Abstract
Acute myeloid leukemia (AML) is defined by the presence of ≥ 20% myeloblasts in the blood or bone marrow. Spontaneous remission (SR) of AML is a rare event, with few cases described in the literature. SR is generally associated with recovery from an infectious or immunologic process, and more recently possibly with clonal hematopoiesis. We review the literature and assess the trends associated with SR, and report a new case of a 58-year-old man with a morphologic diagnosis of AML associated with a severe gastrointestinal (GI) tract infection. The patient had an NF1 variant that was previously unreported in AML as the only clonal abnormality. After treatment of the infection, the increased blast population subsided with no leukemia-directed therapy, and the patient has remained in a continuous, spontaneous complete remission for > 2 years.
Keywords: Acute myeloid leukemia, NF1 alteration, Clonal hematopoiesis, Spontaneous remission, Leukemoid reaction
1. Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease that is fatal in most patients. Without disease-directed therapy, essentially all patients will expire within weeks to months. Spontaneous remission (SR) of AML is a poorly understood and rare event, but it does occur, with multiple cases reported from the 1940’s-present. SR is generally seen in the setting of acute infection, antibiotic use, or blood product transfusion, and an immune-mediated process has been postulated [1]. The time to relapse is generally short, with patients typically requiring standard treatment within a few months.
It is not clear if some SR cases, particularly the more durable ones, were actually a “leukemoid reaction”, a non-malignant process characterized by an exaggerated immune response (usually to infection, e.g., C. difficile colitis) with marked leukocytosis and increased levels of pro-inflammatory cytokines and colony stimualitng factors (G-CSF/GM-CSF) [2,3]. Classically, there is mature neutrophilia in the absence of blasts [4].
In this letter, we present a novel case of AML with NF1 mutation that achieved a durable SR in the setting of GI septicemia. We review the entire body of literature on SR-AML, and analyze the charactetisitcis of SR-AML patients, including those with both brief and prolonged SRs.
2. Case presentation
A 58-year-old Hispanic man with a history of ankylosing spondylitis previously treated with methotrexate and infliximab developed fever, abdominal pain, and hematochezia during a trip to Central America. On return to the United States, blood work revealed 6% circulating blasts, hemoglobin 12.3 g/dL, white blood count (WBC) 2.2 × 103 cells/mm3, 7% neutrophils, 45% lymphocytes, 4% monocytes, 19% eosinophils, and 2% myelocytes. Platelets were 546 × 103/mm3. Bone marrow biopsy demonstrated 40-50% blasts, left-shifted myelopoiesis, and trilineage dysplasia. No Auer rods were seen. The blasts were positive for CD34, CD117, MPO, CD13, and CD33. Cytogenetics were normal. Molecular testing (11-gene AML next generation sequencing [NGS] panel) was negative. His anemia worsened and he required blood transfusions. Intravenous antibiotics were started.
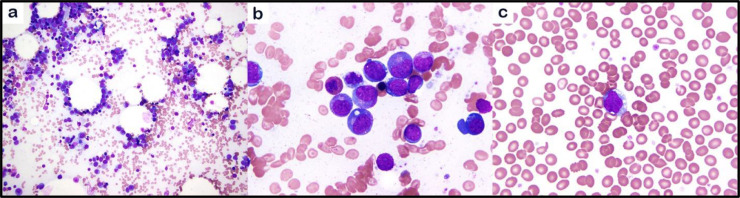
The patient was transferred to our hospital with ongoing bloody diarrhea and hypotension. Computed tomography (CT) imaging showed acute colitis. Upon arrival his WBC was 14 × 103 cells/mm3 with neutrophilia and no circulating blasts, hemoglobin was 7.6 g/dL (transfusion dependent), and platelets were 1,006 × 103/mm3. Repeat bone marrow examination showed 25% blasts with background dysplasia (Fig. 1). AML induction was postponed as he was treated for GI septicemia.
Fig. 1.
Bone marrow biopsy showing acute myeloid leukemia. The bone marrow aspirate smears show left shifted myelopoiesis with increased blasts (a). Blasts comprise 25% of bone marrow cellularity (b), with circulating blasts in peripheral blood (c) (a, 200X, b,c, 1000x; a-c, May-Grünwald Giemsa stain).
