Abstract
Objective
VOLUME is a randomized, open-label, post-approval pragmatic trial aiming to evaluate long-term pulmonary and cardiovascular safety of Exubera® (EXU; insulin human [rDNA origin] Inhalation Powder) in routine clinical practice. The primary study objective is to compare risk of persistent decline in forced expiratory volume in 1 second (FEV1) among patients treated with and without EXU.
Research design and methods
Patients eligible to take EXU per approved local label were randomized to EXU or routine care and followed per usual care, with scheduled FEV1 tests at baseline, 6 months, and yearly.
Randomization halted in October 2007 after Pfizer announced it would stop marketing EXU due to low sales. EXU patients were subsequently transitioned to usual care and all patients were followed for 6 additional months.
Results
Although there was insufficient power to evaluate the primary endpoint (37% of the planned 5,300 were randomized), the study provided important descriptive information.
Per the primary endpoint definition, more EXU group patients (n = 8) experienced a persistent decline in FEV1 (n = 0 in usual care). Using a broader, clinically relevant pre-specified supplementary definition of persistent decline, similar numbers were observed in the EXU (n = 27) and usual care (n = 24) groups. Slightly more pulmonary and allergic serious adverse event composite endpoints were seen in the EXU group. There were no consistent treatment group differences in the cardiovascular composite endpoint, all-cause mortality, or glycemic control.
Conclusions
Clinically important declines in lung function that persisted more than 60 days were uncommon and of similar frequency in Exubera and usual care.
Clinicaltrials.gov
Keywords: Large simple trial, Pragmatic trial, Inhaled insulin, Real World evidence, Safety, Effectiveness
1. Background
Exubera® insulin human [rDNA origin] Inhalation Powder (EXU) is an inhaled rapid-acting human insulin developed by Pfizer in collaboration with Sanofi-Aventis and Nektar Therapeutics to control hyperglycemia in adult patients with type 1 and 2 diabetes It was intended to provide a non-invasive alternative to subcutaneously (SC) injected short-acting insulin [1]. In 2006, the US FDA approved EXU for treatment of diabetes in the United States [2] and the EMA approved use in Europe [3].
At time of approval, approximately 2,000 patients had been treated with EXU for up to two years in controlled Phase 2/3 clinical trials. Data from these studies showed EXU use associated with a small, non-progressive decrease in pulmonary function that was reversible following cessation of treatment [1]. Based on these data, the EXU product label specified recommended time points for healthcare providers to perform pulmonary function testing at baseline, 6 months, 1 year and then yearly. Generalizability of clinical trial data to EXU use in routine practice was limited by rigorous criteria typical of randomized clinical trials, e.g., strict study inclusion criteria, administration by well-trained clinical researchers, and in this case, standardized pulmonary function testing. The ability to monitor or manage pulmonary safety of EXU in routine clinical practice was unknown.
Pfizer committed (to FDA and EMA) to evaluate the pulmonary safety of EXU in adults, as used in routine clinical practice, over a longer duration of exposure than studied in the clinical development program. The aim of this evaluation was to assess whether the label instructions were sufficient to manage pulmonary safety in patients using EXU. Given an association between pulmonary decline and cardiovascular risk [4], evaluation of the cardiovascular safety of EXU was planned.
The Exubera Large Simple Trial (VOLUME) was designed to examine the post-approval safety of EXU in routine clinical practice. As originally planned, 5,300 subjects were to be recruited in 24 countries and followed for at least 5 years. However, in October 2007 (15 months after the first VOLUME subject was randomized), Pfizer announced that it would cease marketing EXU due to low sales. When announced, 1,976 patients were enrolled in the US, Germany, United Kingdom (UK) and Sweden; several other countries were in various phases of start-up.
As EXU would become unavailable, the study could not continue as planned and, in April 2008, a plan to transition subjects to other care was instituted by registered protocol amendment [5]. This amendment aimed to: (1) protect the safety of participating patients, (2) avoid unnecessary long-term burden to patients and investigators, and (3) maintain data integrity.
2. Research design and methods
2.1. Original study design
VOLUME is a randomized, open-label, post-approval, pragmatic, large simple trial, designed to evaluate long-term pulmonary and cardiovascular safety of EXU in routine clinical practice. Two design features were necessary to validly study the safety of EXU: randomization and periodic formal pulmonary function testing (via spirometry). Because EXU was contraindicated for patients with moderate to severe pulmonary function impairment [2,3], it seemed patients with any history of pulmonary function impairment or significant lung disease would likely be prescribed non-EXU usual diabetes care. Since EXU was likely to be selectively used in patients who failed or refused other forms of insulin, it was possible that patients treated with EXU would have more poorly controlled diabetes. The likelihood of confounding by indication/severity would make it difficult to adjust fully for confounding, limiting interpretation of pulmonary and cardiovascular outcomes within a purely observational study. Thus, we designed a post-approval large simple trial, a design that combines the randomization component of a randomized clinical trial with the observational eligibility and follow-up of a typical epidemiology study. This design avoids baseline confounding, while allowing physicians to then treat patients as they would in routine clinical practice.
