Abstract
This paper provides a brief review of various uses of magnetic resonance perfusion imaging in the investigation of brain/language relationships. The reviewed studies illustrate how perfusion imaging can reveal areas of brain where dysfunction due to low blood flow is associated with specific language deficits, and where restoration of blood flow to improve the tissue function results in recovery of those deficits. This sort of evidence helps to reveal areas of the brain that are essential to a particular language task. Other studies have provided clues regarding the mechanisms of early language recovery, or have shown how perfusion imaging can identify patients with chronic hypoperfusion due to cerebrovascular stenosis in whom the BOLD effect in fMRI may be absent or reduced in areas of neural activation.
Keywords: Magnetic resonance imaging, Perfusion-weighted, Stroke, Aphasia
1. Introduction
Imaging blood flow in the brain can provide insights into the brain/language relationships in a number of ways. It has long been recognized that neural activation of a region of brain results in increased regional blood flow in that area. In fact, as early as the 1800s, Mosso (1881) observed through a skull defect in a man who had sustained head injury increased pulsations in the vessels in the brain that were temporally coupled to the church bells ringing at noon (a signal to say the Ave Maria) or to performing calculations. This relationship between cognitive function and blood flow has served as the basis of O15 PET, the BOLD effect in fMRI, and the use of arterial spin labeling (ASL) perfusion MRI activation studies. It has also long been recognized that reduction in regional cerebral blood flow (rCBF) is associated with impairment of neural function in that area of brain. The reduced rCBF (hypoperfusion) can be secondary to dysfunction, as exemplified by the temporal and parietal hypoperfusion seen with PET, SPECT, or ASL studies of patients with Alzheimer’s disease (Grossman, Alsop, & Detre, 2001). Alternatively, the hypoperfusion can be primary—it can cause dysfunction. Adequate blood flow is necessary for both neural function and neural viability. Lassen and colleagues (e.g. Astrup, Symon, Branston, & Lassen, 1977) reported evidence from animal models demonstrating that when regional cerebral blood flow drops to about 30% of normal, neural function is negatively influenced, and that when it drops to about 20% of normal, neural function ceases. It is only when rCBF further drops to about 10% of normal, that there is release of potassium signaling cell death. These data indicate that tissue receiving blood flow that is between 10 and 30% of the normal blood flow rate is getting just enough to survive, but not enough to function. Therefore, imaging of blood flow can reveal areas of dysfunctional tissue that may be responsible for acute language deficits early after stroke. The hypoperfused areas are often due to severe vascular disease—severe narrowing or occlusion of the main vessels that provide blood flow to the brain.
Imaging modalities that have been useful for detecting focal reductions of blood flow include O15 PET, arterial spin labeling (ASL) magnetic resonance perfusion, CT perfusion, and dynamic contrast magnetic resonance perfusion weighted imaging (PWI). The last is also known as “bolus tracking” PWI, because it reveals the relative rate of arrival and clearance of a bolus of contrast, injected intravenously, in each voxel of the image. Several studies have shown that the volume of hypoperfused tissue revealed by PWI is highly correlated with the severity of neurological dysfunction (Barber et al., 1998, Beaulieu et al., 1999, Neumann-Haefelin et al., 2000) or severity of aphasia or neglect (Hillis et al., 2000, Hillis et al., 2003) in acute stroke. Comparison of PWI with structural imaging that reveals the volume of densely ischemic tissue in acute stroke, such as diffusion weighted imaging (DWI), allows one to estimate the volume of hypoperfused but salvageable tissue in acute stroke patients. This assessment of the “diffusion–perfusion mismatch” in acute stroke to identify patients who may benefit from aggressive treatment of stroke has been the predominant use of PWI (Hillis et al., 2002). However, this review article will focus on uses of PWI to reveal areas of brain essential for language functions, although studies utilizing ASL or CT perfusion for the same purpose will also be cited. Evidence that a region of the brain is necessary for a specific language function will consist of data showing that the region of hypoperfusion (dysfunctional tissue) is strongly associated with impairment of that function in the first day of stroke, and data showing that the language function recovers when the neural region is reperfused (functional again).
2. Strengths and weaknesses of lesion studies for identifying brain/language relationships
The basic assumption underlying traditional lesions studies to identify brain/language relationships is that areas of the brain that are most commonly damaged in individuals with chronic impairment in a particular language task (e.g., speech production) must constitute the areas of brain responsible for the function that is impaired. Although this reasoning seems to straightforward, there are a few caveats that must be considered. First, the areas that are most commonly damaged, at least by stroke, are not evenly distributed across the brain. Some areas are more vulnerable than others to the reductions in blood flow that lead to stroke (Caviness et al., 2002). For example, strokes of the anterior, inferior temporal lobe are rare, so that the role of this region in language functions may be underestimated. In contrast, 98% of strokes caused by occlusion of the middle cerebral artery include the insula (Finley et al., 2003). To illustrate this point further, Fig. 1 shows the probability of infarct/dense ischemia in each left hemisphere voxel of diffusion-weighted images of a consecutive series of 11 patients who presented within 24 h of onset of any left hemisphere stroke symptoms (e.g., right sided weakness or sensory loss, language problems, right visual field cut, right facial droop) and had a high resolution MRI that allowed registration of the scans to the Montreal Neurological Institute (MNI) atlas (Bilello, Lao, Krejza, Hillis, & Herskovits, in press). Common areas of infarct included parts of the insula and the “borderzone territory” between the posterior cerebral artery and middle cerebral artery vascular distributions. Many probability maps of various language deficits (either lesion overlap studies or voxel based lesion analyses) look very similar to this map of all left hemisphere stroke patients (unselected for language impairment of any kind). For example, this map shows that 100% of the patients had damage to the anterior insula. This finding may account for the wide variety of deficits that have been attributed to the insula, and in particular, the anterior insula. This limitation of lesion studies can be addressed by determining how often damage to a particular area causes the deficit of interest, in addition to determining how often the deficit is associated with damage in that area of the insula (Hillis et al., 2004).
Fig. 1.
