Abstract
The study of chemical bioactivity in the rhizosphere has recently broadened to include microbial metabolites, and their roles in niche construction and competition via growth promotion, growth inhibition, and toxicity. Several prior studies have identified bacteria that produce volatile organic compounds (VOCs) with antifungal activities, indicating their potential use as biocontrol organisms to suppress phytopathogenic fungi and reduce agricultural losses. We sought to expand the roster of soil bacteria with known antifungal VOCs by testing bacterial isolates from wild and cultivated cranberry bog soils for VOCs that inhibit the growth of four common fungal and oomycete plant pathogens, and Trichoderma sp. Twenty one of the screened isolates inhibited the growth of at least one fungus by the production of VOCs, and isolates of Chromobacterium vaccinii had broad antifungal VOC activity, with growth inhibition over 90% for some fungi. Fungi exposed to C. vaccinii VOCs had extensive morphological abnormalities such as swollen hyphal cells, vacuolar depositions, and cell wall alterations. Quorum-insensitive cviR− mutants of C. vaccinii were significantly less fungistatic, indicating a role for quorum regulation in the production of antifungal VOCs. We collected and characterized VOCs from co-cultivation assays of Phoma sp. exposed to wild-type C. vaccinii MWU328, and its cviR− mutant using stir bar sorptive extraction and comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry (SBSE-GC × GC-TOFMS). We detected 53 VOCs that differ significantly in abundance between microbial cultures and media controls, including four candidate quorum-regulated fungistatic VOCs produced by C. vaccinii. Importantly, the metabolomes of the bacterial-fungal co-cultures were not the sum of the monoculture VOCs, an emergent property of their VOC-mediated interactions. These data suggest semiochemical feedback loops between microbes that have co-evolved for sensing and responding to exogenous VOCs.
Keywords: fungal pathogen antagonism, volatile organic compound (VOC), inter-kingdom communication, semiochemical communication, quorum sensing (QS), emergent properties
Introduction
The health of any soil, and therefore the productivity of the plants growing in it, is dependent on the activity and interactions of the microbes that are present (Berendsen et al., 2012; Xiong et al., 2017; Qiao et al., 2019). Culture-independent data have revealed the depth and diversity of microbial populations in the soil, rhizosphere, and phylosphere (Zou et al., 2007; Wenke et al., 2010; Blom et al., 2011; Bailly and Weisskopf, 2012; Farag et al., 2013; De Vrieze et al., 2015; Giorgio et al., 2015; Schmidt et al., 2016; Mülner et al., 2019), and there is considerable evidence that the host plant plays a role in directing the composition of those populations (Sharifi and Ryu, 2018). Additionally, microbial competition for nutritional and niche resources in these environments results in a form of chemical warfare that ostensibly facilitates the overall co-existence of bacteria and fungi in these environments (Paulitz and Bélanger, 2001; Minuto et al., 2006; Ushio et al., 2013; Sharifi and Ryu, 2018).
The full extent of the nature and influence of the compounds that serve as a means of intra- and inter-kingdom communication among and between plant-associated microbial communities is now being explored (Zhang et al., 2017; Jansson and Hofmockel, 2018). Some microbial secondary metabolites, including antibacterial and antifungal compounds, toxins and bio-surfactants (Raaijmakers et al., 2002), are water soluble and therefore diffusible within the soil/water matrix. Soluble compounds can act as semiochemical signals within the soil if there is a continuous liquid interface connecting microbial communities, and if distances are not too great (Westhoff et al., 2017). In contrast, volatile compounds can act through discontinuous systems, or at greater distances (Barr, 1976; Stotzky et al., 1976; Effmert et al., 2012). There is a long history of observations indicating that plant and soil-associated bacteria produce inorganic and organic volatile compounds with antifungal activity (Dobbs and Hinson, 1953; Barr, 1976; Zygadlo et al., 1994; Zou et al., 2007; Effmert et al., 2012; Audrain et al., 2015; De Vrieze et al., 2015; Schmidt et al., 2016; Mülner et al., 2019), and that fungi produce antibacterial volatile organic compounds (VOCs) (Effmert et al., 2012; Werner et al., 2016). Over the past two decades, investigations into the production and biological activities of VOCs have resulted in a large catalog of compounds that are synthesized by soil microorganisms, often in intriguingly complex and dynamic combinations (Mackie and Wheatley, 1999; Fernando et al., 2005; Zou et al., 2007; Korpi et al., 2009; Insam and Seewald, 2010; Effmert et al., 2012; Penuelas and Terradas, 2014; Lemfack et al., 2017; Rajer et al., 2017; Schulz-Bohm et al., 2017; Yuan et al., 2017), though little is yet known about the semiochemical interactions that are mediated by VOCs, nor how those signals are transduced.
Recently we have begun using Cranberry (Vaccinium macrocarpon Ait.; Ericaceae) as a model system for microbial interactions because it grows in bogs with relatively open, sandy soil architecture (Eck, 1990), which allows for the moderately free movement of bacteria through the soil (Soby and Bergman, 1983); the geographic proximity of cultivated and wild plants; and its importance as a cash crop in several states and provinces. Cranberry bogs are exceptionally acidic (pH ~3.6–4.3), high iron, low-nutrient environments (Gorham, 1991; Keddy, 2010), therefore, the complex multi-species microbial networks in the soil and rhizosphere that determine the health of these plants have adapted to a set of conditions that are in many respects unlike more familiar agronomically important crops. Cultivated cranberry is susceptible to a number of fungal diseases during its growth and development that can cause significant economic losses through reduced yields and postharvest rot (Oudemans et al., 1998; Oudemans, 1999; Wells-Hansen and Mcmanus, 2017). Cranberry fruit rot is caused by a complex of several fungal species, including Phoma sp. (Polashock et al., 2017), Coleophoma empetri (Rostr.) Petr., and Colletotrichum acutatum J. H. Simmonds (Halsted, 1889; Stevens, 1924; Oudemans et al., 1998; Polashock et al., 2017; Wells-Hansen and Mcmanus, 2017). Phytophthora cinnamomi is an important soil-associated oomycete that causes cranberry dieback disorder in cultivated cranberry bogs (Caruso and Wilcox, 1990; Caruso, 2000). These fungi can act separately or in concert to destroy up to 100% of annual yields in the absence of fungicide controls (Oudemans et al., 1998). In contrast, little fruit rot disease is evident in wild cranberry stands (unpublished observations), which may be due to differences in the soil and rhizospheric microbiomes of wild vs. cultivated bogs. This has prompted us to begin exploring the antifungal properties of the plant- and soil-associated bacteria of cranberry bogs.
The objective of this study was to identify cranberry bog soil bacteria that produce antifungal VOCs, and to examine their effects on fungal growth and development. After prescreening for fungal inhibition in a co-culture agar diffusion assay, we used a system that allowed gas exchange while prohibiting the exchange of soluble metabolites to test co-cultures of 21 isolates of bacteria from wild and cultivated cranberry bogs with common cranberry fungal and oomycete pathogens and one biocontrol fungus. Of the bacteria we tested, we found that the VOCs produced by Chromobacterium vaccinii generated the highest degree of fungal growth inhibition. We further characterized the chemical compositions of the inhibitory VOCs, their quorum regulation in C. vaccinii, and the morphological and chemical responses of Phoma sp. upon exposure.
Materials and Methods
Microorganisms and Culture Conditions
Bacteria used in this study were isolated from soil and plant samples collected from wild cranberry bogs in the Cape Cod National Seashore (CCNS) in Truro and Provincetown, MA, USA, and from cultivated bogs maintained by The University of Massachusetts and commercial growers in Plymouth County, MA, USA, as previously described (Soby et al., 2013). A multiyear and multiseason sampling protocol has been employed, starting in 2009, to characterize the microbiota of wild and cultivated cranberry bogs. We have used culture-independent as well as the culture-dependent strategy outlined in this manuscript and elsewhere (see, for example, Ebadzadsahrai and Soby, 2019). In this work we prescreened 68 bacterial isolates from both wild and cultivated bog soils for antifungal activity against Trichoderma sp., Phoma sp., and Colletotrichum sp. using the agar-diffusion assay (Grover and Moore, 1962), and isolates that visually inhibited the growth of any one of the fungi were selected for use in this study. Supplementary Table 1 describes the bacterial and fungal isolates used in this study.
Isolates were identified to at least the genus level by 16S rRNA gene sequencing using 27F and 1525R primers (Nicholson et al., 1994; Frank et al., 2008). Chromobacterium vaccinii strains were isolated from wild (MWU205) and cultivated (MWU328 and MWU300) cranberry bog soils in Massachusetts, and characterized as a new species (Soby et al., 2013). Spontaneous quorum sensing (QS) null mutants lacking a functional acyl-homoserine lactone receptor (CviR−; MWU205W, MWU300W, and MWU328W), and are therefore unable to produce the purple pigment violacein (Mcclean et al., 1997a,b), were isolated as white colonies during routine culture. Chromobacterium subtsugae MWU12-2387 and MWU13-2521 were isolated from wild cranberry bogs in the CCNS and identified using molecular phylogeny of the 16S rRNA gene sequences (Supplementary Figure 1). Pseudomonas chlororaphis 30-84 was obtained from the Pierson lab at Texas A&M (Yu et al., 2018). Trichoderma sp. MWU14-9201 was isolated from wild bog soil, and the berry pathogens Phoma sp. MWU-UMCS9302, Colletotrichum sp. MWU-UMCS9301, and Coleophoma sp. MWU-UMCS9305, were obtained from the University of Massachusetts Cranberry Station. Fungal identification was based on micro-morphological features and verified by 18S and ITS sequencing using the primers NS1 and NS6, and ITS1 and ITS4, respectively (White et al., 1990). Phytophthora cinnamomi R001 was provided by the USDA/ARS Corvallis, OR (Supplementary Table 1).
All bacterial isolates used for experiments were recovered from −80°C glycerol stocks, plated on Kings Medium B (KMB) agar (King et al., 1954) and grown overnight at 25°C before use. The fungi and P. cinnamomi were pre-cultured on Potato Dextrose Agar (PDA) plates and incubated for 6 days at 25°C before assays were performed.