In addition, while patients treated with EXU in clinical practice would receive baseline and follow-up spirometry (assuming the EXU label recommendations were followed), other patients would not, precluding comparison of a spirometry-based endpoint in a purely observational study. Thus, we required spirometry testing for all subjects at intervals consistent with the approved label for EXU. All other patient treatment and follow-up was per routine clinical practice at the discretion of the treating physician.
2.2. Oversight committees
To facilitate scientific oversight and safeguard the well-being of patients, an external governance structure, comprised of experts with extensive knowledge in the areas of pulmonary medicine, endocrinology, cardiology, epidemiology, biostatistics, and immunology was established. Three external committees were formed, the: Scientific Steering Committee (SSC), Data Monitoring Committee (DMC), and Endpoint Committee (EC). While VOLUME was an open-label study, the Pfizer study team, SSC and EC were “blinded”; only DMC members reviewed data by randomization group (or actual treatment status) in closed sessions.
The SSC proposed and reviewed study performance characteristics (e.g., number of subjects randomized, discontinued, lost to follow-up) and study data summarized across randomization groups, and advised the study team on DMC recommendations. The independent DMC was charged with reviewing unblinded interim data, and recommending whether to continue or stop the trial based on those reviews. The DMC and SSC convened semi-annually (in person or via teleconference). The independent EC screened, reviewed, and coded all potential secondary study endpoints, masked to treatment, using pre-specified algorithms built for use in observational data collection. At the first EC meeting, recommended edits were made to these algorithms, and new algorithms were developed when none existed.
2.3. Study subjects and follow-up
Patients with type 1 or 2 diabetes were recruited from primary care centers, diabetes and endocrinology clinics, and academic treatment centers to ensure broad physician and subject representation. We planned to randomize approximately 5,300 patients from 24 countries, with randomization beginning after EXU was approved by Health Authorities in each country. Patients 75 years of age and older were, by design, to comprise at least 5% of the baseline study population. Subjects were randomized 1:1 to EXU or non-EXU usual diabetes care using block randomization by country and site.
Patients were eligible for the study if their treating physician considered EXU to be an appropriate therapy for that patient and was equally confident treating the patient with EXU or other diabetes care. Along with study entry criteria, physicians were provided with the approved local label for EXU to guide enrollment decisions. All study subjects were to be followed for the study outcomes for 5 years, regardless of how long they remained on randomized treatment.
Randomization was halted in October 2007 (with 1,976 of the intended 5,300 subjects enrolled and randomized) after Pfizer announced it would stop marketing EXU due to inadequate sales. EXU patients were subsequently transitioned to usual care and all patients were followed for 6 additional months.
2.4. Objectives
The primary objective of this study is to estimate the relative risk of a persistent decline in forced expiratory volume for EXU compared with patients not randomized to EXU. For both treatment groups, the primary outcome was defined as an observed decline in FEV1 exceeding 20% from baseline, 3 months after a confirmed decline in FEV1 exceeding 20% from baseline (see Supplemental Fig. S1). Per the summary of product characteristics (SmPC)/United States prescribing information (USPI), subjects treated with EXU were to discontinue use of EXU upon a confirmed decline in FEV1 exceeding 20% from baseline. Thus, the primary outcome assessed the relative risk of persistent decline in FEV1 3 months after EXU patients discontinued EXU therapy.
The secondary objectives were to compare subjects randomized to EXU to those not randomized to EXU by estimating the relative risk of: (1) pulmonary serious adverse event (SAE) composite, including: asthma, chronic obstructive pulmonary disease (COPD), pneumonia, or acute bronchitis, (2) all-cause mortality, (3) cardiovascular SAE composite, including: cardiovascular mortality, non-fatal myocardial infarction (MI), or non-fatal stroke, (4) allergic response SAE composite, including: anaphylaxis, angioedema, generalized allergic reaction, and allergic bronchospasm; and (5) by estimating the change in glycosylated hemoglobin (HbA1c) from baseline at Month 6, and Years 1, 2, 3, 4, and 5.
