Abstract
As coronavirus disease 2019 (COVID-19) pandemic poses a substantial global public health threat, traditional Chinese medicine (TCM) was used in 91.50% of the COVID-19 cases in China, showing encouraging results in improving symptom management and reducing the deterioration, mortality, and recurrence rates. A total of 166 modified herbal formulae consisting of 179 single herbal medicines were collected for treating COVID-19 in China. Glycyrrhizae Radix et Rhizome, Scutellariae Radix, and Armeniacae Semen Amarum are the most frequently utilized in clinics, most of which are antipyretic (47, 26.26%), expectorant and cough-suppressing (22, 12.29%), and dampness-resolving (21, 11.73%) from traditional descriptions. A total of 1212 chemical components containing β-sitosterol, stigmasterol, and quercetin were primarily selected. Additionally, using complex system entropy and unsupervised hierarchical clustering, 8 core herbal combinations and 10 new formulae emerged as potentially useful candidates for COVID-19. Finally, following scaffold analysis, self-organizing mapping (SOM) and cluster analysis, 12 clusters of molecules yielded 8 pharmacophore families of structures that were further screened as pharmacological targets in human metabolic pathways for inhibiting coronavirus. This article aims to make more easily accessible and share historical herbal knowledge used in contemporary treatments in a modern manner to assist researchers contain the global spread of COVID-19.
Key words: COVID-19, Traditional Chinese medicine (TCM), Herbal medicines, Clustering analysis, Scaffold analysis
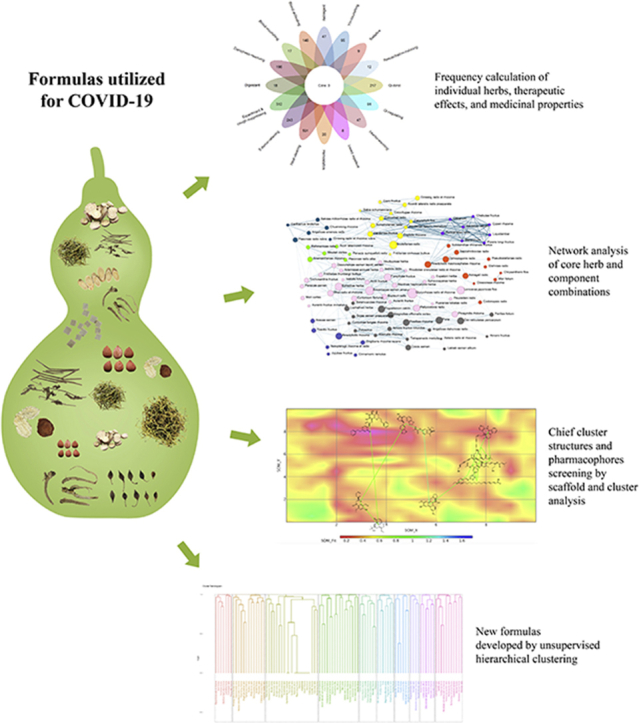
Graphical abstract
Herbal formulae were collected from 26 protocols for treating COVID-19. Using complex system entropy and unsupervised hierarchical clustering, 8 core combinations and 10 formulae emerged as potential candidates. Following scaffold analysis and self organizing mapping, 12 clusters of molecules yielded 8 pharmacophore structures screened as pharmacological targets for inhibiting corona virus.
1. Introduction
While COVID-19 is now being effectively controlled within China, it is increasingly affecting other countries worldwide, most notably the U.S., Iran, and Italy since Feb 25, 20201. World Health Organization (WHO) has officially declared COVID-19 a global pandemic and announced that Europe and the U.S. have now become the epicenter of the pandemic2. Particularly, China used a unique medical guideline for disease management, which combines traditional Chinese medicine (TCM) and Western medicine together. The government of China announced that a total of 74,187 COVID-19 cases (91.50% of the total cases) were treated by combined conventional and TCM approaches with promising results in all infection stages, including significant symptom management, lower rates of deterioration and mortality, faster recovery as well as disease prevention on 23 March 20203. Rather than conventional Western medicine, the use of TCM is mainly based on traditional knowledge and professional experience. In general, TCM can be comprehensively analyzed from several modern approaches, particularly using computational analysis of traditional herbal knowledge with modern pharmacological perspectives. This study utilized both individual herbal medicines and their respective formulae for COVID-19 in China, and proposed new formulae based on our conclusions. The authors hope that this can aid new drug exploration and clinical trial selection for the control and prevention of COVID-19 in the wider global research community.
1.1. Characteristics and experience of TCM in treating epidemic diseases
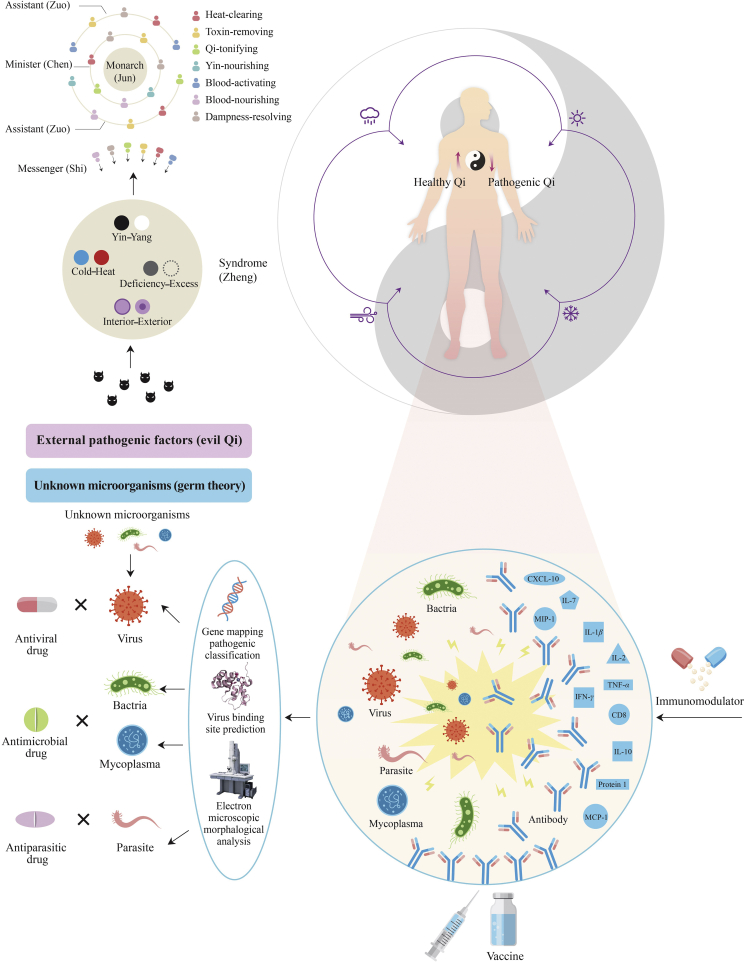
With a long history of combating epidemic diseases with relatively low mortality, TCM has accumulated a rich antiviral herb knowledgebase from clinical observation and pharmacological selection. This is historically based on a phenomenological approach and relies on complex mixtures of herbal medicines, as well as nonpharmacological holistic interventions, such as acupuncture and health lifestyle guidance5. With pandemic diseases, TCM has shown the agility to be administered more quickly and more efficiently than conventional medicine4. It asserts that health is a state of harmony between an individual's internal physiological network of factors and that of an external environmental6. Diagnosis is through syndrome differentiation called “Bian Zheng”, a holistic analysis of clinically observed information that guides personalized treatment options4, which may be adaptably changed dynamically during disease progression7. From point of TCM, epidemic disease is considered caused by an external pathogenic attack (traditionally named pestilent Qi or evil Qi) due to climatic or environmental changes. In the battle between healthy Qi (immunity) and pathogenic Qi (pathogen), a weakened immune system results in disease, while exhaustion of healthy Qi leads to death7. Consequently, different syndromes can manifest as disease progresses. From TCM perspective, the fundamental pathogenic factor of COVID-19 would be termed as “dampness accumulation in the lung”, which invades from the exterior to the interior attacking other organs as disease develops from the early stage to mild, moderate, severe, critical, and recovery stages. According to the syndrome differentiations, the pathogenesis in the mild and moderate stages of COVID-19 is damp accumulation in the lung. During the severe and critical stages, dampness develops to “damp-heat toxin and congests in the lung”. While heat toxin “burns” or rapidly depletes Qi and Yin, this subsequently would appear as what is termed “dual vacuity of Qi and Yin of lung and spleen in the recovery stage”3. It could be otherwise expressed somewhat as exhaustion of the lung system, its moisture, membranes and resource to recover, along with its fundamental immune supporting system. Herbal formulations are then frequently modified as signs and symptoms change along with the diagnosis and corresponding syndrome differentiations. Appropriate herbal formulae are administered, containing herbs composed in a hierarchy called “Jun Chen Zuo Shi”, relating to their primary, secondary, tertiary and quaternary functional class order, respectively8. Through design, herbal formulae guide a combination of different herbal medicines based on the individual synergistic properties of each herb. The Jun (monarch) component is the principal phyto-complex targeting the major symptom of the disease. It synergizes with the Chen (minister) herbs to support its therapeutic effects. The Zuo (assistant) medicinal reduces or eliminates possible adverse or toxic effects. Lastly, the Shi (courier) herbs facilitate the delivery of the principal components to desired sites (e.g., target organ), or facilitate the overall action of the other components. The whole formulae thus strive for a synergistic effect that targets active phytochemicals to their designated sites of action8. Furthermore, herbs can be administered through a diverse number of routes including oral intake, nasal inhalation, moxibustion, and herbal plasters (Fig. 1).
