Abstract
Coronavirus disease 2019 (COVID-19) infection has now reached a pandemic state, affecting more than a million patients worldwide. Predictors of disease outcomes in these patients need to be urgently assessed to decrease morbidity and societal burden. Lactate dehydrogenase (LDH) has been associated with worse outcomes in patients with viral infections. In this pooled analysis of 9 published studies (n = 1532 COVID-19 patients), we evaluated the association between elevated LDH levels measured at earliest time point in hospitalization and disease outcomes in patients with COVID-19. Elevated LDH levels were associated with a ~6-fold increase in odds of developing severe disease and a ~16-fold increase in odds of mortality in patients with COVID-19. Larger studies are needed to confirm these findings.
Keywords: Lactate dehydrogenase, COVID-19, Coronavirus
1. Introduction
The current pandemic of coronavirus disease 2019 (COVID-19) originally emerged from China, but has since then infected >1 million patients worldwide, with over 400,000 cases in the US alone [1]. This condition is associated with high morbidity, leading to significant strain on healthcare infrastructure and resources. The associated fatality rate is also higher than other respiratory viral infections. Hence, it is necessary to urgently identify reliable predictors of disease severity and mortality for careful allocation of healthcare resources and to enable earlier clinical intervention and monitoring to improve clinical outcomes.
Various biomarkers are currently under investigation for their role in determination of prognosis in patients with COVID-19. Lactate dehydrogenase (LDH) is one such biomarker of interest, especially since elevated LDH levels have been associated with worse outcomes in patients with other viral infections in the past [[2], [3], [4]]. Early data in COVID-19 patients has suggested significant differences in LDH levels between patients and without severe disease [5]. Hence, we performed a pooled analysis of the published literature to explore the possible association between increased LDH values and odds of disease severity and mortality in COVID-19 patients.
2. Methods
2.1. Search design
A comprehensive search of literature on online databases Medline (PubMed interface), Web of Science, EMBASE and Scopus, using no language restriction, was conducted with the search terms “lactate dehydrogenase” OR “LDH” AND “COVID-19” OR “coronavirus 2019” OR “SARS-CoV-2” until April 3, 2020. References of all identified studies were investigated to determine other eligible studies. The reporting of this study was performed in compliance with the PRISMA guidelines (Preferred reporting items for systematic reviews and meta-analyses). The PRISMA Checklist is shown in Supplement 1.
2.2. Selection and data collection
All resulting documents were assessed by title, abstract, and full text for observational studies reporting frequency data on LDH values at admission or earliest time point in hospitalization in COVID-19 patients with or without severe disease or in non-survivors and survivors by two independent reviewers. “Severe disease” was clinically defined as patients requiring life support, meeting criteria for acute respiratory distress syndrome (ARDS), need for mechanical ventilation, or intensive care unit (ICU) admission. An acceptable study level definition of elevated LDH with an upper limit cut-off in the range of 240–255 U/L was required. Studies fitting the criteria were included in a pooled analysis. Studies with a higher than 255 U/L cutoff for abnormality were excluded to avoid biasing the analysis via threshold effect. Additional data was sought from study authors when appropriate.
2.3. Statistical analysis
Pooled analysis was performed with MetaXL, software version 5.3 (EpiGear International Pty Ltd., Sunrise Beach, Australia), using an random effects model to estimate the odds ratio (OR) and 95% confidence interval (95% CI) of elevated LDH levels in association with severe versus non-severe COVID-19 and non-survival vs survival. A leave-one-out sensitivity analysis was performed to determine sources of heterogeneity amongst studies. We also performed a meta-regression analysis to assess the impact of age on association of elevated LDH levels with disease severity and mortality. When unavailable, mean and standard deviation of LDH levels were extrapolated from sample size, median and interquartile range (IQR), according to Hozo et al. [6]. Publication bias analysis was performed using funnel plot analysis. The study was carried out in accordance with the declaration of Helsinki and with the terms of local legislation.