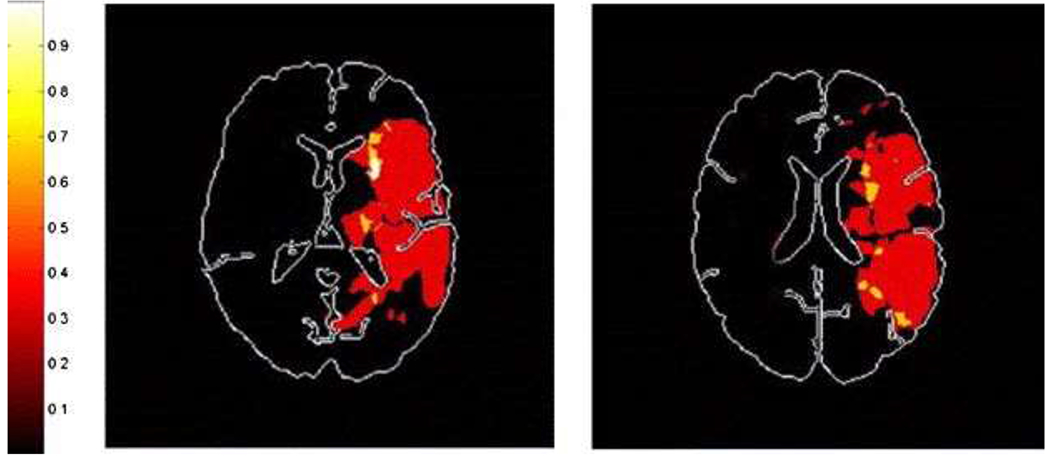
Probability of DWI abnormality in each voxel of diffusion-weighted images of a consecutive series of 11 patients who presented within 24 h of onset of any left hemisphere stroke symptoms and had a high resolution MRI that allowed registration of the scans to the MNI atlas.
However, if patients are studied only months or years after stroke, it is not possible to determine the probability of damage to the area causing the deficit, since many patients who originally had the deficit of interest will no longer have it, due to the extensive reorganization of structure/function relationships that takes place after stroke. So, even if an area of the brain is normally important for a particular function, other areas of the brain may assume that function in the weeks or months after injury (Heiss et al., 1999, Thompson et al., 2000, Thulborn et al., 1999, Warburton et al., 1999, Weiller et al., 1995; see Hillis, in press, for review and alternative accounts of the evidence for reorganization). Because of this potential for reorganization (particularly after small lesions), patients with chronic language deficits generally have quite large lesions that prevent complete functional reorganization. It is difficult to evaluate what part of a large lesion is responsible for any residual chronic deficits. Most investigators have assumed it is the area of greatest overlap among large lesions of patients with a given chronic impairment in language that is responsible for the impaired function (Alexander, 1997, Dronkers, 1996, Naeser and Hayward, 1978). However, as noted above, the area of greatest overlap among large strokes may simply reveal areas that are most vulnerable to ischemia, and may be unrelated to the language function being studied. Some investigators avoid this problem by “subtracting” the lesions of patients who do not have the deficit (e.g., Karnath, 2001) or identifying the voxels with the highest association with the deficit (Bates et al., 2003). However, these methods can also be problematic in studies of patients with chronic lesions because the patients without the deficit of interest may have had the deficit at onset, as noted earlier. Thus, one might “subtract” out areas, or fail to find associations with areas, that initially were important for the function that recovered. For example, patients with damage to the striate cortex reliably have visual field cuts immediately after stroke, but may recover normal visual fields as other areas of cortex “take over” the function of the damaged part in the months to years after stroke (Slotnick, Moo, Krauss, & Hart, 2002). Subtracting lesions of patients without chronic visual field cuts from the overlap of patients with chronic visual field cuts would involve subtracting some infarcts restricted to striate cortex, which might lead to the incorrect conclusion that the striate cortex is not important for vision.
One way to avoid the problem of reorganization is to study patients at onset of brain damage (another way is to study the effects of temporary lesions, e.g., as produced by transcranial magnetic stimulation). Although reorganization might begin immediately after stroke, earlier studies of aphasia recovery indicate that neurovascular changes leading to tissue recovery can account for the spontaneous recovery of language that occurs early after stroke (Hillis & Heidler, 2002). However, most investigators have avoided studying acute patients, because scans could not reveal the entire area of dysfunctional tissue in acute stroke. That is, particularly in acute stroke, there is often a lesion of core infarct, surrounded by an area of hypoperfused tissue getting just enough blood to survive, but not enough to function (the “ischemic penumbra”). Although this limitation of structural imaging is most relevant in acute stroke, PET studies of chronic stroke also demonstrate regions of brain surrounding the infarct that have low blood flow (hypoperfusion) and low metabolism. These areas of dysfunctional tissue are likely to contribute to the person’s language deficits. Therefore, a final limitation of traditional chronic lesion/deficit methodology is that structural imaging may not reveal the entire region of the brain that is dysfunctional and that may contribute to the impairment.
One approach that addresses these limitations of traditional brain/lesion studies involves: (1) identifying the entire area of dysfunctional brain that might be responsible for particular language impairments, using imaging that reveals both infarct and regions of low blood flow; and (2) studying patients at the onset of brain damage, before reorganization, rehabilitation, or recovery. Although PET is the current “gold standard” for identifying dysfunctional brain tissue, it is not generally feasible to obtain PET imaging in all acute stroke patients. Furthermore, there are other drawbacks of PET, such as the high expense, exposure to radiation, and a relatively long duration of the scan. However, magnetic resonance imaging (MRI) is widely used in acute stroke, including DWI to identify regions of dense ischemia or infarct that are unlikely to survive and PWI to rapidly identify regions of low blood flow. Together with conventional MRI scans that reveal areas of old stroke or other brain damage, these scans may reveal the entire area of dysfunctional brain in the first hours after onset of stroke. A recent study demonstrated a high correlation between dysfunctional tissue identified with PWI and dysfunctional tissue identified with PET, when “hypoperfused” tissue on PWI is identified with a threshold of >4 s delay in time to peak arrival of contrast on time-to-peak (TTP) maps (one hemodynamic map generated from PWI; Sobesky et al., 2004)1. This paper illustrates some uses of PWI (using this threshold on TTP maps) for identifying regions of brain that are essential for particular language functions. It will be demonstrated that this methodology (combining DWI, PWI, and language testing at onset of stroke) can be complementary to functional imaging studies in the effort to identify the neural substrates of language processes.
3. Use of PWI to test whether brain regions reliably activated during a task are essential for the task
It is widely agreed that functional imaging (using fMRI, PET, or activation ASL) is ideally suited for identifying the brain regions that are engaged in specific task, but cannot demonstrate whether or not those regions are essential for the task (Chatterjee, 2005, Fellows et al., 2005). Areas of increased BOLD signal correspond to areas in which blood flow (out of proportion to oxygen consumption) is temporally coupled to performance of the task. These areas may be neurally activated (as can be demonstrated with simultaneous electrophysiological studies), actively inhibited, or passively disinhibited during the task, as discussed elsewhere in this special issue. Lesion studies, on the other hand, cannot reveal all of the areas involved in a task, but can provide evidence that a particular region is necessary for the task, by showing that damage/dysfunction of that area disrupts performance of the task. Therefore, it would be reasonable to use functional neuroimaging to generate hypotheses about the areas needed for some function by revealing all of the areas engaged during that function, and then test whether or not these areas are crucial for that function by determining the acute effects of a lesion in that area. If the area is crucial, damage or dysfunction of the area should compromise performance of the task.