2.5. Data collection
Informed consent and alternate contact forms were completed by subjects prior to randomization. The alternate contact form identified the patient's primary care physician and next of kin or any person to be contacted if the subject did not return for follow-up visits. All other forms were completed by the enrolling physician (or his/her qualified representative) including baseline questionnaire, follow-up questionnaires, subject summary, adverse event monitoring forms, and pulmonary consult form. Minimal information was collected at the baseline visit, including: subject's demographic and clinical characteristics, age of onset and type of diabetes, smoking history, FEV1 results (per routine clinical practice, e.g. via handheld spirometry test), prior use of diabetes medications, HbA1c, and history of respiratory, allergic and cardiovascular disease. Follow-up questionnaires were administered at each scheduled study visit, which collected information on the subject's: smoking status, follow-up spirometry test results, HbA1c, continuation/discontinuation of the assigned treatment, EXU use since last scheduled study visit, and other diabetes medication use since the last scheduled study visit.
2.6. Secondary endpoint adjudication
All reported SAEs were reviewed by the EC chair and classified into one of three categories: a “potential” endpoint, an endpoint with “insufficient data”, or not a potential study endpoint. Potential study endpoints were further categorized into one of four categories (cardiovascular, pulmonary, allergic response, or fatal event) and adjudicated by two EC members with expertise in appropriate fields (i.e. pulmonology, cardiology, and immunology/allergy). The adjudicators - masked to treatment allocation - reviewed anonymized records (e.g., medical records, laboratory data, hospital discharge or admission notes, and/or death certificates) specific to each event, and classified the event as a “definite endpoint”, “possible endpoint”, “no endpoint”, or “insufficient data” using pre-specified algorithms. If the two adjudicators did not agree, the event was reviewed by a third adjudicator. If all 3 adjudicators assigned a different classification, the classification was resolved in a tiebreak discussion. All fatal events, including those with unknown cause or a known cause not related to other study endpoints, were adjudicated to confirm the patient died during the study period.
2.7. Amended study design
To ensure a safe and timely transition to usual diabetes care following Pfizer's decision to discontinue marketing EXU, the study was divided into 2 post-randomization follow-up periods: the controlled follow-up period and the observational follow-up period (see Supplemental Fig. S2). For each subject, an index visit was to occur within a 2- to 4-month window after country-specific approval of the study protocol amendment. This visit included transition from EXU to usual care treatment, and final scheduled spirometry (to preserve the primary endpoint definition).
The controlled follow-up period was defined as the time period from randomization to the index visit date, during which subjects received either EXU or usual care treatment and included Month 6, Year 1, and Year 2 visits. Subjects randomized to EXU, who experienced a decline in FEV1 exceeding 20% from baseline at the final scheduled spirometry, were to remain on EXU until a confirmatory test 3–4 weeks later. These subjects were transitioned to usual care treatment on the day of the confirmatory test, regardless of the result. The observational follow-up period was defined as the 6-month period following the index visit, during which subjects received usual care treatment, and secondary endpoint and other SAE data were collected. An additional 6 month follow-up was identified to allow sufficient time to ensure EXU patients were safely transitioned to usual care. At the end of this period, the final study visit occurred (via telephone or in-office visit), and additional data, including subject status and occurrence of severe hypoglycemic events, were collected.
2.8. Amended statistical analysis
To meet the primary objective, VOLUME recruitment was planned to terminate once 106 primary endpoints had occurred, conservatively estimated as when 5300 subjects had been followed for 5 years (estimated such that the 1-sided 97.5% CI would exclude the hazard ratio of 1.8 with 85% power, assuming a true hazard ratio equal to 1.0). Analyses were performed according to the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement [6].
Because the trial stopped earlier than expected (with only 37% of expected subjects randomized), it was underpowered to address the primary endpoint. An addendum to the approved statistical analysis plan detailed relevant changes to the statistical analysis, based on the trial's early stoppage. Assuming primary and secondary endpoints had insufficient power for formal hypothesis testing, each endpoint was evaluated descriptively by randomized group.
Two analyses were performed for tabulation of persistent declines in FEV1:
-
(1)
an analysis based on the original protocol definition, where a confirmed decline was a second consecutive spirometry showing a >20% decline in FEV1, occurring within 14–42 days (inclusive) of the initial decline, and where a persistent decline was a third consecutive spirometry showing a >20% decline in FEV1, occurring within 60–120 days (inclusive) of the confirming (second) decline; and
-
(2)
an analysis using a pre-specified supplemental clinical definition (established with consultation from the independent endpoint committee) where a confirmed decline was a second consecutive spirometry showing a >20% decline in FEV1 occurring ≥14 days after the initial decline, and where a persistent decline was a third consecutive spirometry showing a >20% decline in FEV1 occurring ≥60 days after the initial decline.
For both the original protocol definition and supplemental clinical definition of the primary endpoint, the following were tabulated: a) the number of subjects experiencing an initial FEV1 decline (≥20% change from baseline), b) the number of subjects in (a) but not experiencing a confirmed decline, and c) the number of subjects in (a) who experienced a confirmed decline. For those experiencing a persistent decline, the median time (and 25th and 75th percentiles) from baseline to that persistent decline was estimated.
