A 50 year-old man with controlled hypertension and type II diabetes presented after one week of dyspnea and cough with a blood O2 saturation of 90%. A reverse ttranscriptase-polymerase chain eaction assay on a nasopharyngeal swab specimen confirmed severe acute respiratory syndrome coronavirus 2, and a diagnosis of coronavirus disease 2019 (COVID-19) pneumonia. On initial neurologic examination, the patient was alert and fully oriented with fluent speech and no focal deficits. On the second hospital day and while on 2L of supplemental O2 through nasal cannula, blood O2 saturation dropped to 70% and he was intubated. His hospital course was complicated by acute tubular necrosis requiring temporary hemodialysis, and a Klebsiella bacteremia treated with piperacillin/tazobactam. The patient received a 5-day course of hydroxychroloquine-azithromycin. No antiviral medications were given. O2 saturation remained consistently above 95% on mechanical ventilation. Inflammatory markers and radiographic findings of pneumonia gradually improved over 2 weeks. However, the patient continued to have markedly depressed mental status and occasional diffuse myoclonic movements. Neurologic examination showed preserved brainstem reflexes with equal sluggishly reactive pupils (3mm) but no grimace or response to noxious stimuli in extremities. Electroencephalogram (EEG) showed moderate diffuse slowing without epileptiform discharges. One attempted lumbar puncture was unsuccessful.
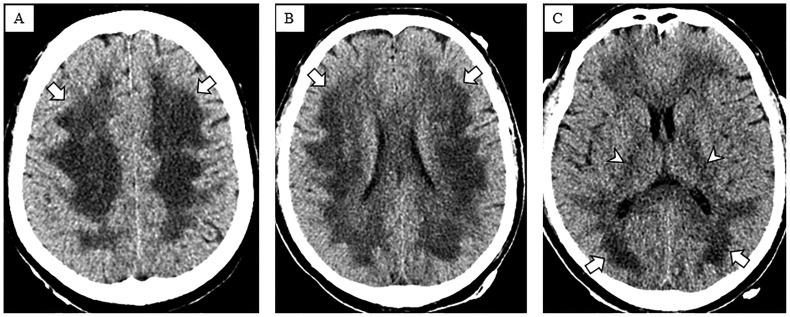
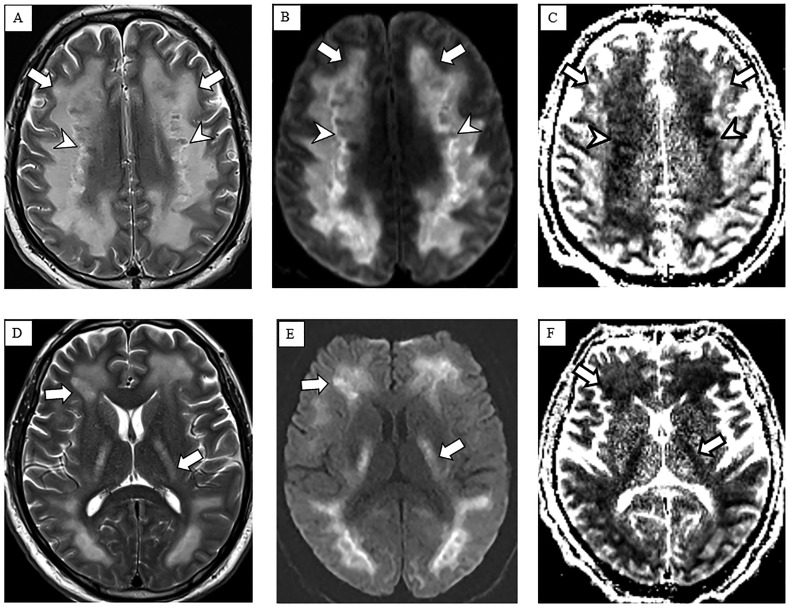
Brain CT on hospital day 17 (Fig. 1 ) showed symmetric confluent hypodensities involving supratentorial white matter. Brain MRI on hospital day 23 (Fig. 2 ) showed symmetric confluent T2 hyperintensities involving the periventricular, deep and subcortical white matter, sparing the U-fibers. There was reduced diffusion in the more central areas of T2 abnormality. Deep gray nuclei, brainstem and cerebellum were spared.