This approach can be illustrated by a recent study of the role of the left midfusiform gyrus in reading. A large number of fMRI studies of reading words, pseudowords, and letters have shown activation of left midfusiform gyrus during the reading task relative to baseline or control conditions (e.g., reading words relative to letter-strings; reading letters relative to shapes; Cohen et al., 2000, Cohen et al., 2002, Dehaene et al., 2002, Gros et al., 2001, Polk and Farah, 2002, Uchida et al., 1999). In fact, activation of this area was so robust across individuals and across studies of reading that Cohen (Cohen et al., 2000, Cohen et al., 2002; see also Cohen & Dehaene, 2004) dubbed the area, “the visual word form area” (VWFA). However, other functional imaging studies have documented activation in essentially the same area during modality-independent lexical processing, such as reading Braille by blind subjects (Buchel, Price, & Friston, 1998). Furthermore, it was argued that lesions isolated to the VWFA do not affect reading (Price and Devlin, 2004, Price et al., 2003). However, since the lesion data were from patients with subacute to chronic stroke, they may have had reading impairments acutely after damage to VWFA, but recovered. Therefore, to test the hypothesis that this region is essential for reading, we studied a variety of reading tasks and other lexical tasks in 80 patients in the first day of stroke. We reasoned that if VWFA is necessary for reading, acute damage or dysfunction (hypoperfusion) of VWFA (with or without damage or dysfunction elsewhere) should disrupt written word comprehension and written lexical decision, two tasks that require access to “visual word forms” but do not require lexical output (Hillis et al., 2005).
Patients for this study (and all of our studies reported in this paper) were studied with bedside language testing and MRI (PWI, DWI, Fluid Attenuated Inversion Recovery or FLAIR, T2, Gradient Echo ± SPGR) within 24 h of stroke onset. A subset of included patients had some sort of intervention to restore blood flow. When possible, patients had repeat imaging and language testing immediately after intervention or at Day 3 to evaluate changes in blood flow that are associated with changes in performance. We used “time to peak” (TTP) maps to identify areas of hypoperfusion, defined as greater than 2.5–4.5 s delay in time to peak arrival of contrast in each region of interest, relative to the homologous region in the intact hemisphere. This threshold has been found to reflect dysfunctional tissue, even if the same threshold does not predict that the area will necessarily go on to die if not reperfused (see Hillis et al., 2004, for discussion and review of the evidence).
Results are shown in Table 1. Acute damage or dysfunction of VWFA was not associated with impaired written word comprehension or written lexical decision. Although there was a slight tendency for more patients with infarct and/or hypoperfusion of VWFA to have impaired word comprehension compared to patients with no infarct in this region (58% versus 41%), a large percentage (42%) of them had normal written word comprehension. However, damage or dysfunction of VWFA was strongly associated with impaired oral and written naming of both pictures and objects named from tactile exploration and impaired oral reading—tasks that require modality-independent lexical output. These results were consistent with previous findings that hypoperfusion and/or infarct of BA 37 was strongly associated with impaired naming, particularly of objects (relative to actions; Hillis, Tuffiash, Wityk, & Barker, 2002; see also Antonucci et al., 2004, Raymer et al., 1997). Furthermore, patients who showed relatively discrete hypoperfusion in left Brodmann’s area 37 (posterior middle and inferior temporal gyrus, including VWFA) showed impaired naming at Day 1 and recovery of naming at Day 3 when this area was reperfused. Written word comprehension was intact both days. Fig. 2 shows DWI and PWI scans of additional patients with hypoperfusion of left BA 37 who had impaired oral and written naming but intact spoken and written word comprehension on Day 1. These data indicate that at least part of left BA 37, perhaps including the area that has been called the VWFA, is essential for accessing modality-independent lexical representations. Moreover, we found no evidence that this area, including VWFA, is necessary for reading comprehension.
Table 1.
Association between damage or dysfunction of VWFA and written word comprehension deficit
| Infarct and/or hypoperfusion including VWFA | No abnormality in VWFA | Total | |
|---|---|---|---|
| Impaired written word comprehension | 31 (58%) | 11 (41%) | 42 |
| Normal written word comprehension | 22 (42%) | 16 (59%) | 38 |
| Total | 53 | 27 | 80 |
Fig. 2.
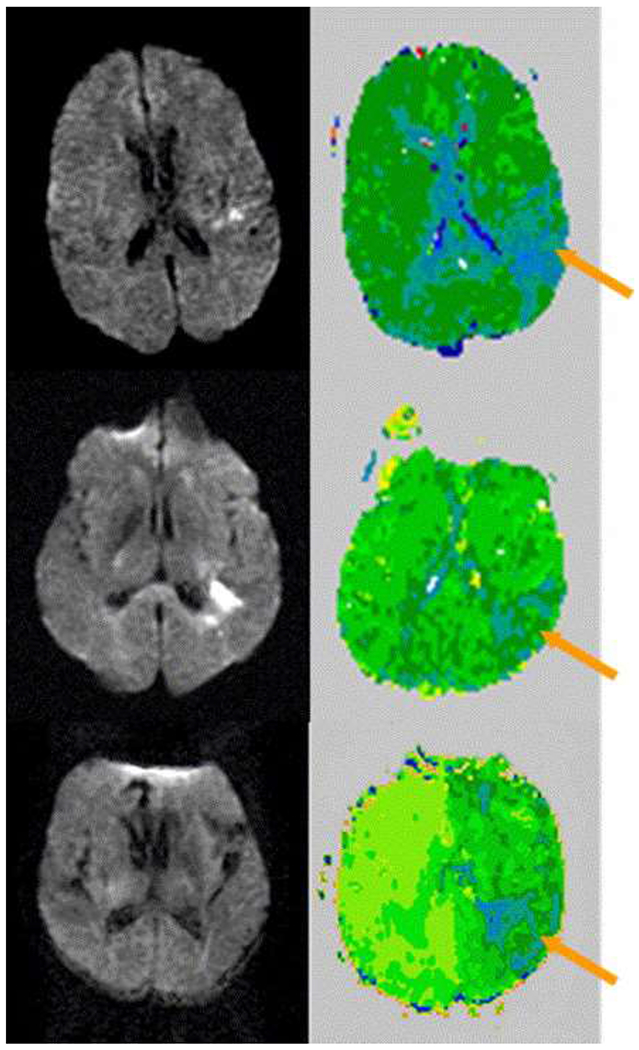
DWI and PWI scans of three patients with hypoperfusion of left BA 37 (arrow) who had impaired oral and written naming but intact spoken and written word comprehension on Day 1. Relatively hypoperfused regions appear dark green or blue.