Antifungal VOC Activity
Twenty one bacterial isolates from 13 genera and three QS mutants of C. vaccinii were tested for effects of bacterial volatile organic compounds (bVOCs) on fungal growth and hyphal development of Trichoderma sp. MWU14-9201, Phoma sp. MWU-UMCS9302, Colletotrichum sp. MWU-UMCS9301, Coleophoma sp. MWU-UMCS9305, and P. cinnamomi R001. The QS mutants of C. vaccinii were included to determine if antifungal activity is dependent on quorum sensing. Fungal isolates were grown on PDA at room temperature (25–26°C) for 24 h for Trichoderma sp., Phoma sp., and P. cinnamomi, and 96 h for Colletotrichum sp. and Coleophoma sp. to accommodate slower growth (Chaurasia et al., 2005), after which 0.5 × 0.5 cm mycelial blocks were harvested from the growing edge of the fungal colonies. Bacterial isolates were grown at 25°C on KMB overnight, then re-streaked on KMB and grown at 25°C for 72 h, after which the lid of the bacterial plate was replaced by a ‘bottom' petri dish plate containing PDA inoculated with a mycelial agar block. This arrangement, which we call a sandwich plate, allows free gas exchange between the fungus and bacterium but no physical contact or exchange of non-volatile metabolites. The plates were joined using sealing tape (Petri-Seal™ Stretch tape, RPI Corp) to contain VOCs and to hold the two petri dish bottoms together. Fungal growth controls were prepared in the same way but without bacteria or agar in the bottom plate. Sealed plate sets were incubated at room temperature and the radii of the fungal colonies were measured after 5 days. bVOC antifungal activity was calculated for each culture expressed as percentage growth inhibition (PGI) (Zygadlo et al., 1994), calculated as PGI (%) = 100 [(GC-GT)/GC], where GC (growth control) represents the mean diameter of fungi grown in PDA, and GT (growth treatment) represents the mean diameter of fungi exposed to bVOCs in the sandwich plate assay. All bacterial isolates were screened using three to four replicates prepared from one overnight culture of the bacterium and mycelial blocks harvested from one fungal culture plate. C. vaccinii isolates were assayed in more detail with three overnight cultures prepared, and each culture was tested in triplicate for fungal inhibition.
Hydrogen Cyanide Production
C. vaccinii MWU205, MWU328, MWU300, the QS mutants MWU205W, MWU300W, and MWU328W, and C. subtsugae MWU12-2387 and MWU13-2521 were grown in 50 mL KMB broth at 26°C with aeration until early stationary phase. C. subtsugae was included as a negative control for hydrogen cyanide (HCN) production based on prior unpublished results. Cell densities were measured as OD600 for normalization between isolates and replicates. Culture supernatants (1 mL) were alkalinized by the addition of 100 μL 1N NaOH (pH ≥ 11), and HCN concentrations in solution were directly measured using a cyanide probe (Lazar Research Laboratories Inc.) attached to a pH meter (Corning Inc. Corning, NY, USA) by a modification of a previously-described method (Zlosnik and Williams, 2004). Direct measurements (mV) were converted to concentration (ppm) by comparison with a standard concentration curve (R2 > 0.99). All experiments were performed at least three times.
Reversibility of Phoma sp. Growth Inhibition
To determine if the inhibition of growth by C. vaccinii bVOCs is reversible, the fast-growing Phoma sp. was grown alone or with C. vaccinii MWU328 or MWU328W in sandwich plates at room temperature, as described above. After 5 days of co-culture, the radii of the fungal colonies were measured and then the sandwich plates were separated from each other and the plates containing bacteria were replaced with fresh lids without bacteria or agar. Fungi were then incubated for an additional 4 days at room temperature, and the radii of the fungal colonies were measured. Growth rates during and after co-culture with bacteria were compared with negative controls that did not contain bacteria.
Light and Transmission Electron Microscopy (TEM)
Mycelial margins of actively growing fungal cultures were excised after 120 h monoculture or co-culture in the sandwich plate format and stained with lactophenol-cotton blue to record hyphal development using light microscopy (Olympus CX41 and CellSens Entry imaging software, Hamburg, Germany). For TEM thin sectioning, hyphal apices of actively growing Phoma sp. in the presence or absence of C. vaccinii MWU328 were excised from colony margins and initially fixed for 2 h at 4°C in 0.1 M potassium phosphate buffer (pH 7.2) with 2% glutaraldehyde and post-fixed at 4°C in 1% osmium tetroxide in the same buffer for 2 h. Overnight en bloc staining was performed in 0.5% aqueous uranyl acetate at 4°C. Samples were dehydrated in a graded ethanol series and transitioned to propylene oxide, then infiltrated with Spurr's epoxy resin (Ann Ellis, 2018), and polymerized at 60°C for 36 h. Sections were cut to 70 nm with a Leica Ultracut-R microtome and mounted on formvar-coated copper slot grids. Grids were post-stained with 2% uranyl acetate in 50% ethanol for 8 min, followed by Sato's lead citrate (Hanaichi et al., 1986) for 4 min. Images were generated using a Philips CM12 TEM at 80 kV and acquired with a Gatan model 791 slow-scan CCD camera (1024 × 1024 pixel resolution).
VOC Collection and Analysis by SBSE-TD-GC × GC-TOFMS
The sandwich plate was modified to capture bacterial and fungal VOCs using stir-bar sorptive extraction (SBSE; Figure 1). Molten KMB agar was poured into a 100 × 21 mm petri dish, and an empty 35 mm petri dish was aseptically embedded in the KMB agar. After cooling, bacteria were spread on the KMB around the smaller petri dish, grown for 24 h, and three polydimethylsiloxane/ethylene glycol-coated SBSE stir bars (32 μl phase volume, 10 mm length; Twister®, Gerstel, US) were aseptically placed in the smaller dish to collect VOCs in technical replicates. The lid of the bacterial plate was replaced by a “bottom” petri dish plate containing PDA inoculated in the center with a mycelial agar block (Chaurasia et al., 2005). The two plates were then sealed with petri-seal tape and incubated at room temperature for 120 h, when the stir bars were removed and placed in individual vials, and stored at 4°C prior to analysis. Each bacterial and fungal monoculture and co-culture combination was independently assayed in six biological replicates.
Figure 1.

Sandwich plate device modified for sampling VOCs.
VOCs collected on the SBSE stir bars were analyzed via thermal desorption (TD; Gerstel MPS Robotic, Maestro software version 1.5.3.2, Linthicum Heights, MD) coupled with comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry (GC × GC-TOFMS; LECO Pegasus 4D and Agilent 7,890 GC, ChromaTOF software version 4.71, LECO Corporation, St. Joseph, MI). The stir bars were dry purged under helium at 2 mL/min for 20 min at 50°C, then desorbed at 220°C for 5 min. Desorbed VOCs were transferred to the cooled injection system with a transfer line temperature of 240°C and cryofocused onto an unpacked glass inlet liner at −80°C. VOCs were injected at 275°C for 3 min, without split. Chromatographic analysis was performed using a 2D column set consisting of an Rxi-624Sil (60 m × 250 μm × 1.4 μm (length × internal diameter × film thickness); Restek, Bellefonte, PA) as the first dimension column, and a Stabilwax (1.3 m × 250 μm × 0.5 μm; Restek) as the second dimension column. The primary oven was initiated at 35°C for 0.5 min, ramped at a rate of 5°C/min to 230°C, and held at that temperature for 5 min. The secondary oven and modulator were maintained at a +5°C and +20°C offset from the primary oven, respectively. A 2 s modulation period (alternating 0.5 s hot and cold pulses) was used with helium as the carrier gas at a flowrate of 2 mL/min. The TOFMS was operated as follows: electron impact at −70 eV; acquisition range: 35–400 m/z; acquisition rate: 100 spectra/s; and ion source temperature: 250°C. An external alkane standards mixture (C8-C20; Sigma-Aldrich, St. Louis, MO), was sampled multiple times for use in determining retention indices. The injection, chromatographic, and mass spectrometric methods for analyzing the alkane standards was as described above for SBSE sample analyses.
Processing and Analysis of Chromatographic Data
Data collection, processing, and alignment were performed using ChromaTOF software version 4.71 with the Statistical Compare package (Leco Corp.). For peak identification, the baseline was set through the middle of the noise, and the signal-to-noise (S/N) cutoff for the initial peak finding was set to 50 for a minimum of two apexing masses. Subpeaks were combined when the mass spectral match score was ≥ 600 (out of 1,000) and the second dimension retention time shift was ≤ 100 ms for subsequent modulation periods.
Peak alignment was performed using the Statistical Compare feature of ChromaTOF. For the alignment of peaks across chromatograms, the maximum first and second dimension retention time deviations were set at 6.0 and 0.2 s, respectively, and the inter-chromatogram spectral match threshold was set at 600. A second round of peak discovery was performed using a reduced S/N of 5 to include low-abundance peaks that appeared in at least one chromatogram at S/N ≥ 50. Peaks were identified by forward and reverse searches of the NIST 2011 library. Peaks were assigned a putative identification based on mass spectral similarity and retention index (RI) data. Peaks with a level 2 or level 3 identification (Sumner et al., 2007) have ≥ 800 mass spectral match by a forward search of the NIST 2011 library. Level 2 peaks also have RIs that are consistent with the midpolar Rxi-624Sil stationary phase, as previously described (Bean et al., 2016), but using a modified RI range of 0–43%. Level 2 and 3 compounds are assigned to chemical functional groups based upon characteristic mass spectral fragmentation patterns and second dimension retention times. Level 4 compounds do not have mass spectral or RI data of sufficient quality for identification, and are reported as unknowns.
Statistical Analyses
Differences between fungal growth inhibition from wild type vs. QS mutant isolates of C. vaccinii were statistically analyzed in each targeted fungus using Kruskal–Wallis and Mann–Whitney U-tests [Statistical Package for the Social Sciences (SPSS) 7 software] and an alpha of 0.05. All statistical analyses of metabolomics data were performed using R version 3.5.3 (R Foundation for Statistical Computing). Before statistical analyses, compounds eluting prior to 358 s (acetone retention time) and siloxanes (i.e., chromatographic artifacts) were removed from the peak table. The relative abundance of compounds across chromatograms was normalized using probabilistic quotient normalization (PQN) (Dieterle et al., 2006). Intraclass correlation coefficients (ICCs) were calculated, using R ICC package version 2.3.0, on samples with three technical replicates; peaks with an ICC <0.4 were not further processed. Analytes were retained for further analysis if the arithmetic means of sample peak areas were two-fold greater in any sample compared to the media-only controls, and significantly greater in abundance using Welch's t-test with an alpha of 0.05. Geometric means of the technical replicates were calculated and used for all subsequent comparisons. Principal component analysis (PCA) was performed with the biological replicates as observations and the absolute peak intensities (mean-centered and scaled to unit variance) as variables.
