Abstract
We describe the institutional guidelines of a major tertiary cancer center with regard to using hypofractionated radiation regimens to treat glioblastoma as a measure to minimize exposure to coronavirus disease 2019 (COVID-19) while not sacrificing clinical outcomes. Our guidelines review level one evidence of various hypofractionated regimens, and recommend a multidisciplinary approach while balancing the risk of morbidity and mortality among individuals at high risk for severe illness from COVID-19 infection. We also briefly outline strategies our department is taking in mitigating risk among our cancer patients undergoing radiation.
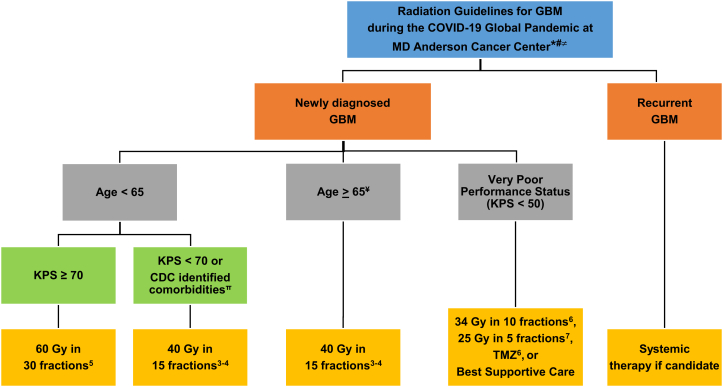
In the midst of the coronavirus disease 2019 (COVID-19) pandemic, minimizing the risk of exposure for both patients and health care personnel is critical. Early data suggest that patients with cancer are particularly susceptible to severe illness due to COVID-19.1 Delivering a course of radiation therapy (RT) involves multiple person-to-person interactions at the individual level over many weeks, including between patients, physicians, nurses, radiation therapists, physicists, and others. Thus, many radiation oncology facilities are identifying measures to reduce exposure of patients and providers to COVID-19. Therefore, radiation oncologists are revisiting the role of hypofractionated RT regimens to decrease treatment time and exposure for all involved. To that end, our central nervous system working group at a large tertiary cancer care center has drafted guidance for selecting patients with glioblastoma (GBM) who would benefit from hypofractionated RT in the era of COVID-19 (Fig 1).
Figure 1.
Algorithm of Radiation Guidelines for GBM during the COVID-19 Global Pandemic at MD Anderson Cancer Center: Radiation recommendations regarding GBM in asymptomatic, COVID-19 negative, and/or patients not requiring self-quarantine at a major tertiary cancer center. ∗: Healthcare guidelines during COVID-19 pandemic are dynamic and application of the guidelines outlined should be in accordance with local institutional, state, and federal guidelines. Consider travel restrictions for new patients and use of a telemedicine platform to monitor patients for side-effects while on treatment. #: recommend multidisciplinary discussion with radiation oncology, neuro-oncology regarding systemic therapy, and neurosurgery. ≠: In patients who have tested COVID-19 negative, we recommend re-testing if the index of suspicion for COVID-19 infection is high. If a patient becomes COVID-19 positive during RT, we recommend holding RT until the patient self-quarantines for 14 days after positive test date. The patient is then screened for symptoms after completion of self-quarantine and the patient’s case undergoes multidisciplinary review to determine if he/she can resume RT. ¥: consider 60 Gy in 30 fractions with excellent performance status and without high risk CDC identified COVID-19 comorbidities. π: consider risk/benefits of hypofractionated RT if patient has the following uncontrolled comorbidities as identified by the CDC: chronic lung disease, moderate to severe asthma, serious heart conditions, immunocompromised, severe obesity, diabetes, chronic kidney disease undergoing dialysis, liver disease. Abbreviations: CDC = Center for Disease Control and Prevention; GBM = Glioblastoma; KPS = Karnofsky Performance Status; RT = Radiation treatment; TMZ = Temozolamide.