We attempted to reconcile the results with results from functional imaging studies that reliably show increased BOLD signal in VWFA during reading by hypothesizing that VWFA does have a role in reading (computing a location-independent spatial representation of the word or pseudoword as proposed by McCandliss, Cohen, & Dehaene, 2003), but that this role can be immediately assumed by the right VWFA. This hypothesis would account for the consistent “activation” of left VWFA (which is usually accompanied by activation of the homologous region on the right). It would also account for the observation that lesions or hypoperfusion of left VWFA alone do not impair reading comprehension or lexical decision. However, these functions might be slower when there is damage to left VWFA. A similar account has been proposed regarding the role of Heschl’s gyrus in auditory perception; damage to either side alone does not significantly disrupt auditory perception, because the opposite Heschl’s gyrus is sufficient to support this function. Furthermore, this hypothesis would account for additional observations that: (1) damage to both left and right VWFA does substantially disrupt reading (just as damage to Heschl’s gyrus bilaterally causes impaired auditory perception), and (2) damage or hypoperfusion of left VWFA and the splenium of the corpus collosum impairs reading (Marsh & Hillis, 2005; see also Cohen et al., 2004; see Dejerine, 1891 for a similar account of alexia). We proposed that when the right VWFA is used for computation of a location-independent spatial representation of the word, this representation must be transferred via the splenium to left hemisphere areas for word comprehension or oral reading. This proposal could explain impaired reading by a patient with a lesion in left VWFA when the splenium was hypoperfused, and recovered reading when the splenium was reperfused (Marsh & Hillis, 2005). It should be noted that computing a location- and font-independent representation of visual word stimuli and early auditory processing entail primary and secondary sensory processes; similar arguments regarding the role of homologous regions in the right hemisphere would probably not apply to more complex cognitive processes.
4. Use of PWI to evaluate mechanisms of acute recovery
In the preceding study it was noted that hypoperfusion of left BA 37 was associated with impaired oral and written naming, and that reperfusion of this region was associated with recovery of oral and written naming. A similar result was reported by Fridriksson et al. (2002) using ASL. They reported a case in which hypoperfusion of left BA 37 was associated with impaired naming in the acute stage, and improvement of naming was documented when left BA 37 was reperfused. These findings indicate that at least part of left BA 37 (perhaps separate from the VWFA; see Cohen, Jobert, Le Bihan, & Dehaene, 2004) is essential for accessing lexical representations for output. Furthermore, these results indicate that reperfusion of specific areas of the brain may be responsible for acute recovery of specific language tasks in the first few days of stroke. This hypothesis was evaluated more explicitly in a study designed to evaluate the mechanisms of acute recovery of spoken word comprehension.
Early studies of aphasia had documented that about 25% of patients with acute impairment of word comprehension show recovery of this function in the first few days after stroke. This recovery often leads to “evolution” of the aphasia syndrome from Global Aphasia to Broca’s Aphasia. Other patients show evolution from Global Aphasia to Wernicke’s Aphasia when speech articulation recovers acutely. Potential mechanisms of this rapid recovery or evolution of aphasia include: resolution of diaschisis (disrupted neural input from the damaged area to a remote area, causing dysfunction of the remote area), recovery of membrane function, rapid reorganization of structure/function relationships, or reperfusion of crucial language areas. We evaluated the extent to which reperfusion, and in what brain regions, could account for acute recovery of word comprehension.
A total of 90 subjects were tested using the methods described earlier, with a battery of language tests at bedside and MRI at Day 1 and Day 3–5 (see Hillis & Heidler, 2002 for details). Of those patients with impaired word comprehension at Day 1, all patients who recovered word comprehension by Day 3–5 showed hypoperfusion of Wernicke’s area at Day 1 and reperfusion of Wernicke’s area at Day 3–5. No patient recovered word comprehension without reperfusion of Wernicke’s area, indicating that rapid reorganization of structure/function relationships and other means of restored tissue function are not common mechanisms of acute recovery. Furthermore, a patient who showed reperfusion of left BA 37 but not Wernicke’s area showed improvement of naming but no improvement of word comprehension by Day 3, providing additional evidence that reperfusion of left BA 37 is an important mechanism of recovery of naming (Hillis et al., 2002).
This proposal was more systematically studied in a subsequent study of 54 patients (Hillis et al., 2005). In this study, we determined the areas where reperfusion contributed to improvement by >10% on picture naming, using linear regression analysis. We found that reperfusion of left BA 37 contributed most to improvement of naming, although reperfusion of Wernicke’s area and Broca’s area also independently contributed to improvement in naming. This result is unsurprising, since naming requires linking words with semantic representations (which likely depends on Wernicke’s area, as confirmed by its essential role in word comprehension) and motor planning and programming of speech articulation (which likely depends on Broca’s area; Hillis et al., 2004). Reperfusion of other areas examined (including angular and supramarginal gyri, other temporal areas) did not independently contribute to recovery of naming.
These studies provide evidence that restoration of blood flow is an important mechanism of recovery of acute aphasia. Similar results have been reported in a recent study using CT perfusion (Croquelois, Wintermark, Reichhart, Meuli, & Bogousslavsky, 2003). These investigators showed that the volume of hypoperfused tissue was correlated with aphasia severity at onset, and that a reduction in the volume of hypoperfused tissue was associated with at least partial resolution of aphasia in the acute stage. These studies indicate that either MR perfusion or CT perfusion can be useful for identifying patients who have the greatest potential for early functional improvement in language with aggressive treatment to restore blood flow (Hillis et al., 2004).
5. Use of PWI to evaluate brain/language relationships in chronic aphasia
Although hypoperfusion in the absence of infarct probably accounts for much of the language impairment in acute aphasia, functionally significant hypoperfusion beyond the infarct is probably uncommon beyond first few days or weeks (but this issue has not been adequately studied). Nevertheless, language deficits caused by chronic hypoperfusion have been reported. Love, Swinney, Wong, and Buxton (2002) report a case of impaired reading in a patient who suffered a stroke many years earlier. An ASL perfusion scan revealed hypoperfusion in the left angular gyrus, although structural imaging showed no infarct in that region. On the basis that previous studies have provided evidence for an association between reading impairment and damage to the left angular gyrus, these authors attributed the patient’s poor reading to hypoperfusion of this region. In another study of a chronic alexic patient, Wityk, Hillis, Beauchamp, Barker, and Rigamonti (2002) reported chronic hypoperfusion of bilateral temporal occipital regions (including BA 37), associated with severely impaired reading and picture naming in a patient with Moya-moya syndrome—a vascular abnormality associated with severe stenosis of the carotid artery. Importantly, her impairment resolved after treatment to restore blood flow to the hypoperfused region in left BA 37 (including the area identified by Cohen as VWFA). In a subsequent study, Hillis, Chang, and Breese (2004) reported a case of persistent hypoperfusion of left Broca’s area (BA 44/45) and BA 6 revealed by PWI that was associated with chronic pure agraphia one year after stroke. The patient’s persistent agraphia was attributed to this chronic hypoperfusion, since this was the only region of dysfunction at the onset of her agraphia one year earlier. Furthermore, other patients with similar patterns of pure agraphia associated with hypoperfusion of these regions showed recovery of writing skills with reperfusion of BAs 44, 45, and 6 (see also Hillis et al., 2003).