Results
Preliminary Evaluation of the Effect of VOCs on the Growth of Fungi
We used an agar-diffusion assay to prescreen 68 bacterial isolates from wild and cultivated cranberry bogs for antifungal activity, and identified 21 bacterial isolates representing 13 genera that could inhibit the growth of Trichoderma sp., Phoma sp., and Colletotrichum sp. (data not shown). These 21 isolates were analyzed for antifungal bacterial volatile organic compounds (bVOC) activity using a co-cultivation method that restricts microbial interactions to the gas phase. We quantified antifungal activity by measuring radial growth inhibition of bVOC-exposed Trichoderma sp., Phoma sp., Colletotrichum sp., Coleophoma sp., and P. cinnamomi colonies compared to unexposed controls. Pseudomonas chlororaphis 30–84 was used as a positive control since it is known to produce antifungal VOCs (Popova et al., 2014). The bacterial isolates varied in antifungal activity between fungi, and fungi responded to each bacterium differently (Table 1). Trichoderma sp., Phoma sp., Colletotrichum sp., Coleophoma sp., and P. cinnamomi exposed to bVOCs displayed mean radial growth inhibition ranging from 3 to 97%, in comparison with the no-bacteria controls. The radial growth of Colletotrichum sp. was inhibited by most of the bacteria, but even in the presence of Burkholderia cepacia, Xylophilus ampelinus, Ewingella americana, Serratia marcescens, and Delftia sp., which did not inhibit radial growth, the mycelium was altered such that the development of normal, high-density hyphae was restricted to near the inoculum block, and only low-density hyphae were evident beyond the inoculation site (data not shown). The greatest fungal inhibitions were a result of Chromobacterium spp. VOCs, particularly C. subtsugae MWU13-2521 and C. vaccinii MWU205, which inhibited Coleophoma sp. growth by 96 and 97%, respectively, and Phoma sp. by 63 and 82%, respectively. In comparison, the P. chlororaphis positive control inhibited the radial growth of the five tested fungi between 10 and 39% (Table 1).
Table 1.
The percentage of growth inhibition of fungi due to exposure to bacterial VOCs vs. the growth control (i.e., negative control).
| Percentage of fungal growth inhibition | |||||
|---|---|---|---|---|---|
| Bacterial species and strains | Trichoderma sp. | Phoma sp. | Colletotrichum sp. | Coleophoma sp. | Phytophthora cinnamomi |
| Pseudomonas chlororaphis 30–84 | 23 ± 8 | 39 ± 8 | 10 ± 1 | 39 ± 3 | 27 ± 4 |
| Bacillus thuringiensis MWU12-2420 | 3 ± 3 | 25 ± 6 | 4 ± 5 | 33 ± 6 | 29 ± 2 |
| Bacillus cereus MWU14-2326 | 8 ± 6 | 20 ± 2 | 14 ± 10 | 18 ± 11 | 7 ± 1 |
| Aquitalea sp. MWU14-241 | 15 ± 12 | 36 ± 10 | 4 ± 9 | 42 ± 3 | 36 ± 4 |
| Pseudomonas sp. MWU13-2590 | 34 ± 19 | 71 ± 4 | 9 ± 3 | 59 ± 4 | 28 ± 4 |
| Pseudomonas sp. MWU12-2517 | 14 ± 5 | 23 ± 2 | 20 ± 14 | 31 ± 5 | 39 ± 3 |
| Pseudomonas sp. MWU15-20650 | 21 ± 4 | 57 ± 5 | 24 ± 6 | 42 ± 2 | 20 ± 7 |
| Burkholderia cepacia MWU13-2092 | 18 ± 4 | 14 ± 7 | −2 ± 7 | 45 ± 6 | 34 ± 3 |
| Burkholderia tropica MWU12-2056 | 34 ± 19 | 33 ± 5 | 31 ± 8 | 52 ± 6 | 50 ± 3 |
| Xylophilus ampelinus MWU14-20187 | 21 ± 7 | 39 ± 6 | −16 ± 2 | 49 ± 22 | 14 ± 2 |
| Acinetobacter calcoaceticus MWU13-2536 | 9 ± 8 | 46 ± 12 | 4 ± 19 | 59 ± 2 | 22 ± 5 |
| Ewingella americana MWU14-20116 | 6 ± 4 | 30 ± 5 | −2 ± 11 | 50 ± 12 | 35 ± 4 |
| Serratia marcescens MWU13-2543 | 9 ± 3 | 35 ± 8 | −1 ± 8 | 35 ± 6 | 23 ± 3 |
| Lysinibacillus sp. MWU14-2414 | 25 ± 9 | 16 ± 5 | 4 ± 7 | 41 ± 2 | 19 ± 4 |
| Paenibacillus sp. MWU13-2602 | 3 ± 5 | 15 ± 2 | 7 ± 13 | 33 ± 3 | 14 ± 2 |
| Enterobacter sp. MWU13-2507 | 11 ± 7 | 28 ± 6 | 10 ± 8 | 48 ± 3 | 30 ± 0 |
| Delftia sp. MWU13-3324 | 13 ± 2 | 32 ± 10 | −13 ± 4 | 69 ± 1 | 22 ± 1 |
| Chromobacterium subtsugae MWU13-2521 | 21 ± 8 | 63 ± 9 | 7 ± 1 | 96 ± 4 | 5 ± 3 |
| Chromobacterium vaccinii MWU205 | 23 ± 5 | 82 ± 4 | 20 ± 10 | 97 ± 3 | 18 ± 1 |
| Chromobacterium vaccinii MWU300 | 16 ± 4 | 81 ± 2 | 10 ± 3 | 77 ± 4 | 26 ± 4 |
| Chromobacterium vaccinii MWU328 | 16 ± 5 | 81 ± 3 | 25 ± 6 | 92 ± 4 | 27 ± 0 |
Values represent mean ± standard error of the mean from three to four replicates.
Antifungal Activity of C. vaccinii VOCs
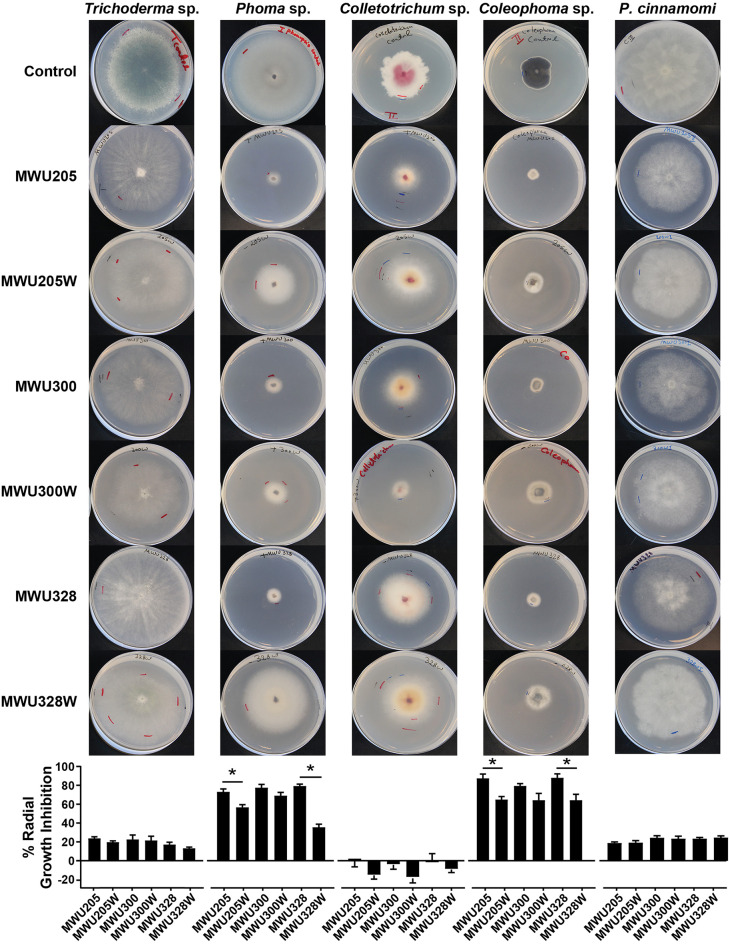
Due to its high levels of fungal inhibition and broad activity against the fungi and oomycete we tested, we selected the recently characterized Gram-negative bog soil β-proteobacterium C. vaccinii (Soby et al., 2013) for further study. This species is notable for its deep purple pigmented phenotype (Soby et al., 2013) and for its ability to kill Aedes sp. mosquito larvae in vitro (Martin and Soby, 2016), with both phenotypes regulated by quorum sensing (QS). We assayed fungal growth inhibition by the VOCs of C. vaccinii wild-type isolates MWU205, MWU300, and MWU328 and their QS-deficient (CviR−) mutants MWU205W, MWU300W, and MWU328W against Trichoderma sp., Phoma sp., Colletotrichum sp., Coleophoma sp., and P. cinnamomi (Figure 2). Radial growth inhibition in the presence of wild-type C. vaccinii MWU205, MWU300, and MWU328 was most pronounced in the fruit pathogens Phoma sp. (74–79%) and Coleophoma sp. (80–88%; Figure 2). The foliar pathogen Colletotrichum sp. was not inhibited by these bacteria as assayed by radial growth, and Trichoderma sp. and P. cinnamomi, two species normally found in the soil, were inhibited by a much smaller amount (13–43%). The QS mutants reduced radial growth of Phoma sp. and Coleophoma sp., but far less than their cognate wild-type strains, with significant differences in all combinations except for the MWU300 and MWU300W pair (Figure 2). There were no significant differences in radial growth between incubation with wild type and QS mutant strains in Trichoderma sp., Colletotrichum sp., or P. cinnamomi, likely because of the small amount of inhibition associated with wild type bVOC production.
Figure 2.
Morphological and fungistatic effects of bVOCs produced by Chromobacterium vaccinii. Wild-type isolates MWU205, MWU300, and MWU328, and quorum sensing mutants MWU205W, MWU300W, and MWU328W were assayed for activity of bVOCs against four fungal isolates from cranberry bogs, Trichoderma sp., Phoma sp., Colletotrichum sp., and Coleophoma sp., and the oomycete Phytophthora cinnamomi. Error bars indicate standard error of the mean (SEM). *p ≤ 0.05.
C. vaccinii bVOCs also affected the colony gross morphology of the exposed fungi. For example, Trichoderma sp., Colletotrichum sp., and Coleophoma sp. showed a reduction in pigmentation in the presence of bVOCs (Figure 2). Although we did not assign pigmentation differences to specific cell types, some of the loss of pigment may have been due to reduction in sporulation, but some was clearly associated with mycelium. For example, Colletotrichum sp. strain MWU-UMCS9301 does not sporulate well on PDA, but there was a visible reduction in pigmentation on that medium. The radial growth of Trichoderma sp., Colletotrichum sp., and P. cinnamomi was not significantly inhibited, and in some cases slightly promoted, but mycelium density was noticeably reduced (Figure 2), suggesting that bVOCs had an effect that resulted in hyphal tip hypertrophy at the expense of normal development. Structural deformations have previously been observed in phytopathogenic fungi exposed to bVOCs (Moore-Landecker and Stotzky, 1973; Chaurasia et al., 2005; Giorgio et al., 2015). In order to evaluate the effect of bVOCs on fungal cellular structures, light microscopy was performed on hyphae of Phoma sp., Colletotrichum sp., Coleophoma sp., and P. cinnamomi after exposure to C. vaccinii MWU328 bVOCs (Figure 3). We selected MWU328 for this analysis because it demonstrated antifungal activity against all fungi and oomycete tested, but not complete growth inhibition. In Coleophoma sp., hyphal cell membranes had pulled away from the cell wall, and there was a higher than normal proportion of empty cells in treated (Figures 3H,I) vs. control hyphae (Figure 3G). There was abnormal swelling and deformation of hyphae in Phoma sp., Colletotrichum sp., and P. cinnamomi (Figures 3B,C,E,F,K,L), as well as an absence of sporangia in P. cinnamomi.