Figure 1.
Comparison between traditional Chinese medicine (TCM) and conventional medicine conceptual bases for treating epidemic disease.
TCM posits that epidemic diseases occur as a result of macroclimate or physical changes, leading to the Yin–Yang imbalance of the body followed by the development of a variety of syndromes. Herbal formulae are then organized based on “Jun Chen Zuo Shi” compatibility in accordance with traditional syndrome differentiations, such as “the eight principles”. In contrast, conventional medicine initially categorizes the microorganisms into virus, bacteria, mycoplasma, parasite, etc. The corresponding anti-microorganism drugs are then given in combination with vaccines and immunomodulators.
China historically has a high national epidemic occurrence frequency, sometimes as much as 25% per annum during the 770 B.C. to 1911 A.D. period9. At least 365 major documented plague outbreaks throughout Chinese history (from 674 B.C. to 1911 A.D.) have been identified. Two particular spikes, documented outbreaks which occurred during the Han dynasty to the Three Kingdoms period (25–255 A.D) and again from the end of the Ming dynasty to the beginning of the Qing dynasty (1622–1656 A.D.)10, 11, 12. Hundreds of years of treating epidemics, TCM epidemiology development has enriched and rapidly advanced. These resultant TCM theories have contributed to a notable decrease in mortality during those times13. A large number of empirical prevention strategies thus developed due to the practical necessities for fighting plague, including advances in the development of preventative herbal formulations, aromatic herb applications, fumigation, quarantine and disinfection (556–559 A.D.)14, vaccination (1695 A.D.)15,16, hygiene, and sanitation maintenance, etc17, 18, 19. More recently, China practiced combining Western and Chinese medicines, which have significantly reduced mortality rates in outbreaks of encephalitis B (1955–1957)20, 21, 22, 23, and severe acute respiratory syndrome (SARS) in 200324,25. The rich experience and herbal knowledgebase in fighting pandemics, are now explored here as a foundation to further explore potential effective components and combinations in herbal medicines using more recent advances in data analysis.
2. Materials and methods
The National Health Commission of China has so far published seven editions of clinical guidelines of Recommended Approaches for COVID-19 Diagnoses and Treatments26 (the fifth edition was applied in this study), while 24 provinces and Wuhan city have also published their recommended protocols for COVID-19, all of which recommended the application of TCM herbal formulae. Here, 166 modified herbal formulae including 99 classical prescriptions and herbal patents were collected from these sources (Supporting Information Table S1). A dataset of standardized names was then compiled, by substituting all of the polysemes, synonyms and acronyms of the herbs according to Chinese Pharmacopoeia (2015 edition)27 and Chinese Materia Medica28. The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform29 (http://tcmspw.com/tcmsp.php) was then utilized for searching the chemical components of 179 single herbs. Only components with oral bioavailability (OB) > 30% and drug-likeness (DL) > 0.18 were included. The frequency of occurrence of herbal formulae and single herbs were calculated, in addition to the frequency of therapeutic effects, categories of medicinal properties, and chemical components.
The core combinations of herbs and new prescriptions were analyzed using modified mutual information30 and unsupervised hierarchical clustering techniques31. Additionally, scaffold analysis, self-organizing mapping (SOM), and cluster analysis [run by custom reconfigured, Datawarrior, in a JAVA runtime framework within a Mac OS Catalina v.10.15.3 (19D76) environment] were employed to classify diverse herbal medicines into families of structures which are distinctly dissimilar for further evaluation as candidates in preventing and treating COVID-19. The 1212 chemical components were converted to Murcko scaffolds, dissecting molecules into ring systems, linkers, side chain atoms, and frameworks, by scaffold analysis32. SOM arranges the position of these structures in two-dimensional space, after which the dataset contains inherently similar structures and is suitable for inclusion and further cluster analysis33. Finally, the cluster map of the complete dataset was computer generated followed by visually assessing the largest common groupings of clusters, by reducing the cluster numbers manually until all the disparate clusters without neighbors were included, but the main clusters remained34.
Figures were plotted using OriginPro 2018C (64-bit) SR1b 9.5.1.195 (One Roundhouse Plaza OriginLab Co., Northampton, MA, USA), R language [no IDE (integrated development environment), R from the Linux terminal R version 3.5.1 (2018-007-02, “Feather Spray”©2018), the R function for Statistic Computing Platform: x86_64-pc-Linux-gnu (64-bit)]35 and Pajek visualization36.
3. Results
3.1. Statistical analysis of herbs and formulae for treating COVID-19 in China
3.1.1. Frequently utilized formulae and individual herbs for treating COVID-19
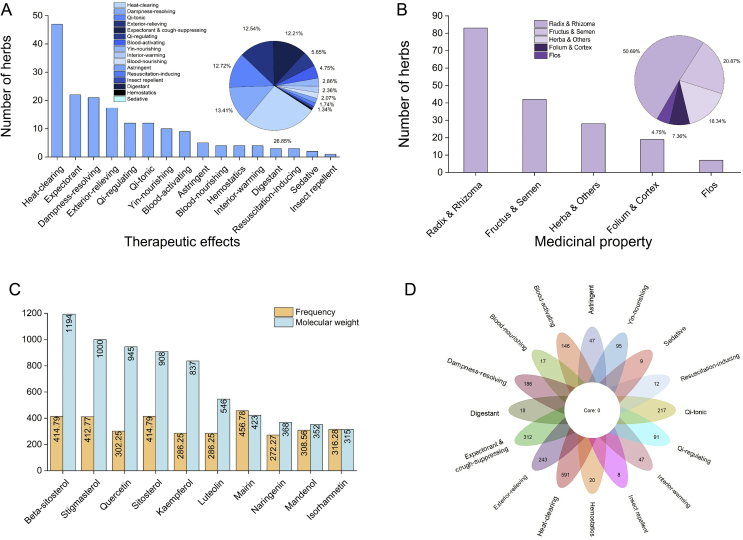
The top 10 most frequently utilized herbal formulae were: An Gong Niu Huang pill, modification of Ephedra and Apricot Kernel decoction (Ma Huang Xing Ren Gan Cao Shi Gao Tang), gypsum and licorice Xuan Bai Cheng Qi decoction, Yin Qiao powder, Zi Xue powder, Xue Bi Jing decoction, Hou Po Xia Ling decoction, Lian Hua Qing Wen decoction, Hou Pu Xia Ling decoction, upbearing and downbearing powder (Sheng Jiang San), and Su He Xiang pill (Supporting Information Table S2). The standardized herb name list consists of 179 herbs (Supporting Information Table S3.1). In total, 99 classical herbal formulae were represented 423 times, and 179 single herbs represented 2760 times. The top 10 most frequently utilized herbs in 166 herbal formulae were Glycyrrhizae Radix et Rhizome (Gan Cao, 139, 83.73%), Scutellariae Radix (Huang Qin, 101, 60.84%), Armeniacae Semen Amarum (Ku Xing Ren, 87, 52.41%), Lonicerae Japonicae Flos (Jin Yin Hua, 82, 49.40%), Forsythiae Fructus (Lian Qiao, 81, 48.80%), Ephedrae Herba (Ma Huang, 74, 44.58%), Poria (Fu Ling, 65, 39.16%), Pogostemon Cablin (Guang Huo Xiang, 63, 37.95%), Citri Reticulatae Pericarpium (Chen Pi, 57, 34.34%), and Platycodonis Radix (Jie Geng, 55, 33.13%, Fig. 2 and Table S3.1). Glycyrrhizae Radix et Rhizoma (Gan Cao) was most frequently utilized in observational, mild, moderate, severe, and critical stages. Scutellariae Radix (Huang Qin) and Armeniacae Semen Amarum (Ku Xing Ren) were most frequently applied in moderate and severe stages, while Scutellariae Radix was also mostly utilized in critical stage and Armeniacae Semen Amarum in mild stage. Other frequently utilized in each clinic stage include Lonicerae Japonicae Flos (Jin Yin Hua, observational stage), Astragali Radix (Huang Qi, observational stage), Gardeniae Fructus (Zhi Zi, critical stage), Platycodonis Radix (Jie Geng, mild stage), Aconiti Lateralis Radix Praeparata (Fu Zi, critical stage), Poria (Fu Ling, recovery and severe stages), and Ophiopogonis Radix (Mai Dong, severe stage). The most frequently applied herbs are antipyretic (47, 26.26%), expectorant and cough-suppressing (22, 12.29%), and dampness-resolving (21, 11.73%) in nature (Fig. 2 and Table S3.2), while the medicinal parts of the most frequently applied herbs are Radix and Rhizome (83, 46.37%), Fructus and Semen (42, 23.46%), and Herba and others (28, 15.64%, Fig. 2 and Tables S3.3 and S3.4). As 23 herbal medicines without fully defined chemical components were excluded, a total of 1212 chemical components with OB>30% and DL > 0.18 were collected from the remaining 156 herbs. The most frequently used chemical components possibly related to the antiviral signing pathway are β-sitosterol (1194), stigmasterol (1000), and quercetin (945, Fig. 2 and Supporting Information Fig. S1). Categorizing a total of 1212 components into 16 groups by traditional therapeutic effects, 591 components belong to the heat-clearing class, 312 belong to expectorant and cough-suppressing, and 243 belong to exterior-relieving class (Fig. 2 and Fig. S1).