6. Use of PWI to evaluate for hypoperfusion that may interfere with the BOLD effect in fMRI
The BOLD effect in fMRI depends on neural activation causing a hemodynamic response that is out of proportion to oxygen extraction in that region. In cases of severe arterial stenosis there can be impaired vascular reactivity because vessels are maximally dilated at rest (see Marshall, 2004 for discussion). In such cases, neural activity may result in increased oxygen extraction without a corresponding increase in blood flow. Thus, there may be no significant BOLD effect, or even a negative BOLD effect (because of the increased oxygen extraction without a hemodynamic response) in response to neural activation in these cases. For example, it has been recently demonstrated that median nerve stimulation resulted in concurrent magnetoencephalographic (MEG-evoked fields) and BOLD responses in 10 normal subjects. However, patients with severe cerebrovascular stenosis showed uncorrelated activation properties, with clear MEG signals in both the affected and unaffected hemispheres, indicating that there was stimulus-locked neural activation in the primary sensorimotor cortex bilaterally, but with no corresponding BOLD response in the affected hemisphere (Rossini et al., 2004). Impaired cerebral vasomotor reactivity measured by transcranial Doppler during CO2 inhalation was strongly related to the lack of BOLD effect in fMRI despite neural activation demonstrated with MEG, in the affected hemisphere. Only one patient—an individual with microangiopathy—showed preserved vasomotor reactivity with absent BOLD bilaterally. However, technical limitations may account for this negative result; a multivariate analysis using a mixture of Gamma functions might have been more successful in modeling the hemodynamic response when there is negative or delayed BOLD response (Fridriksson, Rorden, Morgan, Murrow, & Bayliss, in press).
We have also evaluated the effects of chronic hypoperfusion on the BOLD response in fMRI studies of language in our laboratory (Prabhakaran et al., 2003). Six patients with subcortical stroke at least six weeks earlier with persistent severe intravascular stenosis and six normal control subjects underwent resting perfusion imaging to evaluate for areas of chronic hypoperfusion, followed by an fMRI study of word generation (blocks of overt word generation alternating with blocks of rest). Word generation was selected because it is a complex task that requires semantic processing, word retrieval, searching and response selection (generally considered functions of bilateral posterior frontal areas), speech articulation, and hearing one’s own response, and was therefore expected to be associated with increased activation in bilateral frontal cortex (Brodmann’s areas 44, 45, and 6, thought to be engaged in search and response selection) and throughout perisylvian language cortex in the temporal, posterior frontal, and inferior parietal lobes (BA 44, 45, 21, 22, 37, 40, thought to be involved in semantics and word-retrieval) and bilateral auditory cortex (BA 41/42, involved in auditory perception). All of these areas were analyzed as regions of interest (ROIs). As expected, normal subjects consistently showed significantly increased BOLD effect in a left dominant fronto-temporal-parietal network during the task compared to rest (p < .05, corrected for multiple comparisons across ROIs). Areas that consistently showed significantly increased BOLD in normal subjects included the following ROIs: left BA 6 (dorsal posterior frontal), left BA 22, 37 (posterior temporal areas), left BA 40 (supramarginal gyrus in the parietal lobe), and bilateral BA 44, 45 (posterior, inferior frontal) and 41/42 (auditory cortex), as well as right BA 21 (middle temporal gyrus), similar to previously reported results using this task. All of the patients who showed hypoperfusion (but no infarct) in the regions normally activated showed: (1) decreased BOLD effect in the hypoperfused region and (2) increased BOLD effect in the homologous region of the opposite hemisphere (see example in Fig. 3). These results were consistent with the hypothesis that chronic cortical hypoperfusion due to intracranial stenosis, even in the absence of infarct in the normally activated regions, is sufficient to cause reorganization of cognitive functions (see Krakauer et al., 2004 for similar findings for motor functions).
Fig. 3.
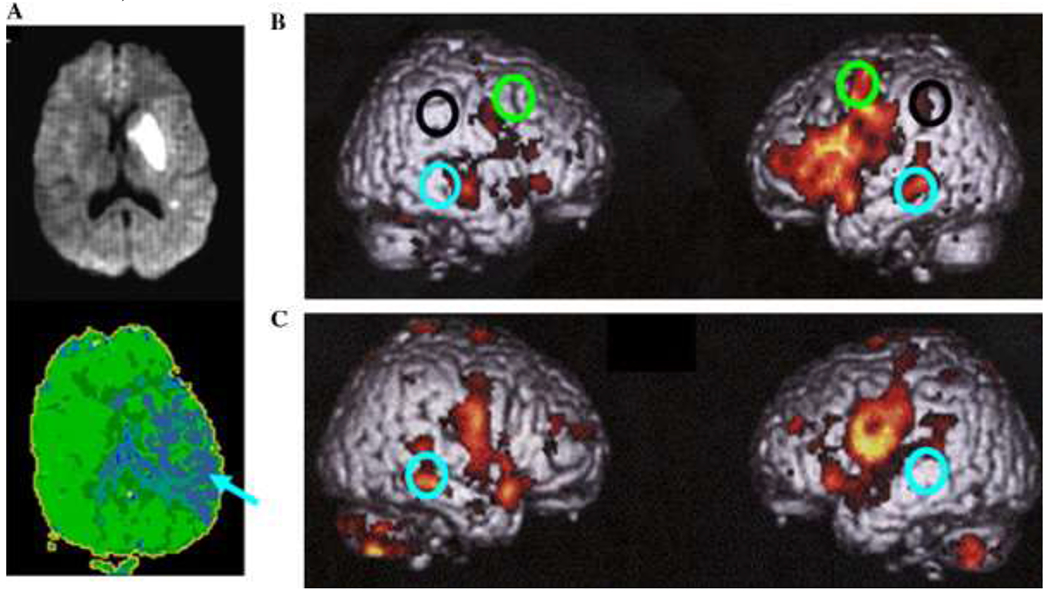
(A) DWI (top) done at Day 1 and PWI done at week 6 (bottom) of a patient with almost complete resolution of initial anomia and comprehension deficits, showing chronic hypoperfusion of left temporal cortex, including BA 37 (arrow). (B) fMRI study of word generation in six healthy control patients, showing significant BOLD effect in left BA 6 (dorsal posterior frontal; green circles), left BA 22, 37 (posterior temporal areas; blue circles), left BA 40 (supramarginal gyrus in the parietal lobe; black circles), and bilateral BA 44, 45 (posterior, inferior frontal) and 41/42 (auditory cortex), as well as right BA 21 (middle temporal gyrus). (C) fMRI study of word generation in the patient whose DWI and PWI are shown, demonstrating absence of the expected BOLD effect in the hypoperfused region (BA 37) and increased BOLD effect in the homologous region of the opposite hemisphere.