Figure 3.
Morphological changes in Phoma sp. (A–C), Colletotrichum sp. (D–F), Coleophoma sp. (G–I) and Phytophthora cinnamomi (J–L), by bVOCs produced by C. vaccinii MWU328. The column ADGJ represents untreated hyphae for each of the fungi. Columns BEHK and CFIL show hyphae from each of the fungi after exposure to bVOCs. (B,D,G,K) Are shown at 40x magnification, and (A,C,E,F,H,I,J, L) are shown at 100x.
VOC Interactions Between C. vaccinii and Phoma sp.
To examine the effects of antifungal bVOC exposure in greater detail, we selected C. vaccinii MWU328, C. vaccinii MWU328W, and Phoma sp. MWU-UMCS9302 for additional analyses. We selected these isolates because Phoma sp. is fast growing, and the wild-type C. vaccinii strain MWU328 did not fully inhibit its growth, which facilitated investigations of fungal morphology, fungal VOCs (fVOCs), and the reversibility of growth inhibition when C. vaccinii VOCs are removed.
Phoma sp. Cellular Structural Abnormalities Caused by C. vaccinii bVOCs
Phoma sp. either exposed or unexposed to C. vaccinii MWU328 bVOCs was examined by transmission electron microscopy (Figure 4). The cytoplasm of bVOC-exposed cells was highly vacuolated with the inclusion of large dark-staining bodies (Figures 4C,E,F), and the presence of abnormally large and disorganized Spitzenkörper (Figure 4D), which are not apparent in untreated control hyphal tips (Figures 4A,B). Treated mycelia were also characterized by the swelling of individual cells (Figures 4E,F), and a dark-staining material of unknown composition associated with the external surface of cell walls (Figure 4F). Untreated controls had an average cell wall thickness of 1.04 mm (SD = 0.23), and treated mycelia had an average cell wall thickness of 1.50 mm (SD = 0.49; p = 0.0002).
Figure 4.
Ultrastructural analysis of Phoma sp. mycelia exposed to the bVOCs of C. vaccinii MWU328 (C–F). Phoma sp. mycelia not exposed to bVOCs were used as controls (A,B).
Reversibility of Phoma sp. Growth Inhibition by C. vaccinii VOCs
To determine if the inhibition of growth by C. vaccinii bVOCs is reversible, we measured the growth of Phoma sp. during and after exposure to MWU328 and MWU328W bVOCs in the sandwich plate assay. Phoma sp. growth rates were significantly different after 5 days exposure to C. vaccinii bVOCs compared to the control (p = 3 × 10−10). Phoma sp. grew at an average rate of 0.5 mm/day in the control compared to 0.1 mm/day in the presence of wild type MWU328, and 0.4 mm/day in the presence of QS mutant MWU328W. After the bacteria cultures and control blank agar were replaced with empty petri plate lids on day five, Phoma sp. that had been previously exposed to MWU328 and MWU328W bVOCs grew at the same rate as the control (MWU328 vs. control, p = 0.083; MWU328W vs control, p = 0.074), showing that the growth inhibition is reversible at the bVOC concentrations produced in these experiments.
Characterizing the VOCs of C. vaccinii and Phoma sp. by SBSE-GC × GC-TOFMS
Stir bar sorptive extraction and comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry (SBSE-GC × GC-TOFMS) was used to collect and analyze the VOCs produced by C. vaccinii strain MWU328 and its less-fungistatic QS mutant MWU328W in the presence or absence of Phoma sp. MWU-UMCS9302. The VOCs of Phoma sp. in monoculture and of media-only controls were also analyzed by SBSE-GC × GC-TOFMS (Supplementary Figure 2). After data alignment and removal of chromatographic artifacts, 1,931 non-redundant peaks were detected across the 84 samples, which included six biological replicates of each experimental combination. The peak list was then filtered to include only compounds that were detected in reproducible intensities across biological replicates and present in the biological samples in at least two-fold greater concentration relative to media controls. This step removed 1,878 peaks. The remaining 53 peaks were relatively evenly distributed across the five microbial samples (Table 2, Supplementary Table 2). Based upon mass spectral and chromatographic data, we were able to assign putative identities to eight VOCs, which included known bioactive compounds such as dimethyl disulfide, dimethyl trisulfide, indole, 1-octanol, and octanoic acid (Kai et al., 2009; Schulz et al., 2010; Forlani et al., 2011; Groenhagen et al., 2013; Giorgio et al., 2015; Lo Cantore et al., 2015). For the unnamed compounds, we were able to assign chemical classifications to 30 of them. The remaining 15 VOCs are classified as unknowns, due to a lack of mass spectral or chromatographic data of sufficient quality for putative identification.
Table 2.
Volatile metabolites that are significantly more abundant than blank media as a function of sample type.
| Compound ID | Cv | P-Cv | P | P-CvW | CvW |
|---|---|---|---|---|---|
| Thiazole | ↓- | ||||
| Disulfide, dimethyl | -↑ | ↑- | |||
| UNK-3 | -↓ | ↓- | |||
| CA-4 | |||||
| CA-5 | -↓ | ||||
| UNK-6 | -↓ | ||||
| HC-7 | |||||
| HC-8 | |||||
| OTH-9 | |||||
| 1,2-Ethanediol, diacetate | -↓ | ||||
| S,N-11 | ↓- | ||||
| Dimethyl trisulfide | ↓- | ↑- | |||
| N-13 | |||||
| CA-14 | |||||
| N-15 | |||||
| EST-16 | -↓ | ||||
| 1-Octanol | ↓- | ||||
| CA-18 | |||||
| S-19 | |||||
| UNK-20 | |||||
| UNK-21 | |||||
| UNK-22 | |||||
| ETH-23 | |||||
| Octanoic acid | |||||
| CA-25 | -↓ | ||||
| HC-26 | |||||
| UNK-27 | |||||
| UNK-28 | ↓↓ | ↓- | |||
| ETH-29 | -↓ | ||||
| ETH-30 | -↓ | ↓- | |||
| ETH-31 | |||||
| Indole | |||||
| EST-33 | ↑- | -↑ | |||
| EST-34 | |||||
| UNK-35 | |||||
| UNK-36 | |||||
| UNK-37 | ↑↑ | ||||
| EST-38 | -↓ | ↓- | |||
| ETH-39 | |||||
| T-40 | |||||
| UNK-41 | -↓ | ||||
| N-42 | |||||
| UNK-43 | |||||
| ARO-44 | |||||
| ARO-45 | -↓ | ↓- | |||
| α-Calacorene | |||||
| UNK-47 | |||||
| EST-48 | ↑- | -↑ | |||
| UNK-49 | ↑- | ↓- | |||
| ETH-50 | |||||
| ARO-51 | |||||
| UNK-52 | ↑- | -↑ | |||
| N-53 | |||||
| C. vaccinii MWU328 monocultures (Cv) | |||||
| Phoma sp., MWU328 co-cultures (P-Cv) | |||||
| Phoma sp. monocultures (P) | |||||
| Phoma sp., MWU328W co-cultures (P-CvW) | |||||
| C. vaccinii MWU328W monocultures (CvW) |
The numbers of replicates the VOCs were detected in are indicated by the color saturation. In co-cultures, the hypothesized source of the VOCs is indicated by color, and arrows indicate which peak intensity significantly (p <0.05) increased (↑) or decreased (↓) in co-culture compared to the two constituent monocultures; a dash (-) indicates no change. Functional classes include: ARO, aromatic hydrocarbon, CA, carboxylic acid, EST, ester, ETH, ether, HC, hydrocarbon, N, nitrogen containing, OTH, other, S, sulfur containing, T, terpene, UNK, unknown. Additional compound information and the peak intensities measured in each experimental condition are provided in Supplementary Table 2.
Among the 53 microbially-derived VOCs, 14 were uniquely produced by Phoma sp. (i.e., detected in a majority of Phoma sp. monocultures and one or zero MWU328 or MWU328W replicates), including several carboxylic acids and aromatic hydrocarbons (Table 2). Eight VOCs were detected that were unique to the bacteria, including indole (Yu et al., 2000; Blom et al., 2011; Lazazzara et al., 2017), which has been previously reported as a bVOC. Among the bVOCs, there were two compounds that were only detected in the wild-type MWU328, CA-18 and UNK-27. Two compounds, N-13 and indole, were solely produced by the QS mutant MWU328W. The detection of indole in the headspace of the unpigmented strain MWU328W is consistent with its cviR mutation, because violacein, the quorum-regulated purple pigment produced by C. vaccinii, is synthesized from two molecules of tryptophan (Momen et al., 1998; Rettori and Duran, 1998). The tryptophan biosynthesis operon is constitutively expressed in violacein-producing species of Chromobacterium at a high level to supply violacein precursors (Antonio and Creczynski-Pasa, 2004). Therefore, down regulation of the violacein synthesis pathway caused by the cviR mutation likely results in excess tryptophan, and thus an increase in its catabolism with the indole side chain released as a byproduct.
To filter the peak list down to potentially biogenic compounds, we took a biologically agnostic approach so as not to restrict our analyses to previously known microbial VOCs; rather we used statistical criteria to determine the list of putatively biogenic compounds by comparing VOC peak intensities in microbial cultures vs. media controls. Aside from the exclusion of siloxanes, we also took a chemically-agnostic approach, and did not exclude a priori compounds such as ethers, which can be generated abiologically via thermal decomposition of the polymers coating the SBSE devices. However, the list of 53 microbial VOCs includes possible analytical artifacts such as a potential phthalate (EST-33) and compounds resembling polyethylene glycol decomposition products (ETH-29, ETH-30, and ETH-39). Unlike typical chemical artifacts, these compounds statistically and reproducibly differed in intensity in the bacterial and fungal samples vs. media controls, and between one experimental biological condition and another. For example, EST-33 was present in higher concentrations in the bacterial-fungal co-cultures than in any of the monocultures, and ETH-39 was specific to cultures containing bacteria. While it is possible that the sources of these compounds are the materials that were used to culture the microbes or to collect the VOCs, our data indicate that there is a biological contribution to their production, such as the microbial degradation of petri dish materials, or the chemical reaction of biological VOCs with the sorbents used to collect them. However, additional analyses (below) indicate that these compounds are not highly relevant to the fungal inhibition and growth phenotypes we observed.