3. Results
3.1. Study identification and characteristics of studies
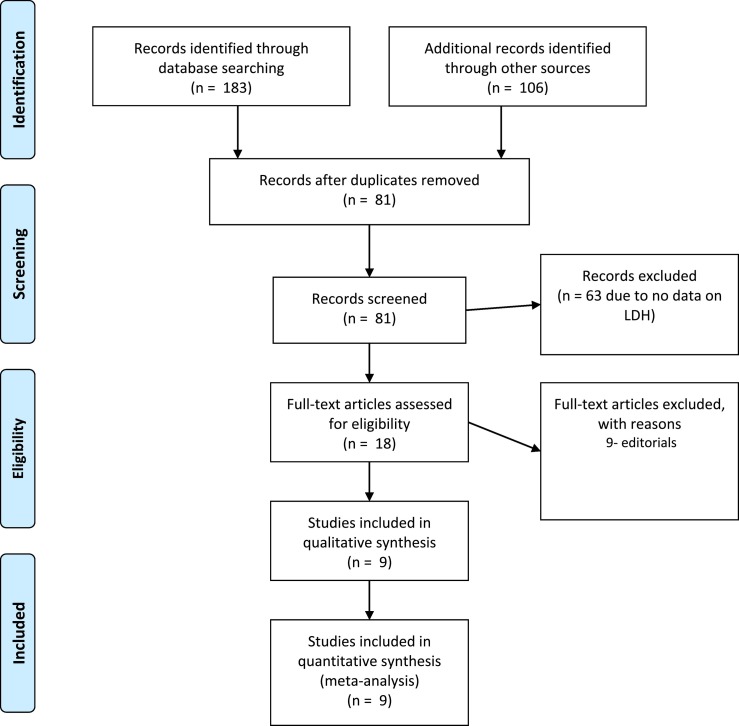
A total of 289 studies were initially found, out of which 208 were excluded due to repetition. Another 63 were removed as they did not report LDH values. 18 studies were left, out of which 9 were removed because they were review articles or editorials. Nine studies (four case-control studies and five retrospective cohort studies) with 1532 patients, were finally used in the pooled analysis [[7], [8], [9], [10], [11], [12], [13], [14], [15]]. One study by Wu et al. reported cohorts of both severity and mortality [13]. All studies were from China and all reported LDH values were measured at time of admission or earliest time point after hospitalization. The PRISMA flow diagram is demonstrated in Fig. 1 . The characteristics of included studies are presented in Table 1 . Five studies did not report LDH values for all included patients; the study level samples used are presented in Table 1.
Fig. 1.
PRISMA Flow diagram.
Table 1.
Characteristics of included studies.
| Study | Setting | Sample size | Outcomes | Severe patients |
Non-severe patients |
||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Age (yrs)⁎ | Elevated LDH (%) | n (%) | Age (yrs)⁎ | Elevated LDH (%) | ||||
| Guan W et al. 2020 | China | 675 | Admission to ICU, MV | 44 (6.5%) | 63 (53–71) | 31 (70.5%) | 631 (93.5%) | 46 (35–57) | 246 (39.0%) |
| Huang C et al. 2020 | China | 40 | ICU Care | 13 (32.5%) | 49 (41–61) | 12 (92.3%) | 27 (67.5) | 49 (41–58) | 17 (63.0%) |
| Liu Y et al. 2020 | China | 12 | Respiratory Failure, MV | 6 (50.0%) | 64 (63–65) | 5 (83.3%) | 6 (50.0%) | 44 (35–55) | 6 (100.0%) |
| Ruan Q et al. 2020 | China | 60 | Death | 60 (42.3%) | 67 (15–81) | 57 (95.0%) | 82 (57.7%) | 50 (44–81) | 48 (58.5%) |
| Wan S et al. 2020 | China | 135 | Respiratory Distress, Admission to ICU | 40 (29.6%) | 56 (52–73) | 30 (75.0%) | 95 (70.4%) | 44 (33–49) | 28 (29.5%) |
| Wang Z et al. 2020 | China | 61 | SpO2 < 90% | 12 (19.7%) | 70.5 (62–77) | 10 (83.3%) | 49 (80.3%) | 37 (32–51) | 15 (30.6%) |
| Wu C et al. 2020 | China | 188 | Admission to ICU | 48 (25.5%) | NR | 46 (95.8%) | 140 (74.5%) | 46.97 ± 11.2 | 80 (57.1%) |
| Wu C et al. 2020 | China | 188 | Death | 43 (22.9%) | NR | 41 (95.3%) | 145 (77.1%) | 46.97 ± 11.2 | 85 (58.6%) |
| Zhang G et al. 2020 | China | 95 | Admission to ICU, MV | 25 (26.3%) | 52 (38–63) | 25 (100.0%) | 70 (73.7%) | 49 (41–56) | 49 (70.0%) |
| Zhou F et al. 2020 | China | 184 | Death | 54 (29.3%) | 69 (63–76) | 53 (98.1%) | 130 (70.7%) | 52 (45–58) | 70 (53.8%) |
Age data presented as median (IQR) or mean (SD). MV — Mechanical Ventilation, ICU –— Intensive Care Unit, NR — Not reported.