Additionally, continuous variables (FEV1 and HbA1c) were presented descriptively at weeks 26, 52 and at the index visit for the 6 month observational follow-up period using boxplot figures. Cumulative incidence estimates (1 minus the Kaplan Meier probability) for time to persistent FEV1 decline between the treatment groups were performed as post-hoc analyses.
The secondary adjudicated endpoints for pulmonary SAE composite score, cardiovascular SAE composite score, and allergic response SAE composite score were tabulated by categories such as definite, possible, non-event, other, or insufficient data; and all-cause mortalities were confirmed and reported.
3. Results
Due to early termination of the study, 1,976 subjects (37.3% of the planned 5,300) were enrolled and randomized. The patient disposition is shown in Supplemental Fig. S3. Among randomized subjects, half were randomized to EXU (N = 987) and half to usual care (N = 989). A total of 1,451 (73.4%) subjects completed the study according to the amendment. More subjects in the EXU group (N = 298, 30.2%) than the usual care group (N = 216, 21.8%) discontinued from the study, largely driven by those no longer willing to participate (n = 143 in EXU; n = 96 in usual care). The mean (SD) duration of randomized treatment was 19.1 (6.9) months for subjects in the EXU group and 19.8 (6.1) months for subjects receiving usual care.
3.1. Baseline data
Selected baseline demographics and clinical characteristics are shown in Table 1. Among the EXU group, the mean age was 57.9 years (SD = 11.3), 44.5% were female, 84.1% were Caucasian, and 11.6% were black. Similarly, among the usual care group, the mean age was 57.6 years (SD = 12.1), 43.9% were female, 85.1% were Caucasian, and 11.0% were black. Treatment groups were fairly balanced with respect to smoking history, body mass index (BMI), diabetes history, and baseline total cholesterol, HbA1c and FEV1.
Table 1.
Baseline demographic and clinical characteristics.
| Exubera N = 987 |
Usual Care N = 989 |
|
|---|---|---|
| Age (years) | 57.9 ± 11.3 | 57.6 ± 12.1 |
| Female sex | 439 (44.5) | 434 (43.9) |
| Race | ||
| White | 830 (84.1) | 842 (85.1) |
| Black | 114 (11.6) | 109 (11.0) |
| Asian | 20 (2.0) | 17 (1.7) |
| Other | 23 (2.3) | 21 (2.1) |
| Smoking classification | ||
| Never smoker | 564 (57.1) | 562 (56.8) |
| Current smoker | 3 (0.3) | 3 (0.3) |
| Ex-smoker | 420 (42.6) | 424 (42.9) |
| BMI (kg/m2) | 33.8 ± 7.4 | 33.5 ± 7.3 |
| Type of diabetes | ||
| Type 1 | 48 (4.9) | 58 (5.9) |
| Type 2 | 938 (95.0) | 931 (94.1) |
| Unspecified | 1 (0.1)a | 0 (0.0) |
| Duration of diabetes (years) | 10.6 ± 8.2 | 10.9 ± 8.8 |
| Total cholesterol (mg/dL)b | 179.0 (149.0, 211.0) | 180.0 (148.0, 213.5) |
| HbA1c (%)c | 8.6 ± 1.9 | 8.5 ± 1.9 |
| FEV1 (L)d | 2.66 ± 0.84 | 2.68 ± 0.89 |
Unless noted, data are means ± SD or n (%).
BMI = body mass index; FEV1 = forced expiratory volume in 1 s; HbA1c = glycosylated hemoglobin; N = number of subjects in the treatment group.
This subject has a missing primary diagnosis and was randomized in error.
Median (25th, 75th percentile) presented. 51 EXU and 49 usual care subjects were missing baseline total cholesterol.
9 EXU and 4 usual care subjects were missing baseline HbA1c.
2 EXU and 2 usual care subjects were missing baseline FEV1.
The number of subjects taking any medication to control hypertension or hyperlipidemia, any beta-blockers, or daily aspirin were similar in the 2 treatment groups (Supplemental Table S1). The most frequent diseases or syndromes reported in the medical history of subjects in both treatment groups were hypertension, high cholesterol, and high triglycerides. Slightly more subjects in the EXU group compared with usual care reported a history of heart attack (9.1% of EXU group versus 7.3% of usual care-treated group), hypertension (79.5% of EXU group versus 77.0% of usual care group), and high triglycerides (55.4% of EXU group versus 53.3% of usual care group).
Within 7 days before randomization, the most frequently used diabetes drug treatments were metformin, insulin glargine, glimepiride, and pioglitazone, and they were used with a similar frequency in both treatment groups. The most frequently used concomitant diabetes drug treatments (those the subject would remain on after randomization), were metformin, insulin glargine, pioglitazone, and glimepiride; which were used with similar frequency across treatment groups.