We used principal components analysis (PCA) for an untargeted approach to evaluate the relationships between the VOCs produced by Phoma sp., C. vaccinii MWU328, and MWU328W in mono- and co-culture (Figure 5, Supplementary Figures 3, 4). Using the 53 microbial VOCs as the variables and the six biological replicates of each culture condition as the observations, the presence or absence of bVOCs vs. fVOCs explains the largest proportion of variance in the model (PC1, 21.2%). Monocultures of MWU328 and MWU328W cluster together along PC1 <0, and away from the Phoma sp. monocultures clustered in PC1 > 0. Importantly, the co-cultures do not fall between the fungal and bacterial monoculture clusters, demonstrating that the VOCs of the co-culture are not a linear combination of fVOCs and bVOCs (Figure 5, Supplementary Figure 3). Further, the two co-cultures do not cluster together as the two monocultures of bacteria do, showing that the volatile metabolomes created by co-culture differ based on the presence or absence of intact C. vaccinii quorum sensing. PCA biplots reiterate many of the observations we made in the analysis of the VOC peak tables (Supplementary Figure 4). For example, most of the VOCs that are only produced by Phoma sp. show strong loadings on PC1 > 0. However, the loadings also reveal additional VOCs that are associated with more than one culture condition. For example, the loadings in PC1 vs. PC3 (Supplementary Figure 4) indicate that three aromatic hydrocarbons—ARO-45, ARO-44, and ARO-51–are detected in Phoma sp. monocultures as well as in Phoma sp. co-culture with MWU328.
Figure 5.
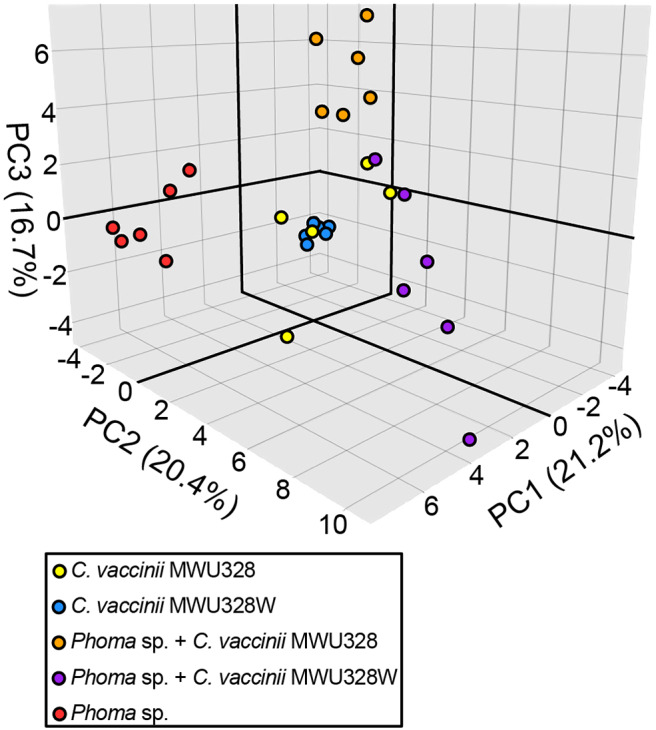
Principal component analysis score plot of monocultures of Phoma sp. (red), C. vaccinii MWU328 (Yellow), and C. vaccinii QS mutant MWU328W (Blue), and co-cultures of Phoma sp. and MWU328 (Orange) and Phoma sp. and MWU328W (Purple), based upon 53 biogenic VOCs. Six biological replicates were performed for each experiment.
The induction or suppression of monoculture VOCs in the co-cultures are evidence of VOC-mediated interactions between C. vaccinii and Phoma sp. For example, in both co-cultures there was a statistically significant increase in the production of UNK-52, a VOC produced in low abundance and low frequency in the bacterial monocultures, but in very high concentrations in the co-cultures (Table 2 and Supplementary Table 2). Phoma VOCs HC-26 and HC-7 increased in concentration more than 10-fold (though not significantly) and α-calacorene was detected with higher frequency in the presence of wild-type C. vaccinii, in spite of suppressed fungal growth. We also observed the inhibition of many of the Phoma sp. VOCs in one or both co-cultures, and the inhibition of wild type and/or mutant bVOCs, such as N-42 and T-40. Some of the most interesting interactions, however, are characterized by VOCs that are not observed in any of the three monocultures (or are observed in very low abundance and frequency), but are robustly detected in the co-cultures (e.g., S-19, ETH-31, UNK-36, and UNK-37), or VOCs detected in both bacterial and fungal monocultures but significantly reduced in co-culture (e.g., UNK-28), suggesting feedback loops between the bacterium and fungus mediated by volatile metabolites. Overall, amongst the 106 possible observations of VOCs in co-cultures (53 VOCs × 2 co-cultures) there were 33 statistically significant changes in VOC concentration compared to monocultures, affirming that both the bacterium and the fungus are altering substantial proportions of their volatile metabolomes in response to one another.
Putative Inhibitory VOCs
To determine which C. vaccinii VOCs may be inhibiting Phoma sp. growth, we took two main approaches. The first approach was to distinguish putative inhibitory compounds that are consistently produced by wild type MWU328 in our experimental system in greater abundance than the QS mutant MWU328W in monoculture, and that are detectable in the MWU328 co-cultures with Phoma sp. The second approach was to evaluate potentially inhibitory VOCs that are absent or produced in low abundance in the MWU328 monoculture, but produced in higher abundance by the bacterium when it detects the presence of Phoma sp. VOCs. We refer to these two sets of VOCs as the consistent and inducible VOCs, respectively.
For inducible VOCs to be considered as candidates for the QS-regulated antifungal VOCs, they should not be detected in the fungus monocultures, they should be detected in higher frequency and/or concentration in wild-type bacteria co-culture vs. the QS mutant co-culture, and they must be absent or detected in low frequency or concentration in MWU328 monoculture vs. co-culture with the fungus. However, because there was some limited inhibition of the fungus by MWU328W, it is possible that the putative fungistatic VOCs are detectable in co-cultures with mutant bacteria, in which case they should be present in substantively lower concentrations than in wild type co-cultures. Of the bVOCs that were induced by the presence of Phoma sp., none fit these criteria. Therefore, we postulate that the QS-regulated fungistatic VOCs are consistently produced by the wild-type bacterium under the conditions we tested.
The qualitative and quantitative data indicate that wild-type C. vaccinii MWU328 produces more VOCs at higher concentrations in monoculture than its QS mutant. Of the 45 VOCs detected in MWU328 or MWU328W in monoculture, 30 were detected in at least two-fold higher concentration in the wild type vs. the quorum-insensitive mutant (Figure 6, Supplementary Table 3). Of these, four compounds were significantly greater in wild type (p < 0.05), and compounds CA-18 and UNK-27 were not detectable in the QS mutant. For compounds to be candidates as fungistatic VOCs, they must be detectable in co-culture, and not produced by the fungus [i.e., not detected in Phoma sp. monocultures, or detected in the same, low concentrations in Phoma sp. monocultures and the media blanks (Supplementary Table 2)]. 1-Octanol, CA-18, and UNK-27 fit these additional criteria, making them candidate inhibitory VOCs that are consistently produced under QS regulation by C. vaccinii.
Figure 6.
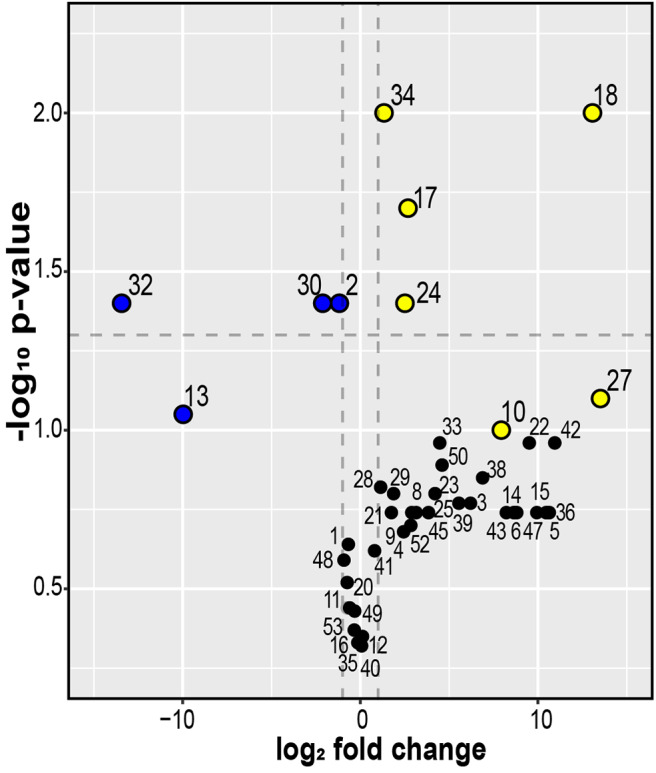
Volcano plot of the log2 fold-change difference in VOC abundance for MWU328 relative to MWU328W in monocultures. Dashed lines indicate p < 0.05 and log2 fold change > 1 or < −1. Metabolites in blue or yellow are more abundant in the mutant or wild type, respectively (p < 0.1). The fold-changes and p-values are provided in Supplementary Table 3. Compound IDs: 2 = dimethyl disulfide; 10 = 1,2-Ethanediol, diacetate; 13 = N-13; 17 = 1-Octanol; 18 = CA-18; 24 = Octanoic acid; 27 = UNK-27; 30 = ETH-30; 32 = Indole; 34 = EST-34.
To address whether hydrogen cyanide, which is undetectable in our GC × GC-TOFMS analysis, is responsible for the fungal inhibition by C. vaccinii VOCs, we quantified the amount of HCN produced by C. vaccinii in KMB broth (Table 3). HCN concentration at early stationary phase ranged from 54 to 63 ppm for C. vaccinii wild-type isolates. The QS mutants also produce HCN, but at far lower concentrations. Under the same conditions, HCN concentrations ranged from 21 to 34 ppm. The negative controls C. subtsugae MWU12-2387 and MWU13-2521 produced 6 ppm and 9 ppm, respectively, which is marginally above the signal-to-noise threshold for the assay, yet this species demonstrated strong inhibition of several of the fungi we tested (Table 1). Therefore, in addition to the three putative inhibitory C. vaccinii VOCs detected by GC × GC-TOFMS, HCN can be contributing to the QS-regulated fungal inhibition we observed, but is unlikely to be wholly responsible.
Table 3.
Concentrations of hydrogen cyanide produced by C. vaccinii and C. subtsugae grown in KMB to early stationary phase.
| Chromobacterium isolate | HCN concentration (ppm) | Number of replicates |
|---|---|---|
| C. vaccinii MWU205 | 54.4 ± 1.8 | 108 |
| C. vaccinii MWU300 | 58.5 ± 1.9 | 36 |
| C. vaccinii MWU328 | 63.3 ± 2.9 | 36 |
| C. vaccinii MWU205W | 23.7 ± 2.3 | 108 |
| C. vaccinii MWU300W | 20.5 ± 1.7 | 36 |
| C. vaccinii MWU328W | 33.5 ± 3.2 | 36 |
| C. subtsugae MWU12-2387 | 6.4 ± 2.4 | 4 |
| C. subtsugae MWU13-2521 | 8.9 ± 0.03 | 3 |
Values represent the mean ± standard error of the mean.