3.2. Pooled analysis of disease severity
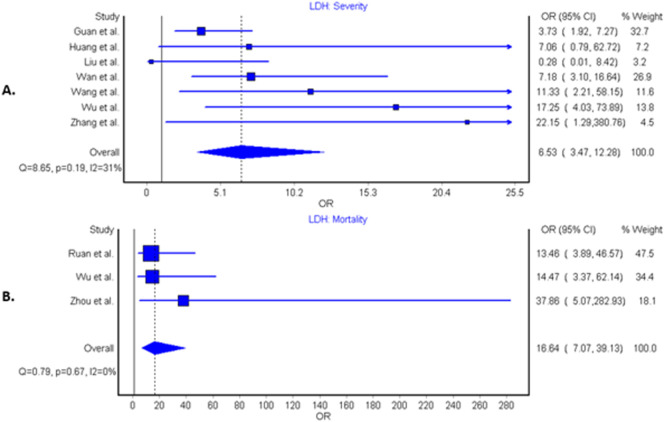
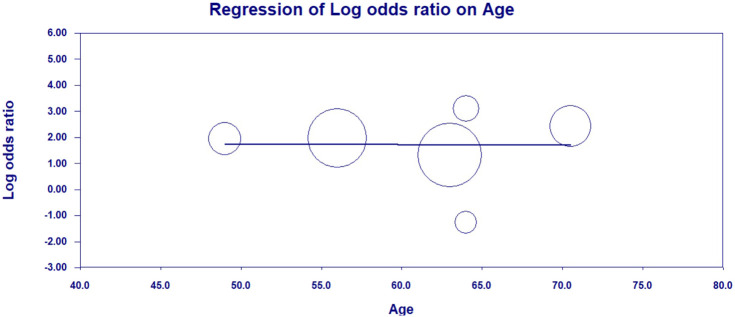
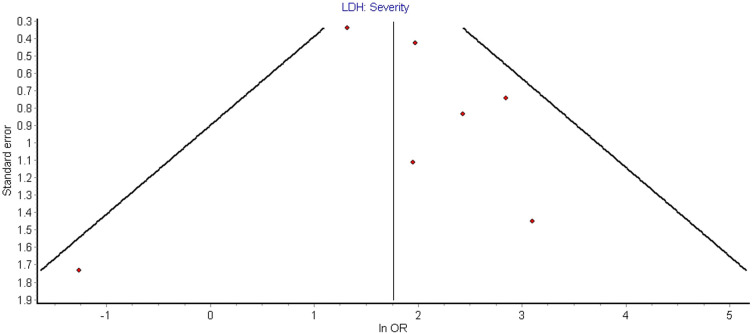
Seven studies compared elevated LDH values in severe vs. non-severe cases in a total of 1206 patients, 188 (15.6%) of whom had severe disease outcome [[7], [8], [9],[11], [12], [13], [14]]. A total of 600 patients (49.8%) presented with elevated LDH values, with 159 severe patients (84.6%) having elevated LDH vs 441 patients (43.3%) in non-severe group. The LDH cutoff in the included studies ranged from 240 to 253.2 U/L. Findings of our pooled analysis are shown in Fig. 2 . Elevated LDH values were found to be associated with an increased odds of severe COVID-19 outcome in all but 2 individual studies [8,9]. Pooled analysis showed about ~6.5-fold increase in odds of developing severe COVID-19 disease (OR: 6.53 [95% CI: 3.47–12.28], I2 = 31%, Cochran's Q, p = 0.19). A leave-one-out sensitivity study did not find any significant differences in association, however, analysis with exclusion of Guan et al. showed a substantially reduced heterogeneity (OR: 8.54 [95% CI: 4.33–16.87], I2 = 9.3%, p = 0.36). LDH was associated with significantly increased odds of severe COVID-19 in both case-control studies (OR: 7.76 [95% CI: 3.64–16.53], I2 = 0%, Cochran's Q, p = 0.75) and retrospective cohort studies (OR: 5.77 [95% CI: 1.82–18.29], I2 = 59%, Cochran's Q, p = 0.06). The results of meta-regression analysis demonstrated no impact of age on the association of elevated LDH levels and disease severity in patients with COVID-19 (correlation coefficient − 0.0027, [95% CI −0.12–0.11], p = 0.96, Fig. 3 ). Funnel plot analysis indicate some asymmetry amongst studies suggestive of publication bias, however, limited studies exclude firm conclusions (Fig. 4 ).