Figure 2.
Frequency of individual herbs by therapeutic effects, medicinal property, and chemical component categories. (A) Application of most frequent herbs found in 16 categories of traditional therapeutic effects. (B) Application of most frequent herbs found with five medicinal properties. (C) Application of most frequent top 10 herbs by chemical component categories and their molecular weights (MW). (D) Frequency of 1212 chemical components in 16 categories of traditional therapeutic effects. The bar chart shows the number of herbs (n = 179) in each category, and the pie chart shows the frequency of each category in a total of 2760 times of herbal applications (n = 2760); 23 herbal medicines were excluded due to incomplete component information in the database.
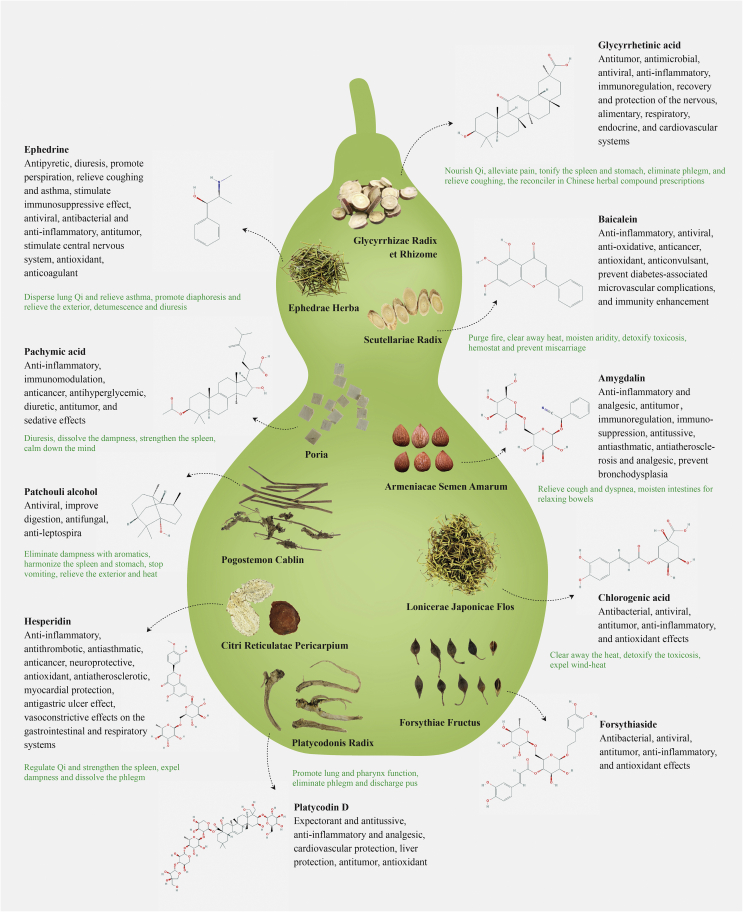
Fig. 3 elucidates the potential therapeutic effects of the 10 most frequently utilized herbal ingredients and their pharmacological activities. These herbal components could provide guidance for future drug discovery to combat COVID-19. Glycyrrhizae Radix et Rhizome (Gan Cao) has long been considered the “excellent reconciler” to assist herb synergy while itself can nourish Qi, alleviate pain, eliminate phlegm and relieve cough38. It contains over 20 kinds of triterpenoids and 300 types of flavonoids. It was reported that its active compounds possess antiviral, antimicrobial, anti-inflammatory, and immunoregulatory activities that contribute to neuro-regenerative and -protective, alimentary, respiratory, endocrine and cardiovascular systems38. Several reports showed that glycyrrhizin, 18β-glycyrrhetinic acid, and other flavonoids isolated from Glycyrrhizae Radix et Rhizome (Gan Cao)possess anti-inflammatory activity. Its extracts support beneficial effects in acute and chronic inflammatory conditions39, 40 and significant antiviral activities against human immunodeficiency virus (HIV), SARS-CoV, herpes simplex virus (HSV), influenza virus (IAV-H3N2), rotavirus, enterovirus, coxsackievirus, varicella zoster virus, and respiratory syncytial virus41, 42, 43, 44. It was accepted that glycyrrhizin and 18β-glycyrrhetinic acid suppress proinflammatory cytokine cyclooxygenase-2 (COX-2), myeloperoxidase, iNOS, TNF-α, NF-κB, HMGP 1, PGE 2, DPPH radicals, IL-6, IL-10, and TGF-β, as well as activation of ABCA145, 46, 47, 48, 49, 50, 51, 52. In vitro studies have shown that glycyrrhizin inhibits influenza virus by decreasing HMGB1 binding and restraining interactions between host proteins and viral macromolecules53, HIV by controlling virus replication54, and H5N1 by controlling H5N1-induced proinflammatory gene expression55. Additionally, glycyrrhizin, 18β-glycyrrhetinic acid, and licochalcone A also presented immunoregulatory activity56, 57, 58, 59, 60, 61. Glycyrrhizin revealed a fine immune stimulant and antiviral effect against duck hepatitis virus (DHV)58. A combination of glutamyl-tryptophan and glycyrrhizin exerts a protective effect in reducing the death of H3N2 IAV-infected mice59. In vivo and clinical studies have shown that these compounds protect mice against disseminated candidiasis62, and relieved experimental autoimmune encephalomyelitis in mice63, increased the endpoint serum antibody titers60, and performed as an immune stimulant against DHV61. All these reports affirmed the immunoregulatory activity of Glycyrrhizae Radix et Rhizome (Gan Cao) and indicated that it might be a candidate for novel immunomodulatory medicine development.
Figure 3.
Top 10 most frequently applied herbs with therapeutic effects and chief chemical components possessing well-defined pharmacological activities. Only the chief chemical components with relatively well-elucidated pharmacological activities are shown. Chemical structures were downloaded from the National Center for Biotechnology Information37. Glycyrrhizae Radix et Rhizome (Gan Cao): glycyrrhetinic acid (C30H46O4, MW = 470.7); Scutellariae Radix (Huang Qin): baicalein (C15H10O5, MW = 270.24); Armeniacae Semen Amarum (Ku Xing Ren): amygdalin (C20H27NO11, MW = 457.48); Lonicerae Japonicae Flos (Jin Yin Hua): fhlorogenic acid (C16H18O9, MW = 354.31); Forsythiae Fructus (Lian Qiao): forsythiaside (C29H36O15, MW = 624.65); Ephedrae Herba (Ma Huang): ephedrine (C10H15NO, MW = 165.23); Poria (Fu Ling): pachymic acid (C33H52O5, MW = 528.85); Pogostemon Cablin (Guang Huo Xiang): patchouli alcohol (C15H26O, MW = 222.37); Citri Reticulatae Pericarpium (Chen Pi): hesperidin (C28H34O15, MW = 610.62); Platycodonis Radix (Jie Geng): platycodin D (C57H92O281, MW = 225.49, Supporting Information Table S9).
Scutellariae Radix (Huang Qin) possesses broad therapeutic effects including antipyretic and hemostatic effect, moistening dryness, and detoxifying toxicosis. Flavonoids are the most abundant constituents, pharmacologically active with great potential in treating inflammation, cancer and virus-related diseases64. Its anti-inflammatory and anti-oxidative effects are possibly related to cytokine inhibition, nitric oxide (NO), chemokine, and growth factor production in macrophages65, 66, 67, as confirmed by in vitro and in vivo studies68, 69. Furthermore, Scutellariae Radix (Huang Qin) can inhibit the replication of the influenza virus in mice70. Baicalin demonstrated its anti-inflammatory effect by inhibiting the binding of chemokines to human leukocytes and cells transfected to express specific chemokine receptors71. Its mechanism was studied on COX-2 gene expression in LPS-induced Raw 264.7 cells, and it might inhibit the binding activity of C/EBPb DNA72. Baicalin can also inhibit human immunodeficiency virus type 173, 74, and block DNA synthesis of human cytomegalovirus75, 76.