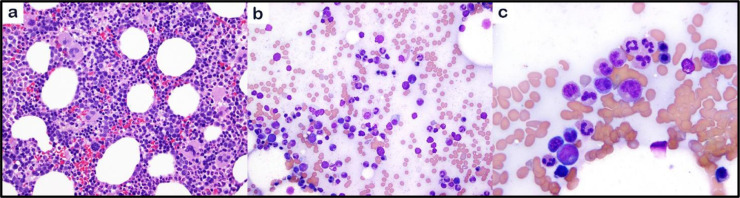
Over the next 2 weeks, the patient's symptoms resolved and his blood counts normalized. He underwent a third bone marrow biopsy ~4 weeks after the initial assessment (Fig. 2), which demonstrated a cellular bone marrow (50-70%), increased megakaryocytes, and mild dyserythropoiesis. Blasts comprised 1% of total cells. The only abnormality was an NF1 mutation (c.4430+delT;splice-region) with variant allele frequency (VAF) 17% on an expanded NGS panel. Induction chemotherapy was deferred, and he was placed on observation.
Fig. 2.
Bone marrow biopsy with no evidence of acute leukemia. The bone marrow core biopsy shows hypercellular bone marrow with increased megakaryocytes (a). The bone marrow aspirate smears show maturing hematopoiesis (b) with no increased blasts (c) (a, 200X, H&E stain; b,c, 1000x, May-Grünwald Giemsa stain)
Follow-up bone marrow biopsy 6 months after achieving SR demonstrated normocellular marrow (20-40%) with erythroid predominance and maturing trilineage hematopoiesis and no evidence of acute leukemia or myeloid neoplasm. NF1 gene reassessment could not be done due to insurance barriers. He remians in continuous SR for >2 years at time of writing.
2.1. Analysis of reported cases
A PubMed search was performed using terms “acute leukemia”, “remission”, “regression”, “spontaneous”; including only articles written in English. Infant and down syndrome cases were excluded. A total of 47 articles were examined, containing 55 cases of acute leukemia with SR. Among the 56 cases studied (including our patient), 33 patients were male (59%) and 23 were female (41%). The median age was 53.5 years. AML comprised 50 cases (89%), acute lymphocytic leukemia 4 cases (7%), and cutaneous myeloid sarcoma 2 cases (4%).
The mean time to relapse was 12.4 months. The median time to relapse was 5 months (range 2 weeks-NE). Sixteen of 56 patients had SR for >12 months (not including 1 patient who received therapy after SR and remained in CR >30 months). Of these 16 patients, 10 relapsed and 6 remained in CR at time of publication. For the 6 patients without relapse, follow-up was 14 months, 18 months, 24 months (our case), 29 months, 4 years, and 10 years. Of these 6 durable CRs, all had monocytic differentiation (M4/M5) except our case (5/6 cases). Five received antibiotics for acute infection (the one that did not received a GnRH agonist for misdiagnosed prostate cancer). Three additional patients had late relapse >2 years after SR. Of note, there were patients in remission for <1 year at date of last follow-up, and their long-term outcome is unknown.
When looking at all 56 cases, almost half were monocytic subtype by FAB (M4/M5). Cytogenetics were available for 42 cases: 15 patients (36%) had a normal karyotype (NK), 5 (12%) trisomy 8, 5 (12%) t(8;21), 4 (9%) 11q23/MLL re-arragenemnt, 2 inv(16), and 2 t(3;3)/EVI1 re-arragenemnt. Ten patients (24%) had other abnormalities. More recently, Grunwald et al., reported an AML patient with NPM1 mutation who had SR with loss of NPM1 mutation, but persistent background mutations such as TET2. His disease relapsed abruptly ~1 year later, with recurrence of NPM1 mutation [5].
Patients were reported to have an associated infection in 76% of cases and blood product transfusion in 45%. Less common associations were G-CSF, steroids, hydroxyurea, termination of pregnancy, GnRH, tumor lysis syndrome, discontinuation of lenalidomide, and Henoch-Schönlein purpura. 9% had no identifiable association. Among 42 cases with a presenting infection, 45% had pneumonia (n=19) and 16% bacteremia (n=7). Other sources included upper respiratory, urinary, GI tract, skin, disseminated tuberculosis, and liver abscess.
3. Discussion
Our patient had histologic diagnosis of AML with >20% myeloblasts on two subsequent marrow examinations. After treatment of concurrent GI sepsis, he entered SR and has been in continuous CR for 24 months. On review of SR in the literature, it is clear the vast majority of patients relapse, with most relapses occurring early (<1 year). Patients were typically younger, de novo, and monocytic. Interestingly, most had a cytogenetic abnormality (e.g. +8, core-binding factor (CBF) fusion, and MLL- and EVI1-rearragements; 36% had NK). Most patients with SR have an associated factor such as infection, but the causality has been opaque.