Fig. 2.
Forest plots demonstrating association of elevated lactate dehydrogenase levels with disease severity (panel A) and mortality (panel B) in patients with coronavirus disease 2019 infection.
Fig. 3.
Meta-regression plot showing no impact of age on association of elevated LDH levels and severity of disease in patients with COVID-19 infection.
Fig. 4.
Funnel plot demonstrating publication bias for studies evaluating association of elevated LDH levels and severity of disease in patients with COVID-19 infection.
3.3. Pooled analysis of mortality
Three studies compared elevated LDH values with survival and non-survival in 514 patients, 157 (30.5%) of whom were non-survivors [10,13,15]. A total of 354 patients (68.9%) had elevated LDH values, of which 151 non-survivors (96.2%) had elevated LDH vs. 203 patients (56.9%) in the survivor group. The LDH cutoff in the included studies ranged from 245 to 253.2 U/L. Elevated LDH value was also found to be associated with significantly increased odds of mortality, displaying over 16-fold increased odds compared to patients with LDH below the cutoff value (OR: 16.64 [95% CI: 7.07–39.13], I2 = 0%, Cochran's Q, p = 0.67) (Fig. 2). Sensitivity analysis noted no difference amongst studies. Limited number of studies prevented a meta-regression analysis.
4. Discussion
The results of our pooled analysis demonstrate an association between elevated LDH values and worse outcomes in patients with COVID-19. Specifically, there was a >6-fold increase in odds of severe disease and a >16-fold increase in odds of mortality in patients with elevated LDH. Furthermore, in all the three studies reporting mortality as an outcome, elevated LDH levels were found in >95% of non-survivors compared to <60% of survivors.
LDH is an intracellular enzyme found in cells in almost all organ systems, which catalyzes the interconversion of pyruvate and lactate, with concomitant interconversion of NADH and NAD+ [16]. The enzyme is composed by two major subunits (i.e., A and B), and is present in humans in five separate isozymes (LDH-1 in cardiomyocytes, LDH-2 in reticuloendothelial system, LDH-3 in pneumocytes, LDH-4 in kidneys and pancreas, and LDH-5 in liver and striated muscle). Although LDH has been traditionally used as a marker of cardiac damage since the 1960s, abnormal values can result from multiple organ injury and decreased oxygenation with upregulation of the glycolytic pathway. The acidic extracellular pH due to increased lactate from infection and tissue injury triggers the activation of metalloproteases and enhances macrophage mediated angiogenesis [17].
Severe infections may cause cytokine-mediated tissue damage and LDH release [17]. Since LDH is present in lung tissue (isozyme 3), patients with severe COVID-19 infections can be expected to release greater amounts of LDH in the circulation, as a severe form of interstitial pneumonia, often evolving into acute respiratory distress syndrome, is the hallmark of the disease. However, the contribution of the different LDH isoenzymes to the LDH elevation observed in COVID-19 has not been determined. Additionally, LDH levels are elevated in thrombotic microangiopathy, which is associated with renal failure and myocardial injury [[18], [19], [20]]. Elevated d-dimer levels and thrombocytopenia in patients with severe COVID-19 have also been reported, which suggests a hypercoagulable state may be contributing to severity of illness and mortality [21,22].
Multiple studies have found LDH to be a predictor of worse outcomes in hospitalized patients [2,23]. Many of the prognosticators and therapies currently being studied for COVID-19 are based on experience with the previous coronavirus outbreak, Severe Acute Respiratory Syndrome (SARS), or with other viral respiratory infections. LDH levels were also found to be elevated in patients with Middle East Respiratory Syndrome (MERS) [24]. Elevated LDH levels seem to reflect that the multiple organ injury and failure may play a more prominent role in this pathology in influencing the clinical outcomes in patients with COVID-19.