3.2. Study endpoints
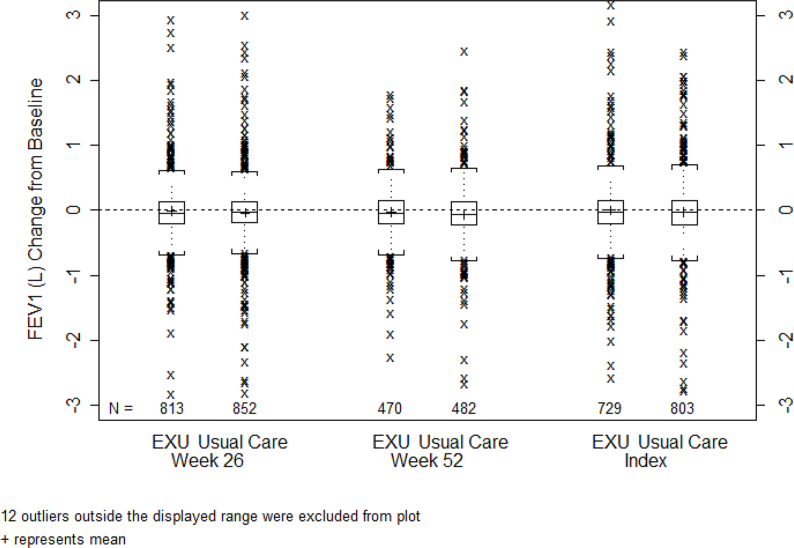
The number of subjects experiencing an initial, confirmed and persistent FEV1 decline is provided in Table 2 using the per protocol and supplemental primary endpoint definitions. Eight subjects (0.8%) in the EXU group and none in the usual care group, experienced a persistent FEV1 decline according to the per protocol definition. For the supplemental definition, 27 subjects (2.7%) in the EXU group versus 24 subjects (2.4%) in the usual care group experienced a persistent FEV1 decline. Of those with a history of smoking, 3.1% experienced the supplemental primary endpoint definition versus 2.2% among those with no smoking history. The change from baseline in FEV1 is presented graphically with a box plot in Fig. 1. Small, non-progressive median reductions from baseline occurred in both treatment groups with similar magnitude at week 26, 52, and the index visits for the 6 month observational follow-up period; there were no clinically meaningful differences between the treatment groups.
Table 2.
Summary of subjects experiencing a FEV1 decline.
| Exubera N = 987 |
Usual Care N = 989 | |
|---|---|---|
| Per Protocol Primary Endpoint Definitiona | ||
| Number (%; CIb) of subjects experiencing an initial FEV1 decline | 100 (10.1; 8.3–12.2) | 112 (11.3; 9.4–13.5) |
| Number (%; CIb) of subjects experiencing a confirmed FEV1 decline | 19 (1.9; 1.2–3.0) | 7 (0.7; 0.3–1.5) |
| Number (%; CIb) of subjects experiencing a persistent FEV1 decline | 8 (0.8; 0.4–1.6) | 0 (0.0; 0.0–0.4) |
| Median time (years) from baseline to persistent declinec | 1.22 (0.96–1.82) | NA |
| Median follow-up time (years) based on reverse Kaplan-Meier method (95% CI) | 1.17 (1.13–1.23) | 1.22 (1.17–1.26) |
| Supplemental Primary Endpoint Definitiond | ||
| Number (%; CIb) of subjects experiencing an initial FEV1 decline | 100 (10.1; 8.3–12.2) | 112 (11.3; 9.4–13.5) |
| Number (%; CIb) of subjects experiencing a confirmed FEV1 decline | 38 (3.9; 2.7–5.2) | 38 (3.8; 2.7–5.2) |
| Number (%; CIb) of subjects experiencing a persistent FEV1 decline | 27 (2.7; 1.8–4.0) | 24 (2.4; 1.6–3.6) |
| Median time (years) from baseline to persistent declinec | 1.28 (1.08, 1.52) | 1.36 (1.10, 1.60) |
| Median follow-up time (years) based on reverse Kaplan-Meier method (95% CI) | 1.18 (1.13–1.23) | 1.22 (1.17–1.26) |
FEV1 = forced expiratory volume in 1 s; N = number of subjects in the treatment group.
The second spirometry that confirmed the FEV1 decline was to occur within 14–42 days (inclusive) of the decline. The spirometry that established persistence was to occur within 60–120 days (inclusive) of the confirming (second) FEV1 decline.
95% confidence intervals on percentage based on Clopper-Pearson method.
Median (25th, 75th percentile) presented.
Any 2 consecutive FEV1 declines that were ≥14 days apart. The spirometry that established persistence was to occur ≥60 days after the initial FEV1 decline.