Fig. 1.
Non-contrast head CT. Axial CT images of the head at the level of the centrum semiovale (A), corona radiata (B), and basal ganglia (C) demonstrate diffuse confluent hypodensities in the supratentorial periventricular, deep and subcortical white matter (arrows) extending into the internal capsules (arrowheads).
Fig. 2.
Non-contrast brain MRI. Multiple axial images including T2-weighted (A and D), diffusion-weighted (B and E) and apparent diffusion coefficient (C and F) images demonstrate symmetric confluent supratentorial white matter T2 hyperintensities extending into the internal capsules (arrows in A, D) sparing the subcortical U fibers and deep gray nuclei. Notably, there is reduced diffusion in the more central white matter (arrowheads in B, C) with geographic margins and corresponding internal T2 heterogeneity (arrowheads in A), suggesting areas of active demyelination and necrosis. Susceptibility-weighted images (not shown) did not show any appreciable blood products.
The presumed diagnosis was delayed post-hypoxic leukoencephalopathy (DPHL), with likely necrotizing components, subsequent to acute hypoxic respiratory failure, potentially aggravated by altered cerebral autoregulation as a result of sepsis. MRI findings reflected extensive areas of white matter demyelination with areas of active demyelination (geographic margins of reduced diffusion) and presumed central regions of necrosis (T2 heterogeneity). These characteristics are similar to what has been described in DPHL [1] and are unusual for posterior reversible encephalopathy syndrome or uremic encephalopathy. Our patient did not receive any potentially neurotoxic medications.
Unlike acute hypoxic-ischemic brain injury, which is related to glutamate excitotoxicity and predominantly involves the deep and cortical gray matter, DPHL is thought to result from necrosis of vulnerable oligodendrocytes in arterial border zones during a hypoxic insult and failed myelin turnover, leading to widespread demyelination with axonal sparing, without myelin vacuolization [2]. Relative deficiency of arylsulfatase A has been suggested as a potential predisposing factor in some DPHL patients (not tested in our patient) [3]. Previously reported cases of DPHL were mostly due to carbon monoxide poisoning, drug overdose or cardiac arrest [1]. Although DPHL was recently described in critically ill COVID-19 patients [5], to our knowledge, this is the first report of a COVID-19-associated presumed necrotizing DPHL. In a COVID-19 patient with persistently depressed mental state despite treated pneumonia and improved respiratory status, DPHL and brain imaging evaluation should be considered [4]. Treatment is mainly supportive, and prognosis is highly variable but usually poor with permanent disability [2]. For our patient, plan of care discussions with the family are ongoing.
References
- 1.Zamora C.A., Nauen D., Hynecek R. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav. 2015;5(8) doi: 10.1002/brb3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King F., Morris N.A., Schmahmann J.D. Delayed Posthypoxic Leukoencephalopathy: improvement with antioxidant therapy. Case Rep Neurol. 2015;7(3):242–246. doi: 10.1159/000441892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottfried J.A., Mayer S.A., Shungu D.C., Chang Y., Duyn J.H. Delayed posthypoxic demyelination: association with arylsulfatase a deficiency and lactic acidosis on proton MR spectroscopy. Neurology. 1997;49:1400–1404. doi: 10.1212/wnl.49.5.1400. [DOI] [PubMed] [Google Scholar]
- 4.kim J.H., Chang K.H., Song I.C. Delayed encephalopathy of acute carbon monoxide intoxication: diffusivity of cerebral white matter lesions. AJNR Am. J. Neuroradiol. 2003;24:1592–1597. [PMC free article] [PubMed] [Google Scholar]
- 5.Radmanesh A., Derman A., Lui Y.W. COVID-19 associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]