Our study has some limitations, such as the small number of studies with limited sample sizes. There was heterogeneity in the LDH data, likely due to the poor standardization of analytical methods and poor description in “Material and Methods” section of the analytical performances, including different methods of measurement. To account for heterogeneity amongst studies, we performed sensitivity analysis. We also performed funnel plot analysis to assess for publication bias. Finally, all the studies were from China and hence the findings may not be applicable to other populations. Larger studies from other countries are needed to confirm our findings. In the meantime, we suggest that LDH level may be used as an important tool in determining prognosis in patients with COVID-19. Since LDH measurement is based on a colorimetric method, quick processing of multiple samples can be done using computer automation which may help in quick triage of COVID-19 patients [25].
5. Conclusion
In our pooled analysis, elevated LDH values were associated with 6-fold increased odds of severe COVID-19 disease. More importantly, elevated LDH was associated with a >16-fold increase in odds of mortality. As such, patients' LDH should be closely monitored for any of signs of disease progression or decompensation. Since the LDH levels used in the study were at admission or earliest time during hospitalization, admission LDH levels could be considered for inclusion in future risk stratification models for COVID-19 severity and mortality. Larger studies are needed to confirm these findings.
The following is the supplementary data related to this article.
PRISMA Checklist.
CRediT authorship contribution statement
Brandon Michael Henry: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Gaurav Aggarwal: Writing - original draft, Investigation, Data curation. Johnny Wong: Methodology, Software. Stefanie Benoit: Conceptualization, Writing - review & editing. Jens Vikse: Conceptualization, Writing - review & editing. Mario Plebani: Conceptualization, Writing - review & editing. Giuseppe Lippi: Conceptualization, Writing - review & editing.
Acknowledgments
Acknowledgement
None.
Funding
None.
Disclosure
None.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Chen C.Y., Lee C.H., Liu C.Y., Wang J.H., Wang L.M., Perng R.P. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68(1):4–10. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang C.H., Shih J.F., Su W.J., Perng R.P. Eight-month prospective study of 14 patients with hospital-acquired severe acute respiratory syndrome. Mayo Clin Proc. 2004;79(11):1372–1379. doi: 10.4065/79.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao R.J., Luo X.L., Xu W. Viral infection in community acquired pneumonia patients with fever: a prospective observational study. J Thorac Dis. 2018;10(7):4387–4395. doi: 10.21037/jtd.2018.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry B, De Olivera MHS, S. B, M. P, G. L Hematologic, biochemical and immune marker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID 19): a meta-analysis. Clin Chem Lab Med. 2020. [DOI] [PubMed]
- 6.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. May 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.25783. [Epub ahead of print March 21, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C., Hu X., Song J. 2020. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) medRxiv. 2020.2002.2026.20028589. [Google Scholar]
- 14.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu P.P., Sabatini D.M. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Outschoorn U.E., Prisco M., Ertel A. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via metabolo-genomics. Cell Cycle. 2011;10(8):1271–1286. doi: 10.4161/cc.10.8.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan B., Meier-Kriesche H.U. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2(10):970–974. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 19.Patschan D., Witzke O., Duhrsen U., Erbel R., Philipp T., Herget-Rosenthal S. Acute myocardial infarction in thrombotic microangiopathies — clinical characteristics, risk factors and outcome. Nephrol Dial Transplant. 2006;21(6):1549–1554. doi: 10.1093/ndt/gfl127. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T., Chen H., Liang S. A non-invasive laboratory panel as a diagnostic and prognostic biomarker for thrombotic microangiopathy: development and application in a Chinese cohort study. PLoS One. 2014;9(11):e111992. doi: 10.1371/journal.pone.0111992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. May 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erez A., Shental O., Tchebiner J.Z. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J. 2014;16(7):439–443. [PubMed] [Google Scholar]
- 24.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjeld M. An automated colorimetric method for the estimation of lactate dehydrogenase activity in serum. Scand J Clin Lab Invest. 1972;29(4):421–425. doi: 10.3109/00365517209080261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.