Discussion
Twenty one of 68 bacterial isolates we pre-screened from wild and cultivated cranberry bogs had significant antifungal bVOC activity against Trichoderma sp., several important plant pathogenic fungi, and the oomycete P. cinnamomi, indicating that VOC-mediated semiotic interactions between kingdoms is fairly widespread among soil bacteria. The effects on fungi were wide-ranging, and they reflected some degree of pairwise specificity in bacterial-fungal interactions. Even when growth inhibition was not observed, changes in gross fungal colony morphology, such as reduced sporulation, pigmentation, and hyphal density were common. The combination of fungal growth inhibition and morphology changes we observed demonstrate that there is broad biological activity across the bacterial volatile metabolome.
The strongest fungal growth inhibition was produced by C. vaccinii bVOCs against Phoma sp. and Coleophoma sp. Because Phoma sp. was not completely inhibited by C. vaccinii, we could use this pairing as a model to characterize the cellular impacts of antifungal bVOCs. We observed cytological abnormalities such as thickened cell walls, increased vacuolization, intracellular walls with occasional breakage or incomplete development, electron-dense inclusions within the cytoplasm and vacuoles, the presence of disorganized Spitzenkörper, and the accumulation of an unknown extracellular electron-dense material indicating that the fungus is under severe physiological stress. The fact that no mitochondrial abnormalities occur in Phoma sp. may offer a clue as to what other cellular processes are affected by the bVOCs, but are likely unrelated to cellular energy production via the electron transport chain. We observed that once the C. vaccinii bVOCs are removed, the growth rate of Phoma sp. is the same rate as the untreated control, suggesting that there is not long-term damage to the fungus. However, the disorganized Spitzenkörper and other cytological changes in the presence of bVOCs suggest that recovery from growth inhibition may not be as simple as resuming mitosis and cell elongation, and recovery may be a dose-dependent phenomenon, which we have not yet tested. The dose effects and the mechanism (or mechanisms) involved in fungal growth inhibition by C. vaccinii bVOC are the subjects of ongoing work.
The involvement of quorum sensing in the regulation of some component of the VOC suite produced by C. vaccinii suggests that the set of VOCs produced at any given time is influenced by the relative population density of members of the same species, of other species that produce the same quorum sensing autoinducer, or the presence of quorum quenching fVOCs. Based on our comparative analyses of the VOCs from mono- and co-cultures with C. vaccinii MWU328 and MWU328W, we identified four candidate fungistatic VOCs, 1-octanol, CA-18, UNK-27, and HCN, which we presume to be under quorum regulation. More than a dozen alcohols and carboxylic acids have been previously identified as fungistatic or fungicidal (Giorgio et al., 2015; Alijani et al., 2019; Osaki et al., 2019), lending precedence to the putative antifungal VOCs 1-octanol and CA-18, though the link between quorum regulation and antifungal alcohol or carboxylic acid bVOCs has not been established. However, the quorum regulation of antifungal VOCs has been previously demonstrated with acetophenone and 2-aminoacetophenone, quorum-sensing-related molecules produced by Pseudomonas sp. (Kesarwani et al., 2011), which have been shown to significantly inhibit Phytophthora infestans direct sporangial germination (De Vrieze et al., 2015).
Additional known fungistatic VOCs were detected in our analyses, such as dimethyl trisulfide (Barbieri et al., 2005; Fernando et al., 2005; Kai et al., 2009; Groenhagen et al., 2013; De Vrieze et al., 2015; Giorgio et al., 2015) and octanoic acid (Schulz et al., 2010), but they were not differentially expressed between MWU328 and its QS mutant, and therefore were not identified as the QS-regulated fungistatic VOCs in our experiments. We note that MWU328W mildly inhibited Phoma sp. growth, which may have been due to one, some, or all of these known bioactive VOCs. Additionally, a third to a half of HCN production is maintained in the QS mutants, so some of the residual fungal growth inhibition observed in these mutants may be due to HCN. However, C. subtsugae does not produce appreciable amounts of HCN, yet it has good growth inhibition properties (Table 1). Therefore, we cannot attribute all of the Chromobacterium sp. inhibition to HCN alone. It has long been known that microbial VOCs are produced in combinations, the sum of which are responsible for antifungal activity (Moore-Landecker and Stotzky, 1973, 1974). Although we have attempted to identify individual fungistatic compounds, it should be understood that it is likely a suite of VOCs that is responsible for the gross morphological and growth inhibition effects we observed. Determining the degree to which the four candidate fungistatic VOCs can inhibit growth independently, in combination, or in complex with the many other VOCs produced by C. vaccinii will require positive identification of the QS-regulated bVOCs via authentic standards, followed by bioassays against Phoma sp. and other fungi. These experiments are the subjects of ongoing work.
The variation in growth inhibition, and in phenotypic and morphological changes among fungi we tested suggests varying levels of fungal susceptibility to the types and concentrations of bVOCs produced. The soil and rhizosphere genera Chromobacterium and Pseudomonas were particularly effective at inhibiting fungal growth, and both of these genera include species with biocontrol activities against insects, fungi, and oomycetes (Siddiqui and Shaukat, 2004; Durán et al., 2007, 2016; Martin et al., 2007; De Vrieze et al., 2015; Hunziker et al., 2015; Ossowicki et al., 2017; David et al., 2018). The only species we tested from both wild and cultivated bogs was C. vaccinii, but the numbers of isolates were insufficient to test hypotheses related to antifungal VOCs, cultivation practices, and fungal disease burden. However, we noted that all three C. vaccinii wild-type isolates MWU205, MWU300, and MWU328 were able to limit the growth of Phoma sp. and Coleophoma sp.—two fruit rot pathogens—to <3% of the control growth rate. In contrast, Trichoderma sp. and P. cinnamomi, two species normally found in the soil where Chromobacterium sp. dwell, were far less susceptible to bVOC-induced inhibition for reasons that are as yet unknown. The observation that fungi isolated from, and primarily associated with the above-ground parts of the plant are more sensitive to stasis and morphological changes induced by exposure to bVOCs raises the possibility that there has been co-evolution of the soil fungi with bacteria that occupy the same niches, resulting in fungal resistance to the effects of the bVOCs. We think this hypothesis is worthy of testing and may lead to a broader understanding of semiochemical interactions in natural and engineered microbial communities.
A significant and unexpected finding from our VOC analyses is that the volatile metabolomes of both the fungus and the bacteria are altered in co-culture compared to their respective monocultures. We measured statistically significant increases in concentration of three bVOCs and an increase in detection rates of three fVOCs in co-culture compared to monoculture. Three additional VOCs were robustly detected only in co-cultures and 17 bVOCs and/or fVOCs were significantly inhibited in co-culture vs. monoculture. Intriguingly, we detected co-culture-induced fVOCs in spite of fungal growth inhibition, while also measuring statistically significant decreases in the antifungal bVOCs dimethyl trisulfide and 1-octanol, which together suggests that Phoma sp. produces defensive VOCs to reduce the production of antifungals by C. vaccinii. To our knowledge only two other studies have reported similar VOC-mediated inductions of microbial volatile metabolites. Azzollini and colleagues identified sixteen fVOCs that were induced when two wood-decaying fungi exchanged VOCs, and among them was 2-nonanone, an antifungal VOC that could suppress the growth of both fungi (Azzollini et al., 2018). Schmidt and colleagues showed that exposure to Fusarium culmorum VOCs could induce the production of a terpene in Serratia plymuthica (Schmidt et al., 2017). Our data provide an example of volatile metabolites shaping inter-kingdom microbial interactions via bVOC-fVOC feedback loops, resulting in a co-culture volatile metabolome with emergent properties.
In these experiments we have characterized the interactions between single bacterial isolates with single fungi growing in pure cultures. The reality in the soil and rhizosphere is that there are many hundreds of types of microbes present, and the simplicity of our model test system limits its direct translatability. However, based on our preliminary assays, many genera of cultivable bacteria from those environments produce fungal inhibitory bVOCs, and in the case of Chromobacterium, some are under quorum regulation. Thus, the soil and nearby rhizosphere are likely to be rich, dynamic, and highly biologically active signaling environments. The overall effect of bVOCs produced by soil microbes on plants, nematodes, insect larvae, and other microbes is likely to be complex, particularly because the VOCs produced by bacteria and fungi at any given time seems to depend on the VOC signals they are receiving. Except for a single Trichoderma sp. isolate from cultivated cranberry bogs, we have not examined the effect of bVOCs on “beneficial” fungi in the soil, such as ericoid mycorrhizal fungi, which are critical for nitrogen and phosphorous nutrition in cranberry (Kosola et al., 2007; Martin and Nehls, 2009). These studies remain for the future.
Conclusions
We investigated VOCs produced by bacteria isolated from the rough-and-tumble competitive environment of soil as potential biological control agents of fungi. Our finding that C. vaccinii produces VOCs that can inhibit fungal growth by more than 80% holds the potential for controlling fungal growth in a wide range of applications. One of the most significant findings from this study is that the co-cultures of bacteria and fungi have emergent volatile metabolome properties. That is, the metabolome of the co-culture cannot be predicted by the sum of its constituents in monoculture. Undoubtedly the metabolomes we detected in co-culture will not fully extrapolate to more complex systems involving VOCs from plants and other plant and soil-associated microbes. Hence, a systems biology approach is required to characterize and ultimately predict the emergent properties of microbiomes in natural and engineered systems, and to discover VOCs for safe and effective control of plant, human, and animal mycoses.
Data Availability Statement
The datasets generated for this study can be found in the NCBI: MT150599, MT150598, MT227805, MT150597, MT101734, MT101742, MT101743, MT101740, MT101737, MT101748, MT101735, MT101733, MT101747, MT101738, MT101745, MT101739, MT101744, MT101741, MT101736, MT101746, MT158224, MT215537.
Author Contributions
SS and HB conceived of the project. HB and GE designed the experiments. GE and EH collected and analyzed the data. All authors wrote and edited the manuscript and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
P. chlororaphis 30-84 was generously provided by Leland (Sandy) Pierson at Texas A&M, Phytophthora cinnamomi R001 was generously provided by Jerry Weiland at Oregon State University/USDA/ARS. Trenton Davis provided statistical assistance for the analysis of GC × GC-TOFMS data. Alisha Harrison conducted HCN assays and provided technical assistance on maintenance, identification, and sequencing of microbial isolates. The authors would like to especially thank David Lowry, Arizona State University for TEM images.