Armeniacae Semen Amarum (Ku Xing Ren) has been used for the treatment of pain and inflammatory diseases such as asthma, bronchitis, emphysema, constipation, nausea, leprosy and leukoderma77. The herb has been used to reduce fever, relieve cough and quench thirst. Amygdalin is the major compound, which has been reported to have anti-inflammatory and analgesic effects in the inhibition of the COX-2 pathway77. Ephedrae Herba (Ma Huang) has traditionally been used for coughs, colds, bronchial asthma, flu, fever, edema, and arthralgias. Ephedrine is the major isomer comprising 30%–90% of the total alkaloids78. It enhances the release of norepinephrine from sympathetic neurons. The vasoconstrictor and bronchodilator effects explain the traditional use of Ephedraas a nasal decongestant and anti-asthmatic78. It was reported that the growth of H1N1 virus was inhibited when the cells were treated with its extract immediately after infection. Its inhibitory effect was completely or partially reversed by FeCl3, a tannin-reactive agent, suggesting that tannin is one of the active components in the extract78.
Citri Reticulatae Pericarpium (Chen Pi) promotes the circulation of “Qi” and is widely taken for coughing by drying dampness and expectorating phlegm79. Its main components are volatile oils, flavonoids, and alkaloids, possessing wide pharmacological and beneficial effects on the digestive and respiratory systems. Research has shown it having antitumor, antioxidant and anti-inflammatory properties79. Its anti-inflammatory effects are mediated by reducing the secretion of proinflammatory cytokines including NO, TNF-α, IL-1β, and IL-680, 81. Hesperidin has been shown to inhibit inflammatory cell infiltration. The co-administration of hesperidin and naringenin can ameliorate airway structural remodeling and relieving pulmonary symptoms79. It was found that extracts from Glycyrrhizae Radix et Rhizome (Gan Cao), Forsythiae Fructus (Lian Qiao), and Andrographis Paniculata (Chuan Xin Lian) suppressed influenza A virus-induced rantes secretion in human bronchial epithelial cells. It was thereby suggested that these herbs could be effective for chronic inflammatory disorders caused by viral infection81 (Fig. 3 and Supporting Information Tables S5.1 and S5.2).
3.1.2. Core combination of herbs and chemical components by cluster analysis
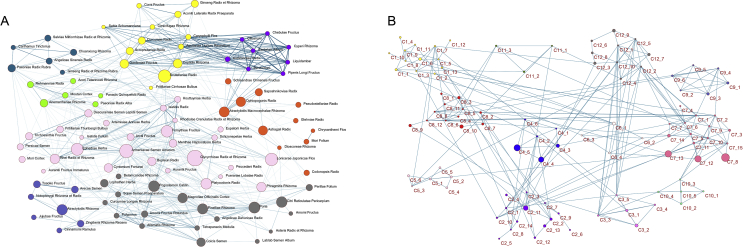
We have explored 8 groups of core combinations by hierarchical clustering with 98 individual herbs whose modified mutual information is over 0.03, drawn from the 166 collected formulae for COVID-19. The first group consists of Fritillariae Cirrhosae Bulbus (Chuan Bei Mu) which are antitussive antiasthmatics, Qi-reinforcing Ginseng Radix et Rhizome (Ren Shen), antipyretic and detoxifying Scutellariae Radix (Huang Qin), and other 10 herbs. The second group includes antipyretic and detoxifying Moutan Cortex (Mu Dan Pi), blood- and liver-nourishing analgesic Paeoniae Radix Alba (Bai Shao), Qi and Yin nourishing and antipyretic Panacis Quinquefolii Radix (Xi Yang Shen). The third core combination contains Citri Reticulatae Pericarpium (Chen Pi), which can strengthen the spleen, harmonize the stomach, move Qi and clear phlegm, Pogostemon Cablin (Guang Huo Xiang) which can harmonize the spleen and stomach, and Poria (Fu Ling) which can strengthen the spleen, dissolve dampness, and calm the mind. The fourth group consists of blood-activating Angelicae Sinensis Radix (Dang Gui), Carthamus Tinctorius (Hong Hua), and Chuanxiong Rhizome (Chuan Xiong), blood-regulating Ginseng Radix et Rhizoma Rubra (Hong Shen), blood-cooling and heat-clearing Paeoniae Radix Rubra (Chi Shao), and blood-cooling and stasis-removing Salviae Miltiorrhizae Radix et Rhizome (Dan Shen). The fifth group is made of antitussive antiasthmatics Armeniacae Semen Amarum (Ku Xing Ren), antipyretic and detoxifying Forsythiae Fructus (Lian Qiao), and diuretic and exterior-releasing Ephedrae Herba (Ma Huang). The sixth group focuses on expelling the pathogen and strengthening the spleen. The seventh group aims at tonifying Qi and nourishing Yin. The last one regulates Qi, warms the interior, and relieves pain (Fig. 4A and Supporting Information Table S6.1). The core combinations found here correspond with the traditional rules of herbal medicine composition and compatibility to reinforce particular functions in the treatment of COVID-19. For core combinations of chemical components, only herbs appeared twice or above and with modified mutual information over 0.1 were included, at last 12 core combinations were obtained. Some chemical components share the same category of traditional therapeutic effect (Fig. 2D), which may signify potential synergistic effects. There are mutual groupings within these core combinations of herbal pairs and chemical components, thus supporting that the herbs and formulae containing these properties may act as potential candidates for COVID-19 treatment (Fig. 4B and Table S6.2).
Figure 4.
Network analyses of core herbal combinations and chemical components. (A) Network analysis of 8 core combinations of herbs. (B) Network analysis of 12 core combinations of chemical components.
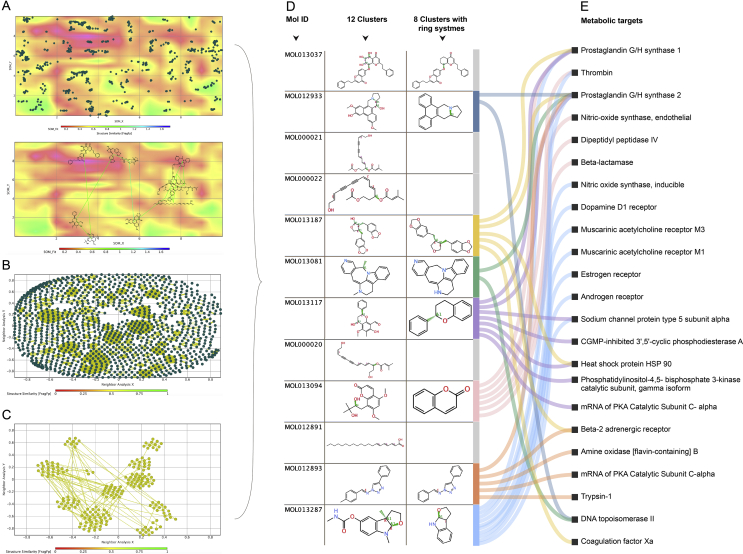
3.1.3. Clusters of structures and active pharmacophores yielded by scaffold and cluster analyses
1164 Murcko scaffolds and 40 aliphatic straight chain fragments were removed from the data and not considered further as they produced useless ring structures (Fig. 5A). SOM resulted in distinctly dispersed map of structural similarly components. Therefore, the dataset is diverse yet contains inherently similar structures suitable for inclusion and further cluster analysis. The clusters were self-organized (SOM) based on the ring system (Fig. 5B). Analysis mapping of the nearest neighbours visually exhibited both diverse clusters and clearly associated families of structures (Fig. 5C). Finally, 12 significant clusters with distinctly different structures were discovered, from which one representing each family were screened in silico for coronavirus activity, against approximately two million molecules using ChEMBL online database82 and their associated pharmacological targets in human metabolic pathways. Eight active pharmacophores were yielded (Fig. 5D and E, and Supporting Information Table S8 and S9) and further scaffold analysis of which yielded a total of 1103 similar-type molecules. Further metabolic targeting of these in silico resulted in 575 (52%) possessing antiviral activity against coronaviruses: corona generally (2%), feline corona (19%), human corona (4%) and SARS (74%), possessing published antiviral assay properties of: inhibition (31%), IC50 (31%), and EC50 (30%). Positive results specifically in the human corona category were for: human coronaviruses, OC43 (12%), 229 E (16%) and NL63 (72%). Herbs containing the same pharmacophore clusters above, due to their chemical scaffold similarity and nearest neighbours, are more likely to behave pharmacologically similar. Therapeutically they may possess synergistic properties when used in combinations due to possessing chemical similarities, yet different when act on similar metabolic targets in slightly a different manner. This is the inverse analog of “shotgun chemistry” where similar compounds are applied en masse, often indiscriminately in either chemical, biological, DNA analysis or in drug discovery83. Further pharmacophore synergy investigations are encouraged and could be thought of “selective shotgun” chemistry based on these findings.