Fig. 1.
Change from baseline in FEV1 (L).
Supplemental Fig. S4 shows the estimated cumulative incidence curves for time to persistent FEV1 decline by treatment group. Applying the protocol primary endpoint definition, the estimated cumulative incidence of persistent FEV1 decline in the EXU group consistently exceeds that of the usual care group throughout the 30 months of follow-up. At approximately 18 months on treatment, the risk of persistent FEV1 decline was approximately 1.3% in EXU users compared with 0% in usual care users. When the supplemental definition of the primary endpoint is used, the disparity in persistent FEV1 decline risk for EXU versus usual care users disappears. Using the same example of 18 months on treatment, the risk of persistent FEV1 decline was approximately 4.3% for EXU users compared with about 3.8% in usual care users. After 2 years, the curves are difficult to interpret due to the small risk sets at these later time-points.
A summary of subjects meeting the secondary endpoints after treatment-masked adjudication by the study endpoint committee is provided in Table 3. Seven subjects (0.7%) in the EXU group met the pulmonary SAE composite endpoint compared with 3 subjects (0.3%) in the usual care group. Twelve EXU (1.2%) versus 11 (1.1%) usual care subjects met the cardiovascular SAE composite endpoint, and 2 (0.2%) EXU versus 0 usual care subjects met the allergic response SAE composite endpoint. Lastly, 12 (1.2%) EXU versus 9 (0.9%) usual care subjects died from any cause.
Table 3.
Pulmonary, cardiovascular, allergic reaction and all-cause mortality secondary endpoints.
| Exubera N = 987 |
Usual Care N = 989 | |
|---|---|---|
| Pulmonary SAE Composite | ||
| Number of events adjudicated | 16 | 10 |
| Classification (%; CIa) | ||
| Met endpoint definitionb | 7 (0.7; 0.3–1.5) | 3 (0.3; 0.1–0.9) |
| Did not meet endpoint definition | 5 (0.5; 0.2–1.2) | 5 (0.5; 0.2–1.2) |
| Insufficient data | 4 (0.4; 0.1–1.0) | 2 (0.2; 0.0–0.7) |
| Cardiovascular SAE Composite | ||
| Number of events adjudicated | 46 | 40 |
| Classification (%; CIa) | ||
| Met endpoint definitionc | 12 (1.2; 0.6–2.1) | 11 (1.1; 0.6–2.0) |
| Did not meet endpoint definition | 25 (2.5; 1.6–3.7) | 22 (2.2; 1.4–3.3) |
| Insufficient data | 9 (0.9; 0.4–1.7) | 7 (0.7; 0.3–1.5) |
| Allergic Response SAE Composite | ||
| Number of events adjudicated | 6 | 1 |
| Classification (%; CIa) | ||
| Met endpoint definitiond | 2 (0.2; 0.0–0.7) | 0 (0.0; 0.0–0.4) |
| Did not meet endpoint definition | 1 (0.1; 0.0–0.6) | 1 (0.1; 0.0–0.6) |
| Insufficient data | 3 (0.3; 0.1–0.9) | 0 (0.0; 0.0–0.4) |
| All-Cause Mortality | ||
| Confirmed deathse | 12 (1.2; 0.6–2.1) | 9 (0.9; 0.4–1.7) |
COPD = chronic obstructive pulmonary disease, MI = myocardial infarction, N = number of subjects in the treatment group, SAE = serious adverse event.
95% confidence intervals on percentage based on Clopper-Pearson method.
Met endpoint definition = events classified as definite/possible pneumonia, definite/possible COPD, definite/possible asthma, probable obstructive lung disease not otherwise specified or probable acute bronchitis.
Met endpoint definition = events classified as definite/possible stroke, definite/possible MI, other (non-MI, non-stroke) cardiovascular/cerebrovascular death.
Met endpoint definition = events classified as anaphylaxis, angioedema/urticaria, bronchospasm or possible allergic reaction NOS.
Confirmed death by medical records or death certificate.
Although overall numbers of relevant events were small, numerically more events were adjudicated as meeting the pulmonary SAE composite and allergic response SAE composite endpoints in the EXU group compared with the usual care group. There were no consistent treatment group differences in events adjudicated to meet the cardiovascular SAE composite endpoint, or all-cause mortality endpoint. Sensitivity analyses, in which fractions (25, 50, 75, and 100%) of the events adjudicated as having insufficient information for classification were included with the definite and possible cases, generally supported these results. Owing to small numbers of events, no definitive conclusions regarding treatment effect could be made.