Footnotes
Funding. Support for the project came from Midwestern University and Arizona State University internal funds. Open access publication fees came from the Biomedical Sciences Program at Midwestern University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01035/full#supplementary-material
References
- Alijani Z., Amini J., Ashengroph M., Bahramnejad B. (2019). Antifungal activity of volatile compounds produced by Staphylococcus sciuri strain MarR44 and its potential for the biocontrol of Colletotrichum nymphaeae, causal agent strawberry anthracnose. Int. J. Food Microbiol. 307:108276. 10.1016/j.ijfoodmicro.2019.108276 [DOI] [PubMed] [Google Scholar]
- Ann Ellis E. (2018). Solutions to the problem of substitution of ERL 4221 for vinyl cyclohexene dioxide in spurr low viscosity embedding formulations. Microscopy Today 14, 32–33. 10.1017/S1551929500050252 [DOI] [Google Scholar]
- Antonio R. V., Creczynski-Pasa T. B. (2004). Genetic analysis of violacein biosynthesis by Chromobacterium violaceum. Genet. Mol. Res. 3, 85–91. [PubMed] [Google Scholar]
- Audrain B., Farag M. A., Ryu C. M., Ghigo J. M. (2015). Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 39, 222–233. 10.1093/femsre/fuu013 [DOI] [PubMed] [Google Scholar]
- Azzollini A., Boggia L., Boccard J., Sgorbini B., Lecoultre N., Allard P. M., et al. (2018). Dynamics of metabolite induction in fungal co-cultures by metabolomics at both volatile and non-volatile levels. Front. Microbiol. 9:72. 10.3389/fmicb.2018.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly A., Weisskopf L. (2012). The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal Behav. 71, 79–85. 10.4161/psb.7.1.18418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri E., Gioacchini A. M., Zambonelli A., Bertini L., Stocchi V. (2005). Determination of microbial volatile organic compounds from Staphylococcus pasteuri against Tuber borchii using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 19, 3411–3415. 10.1002/rcm.2209 [DOI] [PubMed] [Google Scholar]
- Barr J. G. (1976). Effects of volatile bacterial metabolites on the growth, sporulation and mycotoxin production of fungi. J. Sci. Food Agr. 27, 324–330. 10.1002/jsfa.2740270405 [DOI] [PubMed] [Google Scholar]
- Bean H. D., Rees C. A., Hill J. E. (2016). Comparative analysis of the volatile metabolomes of Pseudomonas aeruginosa clinical isolates. J. Breath Res. 10, 047102. 10.1088/1752-7155/10/4/047102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen R. L., Pieterse C. M. J., Bakker P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. 10.1016/j.tplants.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Blom D., Fabbri C., Connor E. C., Schiestl F. P., Klauser D. R., Boller T., et al. (2011). Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 13, 3047–3058. 10.1111/j.1462-2920.2011.02582.x [DOI] [PubMed] [Google Scholar]
- Caruso F. L. (2000). Phytophthora Root Rot, Phytophthora Cinnamomi. (East Wareham, MA: University of Massachusetts, Cranberry Experiment Station; ). [Google Scholar]
- Caruso F. L., Wilcox W. F. (1990). Phytophthora cinnamomi as a cause of root rot and dieback of cranberry in Massachusetts. Plant Dis. 74, 664–667. 10.1094/PD-74-0664 [DOI] [Google Scholar]
- Chaurasia B., Pandey A., Palni L. M. S., Trivedi P., Kumar B., Colvin N. (2005). Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol. Res. 160, 75–81. 10.1016/j.micres.2004.09.013 [DOI] [PubMed] [Google Scholar]
- David B. V., Chandrasehar G., Selvam P. N. (2018). Pseudomonas fluorescens: a plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops, in New and Future Developments in Microbial Biotechnolgy and Bioengineering: Crop Improvement Through Microbial Biotechnology, eds. Prasad R., Gill S.S., Tuteja N. (Amsterdam: Elsevier; ), 221–243. 10.1016/B978-0-444-63987-5.00010-4 [DOI] [Google Scholar]
- De Vrieze M., Pandey P., Bucheli T. D., Varadarajan A. R., Ahrens C. H., Weisskopf L., et al. (2015). Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 6:1295. 10.3389/fmicb.2015.01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle F., Ross A., Schlotterbeck G., Senn H. (2006). Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290. 10.1021/ac051632c [DOI] [PubMed] [Google Scholar]
- Dobbs C. G., Hinson W. H. (1953). A widespread fungistasis in soils. Nature 172, 197–199. 10.1038/172197a0 [DOI] [PubMed] [Google Scholar]
- Durán N., Justo G. Z., Durán M., Brocchi M., Cordi L., Tasic L., et al. (2016). Advances in Chromobacterium violaceum and properties of violacein-its main secondary metabolite: a review. Biotechnol. Adv. 34, 1030–1045. 10.1016/j.biotechadv.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Durán N., Justo G. Z., Ferreira C. V., Melo P. S., Cordi L., Martins D. (2007). Violacein: properties and biological activities. Biotechnol. Appl. Bioc. 48, 127–133. 10.1042/BA20070115 [DOI] [PubMed] [Google Scholar]
- Ebadzadsahrai G., Soby S. (2019). 16S rRNA amplicon profiling of cranberry (Vaccinium macrocarpon Ait.) flower and berry surfaces. Microbiol. Resour. Announc. 8, e01479–e01418. 10.1128/MRA.01479-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck P. (1990). The American Cranberry. (New Brunswich, NJ: Rutgers University Press; ). [Google Scholar]
- Effmert U., Kalderas J., Warnke R., Piechulla B. (2012). Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 38, 665–703. 10.1007/s10886-012-0135-5 [DOI] [PubMed] [Google Scholar]
- Farag M. A., Zhang H. M., Ryu C. M. (2013). Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 39, 1007–1018. 10.1007/s10886-013-0317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando W. G. D., Ramarathnam R., Krishnamoorthy A. S., Savchuk S. C. (2005). Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 37, 955–964. 10.1016/j.soilbio.2004.10.021 [DOI] [Google Scholar]
- Forlani G., Occhipinti A., Bossi S., Bertea C. M., Varese C., Maffei M. E. (2011). Magnaporthe oryzae cell wall hydrolysate induces ros and fungistatic VOCs in rice cell cultures. J. Plant Physiol. 168, 2041–2047. 10.1016/j.jplph.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Frank J. A., Reich C. I., Sharma S., Weisbaum J. S., Wilson B. A., Olsen G. J. (2008). Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 74, 2461–2470. 10.1128/AEM.02272-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., De Stradis A., Lo Cantore P., Iacobellis N. S. (2015). Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 6:1056. 10.3389/fmicb.2015.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham E. (1991). Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1, 182–195. 10.2307/1941811 [DOI] [PubMed] [Google Scholar]
- Groenhagen U., Baumgartner R., Bailly A., Gardiner A., Eberl L., Schulz S., et al. (2013). Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 39, 892–906. 10.1007/s10886-013-0315-y [DOI] [PubMed] [Google Scholar]
- Grover R., Moore J. (1962). Toximetric studies of fungicides against brown rot organisms, Sclerotinia fructiocola and S. laxa. Phytopathology 52, 876–879. [Google Scholar]
- Halsted B. D. (1889). Some Fungus Diseases of the Cranberry. (New Brunswick, NJ: New Jersey Agricultural Collage Experiment Station; ). [Google Scholar]
- Hanaichi T., Sato T., Iwamoto T., Malavasi-Yamashiro J., Hoshino M., Mizuno N. (1986). A stable lead by modification of Sato's method. Microscopy 35, 304–306. [PubMed] [Google Scholar]
- Hunziker L., Bönisch D., Groenhagen U., Bailly A., Schulz S., Weisskopf L. (2015). Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 81, 821–830. 10.1128/AEM.02999-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insam H., Seewald M. S. A. (2010). Volatile organic compounds (VOCs) in soils. Biol. Fert. Soils 46, 199–213. 10.1007/s00374-010-0442-3 [DOI] [Google Scholar]
- Jansson J. K., Hofmockel K. S. (2018). The soil microbiome-from metagenomics to metaphenomics. Curr. Opin. Microbiol. 43, 162–168. 10.1016/j.mib.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Kai M., Haustein M., Molina F., Petri A., Scholz B., Piechulla B. (2009). Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81, 1001–1012. 10.1007/s00253-008-1760-3 [DOI] [PubMed] [Google Scholar]
- Keddy P. A. (2010). Wetland Ecology: Principles and Conservation. (Cambridge, MA: Cambridge University Press; ). 10.1017/CBO9780511778179 [DOI] [Google Scholar]
- Kesarwani M., Hazan R., He J., Que Y., Apidianakis Y., Lesic B., et al. (2011). A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. PLoS Pathog. 7:e1002192. 10.1371/journal.ppat.1002192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. O., Ward M. K., Raney D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescin. Transl. Res. 44, 301–307. [PubMed] [Google Scholar]
- Korpi A., Järnberg J., Pasanen A.-L. (2009). Microbial volatile organic compounds. Crit. Rev. Toxicol. 39, 139–193. 10.1080/10408440802291497 [DOI] [PubMed] [Google Scholar]
- Kosola K. R., Workmaster B. A. A., Spada P.A. (2007). Inoculation of cranberry (Vaccinium macrocarpon) with the ericoid mycorrhizal fungus Rhizoscyphus ericae increases nitrate influx. New Phytol. 176, 184–196. 10.1111/j.1469-8137.2007.02149.x [DOI] [PubMed] [Google Scholar]
- Lazazzara V., Perazzolli M., Pertot I., Biasioli F., Puopolo G., Cappellin L. (2017). Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiol. Res. 201, 52–62. 10.1016/j.micres.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Lemfack M. C., Gohlke B.-O., Toguem Serge m t., Preissner S., Piechulla B., Preissner R. (2017). mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res. 46, D1261–D1265. 10.1093/nar/gkx1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Cantore P., Giorgio A., Iacobellis N. S. (2015). Bioactivity of volatile organic compounds produced by Pseudomonas tolaasii. Front. Microbiol. 6, 1082. 10.3389/fmicb.2015.01082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie A. E., Wheatley R. E. (1999). Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol. Biochem. 31, 375–385. 10.1016/S0038-0717(98)00140-0 [DOI] [Google Scholar]
- Martin F., Nehls U. (2009). Harnessing ectomycorrhizal genomics for ecological insights. Curr. Opin. Plant Biol. 12, 508–515. 10.1016/j.pbi.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Martin P. A., Soby S. D. (2016). United States Patent No.: US009339039B1. [Google Scholar]
- Martin P. A. W., Gundersen-Rindal D., Blackburn M., Buyer J. (2007). Chromobacterium subtsugae sp nov., a betaproteobacterium toxic to colorado potato beetle and other insect pests. Int. J. Syst. Evol. Microbiol. 57, 993–999. 10.1099/ijs.0.64611-0 [DOI] [PubMed] [Google Scholar]