Figure 5.
Scaffold analysis, self-organizing mapping (SOM), and cluster analysis of chemical components. (A) The clusters of chemical component structures self-organized by SOM based on ring system. (B) Cluster similarity analysis of nearest neighbours and clearly associated families of structures. (C) Clusters analysis using manual visual reduction and optimization. (D) Twelve cluster structures and 8 cluster families with ring systems were yielded. (E) Representative metabolic pathways of 8 pharmacophores were screened. The representative pathways were collected from TCMSP29.
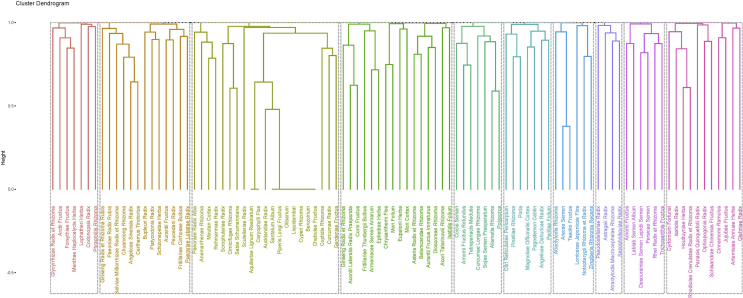
3.1.4. Ten new formulae developed by unsupervised hierarchical clustering
A total of 10 new prescriptions were developed through the unsupervised hierarchical clustering technique from the 166 herbal formulae for COVID-19. The first formula targets (from a TCM perspective) regulating Qi, relieving cough and shortness of breath. One difference in this formula from the others is the inclusion of warm and Qi-tonic herbs, Ginseng Radix et Rhizome (Ren Shen) and Aconiti Lateralis Radix Praeparata (Fu Zi), supporting that this formula can strengthen the healthy Qi and expel pathogenic factors simultaneously. According to the compatibility rules of herbal formulae, Armeniacae Semen Amarum (Ku Xing Ren) and Asteris Radix et Rhizome (Zi Wan) function as the Jun (monarch) to relieve asthma and expel the phlegm; Belamcandae Rhizoma (She Gan) and Chrysanthemi Flos (Ju Hua) function as Chen (minister) to clear the heat and remove the toxin; assisted by Corni Fructus (Shan Zhu Yu) and Dioscoreae Rhizoma (Shan Yao) to strengthen the kidney and spleen; and, Ephedrae Herba (Ma Huang) function as Shi (courier) disperse lung Qi. The second formula mostly consists of diuretic dampness-resolving herbs. Alismatis Rhizoma (Ze Xie) and Polyporus (Zhu Ling) work as Jun to remove dampness; with Tetrapanacis Medulia (Tong Cao) and Coicis Semen (Yi Yi Ren) as Chen to support the diuretic effect of Jun herbs; Amomi Fructus Rotundus (Dou Kou) and Sojae Semen Praeparatum (Dan Dou Chi) as Zuo to dissolve the exterior and dampness; and Curcumae Longae Rhizoma (Jiang Huang) as Shi to regulate Qi and activate blood. The third formula focuses on relieving cough and expelling phlegm. Amomi Fructus (Sha Ren) and Descurainiae Semen Lepidii Semen (Ting Li Zi) are the Jun hers, regulating Qi and relieving asthma; with Lablab Semen Album (Bai Bian Dou) as Chen herb to harmonize spleen and stomach; Trichosanthis Fructus (Gua Lou) and Persicae Semen (Tao Ren) as Zuo herb to expel sputum and remove stasis; and Rhei Radix et rhizoma (Da Huang) as Shi herb to clear heat and remove toxins. The fourth is a modification of classic formula Su He Xiang pill, which mainly consists of aromatic herbs to regulate Qi and relieve pain. Liquidambar (Su He Xiang) and Benzoinum (An Xi Xiang) act as Jun herbs to aid recovery of the mind, dispel cold and relieve pain; Aucklandiae Radix (Mu Xiang) and Caryophylli Flos (Ding Xiang) as Chen herbs to regulate Qi and activate blood; assisted by Piperis Longi Fructus (Bi Ba) and Atractylodis Macrocephalae Rhizoma (Bai Zhu) to strengthen spleen and expel cold; with astringent Chebulae Fructus (He Zi) as Shi herb to balance the formula by preventing excess dispersing. The fifth is a modification of the classic formula Huo Xiang Zheng Qi powder, which releases the exterior and dissolves dampness. Herba Agastaches (Huo Xiang) is an antiperspirant herb, acting as the Jun to dissolve the exterior and expel wind cold; Perillae Folium (Zi Su Ye) and Angelicae Dahuricae Radix (Bai Zhi) act as Chen herbs to strengthen the effect of expelling wind cold, and to regulate Qi and activate blood; Zuo herbs Magnoliae Officinalis Cortex (Hou Po) and Citri Reticulatae Pericarpium (Chen Pi) can regulate Qi, strengthen spleen, and dissolve phlegm; with Poria (Fu Ling) as Shi herb to help dissolving dampness. The sixth is a modification of classic formula Ren Shen Bai Du powder, which releases the exterior and clears internal heat with antipyretic herbs. The antipyretic Jun hers Puerariae Lobatae Radix (Ge Gen) and Bupleuri Radix (Chai Hu) work together to release the exterior; Chen herbs Aurantii Fructus (Zhi Qiao) and Peucedani Radix (Qian Hu) are together to clear heat, expel wind, and dissolve phlegm; Zuo herbs Angelicae Sinensis Radix (Dang Gui) and Chuanxiong Rhizoma (Chuan Xiong) can regulate and activate blood, with Shi herb Platycodonis Radix (Jie Geng) to reinforce the effect of expelling phlegm. The seventh is a modification of classic formula Yin Qiao powder, which emphasizes on clearing heat and releasing the exterior. Jun herb Forsythiae Fructus (Lian Qiao) can release the exterior, clear heat and remove toxin; Chen herbs Lophatheri Herba (Dan Zhu Ye) and Phragmitis Rhizoma (Lu Gen) together reinforce the antipyretic effect of Forsythiae Fructus; assisted by Menthae Haplocalycis Herba (Bo He) and Codonopsis Radix (Dang Shen) to expel wind heat and relieve the throat; with Shi herb Glycyrrhizae Radix et Rhizoma (Gan Cao) to harmonize the whole formula. The eighth formula regulates Qi, releases the exterior and removes dampness. Jun herb Atractylodis Rhizoma (Cang Zhu) can strengthen spleen to dissolve dampness, Chen herbs Tsaoko Fructus (Cao Guo) and Notopterygii Rhizoma et Radix (Qiang Huo) can expel wind damp, regulate Qi, and dispel pathogenic factors; assisted by Arecae Semen (Bing Lang) and Lonicerae Japonicae Flos (Jin Yin Hua) to clear heat toxin and remove dampness; Shi herb Zingiberis Rhizoma Recens (Sheng Jiang) can reinforce spleen, activate blood, and expel cold. The ninth formula nourishes Yin and Qi, reinforces the lung and relieves cough. Jun herbs Panacis Quinquefolii Radix (Xi Yang Shen) and Glehniae Radix (Bei Sha Shen) nourish the Qi and Yin of lung; with Chen herbs Schisandrae Chinensis Fructus (Wu Wei Zi) to nourish Yin and protect the liver and Houttuyniae Herba (Yu Xing Cao) to clear heat toxin of lung; assisted by Cyrtomium Fortune (Guan Zhong) to reinforce the effect of heat toxin removing and diaphoretic herb Cinnamomi Ramulus (Gui Zhi) to release the exterior; Shi herb Jujubae Fructus (Da Zao) reinforces Qi and nourishes blood. The last one is a modification of classic formula Yu Pin Feng powder, which can enhance the Qi and consolidate the exterior. Jun herb Astragali Radix (Huang Qi) consolidates the exterior and reinforces Qi; Chen herb Atractylodis Macrocephalae Rhizoma (Bai Zhu) strengthens spleen and dissolves dampness, Zuo herb Saposhnikoviae Radix (Fang Feng) expels wind and releases the exterior; with Shi herb Pseudostellariae Radix (Tai Zi Shen) to nourish Yin and help strengthen Qi. The 10 formulae developed here may serve as a reference for herbal clinical applications or be used for COVID-19 prevention (Fig. 6 and Table S7).
Figure 6.
New formulae developed by unsupervised hierarchical clustering.