Glycemic control, as defined by HbA1c(%), was maintained similarly in both treatment groups at all visits (e.g. mean [median] HbA1c was 8.0 [7.5] in EXU and usual care at 26 weeks, 7.8 [7.4] in EXU and 7.7 [7.3] in usual care at 52 weeks, and 8.1 [7.6] in EXU and 8.0 [7.3] in usual care at the index visit). Fewer EXU subjects (n = 52, 5.3%) had severe hypoglycemic events than usual care subjects (n = 66, 6.7%); there were 180 events in the EXU group with an event rate of 0.95 events/100 person-months, versus 240 events in the usual care group with an event rate of 1.23 events/100 person-months.
In both treatment groups, a similar number of subjects experienced any SAE (124 subjects in the EXU group and 109 subjects in the usual care group), and the highest numbers of reported SAEs were related to cardiac disorders, nervous system disorders, and metabolism and nutrition disorders.
3.3. Post amendment observational follow-up
During the 6-month observational period following transition of EXU subjects to usual care, only a small number of potential secondary endpoints were reported. One pulmonary SAE composite event was reported in the EXU group but after adjudication did not meet the endpoint definition, and none were reported in the usual care group. Of reported cardiovascular SAE composite events (2 in the EXU group and 4 in the usual care group), one event (0.1%) was adjudicated as definite in the EXU group and one event (0.1%) was adjudicated as a possible in the usual care group. Among reported deaths (3 in the EXU group and 4 in the usual care group), 0 in the EXU group and 1 event in the usual care group were confirmed via medical records (e.g., death certificate) during adjudication. No allergic reaction SAE composite events were reported.
4. Conclusions
EXU is the first inhaled insulin approved for the treatment of diabetes. The EXU Large Simple Trial (VOLUME) was designed to examine the post-approval safety of EXU in usual clinical practice. According to the original protocol, 5,300 subjects were to be recruited in 24 countries and followed for at least 5 years. However, VOLUME was terminated early (when 1,976 patients were randomized) in accordance with a protocol amendment implemented after EXU was voluntarily withdrawn from the market. Thus, the study had insufficient power to address the primary endpoint in the absence of any unanticipated large effects and no formal hypothesis testing could be performed. Instead, the risk of each endpoint was estimated for subjects randomized to EXU or to usual care, using rigorous, predefined descriptive measures.
Observed treatment group differences in change from baseline in FEV1 are unlikely to be clinically meaningful given the magnitude of the differences and the variability in the FEV1 data. The number of subjects experiencing a persistent decline in FEV1 exceeding 20% from baseline was slightly higher in the EXU group compared with subjects in the usual care group, using the original protocol definition of the primary endpoint, and similar for subjects in both treatment groups using the pre-specified supplemental definition of the primary endpoint. The supplemental definition, which did not include specific time windows for the confirmatory FEV1 measurements was intended to capture more endpoints and was deemed more clinically relevant by pulmonologists advising the study conduct.
Although overall numbers of relevant events were small, numerically more events were adjudicated as meeting the pulmonary and allergic response SAE composite endpoints in the EXU group compared with the usual care group. Increased reporting of SAEs among the EXU group cannot be ruled out due to the observational, open-label nature of the follow-up, and labelling for EXU. There were no consistent treatment group differences in events adjudicated to meet the cardiovascular SAE composite endpoint, or all-cause mortality, but the low number of events precluded drawing firm conclusions.
In both treatment groups a similar number of subjects experienced SAEs and deaths.
Glycemic control was maintained similarly in both treatment groups and fewer subjects in the EXU group compared with the usual care group had severe hypoglycemic events. During the 6-month observational period following transition of EXU subjects to usual care, there were no marked differences between the former EXU and usual care groups.
In June 2014, another inhaled insulin, Afrezza®, was approved in the US for treatment of diabetes mellitus among adults. The Afrezza® prescribing information [7] has several similarities to the EXU USPI including clinical trial findings regarding lung function declines and recommendations for spirometry at baseline, 6 months and then yearly. Like EXU, the Afrezza® US approval is predicated on a post-approval commitment to further study its safety with regard to lung function, cardiovascular outcomes and lung cancer. While EXU has not been available since 2008, the results from this study add to the growing body of safety data regarding inhaled insulin for treatment of adults with diabetes mellitus. Moreover, the VOLUME study serves as an example how a large, simple, pragmatic trial, designed to test the value of surveillance testing can inform the pulmonary safety of other inhaled agents.