However, two of the six patients showed another striking result: increased BOLD effect during rest compared to task, in the regions that were hypoperfused at baseline and that showed activation in normal subjects. For example, a patient with severe and widespread hypoperfusion in right frontal and parietal cortex showed no significant BOLD effect during the task compared to rest in the right hemisphere, but showed significantly increased BOLD effect in the right hemisphere during rest compared to task in the regions expected to show BOLD effect during task (Fig. 4). One possible explanation of the increased BOLD effect for rest relative to task was that the task resulted in increased neural activation in the right frontal and temporal regions activated in normal subjects, but the neural activation was associated with a increased oxygen extraction without a corresponding hemodynamic response (because of the arterial stenosis and probable loss of vascular reactivity), resulting in a negative BOLD effect during task. Such a negative BOLD effect in regions of chronic hypoperfusion have been observed during contralateral motor tasks as well (Rother et al., 2002; see also Carusone et al., 2002, Roc et al., 2006). One recent investigation of both PET and MRI within 24 h of stroke demonstrated areas of low regional cerebral blood flow (rCBF) with high oxygen extraction fraction (OEF), as well as areas of low CBF with low OEF, and still other areas of high rCBF with low OEF in areas of the brain that were normal or abnormal on DWI (Guadagno et al., 2005). Together, these results underscore a potential confound in interpreting results of fMRI BOLD studies of patients with cerebrovascular disease (e.g., studies of stroke recovery). However, many stroke patients show a normal hemodynamic response in undamaged tissue. Studies of vascular reactivity (Marshall, 2004) or resting perfusion scans can be used to exclude patients whose results might be complicated by chronic hypoperfusion, or one can model the negative hemodynamic response in cases of hypoperfusion (Fridriksson et al., in press).
Fig. 4.
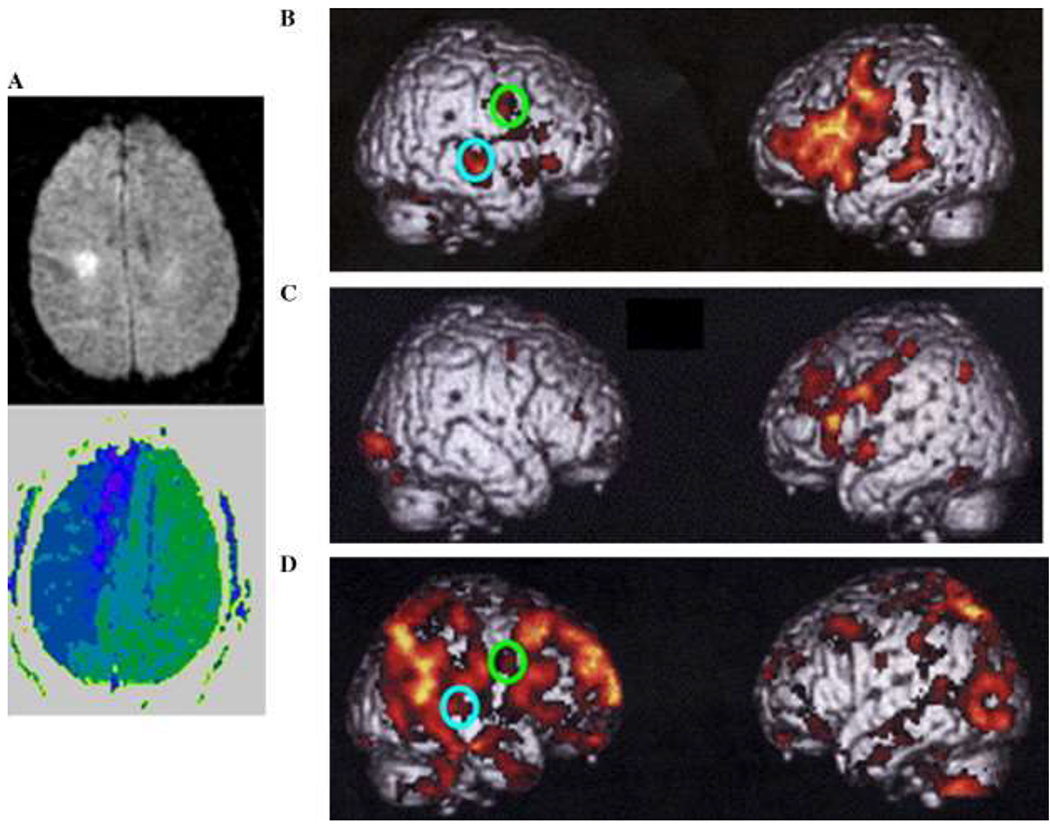
(A) DWI (top) and PWI (bottom) of a patient with hemispatial neglect and inattention, showing extensive hypoperfusion in right frontoparietal cortex. (B) fMRI study of word generation in six healthy control patients, showing significant BOLD effect in six healthy control patients in an extensive left dominant fronto-temporal-parietal network. (C) fMRI study of word generation (task minus rest) in the patient whose DWI and PWI are shown in (A), demonstrating absent of BOLD effect in the hypoperfused regions of the right frontoparietal cortex and relatively normal BOLD effect in the intact left hemisphere. (D) fMRI study of word generation (rest minus task) in the same patient, showing increased BOLD effect in the right, hypoperfused areas during rest relative to task in ROIs that showed right hemisphere BOLD signal in normal subjects in the opposite contrast (task relative to rest).
7. Summary
Magnetic resonance perfusion imaging and CT perfusion imaging provide new tools for evaluating changes in blood flow associated with changes in language. While ASL can also be used to identify areas of neural activation in normal subjects (Aguirre, Detre, Zarahn, & Alsop, 2002), bolus-tracking PWI and CT perfusion are more useful for identifying areas of hypoperfusion that correspond to areas of dysfunction or areas where there may be a reduced hemodynamic response. The latter imaging techniques are inappropriate for activation studies because each repetition of the scan requires a bolus of contrast. PWI and CT perfusion have also been used to evaluate mechanisms of early recovery of language, which occurs in response to restored blood flow to critical areas for language comprehension or production. These imaging techniques have provided insights into the areas of brain that are essential to particular language functions, and have thus provided a method for evaluating whether areas that show “activation” in fMRI during some task are actually necessary for that task. Therefore, perfusion imaging represents a method that is complementary to functional imaging for evaluating the neural substrates of language.
Acknowledgments
This work was supported by NIH (NIDCD) through R01 DC05375.
The research reported in this paper was supported by NIH, through an NIDCD funded grant, RO1 DC05375. The author is grateful to Julius Fridriksson, John Sidtis, and an anonymous reviewer for very helpful suggestions on an earlier draft of this paper.