Of the 16 known patients with durable SR for >12 months, 6 (40%) have not relapsed. Of these 6, 5 had monocytic subtype and 5 had a concomitant infection at diagnosis. Three had a NK and 2 MLL-AF9 fusion (1 did not have cytogenetics available). This raises the question: are durable SRs attributable to: (1) driver-mutated AML undergoing SR via unknown mechanism (e.g. the MLL-rearranged cases), or (2) exaggerated, blastic “leukemoid reaction” in the setting of CH. Microbial products, such as endotoxin and nucleic acids, are potent stimuli for CSF production [6], and pharmacologic CSF exposure can induce a blastic marrow response [7].
Our patient presented with GI sepsis and hemorrhagic colitis, a known cause of leukemoid response [8]. Interestingly, he did not present with leukocytosis, the sine-qua-non of leukemoid reaction, but rather with leukopenia, although he did have a left-shift and marked thrombocytosis. Our patient's self-resolving blast increase and dysplastic features, normal cytogenetics, and long duration of SR, support that he may have had an atypical marrow stress response in the setting of isolated NF1 mutation.
The NF1 gene is a tumor suppressor and negative regulator of RAS. The canonical hereditary mutation is associated with neurofibromatosis Type 1, where the risk of myeloid leukemias is 200 – 500 times higher than the general population [9,10]. Somatic NF1 mutations are found in ~5-7% of de novo AML, and are associated with poor prognosis [11], [12], [13], [14]. Reports of high VAF and presence of the mutation in hematopoietic stem cells (HSCs) suggests that NF1 may act as a driver or founder mutation in some AML patients and it is not a common CH gene [11,12,15]. NF1 mutations occur throughout the gene and consist primarily of truncating frameshift mutations but also missense, nonsense, and indels with a recent hotspot mutation characterized in 27% of AML NF1 mutants at Threonine 676, which leads to nonsense-mediated mRNA decay [12]. The NF1 mutation in our patient at c.4430+delT targets Arginine 1477 with a deletion causing frame-shift in a splice region, which would be expected to cause premature termination and truncated protein sequence lacking the c-terminal nuclear localization signal. Missense and splice mutations at R1477 in NF1 have been previously identified in 7 patients with solid tumors and are predicted to be pathogenic (COSMIC); however, this is the first time it has been reported in AML.
We report a novel NF1 mutation in AML and one of the first cases of AML-SR with NGS data available. Whether our patient had self-limited blast proliferation/self-renewal in the setting of CH, or de novo AML with true SR, it is important to consider both possibilities when triaging leukemic patients presenting with intercurrent infection and reactive blood counts/unexplained count recovery. In the >50 cases we analyzed, while most SRs occured in the setting of severe physiologic stress, over half also had a recurrent cytogenetic abnormality (including 11 patients with AML-defining gene fusion), implicating an autologous mechanism than can induce remission in frank AML, although this is rarely durable, Table 1.
Table 1.
Summary of our case and all cases reported in the literature
| Year/ First author | Age/Gender | FAB Subtype | Cytogenetics/Mutations | Associated factors or characteristic | Duration of remission |
|---|---|---|---|---|---|
| 1. 1949 – Birge | 33 F | AML-M5b | Not disclosed | Eclampsia, termination of pregnancy | 22 months |
| 2. 1979 – Lanchant | 67 F | AML-M1 | Not disclosed | Pneumonia | 17 months |
| 3. 1982 – Ruutu – 35 | 34 M | AML-M5b | Normal | Fever | 2 months |
| 4. 1985 – Ifrah | 56 M | AML-M1 | 50 XXY, +4, +8, +14, +t(21q,22q), -21, -22 | Disseminated tuberculosis, blood transfusion, leukocyte transfusion | 34 months |
| 5. 1986 – Jehn | 34 M | AML-M4 | Partial del(16) | Pneumonia, ear infection, blood transfusion | 5 months |
| 6. 1988 – Kizaki | 53 F | AML – hypoplastic | Normal | Fever, antibiotic use | 5 months |