Conflicts of interest
Drs. Bracken, Koch, Wise, and Cohen were paid consultants to Pfizer for their roles on the VOLUME steering committee. Dr. Bracken has served as a paid consultant to Pfizer, Forest Labs, GlaxoSmithKline, Lilly, Procter and Gamble, and Sanofi-Aventis. Dr. Wise has served as a paid consultant to Pfizer, Mannkind, and Sanofi-Aventis. Dr. Cohen has served as a paid consultant to Pfizer for work related to clinical trials. Dr. Koch is the principal investigator of a biostatistical agreement between Pfizer and the University of North Carolina at Chapel Hill, and that agreement provided the structure for his activity on the Steering Committee of the study reported in this manuscript. He is also the principal investigator of many such biostatistical agreements with other biopharmaceutical sponsors, including ARENA, AstraZeneca, Eli Lilly and Company, Forest Research Institute (Allergan), GlaxoSmithKline, Merck, Novartis, and Sanofi, although his activities for those other sponsors are not related to the content of this manuscript. Information concerning all biostatistical agreements for which Dr. Koch is the principal investigator is publicly available through the University of North Carolina at Chapel Hill. Dr. Koch has no conflicts to report.
Drs. Duggan, Gatto, and Ms. Kolitsopoulos are full time employees and stock shareholders of Pfizer. At the time of study conduct, Dr. Jackson was a full-time employee and stock shareholder of Pfizer.
Acknowledgements
This study was sponsored by Pfizer. We are indebted to the 1,976 participants in the VOLUME Study; to the VOLUME Data Monitoring Committee (Peter Lange, Martin Simoons, and Mark Buyse); to the VOLUME Scientific Steering Committee (Michael Bracken, Chair; James Goldstein, Gary Koch, Robert Wise, Robert Ratner); to the VOLUME Endpoint Committee (Michael Lewis, Chair; Atul Malhotra; Richard deShazo; Sherryn Roth; Cecilia Bahit; Paul Hauptman; Richard Leung; Raphael Heinzer; Phil Lieberman; Stephen Kemp); to Pfizer Medical, Research and Development, and Safety colleagues: Robert Reynolds, Sol Klioze, Richard Riese, Pamela Schwartz, Anne Cropp, Susan DeCorte, Susan Gannon, Valerie Vandevoorde, Kevin Sweetland, and Vivianne Dillon. Study management was provided by Claudia Schaefer/Vera Weilburg (in Germany), Glenn Hare/Dean Spurden (in the United Kingdom), and Tina Ljungh (in Sweden), and by United Biosource Corporation (UBC) (in the US) under Bruce Smith, and was funded by Pfizer. Statistical support was provided by Inventiv Clinical under Eugenia and Earl Webb Henry. We thank all of the investigators and coordinators who took part in this study (a complete list of whom can be found in the appendices).
Dr. Gatto (guarantor) led the study design and implementation, and drafted/revised the manuscript. Dr. Bracken chaired the scientific steering committee, and was involved in implementation, data assessment and manuscript review/editing. Dr. Koch was a member of the Scientific Steering Committee, contributed to amending the SAP, and contributed to manuscript review/editing. Dr. Wise served on the Scientific Steering Committee and was involved in the protocol design, implementation, data assessment and manuscript review/editing. Mrs. Kolitsopoulos served as project manager and was involved in protocol design, implementation, data assessment and manuscript drafting/review/editing. Dr. Duggan contributed to amending the SAP, producing final tables, producing post-hoc analyses, and reviewing/editing manuscript. Dr. Jackson was involved in protocol design, implementation, study governance, data assessment and manuscript review.
The design and results of VOLUME were previously presented in poster format at the International Society of Pharmacoepidemiology annual meetings in August 2008 and August 2010 (respectively) with the accompanying abstracts published in Pharmacoepidemiology and Drug Safety (Volume 17, Supplement S1, 13 Aug 2008 and Volume 19, Supplement S1, 24 Aug 2010). The basic study design and results are posted on clinicaltrials.gov (identifier: NCT00359801).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100427.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Rosenstock J. Two-year pulmonary safety and efficacy of inhaled human insulin (Exubera) in adult patients with type 2 diabetes. Diabetes Care. 2008;31(9):1723–1728. doi: 10.2337/dc08-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NDA 21-868/EXUBERA, US Package Insert. 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021868lbl.pdf 1.31.2018. Available from: [Google Scholar]
- 3.Exubera- Annex 1 . 2006. Summary of Product Characteristics.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000588/WC500054704.pdf 1.31.2018. Available from: [Google Scholar]
- 4.Sin D.D., Wu L., Man S.F. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 5.Exubera Pfizer. 2006. https://clinicaltrials.gov/ct2/show/NCT00359801?term=exubera&rank=3 (Large Simple Trial to Evaluate Long-Term Pulmonary and Cardiovascular Safety). 1.31.2018. Available from: [Google Scholar]
- 6.Moher D., Schulz K.F., Altman D.G. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Clin. Oral Investig. 2003;7(1):2–7. doi: 10.1007/s00784-002-0188-x. [DOI] [PubMed] [Google Scholar]
- 7.AFREZZA® (insulin human) inhalation powder Prescribing Information. 2018. https://www.afrezza.com/pdf/Prescribinginfo.pdf Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.