4. Discussion
TCM asserts that pandemic diseases occur naturally and periodically as the climate or geographic situations change. TCM also emphasizes the enhancement of immune function to encourage the body to expel external pathogens. This is also done through the use of herbal formulations derived from careful analysis of syndromes likely to arise in a given year or season. This preventative strategy has also been useful in the treatment of COVID-19. In addition to assisting in the relief of symptoms for most patients in all stages, this approach is especially appealing due to the strong emphasis on both prevention and participation in the recovery of various disorders79. The prevention strategy appears in three parts: preventing occurrence, deterioration, and reoccurrence. TCM seems to have shown encouraging results in reducing the rate of mild illness, deterioration to severe stage and overall mortality as well as shortening total disease duration. When combined with modern biomedicine, herbal medicines could relieve hypoxemia and chronic obstructive pulmonary disease (COPD)84, exert antiviral, anti-inflammatory, and immunoregulatory activities.
The therapeutic effects of TCM in treating COVID-19 have recently been explored by initial clinical trials. It was reported that 139 clinical trials including 47 programs (33.8%) involving TCM interventions have been registered either on Chinese Clinical Trial Registry (www.chictr.org.cn) or the American Clinical Trial Registry (ClinicalTrials.gov)85, 86, 87. An initial randomized controlled trial (RCT) with a total of 52 cases was conducted in a Wuhan hospital at the epicenter of the outbreak. The TCM treatment group (34 cases) showed positive results in symptom relief, reduction of body temperature, length of the average hospital stay in reductions of incidences of middle stage transforming to the severe stage and increased recovery rates, yet a larger number of cases needs to further justify the results87. It was reported that 33 out of 86 COVID-19 cases (including 65 severe cases and 31 critical cases) were cured with integrative treatments by relieving symptoms and preventing the deterioration to severe stage88. The Chinese herbal formula Lian Hua Qing Wen decoction significantly improved symptoms of fever, cough, fatigue and shortness of breath89. Further evidence-based studies are needed to prove the therapeutic effects of TCM in defeating COVID-19.
TCM is a combination of abundant co-occurring bioactive compounds, an invaluable source of therapeutic agents used over a relatively long period in history90. Medicinal compatibility models can now reduce the number of targets for the identification of active ingredients, which makes the arbitrary nature of the drug discovery process more efficient and effective7. Plant-based medicines remain the most principle source of therapeutics for much of the world's population, and plant-derived products remain important sources for much of the world's population. The development of artemisinin-based antimalarials represents one of the great recent achievements for ethnomedicine91. Many initial RCTs have been conducted proving the effects of herbal formulae for treating infectious diseases with encouraging results92. In 2011, a clinical trial was conducted comparing the effects between oseltamivir and Ma Xing Shi Gan-Yin Qiao San decoction for H1N1, which suggested that Ma Xing Shi Gan-Yin Qiao San could be used as an alternative treatment of H1N1 influenza virus infection93.
This study relies on computational analysis, which has the inherent weakness of not possessing aspects of qualitative judgment and “common sense”, that human conducted analysis possesses. To offset this as much as practically possible, this study's scope only focused on herbal medicines which have been developed, selected and proficiently used in practice over thousands of years. Additionally, it included herbs only which have been well studied, possessing “drug like” bioavailability, further bringing a “real-world”, pharmacological information to the initial dataset. The application of relatively novel computational, pattern recognition and in silico methodologies to a preselected knowledge base of herbal formulae most frequently used, in-part attempts to overcome the shortcomings of the network analysis and computational analysis approach. The 8 resulting pharmacophores and core combinations were based on firstly traditional use, secondly “drug like” characteristics and thirdly reported in silico bioactive pharmacophore structure. Therefore, we propose they present the most likely candidates, taking the constraints of this study into account, to be included, in few multiples or in combination to support the composition of completely novel COVID-19 herbal formulae. The strengths of computational methods far outweigh their weaknesses. They bestow the ability to recognize and extrapolate useful patterns from herbal datasets which would otherwise take many years, if at all possible for human researchers to explore and uncover. The combination of this blended approach, as presented here, utilizes the combined advantages of both, to enable new useful knowledge obtained from a meaningful practical foundation.
The pharmacologically active ingredients of a phyto-complex are not always the original molecules in its natural state, but maybe their host-specific metabolites or molecular complexes formed following co-administration with other herbs. The multicomponent nature of traditional medicines leads to multiple potential molecular interactions, multiple targets, and a myriad of metabolic byproducts, which requires a more network-oriented and holistic approach94. The approach could be further refined by the application of omics technologies to optimize the synergistic effect of herbal remedies. With clinicians and basic researchers creating a database of personal therapeutic responses, continual improvements to the evolution and creation of herbal formulations would become possible5. Integration of Western and Chinese medical conceptual perspectives has enormous potential for constructing modern technological and social innovations. They are poised to merge within the arena of personalized medicine systems, wherein patients can take a greater role in managing their health and wellness95. In a time of increased international awareness of the environment, health and personal responsibility for mankind, an integration of East and West should most likely be mutually beneficial91. As the global spread of COVID-19 continues to worsen, it is necessary to disseminate the many advantages of traditional medicines for the prevention and treatment of COVID-19. Bridging the gap between traditional and conventional medicine during this global COVID-19 spread is both timely and crucial. We sincerely hope that this study will serve as a reference to the global COVID-19 research community.
Acknowledgments
The authors thank Dr. Zheng Yu, Dr. Jialie Sun, Dr. Changhe Yu, Dr. Jason D. Robertson, Dr. Jonathan Chang, Dr. Lei Fan, Dr. Xu Zhang, Jiaxing Liu, and Xin Xiong for their contributions to this article.
Author contributions
Shilin Chen conceived the study. Weifeng Hu, Yumei Zhou, and Lu Luo collected the COVID-19 protocols and standardized the lists. Weifeng Hu, Jingwen Jiang, Martin Fitzgerald and Lu Luo analyzed the data. Jingwen Jiang, Cheng Wang, Martin Fitzgerald, Hui Zhang, and Lu Luo plotted the figures. Shilin Chen and Lu Luo drafted the first version of the manuscript, and all authors proofread the final text.
Conflicts of interests
All authors declare no conflict of interests.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.05.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus disease (COVID-2019) situation report-36. 25 Feb 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200225-sitrep-36-covid-19.pdf?sfvrsn=2791b4e0_2 Available from: Geneva, Switzerland.
- 2.Ryan J. CNET; USA: 11 March 2020. WHO declares coronavirus outbreak a pandemic.https://www.cnet.com/news/who-declares-coronavirus-outbreak-a-pandemic/ Available from: [Google Scholar]
- 3.CNR News National Administration of Traditional Chinese Medicine: the total effective rate of traditional Chinese medicine is over 90% 23 Mar 2020. https://baijiahao.baidu.com/s?id=1661946820250968958&wfr=spider&for=pc Available from: China.
