace, Naming the coronavirus disease (COVID-19) and the virus that causes it. Geneva: World Health Organization, Council for International Classification of Diseases (ICD); [Accessed May 4, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it . [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-2019) situation reports. [Accessed Apr 23, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 4.World Health Organization. Coronavirus disease (COVID-2019) situation reports, February 20, 2020. [Accessed May 4, 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200220-sitrep-31-covid-19.pdf?sfvrsn=dfd11d24_2 .
- 5.Official:COVID-19 kills 90 more in Iran;Epidemiological Report of COVID19 Disease in Iran. Iranian Ministry of Health and Medical Education. [Accessed Apr 23, 2020]. Available at: https://en.irna.ir/news/83762127/Official-COVID-19-kills-90-more-in-Iran .
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 8.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–51. [Google Scholar]
- 9.Kilic AU, Kara F, Alp E, Doganay M. New threat:2019 novel Coronavirus infection and infection control perspective in Turkey. North Clin Istanb. 2020;7:95–8. doi: 10.14744/nci.2020.38159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406–7. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections:Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Surveillance for human infection with coronavirus disease (COVID-19) [Accessed Mar 25, 2020]. Available at: https://www.who.int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov)
- 14.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020 Apr 28; doi: 10.1097/RTI.0000000000000524. [Epub ahead of print], doi:10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Özdemir Ö. Coronavirus Disease 2019 (COVID-19):Diagnosis and Management (narrative review) Erciyes Med J. 2020:42. [Google Scholar]
- 16.Karimi A, Rafiei Tabatabaei S, Rajabnejad M, Pourmoghaddas Z, Rahimi H, Armin S, et al. An Algorithmic Approach to Diagnosis and Treatment of Coronavirus Disease 2019 (COVID-19) in Children:Iranian Expert's Consensus Statement. Arch Pediatr Infect Dis. 2020;8:e102400. [Google Scholar]
- 17.Center for Disease Control and Prevention (CDC) Laboratory Procedure Manual, Lactate Dehydrogenase (LDH) 2019. [Accessed May 4, 2020]. Available at: https://www.cdc.gov/
- 18.Junpaparp P, Staros EB. Total creatine phosophokinase (CPK), Medscape Reference Range. [Accessed May 4, 2020]. Available at: https://emedicine.medscape.com/article/2074023-overview .
- 19.Junpaparp P, Staros EB. Alanine Aminotransferase, Medscape Reference Range. [Accessed May 4, 2020]. Available at: https://emedicine.medscape.com/article/2087247-overview .
- 20.Devaraj S, Wheeler TM. Aspartate Aminotransferase, Medscape Reference Range. [Accessed May 4, 2020]. Available at: https://emedicine.medscape.com/article/2087224-overview .
- 21.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 Mar 11; doi: 10.1001/jama.2020.3786. [Epub ahead of print], doi:10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection:Different points from adults. Pediatr Pulmonol. 2020;55:1169–74. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China:an observational cohort study. Lancet Infect Dis. 2020 Mar 25; doi: 10.1016/S1473-3099(20)30198-5. [Epub ahead of print], doi:10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–6. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Recommendations for management of common childhood conditions. [Accessed May 4, 2020]. Available at: https://www.who.int/maternal_child_adolescent/documents/management_childhood_conditions/en/ [PubMed]
- 26.World Health Organization. Revised WHO classification and treatment of pneumonia in children at health facilities. [Accessed May 4, 2020]. Available at: http://apps.who.int/iris/bitstream/10665/137319/1/9789241507813_eng.pdf?ua=1 . [PubMed]
- 27.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaseghi G, Mansourian M, Karimi R, Heshmat-Ghahdarijani K, Baradaran Mahdavi S, Pezeshki A, et al. Clinical characterization and chest CT findings in laboratory-confirmed COVID-19:a systematic review and meta-analysis. medRxiv. 2020.03.05.20031518. [Google Scholar]
- 29.Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, Navaeifar MR, Enayati AA, Manafi Anari A, et al. COVID-19 Infection in Iranian Children:A Case Series of 9 Patients. Journal of Pediatrics Review. 2020;8:139–44. [Google Scholar]
- 30.Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19:An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355–68. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai WC, Zhang HW, Yu J, Xu HJ, Chen H, Luo SP, et al. CT Imaging and Differential Diagnosis of COVID-19. Can Assoc Radiol J. 2020;71:195–200. doi: 10.1177/0846537120913033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID-19 in Children in the United States:Intensive Care Admissions, Estimated Total Infected, and Projected Numbers of Severe Pediatric Cases in 2020. J Public Health Manag Pract. 2020 Apr 16; doi: 10.1097/PHH.0000000000001190. [Epub ahead of print], doi:10.1097/PHH.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]