Footnotes
Other hemodynamic maps that can be generated with PWI include cerebral blood volume (CBV) maps that show the total blood volume in each voxel of the image and mean time to transit (MTT) maps that show the time of arrival and clearance of the contrast in each voxel. Additionally, cerebral blood flow maps can be computed by dividing the CBV by the MTT, or can be calculated from an arterial input function. We used TTP maps because they have been found in previous studies to reflect dysfunctional tissue as indicated by a high correlation between volume of abnormality and severity of dysfunction measured with behavioral/cognitive tests or other tests of neurological function (Hillis et al., 2003, Reineck et al., 2005; see also Barber et al., 1998), as well as a high correlation with PET studies (Sobesky et al., 2004). Furthermore, the severity of delay in TTP has been shown to be highly correlated with the severity of impairment. For example, the delay in TTP within Wernicke’s area was strongly and linearly related to the rate of errors in a spoken word comprehension task (Hillis et al., 2001). However, other hemodynamic maps are likely to be better for estimating the area of tissue that will progress to infarct if blood flow is not restored (Thijs et al., 2001). CBF maps from CT perfusion and ASL provide an absolute measure of blood flow, and should be at least as accurate in identifying dysfunctional tissue.
References
- Aguirre JK, Detre JA, Zarahn E, D.C. Experimental design and the relative sensitivity of BOLD and perfusion fMRI Neuroimage, 15 (3) (2002), pp. 488–500 [DOI] [PubMed] [Google Scholar]
- Alexander MP Aphasia: clinical and anatomical aspects Feinberg TE, Farah MJ (Eds.), Behavioral neurology and neuropsychology, McGraw Hill, New York: (1997), pp. 133–150 [Google Scholar]
- Antonucci SM, Beeson PM, Rapcsak SZ Anomia in patients with left inferior temporal lobe lesions Aphasiology, 18 (2004), pp. 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Symon L, Branston NM, N.A. LassenCortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia Stroke, 8 (1977), pp. 51–57 [DOI] [PubMed] [Google Scholar]
- Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI Neurology, 51 (1998), pp. 418–426 [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel based lesion-symptom mapping Nature Neuroscience, 6 (5) (2003), pp. 448–450 [DOI] [PubMed] [Google Scholar]
- Beaulieu C, deCrespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome Annals of Neurology, 46 (1999), pp. 568–578 [DOI] [PubMed] [Google Scholar]
- Bilello M, Lao Z, Krejza J, Hillis AE, & Herskovits EH (in press). Statistical atlas of acute stroke from MR diffusion-weighted-images of the brain. Neuroinformatics [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K A multimodal language region in the ventral visual pathway Nature, 394 (1998), pp. 274–277 [DOI] [PubMed] [Google Scholar]
- Carusone LM, Srinivasan J, Gitelman DR, Mesulam MM, Parrish TB Hemodynamic response changes in cerebrovascular disease: implications for functional MR imaging American Journal of Neuroradiology, 23 (2002), pp. 1222–1228 [PMC free article] [PubMed] [Google Scholar]
- Caviness V, Makris N, Montinaro E, Sahin N, Bates J, Schwamm L, et al. Anatomy of stroke, Part I: An MRI-based topographic and volumetric system of analysis Stroke, 33 (2002), pp. 2549–2556 [DOI] [PubMed] [Google Scholar]
- Chatterjee A A madness to the methods in cognitive neuroscience? Journal of Cognitive Neuroscience, 17 (2005), pp. 847–849 [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S Specialization within the ventral stream: the case for the visual word form area NeuroImage, 22 (2004), pp. 466–476 [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff M-A, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients Brain, 123 (2000), pp. 291–307 [DOI] [PubMed] [Google Scholar]
- Cohen L, Henry C, Dehaene S, Martinaud O, Lehericy S, Lemer C, et al. The pathophysiology of letter-by-letter reading Neuropsychologia, 42 (13) (2004), pp. 1768–1780 [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S Distinct unimodal and multimodal regions for word processing in the left temporal cortex NeuroImage, 23 (2004), pp. 1256–1270 [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S Language-specific tuning of visual cortex? Functional properties of the visual word form area Brain, 125 (2002), pp. 1054–1069 [DOI] [PubMed] [Google Scholar]
- Croquelois A, Wintermark M, Reichhart M, Meuli R, Bogousslavsky J Aphasia in hyperacute stroke: language follows brain penumbra dynamics Annals of Neurology, 54 (2003), pp. 321–329 [DOI] [PubMed] [Google Scholar]
- Dehaene S, LeClec’H G, Poline JB, Le Biha D, Cohen L The visual word form area: a prelexical representation of visual words in the fusiform gyrus Neuroreport, 13 (2002), pp. 321–325 [DOI] [PubMed] [Google Scholar]
- Dejerine J Sur un cas de cécité verbale avec agraphie, suivi d’autopsie Comptes Rendus Hebdomadaires des Séances et Mémoires de la Société de Biologie, Ninth Series, 3 (1891), pp. 197–201 [Google Scholar]
- Dronkers NF A new brain region for coordinating speech articulation Nature, 384 (1996), pp. 159–161 [DOI] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH Method matters: an empirical study of impact in cognitive neuroscience Journal of Cognitive Neuroscience, 17 (2005), pp. 850–858 [DOI] [PubMed] [Google Scholar]
- Finley A, Saver J, Alger J, Pregenzer M, Leary M, Ovbiagele B, et al. Diffusion weighted imaging assessment of insular vulnerability in acute middle cerebral artery infarctions [abstract] Stroke, 34 (2003), p. 259 [Google Scholar]
- Fridriksson J, Holland A, Coull BM, Plante E, Trouard TP, Beeson P Aphasia severity: association with cerebral perfusion and diffusion Aphasiology, 16 (2002), pp. 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Morgan PS, Murrow KL, & Bayliss GC (in press). Measuring the hemodynamic response in chronic hypoperfusion. Neurocase. [DOI] [PubMed] [Google Scholar]
- Gros H, Boulanouar K, Viallard G, Cassol E, Celsis P Event-related functional magnetic resonance imaging study of the extrastriate cortex response to a categorically ambiguous stimulus primed by letters and familiar geometric figures Journal of Cerebral Blood Flow and Metabolism, 21 (2001), pp. 1330–1341 [DOI] [PubMed] [Google Scholar]
- Grossman M, Alsop DC, Detre JA Perfusion fMRI using arterial spin labeling in Alzheimer’s Disease and Frontotemporal Dementia: correlations with language Brain and Language, 79 (2001), pp. 94–95 [Google Scholar]
- Guadagno JV, Warburton EA, Jones PS, Fryer TD, Day DJ, Gillard JH, et al. The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography Cerebrovascular Disease, 19 (2005), pp. 239–246 [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia Annals of Neurology, 45 (1999), pp. 430–438 [DOI] [PubMed] [Google Scholar]
- Hillis AE (in press). The right place at the right time? Brain. [Google Scholar]
- Hillis AE, Heidler J Mechanisms of early aphasia recovery: evidence from MR perfusion imaging Aphasiology, 16 (2002), pp. 885–896 [Google Scholar]