- 4.He W., Han H., Wang W., Gao B. Anti-influenza virus effect of aqueous extracts from dandelion. Virol J. 2011;8:538. doi: 10.1186/1743-422X-8-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witt C.M., Liu J., Robinson N. Combining omics and comparative effectiveness research: evidence-based clinical research decision-making for Chinese medicine. Science. 2015;346:S10–S12. [Google Scholar]
- 6.Leung E., Wong V., Jiang Z., Li T., Liu L. Integrated network-based medicine: the role of traditional Chinese medicine in developing a new generation of medicine. Science. 2014;346:S16–S19. [Google Scholar]
- 7.Wang Y., Xu A., Zheng A systems biology approach to diagnosis and treatments. Science. 2014;346:S13–S15. [Google Scholar]
- 8.Zhao X., Zheng X., Fan T.P., Li Z., Zhang Y., Zheng J. A novel drug discovery strategy inspired by traditional medicine philosophies. Science. 2015;347:S38–S40. [Google Scholar]
- 9.Gong S. Changes of the temporal-spatial distribution of epidemic disasters in 770 B.C.−1911 A.D. China. Acta Geograph Sin. 2003;58:878. [Google Scholar]
- 10.Wang Y. Chronology of epidemic diseases in ancient China (674 B.C.−1911 A.D.) J Tianjin Uni Tradit Chin Med. 2003;3:84–88. [Google Scholar]
- 11.Wang Y. Chronology of epidemic diseases in ancient China (674 B.C.−1911 A.D.) J Tianjin Uni Tradit Chin Med. 2003;4:33–36. [Google Scholar]
- 12.Zhang Z. Fujian Science and Technology Press; Fujian: 2007. Epidemic chronology of ancient China. [Google Scholar]
- 13.Liang F. Zhonghua Book Company; Beijing: 2008. Statistics of household registration, land and tax in the ancient dynasties of China. [Google Scholar]
- 14.Liang Z., Zhao W. A textual research on the origin of “rooms for leprosy” in China and an investigation of its sites. Chin J Leprosy Skin Dis. 1985;6:79–83. [Google Scholar]
- 15.Zhang L. China Press of Traditional Chinese Medicine; Beijing: 1995. Dr. Zhang's medical treatment (Zhang Shi Yi tong) [Google Scholar]
- 16.Yu T. Engraved edition of Li Songshou. Directorate of Imperial Academy; Beijing: 1876. Solution to pox family diseases (Dou Ke Jin Jing Fu Ji Jie) [Google Scholar]
- 17.Li S. Beijing Publishing House; Beijing: 2007. Compendium of Materia Medica. [Google Scholar]
- 18.Sun S. Traditional Chinese Medicine Classics Press; Beijing: 1999. Invaluable prescriptions for ready reference. [Google Scholar]
- 19.Zhangsun W. Shanghai Classics Publishing House; Shanghai: 2013. Comment on the law in Tang dynasty. [Google Scholar]
- 20.Han D. A preliminary report on 102 cases of epidemic encephalitis B treated by TCM in rural areas. Shanghai J Tradit Chin Med. 1958;8:8–11. [Google Scholar]
- 21.Wang H., Li C. A report on the therapeutic effect of traditional Chinese medicine on empidemic encephalitis B—summary of TCM treatment group in 1956. J Zhengzhou Univ (Med Sci) 1958;1:1–7. [Google Scholar]
- 22.Unnited TCM hospital of Quanzhou, Fujian provice. A preliminary report on 83 cases of epidemic encephalitis B treated by traditional Chinese medicine. Fujian J Tradit Chin Med. 1957;2:8–10. [Google Scholar]
- 23.Cai Y. Clinical report of ten cases of epidemic encephalitis B treated by traditional Chinese medicine. Fujian J Tradit Chin Med. 1956;2:13–16. [Google Scholar]
- 24.Liu J., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Alter Complem Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 25.WHO . 8–10 October 2003. SARS: clinical trials on treatment using a combination of traditional Chinese medicine and Western medicine: report of the WHO International Expert Meeting to review and analyse clinical reports on combination treatment for SARS. Beijing, China. [Google Scholar]
- 26.National Health and Health Commission Notice on COVID-19 diagnosis and treatment plan (the sixth version) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml Beijing, China. 19 Feb 2020. Available from:
- 27.National Pharmacopoeia Commission . China Medical Science Press; Beijing: 2015. Chinese Pharmacopoeia. Part IV. [Google Scholar]
- 28.State Administration of Traditional Chinese Medicine (SATCM) Shanghai Scientific & Technical Publishers; Shanghai: 1999. Chinese Materia Medica. [Google Scholar]
- 29.Lab of Systems Pharmacology . Nov 2013. Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform.http://tcmspw.com/tcmsp.php Available from: [Google Scholar]
- 30.Tang S., Chen J., Geng L., Wu H., Chen C., Zhang N. Research on component law of Chinese patent medicine for anti-influenza and development of new recipes for anti-influenza by unsupervised data mining methods. J Tradit Chin Med. 2010;30:288–293. doi: 10.1016/s0254-6272(10)60058-1. [DOI] [PubMed] [Google Scholar]
- 31.Aghagolzadeh M., Soltanian-Zadeh H., Araabi B., Aghagolzadeh A. A hierarchical clustering based on mutual information maximization. Proc Int Conf Image Process, ICIP. 2007;7:277–280. doi: 10.1109/ICIP.2007.4378945. September 16–19, San Antonio, TX, USA. Institute of Electrical and Electronics Engineers. Available from: [DOI] [Google Scholar]
- 32.Yongye A.B., Waddell J., Medina-Franco J.L. Molecular scaffold analysis of natural products databases in the public domain. Chem Biol Drug Des. 2012;80:717–724. doi: 10.1111/cbdd.12011. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Li L., Yu C., Yan A., Zhao Z., Zhang G. Classification of mixtures of Chinese herbal medicines based on a self-organizing map (SOM) Mol Inform. 2016;35:109–115. doi: 10.1002/minf.201500115. [DOI] [PubMed] [Google Scholar]
- 34.Kunimoto R., Bajorath J. Combining similarity searching and network analysis for the identification of active compounds. ACS Omega. 2018;3:3768–3777. doi: 10.1021/acsomega.8b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The R Core Team R: a language and environment for statistical computing. https://cran.r-project.org/doc/manuals/fullrefman.pdf Version 4.5.2., 22 Jun 2020. Copyright© 1999–2012 R Foundation for Statistical Computing. Jun 2019. Available from:
- 36.Mrvar A., Batagelj V. Programs for analysis and visualization of very large networks. Pajek. 2018;5:3–108. [Google Scholar]
- 37.NIH . 2020. U.S. national library of medicine, national center for biotechnology information.https://pubchem.ncbi.nlm.nih.gov Available from: Bethesda, USA. [Google Scholar]
- 38.Yang R., Wang L.Q., Yuan B.C., Liu Y. The pharmacological activities of licorice. Planta Med. 2015;81:1654–1669. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- 39.Nirmala P., Selvaraj T. Anti-inflammatory and anti-bacterial activities of Glycyrrhiza glabra L. Int J Agric Technol. 2011;7:815–823. [Google Scholar]
- 40.Wu T., Khor T., Saw C., Loh S., Chen A., Lim S. Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13:1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J Am Med Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 42.Wang J., Chen X., Wang W., Zhang Y., Yang Z., Jin Y. Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J Ethnopharmacol. 2013;147:114–121. doi: 10.1016/j.jep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon H.J., Kim H.H., Ryu Y.B., Kim J.H., Jeong H.J., Lee S.W. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg Med Chem. 2010;18:7668–7674. doi: 10.1016/j.bmc.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 44.Shebl R.I., Amin M.A., Emad-Eldin A., Bin D.S., Mostafa A.S., Ibrahim E.H. Antiviral activity of liquorice powder extract against varicella zoster virus isolated from Egyptian patients. Chang Gung Med J. 2012;35:231–239. doi: 10.4103/2319-4170.106149. [DOI] [PubMed] [Google Scholar]
- 45.Luo L., Jin Y., Kim I.D., Lee J.K. Glycyrrhizin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Exp Neurobiol. 2013;22:107–115. doi: 10.5607/en.2013.22.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai K., Takagi Y., Iwazaki A., Nakanishi K. Radical scavenging ability of glycyrrhizin. Free Radic Antioxidants. 2013;3:40–46. [Google Scholar]
- 47.Fu Y., Zhou E., Wei Z., Liang D., Wang W., Wang T. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014;281:2543–2557. doi: 10.1111/febs.12801. [DOI] [PubMed] [Google Scholar]
- 48.Ni Y.F., Kuai J.K., Lu Z.F., Yang G.D., Fu H.Y., Wang J. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cycloox-ygenase-2 and inducible nitric oxide synthase expression. J Surg Res. 2011;165:e29–e35. doi: 10.1016/j.jss.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Wang C.Y., Kao T.C., Lo W.H., Yen G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acidmodulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem. 2011;59:7726–7733. doi: 10.1021/jf2013265. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharjee S., Bhattacharjee A., Majumder S., Majumdar S.B., Majumdar S. Glycyrrhizic acid suppresses Cox-2-mediated anti-inflammatory responses during Leishmania donovani infection. J Antimicrob Chemother. 2012;67:1905–1914. doi: 10.1093/jac/dks159. [DOI] [PubMed] [Google Scholar]
- 51.Ishida T., Miki I., Tanahashi T., Yagi S., Kondo Y., Inoue J. Effect of 18β-glycyrrhetinic acid and hydroxypropyl γ-cyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur J Pharmacol. 2013;714:125–131. doi: 10.1016/j.ejphar.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Oztanir M.N., Ciftci O., Cetin A., Durak M.A., Basak N., Akyuva Y. The beneficial effects of 18betaβ-glycyrrhetinic acid following oxidative and neuronal damage in brain tissue caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol Sci. 2014;35:1221–1228. doi: 10.1007/s10072-014-1685-9. [DOI] [PubMed] [Google Scholar]