According to the Centers for Disease Control and Prevention, patients aged ≥65 years and those with uncontrolled comorbidities (primarily cardiopulmonary conditions; Fig. 1) are considered at risk for severe illness from COVID-19.2 Based on these risk factors, we recommend that patients aged ≥65 years with GBM who present for first-course RT should strongly be considered for hypofractionated RT regimens, in particular 40 Gy in 15 fractions, as supported by phase 3 data (Fig 1).3,4 Special considerations can be made if these older patients have excellent performance status (PS) and are without major medical comorbidities. For patients age <65 years with good PS (Karnofsky PS ≥70), we continue to favor standard fractionation with 60 Gy in 30 fractions.5 However, individualized risk/benefit considerations should be made for younger patients, even those with a satisfactory PS, if they have risk factors associated with increased COVID-related morbidity and mortality as identified by the Centers for Disease Control and Prevention. This can be made in a shared decision-making model with patients (Fig 1). Together, for patients who present for first-course RT in the treatment of GBM, our algorithm broadly favors the use of hypofractionated RT, halving the total treatment delivery time for many patients with GBM.
For patients with very poor PS (Karnofsky PS <50), palliative regimens of either 34 Gy in 10 fractions6 or 25 Gy in 5 fractions,7 each supported by prospective trial data, can be used for patients considered for first-course RT. Alternatively, best supportive care or temozolomide with the omission of RT is reasonable (Fig. 1). For patients with recurrent GBM, we do not generally recommend reirradiation, and instead favor consideration of systemic therapies if considered reasonable (Fig 1). These therapies may include, but are not limited to, temozolomide, bevacizumab, and lomustine.
Within our department, a daily multidisciplinary group provides guidance to radiation oncologists on decision making given the rapidly changing evidence and protocols in response to the COVID-19 pandemic. This group assesses the appropriateness of safely delaying consultations or treatment, referring patients to local providers as indicated, utilization of telemedicine, and handling of COVID-19-positive patients who require RT. Given the aggressive nature of GBM, we recommend that patients who are asymptomatic, COVID-19-negative, and do not meet the criteria for self-quarantine receive RT in a manner that decreases exposure without compromising clinical outcomes as outlined (Fig 1). For patients who test negative for COVID-19, we recommend retesting if the index of suspicion for COVID-19 infection remains high given reports of high false-negative rates (approximately 30%) with current COVID-19 testing.8 If a patient tests positive for COVID-19 during RT, we recommend holding RT until the patient self-quarantines for 14 days after the positive test date. The patient is then screened for symptoms after completion of the self-quarantine and the patient’s case undergoes multidisciplinary review to determine whether they can resume RT. Since there is no vaccine, standardized therapy, known immunity, or cure for COVID-19, the rationale for holding oncologic treatment for patients with GBM who test positive for COVID-19 is multifactorial, including known adverse outcomes of individuals with a cancer diagnosis and COVID-19 infection,1 increased risk of exposure of other COVID-19 negative individuals (other immunocompromised patients with cancer, RT providers, and front-line staff), or depletion of limited personal protective equipment resources. Thus, we believe the decision to resume RT in a clinically recovered, COVID-19-positive patient with an anticipated life expectancy >6 months would require self-quarantine for 14 days after the test date, no COVID-19–related symptoms after self-quarantine, and multidisciplinary panel review of the patient’s case to determine risk-to-benefit ratio of proceeding with oncologic therapy with consideration of hypofractionated RT as clinically feasible.
In conclusion, the COVID-19 pandemic is likely to impact oncologic management in a lasting way, and the recommendations provided herein may evolve over time. Other institutions should consider implementing these guidelines in accordance with local institutional, state, and federal regulations and with multidisciplinary discussions with radiation oncology, neurooncology, and neurosurgery. Collectively, we believe these recommendations allow for safe, effective, and expedient RT for patients with GBM in the era of COVID-19.
Footnotes
Sources of support: No funding.
Disclosures: Dr. Caroline Chung reports research funding from RaySearch Laboratories and Siemens. Dr. Prajnan Das reports honorarium from Adlai Nortye and the MD Anderson Cancer Center in Madrid, Spain. Dr. Albert C. Koong is a stockholder of Aravive, Inc. All other authors have no conflicts of interest to report.
References
- 1.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention People who are at higher risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fspecific-groups%2Fpeople-at-higher-risk.html Available at:
- 3.Roa W., Brasher P.M.A., Bauman G. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 4.Perry J.R., Laperriere N., O’Callaghan C.J. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Malmström A., Grønberg B.H., Marosi C. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 7.Roa W., Kepka L., Kumar N. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33:4145–4150. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Yang M., Shen C. medRxiv; 2020. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. [Google Scholar]