- Mcclean K. H., Winson M. K., Fish L., Taylor A., Chhabra S. R., Camara M., et al. (1997a). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143, 3703–3711. 10.1099/00221287-143-12-3703 [DOI] [PubMed] [Google Scholar]
- Mcclean K. H., Winson M. K., Fish L., Taylor A., Chhabra S. R., Camara M., et al. (1997b). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143, 3703–3711. 10.1099/00221287-143-12-3703 [DOI] [PubMed] [Google Scholar]
- Minuto A., Spadaro D., Garibaldi A., Gullino M. (2006). Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot. 25, 468–475. 10.1016/j.cropro.2005.08.001 [DOI] [Google Scholar]
- Momen A. Z. M. R., Mizuoka T., Hoshino T. (1998). Studies on the biosynthesis of violacein. Part 9. Green pigments possessing tetraindole and dipyrromethane moieties, chromoviridans and deoxychromoviridans, produced by a cell-free extract of Chromobacterium violaceum and their biosynthetic origins. J. Chem. Soc. Perkin Trans. I 1, 3087–3092. 10.1039/a803617i [DOI] [Google Scholar]
- Moore-Landecker E., Stotzky G. (1973). Morphological abnormalities of fungi induced by volatile microbial metabolites. Mycologia 65, 519–530. 10.1080/00275514.1973.12019467 [DOI] [PubMed] [Google Scholar]
- Moore-Landecker E., Stotzky G. (1974). Effects of concentration of volatile metabolites from bacteria and germinating seeds on fungi in the presence of selective absorbents. Can. J. Microbiol. 20, 97–103. 10.1139/m74-015 [DOI] [PubMed] [Google Scholar]
- Mülner P., Bergna A., Wagner P., Sarajlić D., Gstöttenmayr B., Dietel K., et al. (2019). Microbiota associated with sclerotia of soilborne fungal pathogens - a novel source of biocontrol agents producing bioactive volatiles. Phytobiomes J. 3, 125–136. 10.1094/PBIOMES-11-18-0051-R [DOI] [Google Scholar]
- Nicholson L. A., Morrow C. J., Corner L. A., Hodgson A. L. (1994). Phylogenetic relationship of Fusobacterium necrophorum A, AB, and B biotypes based upon 16S rRNA gene sequence analysis. Int. J. Syst. Bacteriol. 44, 315–319. 10.1099/00207713-44-2-315 [DOI] [PubMed] [Google Scholar]
- Osaki C., Yamaguchi K., Urakawa S., Nakashima Y., Sugita K., Nagaishi M., et al. (2019). The bacteriological properties of Bacillus strain TM-I-3 and analysis of the volatile antifungal compounds emitted by this bacteria. Biocontrol Sci. 24, 129–136. 10.4265/bio.24.129 [DOI] [PubMed] [Google Scholar]
- Ossowicki A., Jafra S., Garbeva P. (2017). The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 12:e0174362. 10.1371/journal.pone.0174362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudemans P. V. (1999). Phytophthora species associated with cranberry root rot and surface irrigation water in New Jersey. Plant Dis. 83, 251–258. 10.1094/pdis.1999.83.3.251 [DOI] [PubMed] [Google Scholar]
- Oudemans P. V., Caruso F. L., Stretch A. W. (1998). Cranberry fruit rot in the northeast: a complex disease. Plant Dis. 82, 1176–1184. 10.1094/PDIS.1998.82.11.1176 [DOI] [PubMed] [Google Scholar]
- Paulitz T. C., Bélanger R. R. (2001). Biological control in greenhouse systems. Annu. Rev. Phytopathol. 39, 103–133. 10.1146/annurev.phyto.39.1.103 [DOI] [PubMed] [Google Scholar]
- Penuelas J., Terradas J. (2014). The foliar microbiome. Trends Plant Sci. 19, 278–280. 10.1016/j.tplants.2013.12.007 [DOI] [PubMed] [Google Scholar]
- Polashock J. J., Caruso F. L., Averill A. L., Schilder A. C. (2017). Compendium of Blueberry, Cranberry, and Lingonberry Diseases and Pests, 2nd Edn. St. Paul, MN: The American Phytopathological Society; 10.1094/9780890 [DOI] [Google Scholar]
- Popova A. A., Koksharova O. A., Lipasova V. A., Zaitseva J. V., Katkova-Zhukotskaya O. A., Eremina S. I., et al. (2014). Inhibitory and toxic effects of volatiles emitted by strains of pseudomonas and serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. BioMed. Res. Int. 11:e125704 10.1155/2014/125704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Penton C. R., Xiong W., Liu C., Wang R., Liu Z., et al. (2019). Reshaping the rhizosphere microbiome by bio-organic amendment to enhance crop yield in a maize-cabbage rotation system. Appl. Soil Ecol. 142, 136–146. 10.1016/j.apsoil.2019.04.014 [DOI] [Google Scholar]
- Raaijmakers J. M., Vlami M., De Souza J. T. (2002). Antibiotic production by bacterial biocontrol agents. Anton. Leeuw. Int. J. G. 81, 537. 10.1023/A:1020501420831 [DOI] [PubMed] [Google Scholar]
- Rajer F. U., Wu H., Xie Y., Xie S., Raza W., Tahir H., et al. (2017). Volatile organic compounds produced by a soil-isolate, Bacillus subtilis FA26 induce adverse ultra-structural changes to the cells of Clavibacter michiganensis ssp. sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 163, 523–530. 10.1099/mic.0.000451 [DOI] [PubMed] [Google Scholar]
- Rettori D., Duran N. (1998). Production, extraction and purification of violacein: an antibiotic pigment produced by Chromobacterium violaceum. World J. Microb. Biot. 14, 685–688. 10.1023/A:1008809504504 [DOI] [Google Scholar]
- Schmidt R., Etalo D. W., De Jager V., Gerards S., Zweers H., De Boer W., et al. (2016). Microbial small talk: volatiles in fungal-bacterial interactions. Front. Microbiol. 6:1495. 10.3389/fmicb.2015.01495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Jager V., Zuhlke D., Wolff C., Bernhardt J., Cankar K., et al. (2017). Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 7:862. 10.1038/s41598-017-00893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Dickschat J. S., Kunze B., Wagner-Dobler I., Diestel R., Sasse F. (2010). Biological activity of volatiles from marine and terrestrial bacteria. Mar. Drugs 8, 2976–2987. 10.3390/md8122976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Bohm K., Martin-Sanchez L., Garbeva P. (2017). Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 8:2484. 10.3389/fmicb.2017.02484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi R., Ryu C. M. (2018). Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 44, 88–97. 10.1016/j.pbi.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Siddiqui I. A., Shaukat S. S. (2004). Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. J. Phytopathol. 152, 48–54. 10.1046/j.1439-0434.2003.00800.x [DOI] [Google Scholar]
- Soby S., Bergman K. (1983). Motility and chemotaxis of rhizobium-meliloti in soil. Appl. Environ. Microbiol. 46, 995–998. 10.1128/AEM.46.5.995-998.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soby S. D., Gadagkar S. R., Contreras C., Caruso F. L. (2013). Chromobacterium vaccinii sp. nov., isolated from native and cultivated cranberry (Vaccinium macrocarpon Ait.) bogs and irrigation ponds. Int. J. Syst. Evol. Microbiol. 63, 1840–1846. 10.1099/ijs.0.045161-0 [DOI] [PubMed] [Google Scholar]
- Stevens N. E. (1924). Notes on cranberry fungi in Massachusetts. Phytopatholoy 14, 101–107. [Google Scholar]
- Stotzky G., Schenck S., Papavizas G. C. (1976). Volatile organic compounds and microorganisms. Crit. Rev. Microbiol. 4, 333–382. 10.3109/10408417609102303 [DOI] [PubMed] [Google Scholar]
- Sumner L. W., Amberg A., Barrett D., Beale M. H., Beger R., Daykin C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 3, 211–221. 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio M., Miki T., Balser T. C. (2013). A coexisting fungal-bacterial community stabilizes soil decomposition activity in a microcosm experiment. PLoS ONE 8:e80320. 10.1371/journal.pone.0080320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Hansen L. D., Mcmanus P. S. (2017). Year-to-year incidence of cranberry fruit rot and persistence of fungal pathogens. Plant Health Prog. 18, 114–119. 10.1094/PHP-12-16-0073-RS [DOI] [Google Scholar]
- Wenke K., Kai M., Piechulla B. (2010). Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231, 499–506. 10.1007/s00425-009-1076-2 [DOI] [PubMed] [Google Scholar]
- Werner S., Polle A., Brinkmann N. (2016). Belowground communication: impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 100, 8651–8665. 10.1007/s00253-016-7792-1 [DOI] [PubMed] [Google Scholar]
- Westhoff S., Van Wezel G. P., Rozen D. E. (2017). Distance-dependent danger responses in bacteria. Curr. Opin. Microbiol. 36, 95–101. 10.1016/j.mib.2017.02.002 [DOI] [PubMed] [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in PCR Protocols - A Guide to Methods and Applications, eds. Innis M.A., Gelfand D.H., Sninsky J.J., White T.J. (San Diego, CA: Academic Press, Inc.). 10.1016/B978-0-12-372180-8.50042-1 [DOI] [Google Scholar]
- Xiong W., Li R., Ren Y., Liu C., Zhao Q., Wu H., et al. (2017). Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 107, 198–207. 10.1016/j.soilbio.2017.01.010 [DOI] [Google Scholar]
- Yu J. M., Wang D., Pierson L. S., 3rd, Pierson E. A. (2018). Effect of producing different phenazines on bacterial fitness and biological control in Pseudomonas chlororaphis 30-84. Plant Pathol. J. 34, 44–58. 10.5423/PPJ.FT.12.2017.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Hamilton-Kemp T. R., Archbold D. D., Collins R. W., Newman M. C. (2000). Volatile compounds from Escherichia coli O157:H7 and their absorption by strawberry fruit. J. Agric. Food Chem. 48, 413–417. 10.1021/jf990576b [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhao M., Li R., Huang Q., Raza W., Rensing C., et al. (2017). Microbial volatile compounds alter the soil microbial community. Environ. Sci. Pollut. Res. 24, 22485–22493. 10.1007/s11356-017-9839-y [DOI] [PubMed] [Google Scholar]
- Zhang R., Vivanco J. M., Shen Q. (2017). The unseen rhizosphere root-soil-microbe interactions for crop production. Curr. Opin. Microbiol. 37, 8–14. 10.1016/j.mib.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Zlosnik J. E. A., Williams H. D. (2004). Methods for assaying cyanide in bacterial culture supernatant. Lett. Appl. Microbiol. 38, 360–365. 10.1111/j.1472-765X.2004.01489.x [DOI] [PubMed] [Google Scholar]
- Zou C.-S., Mo M.-H., Gu Y.-Q., Zhou J.-P., Zhang K.-Q. (2007). Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol. Biochem. 39, 2371–2379. 10.1016/j.soilbio.2007.04.009 [DOI] [Google Scholar]
- Zygadlo J., Guzman C., Grosso N. (1994). Antifungal properties of the leaf oils of Tagetes minuta L. And T. filifolia Lag. J. Essent. Oil Res. 6, 617–621. 10.1080/10412905.1994.9699353 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the NCBI: MT150599, MT150598, MT227805, MT150597, MT101734, MT101742, MT101743, MT101740, MT101737, MT101748, MT101735, MT101733, MT101747, MT101738, MT101745, MT101739, MT101744, MT101741, MT101736, MT101746, MT158224, MT215537.