- 53.Moisy D., Avilov S.V., Jacob Y., Laoide B.M., Ge X., Baudin F. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. J Virol. 2012;86:9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2002;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 55.Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med Microbiol Immunol. 2010;199:291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M.E., Kim H.K., Kim D.H., Yoon J.H., Lee J.S. 18β-Glycyrrhetinic acid from licorice root impairs dendritic cells maturation and Th1 immune responses. Immunopharmacol Immunotoxicol. 2013;35:329–335. doi: 10.3109/08923973.2013.768636. [DOI] [PubMed] [Google Scholar]
- 57.Ma C., Ma Z., Liao X.L., Liu J., Fu Q., Ma S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4+ CD25+ Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J Ethnopharmacol. 2013;148:755–762. doi: 10.1016/j.jep.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 58.Li J., Tu Y., Tong L., Zhang W., Zheng J., Wei Q. Immunosuppressive activity on the murine immune responses of glycyrol from Glycyrrhiza uralensis via inhibition of calcineurin activity. Pharm Biol. 2010;48:1177–1184. doi: 10.3109/13880200903573169. [DOI] [PubMed] [Google Scholar]
- 59.Lee J.Y., Lee J.H., Park J.H., Kim S.Y., Choi J.Y., Lee S.H. Liquiritigenin, a licorice flavonoid, helps mice resist disseminated candidiasis due to Candida albicans by Th1 immune response, whereas liquiritin, its glycoside form, does not. Int Immunopharm. 2009;9:632–638. doi: 10.1016/j.intimp.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Fontes L.B., Dos Santos Dias D., Carvalho L.S., Mesquita H.L., Silva Reis L., Dias A.T. Immunomodulatory effects of licochalcone A on experimental autoimmune encephalomyelitis. J Pharm Pharmacol. 2014;66:886–894. doi: 10.1111/jphp.12212. [DOI] [PubMed] [Google Scholar]
- 61.Hendricks J.M., Hoffman C., Pascual D.W., Hardy M.E. 18β-Glycyrrhetinic acid delivered orally induces isolated lymphoid follicle maturation at the intestinal mucosa and attenuates rotavirus shedding. PloS One. 2012;7 doi: 10.1371/journal.pone.0049491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soufy H., Yassein S., Ahmed A.R., Khodier M.H., Kutkat M.A., Nasr S.M. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. Afr J Tradit, Complementary Altern Med. 2012;9:389–395. doi: 10.4314/ajtcam.v9i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smirnov V.S., Zarubaev V.V., Anfimov P.M., Shtro A.A. Effect of a combination of glutamyl-tryptophan and glycyrrhizic acid on the course of acute infection caused by influenza (H3H2) virus in mice. Vopr Virusol. 2012;57:23–27. [PubMed] [Google Scholar]
- 64.Li C., Lin G., Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32:427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 65.Yoon S.B., Lee Y.J., Park S.K., Kim H.C., Bae H., Kim H.M. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009;125:286–290. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 66.Chen C.S., Chen N.J., Lin L.W., Hsieh C.C., Chen G.W., Hsieh M.T. Effects of Scutellariae Radix on gene expression in HEK 293 cells using cDNA microarray. J Ethnopharmacol. 2006;105:346–351. doi: 10.1016/j.jep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Choi J., Conrad C.C., Malakowsky C.A., Talent J.M., Yuan C.S., Gracy R.W. Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. Biochim Biophys Acta. 2002;1571:201–210. doi: 10.1016/s0304-4165(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 68.Kang K., Oh Y.K., Choue R., Kang S.J. Scutellariae radix extracts suppress ethanol-induced caspase-11 expression and cell death in N2a cells. Brain Res Mol Brain Res. 2005;142:139–145. doi: 10.1016/j.molbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Schinella G.R., Tournier H.A., Prieto J.M., Mordujovich de Buschiazzo P., Rios J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 70.Chu M., Chu Z.Y., Wang D.D. The extract of compound Radix Scutellariae on mRNA replication and IFN expression of influenza virus in mice. Zhong Yao Cai. 2007;30:63–65. [PubMed] [Google Scholar]
- 71.Li B.Q., Fu T., Gong W.H., Dunlop N., Kung H., Yan Y. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000;49:295–306. doi: 10.1016/s0162-3109(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 72.Woo K.J., Lim J.H., Suh S.I., Kwon Y.K., Shin S.W., Kim S.C. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPβDNA-binding activity. Immunobiology. 2006;211:359–368. doi: 10.1016/j.imbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Ahn H.C., Lee S.Y., Kim J.W., Son W.S., Shin C.G., Lee B.J. Binding aspects of baicalein to HIV-1 integrase. Mol Cell. 2001;12:127–130. [PubMed] [Google Scholar]
- 74.Kitamura K., Honda M., Yoshizaki H., Yamamoto S., Nakane H., Fukushima M. Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res. 1998;37:131–140. doi: 10.1016/s0166-3542(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 75.Evers D.L., Chao C.F., Wang X., Zhang Z., Huong S.M., Huang E.S. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antivir Res. 2005;68:124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shao Z.H., Li C.Q., Vanden Hoek T.L., Becker L.B., Schumacker P.T., Wu J.A. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 1999;31:1885–1895. doi: 10.1006/jmcc.1999.1021. [DOI] [PubMed] [Google Scholar]
- 77.Jung H.J., Kim Y.S., Shin M.S., Kim C.J., Kim Y.S. Effects of Armeniacae Semen and amygdalin on the lipopolysaccaride-induced prostaglandin E2 synthesis and nitric oxide production in mouse BV2 microglial cells. Exp Neurobio. 2008;17:71–78. [Google Scholar]
- 78.Abourashed E.A., El-Alfy A.T., Khan I.A., Walker L. Ephedra in perspective—a current review. Phytother Res. 2003;17:703–712. doi: 10.1002/ptr.1337. [DOI] [PubMed] [Google Scholar]
- 79.Yu X., Sun S., Guo Y. Citri Reticulatae Pericarpium (Chen Pi): botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J Ethnopharmacol. 2018;220:265–282. doi: 10.1016/j.jep.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 80.Li S., Sang S., Pan M.H., Lai C.S., Lo C.Y., Yang C.S., Ho C.T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg Med Chem Lett. 2017;18:5177–5181. doi: 10.1016/j.bmcl.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 81.Lee Y.Y., Lee E.J., Park J.S., Jang S.E., Kim D.H., Kim H.S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J Neuroimmune Pharmacol. 2011;2:294–305. doi: 10.1007/s11481-016-9657-x. [DOI] [PubMed] [Google Scholar]
- 82.Gaulton A., Hersey A., Nowotka M., Bento A.P., Chambers J., Mendez D. The ChEMBL database in 2017. Nucleic Acids Res. 2017;45:D945–D954. doi: 10.1093/nar/gkw1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGhee J.J., Rawson N., Bailey B.A., Fernandez-Guerra A., Sisk-Hackworth L., Kelley S.T. Meta-SourceTracker: application of Bayesian source tracking to shotgun metagenomics. PeerJ. 2020;8 doi: 10.7717/peerj.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Shi J., Liu Y., Zhou H., Cai X., Wen J. Exploration of potential clinical application value and mechanism of Chinese Materia Medica for tonifying qi and activating blood in COVID-19 with hypoxemia. Chin Tradit Herb Drugs. 2020;6:1435–1442. [Google Scholar]
- 85.Han Y., Zhao M., Shi Y., Song Z., Zhou S., He Y. Application of integrative medicine protocols on treatment of coronavirus disease 2019. Chin Tradit Herb Drugs. 2020;2:1–5. [Google Scholar]
- 86.Xie Y., Zhang B., Zhao H. Characteristic analysis on international clinical trial registry of COVID-19. J Chin Med. 2020;3:1–5. [Google Scholar]
- 87.Deng H., Zhao Y., Xu J. Analysis report of evidence-based registered clinical trials of TCM treatment for COVID-19. SH J TCM. 2020;54:14. [Google Scholar]
- 88.Xia W., An C., Zheng C., Zhang J., Huang M., Wang Y. Clinical study on 34 COVID-19 cases treated with integrated medicine. J Chin Med. 2020;2:1–7. [Google Scholar]
- 89.Lv R., Wang W., Li X. COVID-19 suspected cases treated with Lianhua Qingwen Decoction: a clinical observation of 63 cases. J Chin Med. 2020;2:1–5. [Google Scholar]
- 90.Kaptchuk T.J. Contemporary; Chicago: 2000. The web that has no weaver—understanding Chinese medicine. [Chapter 1]: medicine east and west: two ways of seeing, two ways of thinking. [Google Scholar]
- 91.Briggs J.P. A global scientific challenge: learning the right lessons from ancient healing practices. Science. 2014;346:S7–S9. [Google Scholar]
- 92.Luo H., Tang Q., Shang Y., Liang S., Yang M., Robinson Nicola Can Chinese medicine be used for prevention of coronavirus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Intern Med. 2020;1:8. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C., Cao B., Liu Q., Zou Z., Liang Z., Gu L. Oseltamivir compared with the Chinese traditional therapy Maxingshigan–Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011;155:217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 94.Liu R., Runyon R.S., Wang Y., Oliver S.G., Fan T.P., Zhang W. Deciphering ancient combinatorial formulae: the Shexiang Baoxin pill. Science. 2015;347:S40–S42. [Google Scholar]
- 95.Hu Q., Schaufeli W., Taris T., Hessen D., Hakanen J., Salanova M. East is east and west is west and never the twain shall meet: work engagement and workaholism across eastern across Eastern and Western cultures. J Behav Social Sci. 2014;1:6–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.