- Hillis AE, Barker P, Beauchamp N, Gordon B, Wityk R MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect Neurology, 55 (2000), pp. 782–788 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Chang S, Breese E The crucial role of posterior frontal regions in modality specific components of the spelling process Neurocase, 10 (2004), pp. 157–187 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker P, Beauchamp N, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke Brain and Language, 79 (2002), pp. 495–510 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits E, Degaonkar M The roles of the “visual word form area” in reading NeuroImage, 24 (2005), pp. 548–559 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Wityk RJ, Barker PB Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke Cognitive Neuropsychology, 19 (2002), pp. 523–534 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk R, Barker PB, Caramazza A Neural regions essential for writing verbs Nature Neuroscience, 6 (2003), pp. 19–20 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Ulatowski JA, Jacobs MA Change in perfusion in acute nondominant hemisphere stroke may be better estimated by tests of hemispatial neglect than by the NIHSS Stroke, 34 (2003), pp. 2392–2398 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, et al. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke Annals of Neurology, 50 (2001), pp. 561–566 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Ulatowski JA, Beauchamp N, Jacobs M, Barker PB MR perfusion weighted imaging as a marker of treatment response in acute and subacute stroke Neuroradiology, 46 (2004), pp. 31–39 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Breese EL, Barker PB, Jacobs MA, Maurer K Re-examining the brain regions crucial for orchestrating speech articulation Brain, 127 (2004), pp. 1479–1487 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Vimal S, Newhart M, Aldrich E, Heidler J, Ken J Reperfusion of selective areas is associated with improved naming in acute stroke Brain and Language, 95 (2005), pp. 100–101 [abstract]. [Google Scholar]
- Karnath H New insights into the functions of the superior temporal cortex Nature Reviews. Neuroscience, 2 (2001), pp. 568–576 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Radoeva PD, Zarahn E, Wydra J, Lazar RM, Hirsch J, et al. Hypoperfusion without stroke alters motor activation in the opposite hemisphere Annals of Neurology, 56 (6) (2004), pp. 796–802 [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Wong E, Buxton R Perfusion imaging and stroke: a more sensitive measure of the brain bases of cognitive deficits Aphasiology, 16 (2002), pp. 873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE Cognitive and neural mechanisms underlying reading and naming: evidence from letter-by-letter reading and optic aphasia Neurocase, 11 (2005), pp. 318–325 [DOI] [PubMed] [Google Scholar]
- Marshall RS The functional relevance of cerebral hemodynamics: why blood flow matters to the injured and recovering brain Current Opinion in Neurology, 17 (2004), pp. 705–709 [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S The visual word form area: expertise for reading in the fusiform gyrus Trends in Cognitive Science, 7 (2003), pp. 293–299 [DOI] [PubMed] [Google Scholar]
- Mosso A Ueber den Kreislauf des Blutes im Mensclichen Gehrin Verlag von Veit & Co., Leipzig: (1881) [Google Scholar]
- Naeser MA, Hayward RW Lesion localization in aphasia with cranial computed tomography and Boston Diagnostic Aphasia Examination Neurology, 28 (1978), pp. 545–551 [DOI] [PubMed] [Google Scholar]
- Haefelin Neumann T., Wittsack HJ, Wenserski F, Li TQ, Moseley ME, Siebler M, et al. Diffusion- and perfusion-weighted MRI in a patient with a prolonged reversible ischaemic neurological deficit Neuroradiology, 42 (2000), pp. 444–447 [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ Functional MRI evidence for an abstract, not perceptual, word-form area Journal of Experimental Psychology: General, 131 (2002), pp. 65–72 [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Raman S, Grunwald M, Mahadevia A, Hussain N, Phillipose L, et al. (2003). Localization of cognitive processes using stroke patients and fMRI. Presented at Society for Neuroscience. [Google Scholar]
- Price CJ, Devlin JT The pros and cons of labeling a left occipitotemporal region: “the visual word form area” NeuroImage, 22 (2004), pp. 477–479 [DOI] [PubMed] [Google Scholar]
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U Cortical localization of the visual and auditory word form areas: A reconsideration of the evidence Brain and Language, 86 (2003), pp. 272–286 [DOI] [PubMed] [Google Scholar]
- Raymer A, Foundas AL, Maher LM, Greenwald ML, Morris M, Rothi LG, et al. Cognitive neuropsychological analysis and neuroanatomical correlates in a case of acute anomia Brain and Language, 58 (1997), pp. 137–156 [DOI] [PubMed] [Google Scholar]
- Reineck L, Agarwal S, Hillis AE. The “diffusion-clinical mismatch” predicts early language recovery in acute stroke Neurology, 64 (2005), pp. 828–833 [DOI] [PubMed] [Google Scholar]
- Roc AC, Wang J, Ances BM, Liebeskind DS, Kasner SE, Detre JA Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenosis Stroke, 2 (2006), pp. 382–387 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferretti A, Vernieri F, Zappasodi F, Caulo M, et al. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain, 127 (1) (2004), pp. 99–110 [DOI] [PubMed] [Google Scholar]
- Rother J, Knab R, Hamzei F, Fiehler J, Reichenbach JR, Buchel C, et al. Negative dip in BOLD fMRI is caused by blood flow–oxygen consumption uncoupling in humans Neuroimage, 15 (2002), pp. 98–102 [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Krauss G, Hart J Jr. Large-scale cortical displacement of a human retinotopic map NeuroReport, 13 (1) (2002), pp. 41–46 [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, et al. time-to-peak threshold best identifies penumbral flow? A comparison of perfusionweighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke Stroke, 35 (2004), pp. 2843–2847 [DOI] [PubMed] [Google Scholar]
- Thijs VN, Adami A, NeumannHaefelin T, Moseley ME, Marks MP, Albers GW Relationship between severity of MR perfusion deficit and DWI lesion evolution Neurology, 57 (2001), pp. 1205–1211 [DOI] [PubMed] [Google Scholar]
- Thompson CK, Fix SC, Gitelman DR, Parrish TB, Mesulam MM FMRI studies of agrammatic sentence comprehension before and after treatment Brain and Language, 74 (2000), pp. 387–391 [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA Plasticity of language-related brain function during recovery from stroke Stroke, 30 (1999), pp. 749–754 [DOI] [PubMed] [Google Scholar]
- Uchida I, Kikyo H, Nakajima K, Konishi S, Sekihara K, Miyashita Y Activation of lateral extrastriate areas during orthographic processing of Japanese characters studied with fMRI NeuroImage, 9 (1999), pp. 208–215 [DOI] [PubMed] [Google Scholar]
- Warburton E, Swinburn K, Price CJ, Wise R Mechanisms of recovery from aphasia: evidence from positron emission tomographic studies Journal of Neurology, Neurosurgery and Psychiatry, 66 (1999), pp. 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, et al. Recovery from Wernicke’s aphasia: a positron emission tomographic study Annals of Neurology, 37 (1995), pp. 723–732 [DOI] [PubMed] [Google Scholar]
- Wityk R, Hillis AE, Beauchamp N, Barker PB, Rigamonti D Perfusion-weighted MRI in adult moyamoya syndrome: characteristic patterns and change after surgical intervention: case report Neurosurgery, 51 (2002), pp. 1499–1506 [PubMed] [Google Scholar]