| 7. 1989 - Antunez de Mayolo | 28 F | AML-M3 | Aneuploidy (with extra chromosome in group C) | Fever, antibiotic treatment, blood transfusion | 3 months |
| 8. 1990 – Spadea | 69 M | AML-M5a | Not disclosed | None | 3 months |
| 9. 1991 – Narayanan | 64 M | AML-M4 | 46XY, del(5)(q13;q31) | Blood transfusion, S. aureus bacteremia | 8 months |
| 10. 1993 – Jimenez | 72 F | AML-M0 | 3n hyperploid | Pneumonia, S. epidermidis bacteremia, blood transfusion, remote history of CHT for AML (ineffective) | 5 months |
| 11. 1993 – Kang | 19 M | AML-M3 | Not disclosed | Purulent cellulitis | 7months |
| 12. 1993 – Kang | 19 F | AML-M3 | Not disclosed | Tuberculosis pneumonia | 14 months |
| 13. 1994 – Paul | 74 F | AML-M5 | Two clones: {1}46XX, t(9;11)(p22;q23) {2}52XX, +3, +8, +8, +14, +19, +t(9;11) (p22;q23) |
None | 7 months |
| 14. 1994 – Musto | 49 F | AML-M5a | Not disclosed | Concomitant Henoch-Schönlein syndrome. | 6 months |
| 15. 1994 – Delmer | 48 M | AML-M2 | 45 × 0, t(8;21) | Gram-negative and Candida albicans sepsis, blood transfusion | 36 months |
| 16. 1994 – Delmer | 41 F | AML-M5 | Normal | Prolonged fever of unknown origin, blood transfusion | 14 months |
| 17. 1994 – Delmer | 54 M | AML-M2 | Normal | Gram-negative sepsis, blood transfusion | 3 months |
| 18. 1996 – Mitterbauer | 64 M | AML-M5b | Not disclosed | Sepsis, E. faecium bacteremia, hydroxyurea, blood transfusion | > 14 months |
| 19. 1996 – Mitterbauer | 83 M | AML-M2 | t(8;21)(q22;q22) AML1/ETO, del(7)(q22) | Pneumonia, G-CSF, blood transfusion | 1 month |
| 20. 1997 – Takahashi | 64 M | Unclear | Not disclosed | Pneumonia, G-CSF | 4 months |
| 21. 1997 – Takahashi | 54 M | Unclear | 47 XX, +8 | Pneumonia, G-CSF | 3 months |
| 22. 1997 – Takahashi | 70 M | Unclear | Not disclosed | G-CSF, blood transfusion | 17 months |
| 23. 2000 – Takezako | 79 F | ALL-T | Not disclosed | Pneumonia, antibiotic use | 1 year |
| 24. 2000 – Martelli | 26 F | AML-M4E | 46 XX, inv(16)(p13q22), CBFB/MYH11 + | Interstitial pneumonia, antibiotics, hydroxyurea, blood transfusion | 1 month (patient received CHT and relapsed 25 months later) |
| 25. 2001 – Tzankov | 60 F | AML – M1 | Normal | Acute tonsillitis, pneumonia, G-CSF, blood transfusion, | 10 months |
| 26. 2001 – Shimohakamada | 71 F | AML-M2 | 45 × 0, -1, +4(q31), t(8;21)(q22;q22), AML1/MTG8 | Pneumonia, blood transfusion, high-dose methylprednisolone | 4 months then lost follow-up |
| 27. 2004 – Mayald | 31 M | AML-M5a | Normal | Fever, group B streptococci bacteremia, antibiotic treatment | 2 months |
| 28. 2004 – Müller | 61 M | AML-M5a | T(9;11)(q22;q23); MLL/AF9 fusion. | Fever, antibiotic treatment | > 29 months |
| 29. 2004 – Fozza | 72 M | AML-M0 | 48 XY, del(6)(p22-pter), +13, +14 | Pneumonia, sputum positive for coagulase-negative S. aureus and Candida spp. Blood transfusion, steroids | 5 months |
| 30. 2006 – Tsavaris | 64 M | AML-M4 | Normal | GnRH agonist therapy | > 4 years |
| 31. 2006 – Al-Tawfiq | 47 M | AML-M5b | Normal | Perforated bowel, Clostridium septicum bacteremia | 4 months |
| 32. 2007 – Trof | 29 M | AML-M2 | 45 × 0, t(8;21) | Infection, antibiotic use, blood transfusion | 3 months |
| 33. 2007 – Trof | 28 M | AML-M5b | Normal | Beta-hemolytic Streptococci bacteremia, blood transfusions | Received consolidation CHT after SR. Relapse 4 weeks after SCT. |
| 34. 2007 – Daccache | 83 F | AML-M5b | 47 XX, trisomy 8 | Antibiotics for possible UTI; blood transfusion | 2 weeks |
| 35. 2007 – Hudecek | 35 F | AML-M1 | 48 XX, del(3)(q21), +6, t(11;15)(q23;q15), +21. 11q23/MLL abnormality | Blood transfusion, prophylactic antibiotics | > 8 months |
| 36. 2008 – Yoruk | 4 F | T-ALL | Not disclosed | Fever, possible pneumonia versus upper respiratory infection | 4 weeks |
| 37. 2008 – Jain | 66 F | AML-M4 | Trisomy 8 | Candida pneumonia | 29 months |
| 38. 2008 – Jain | 72 F | AML-M5b | Not available | None | 5 months |
| 39. 2008 – Jain | 46 M | AML-M5b | Not available | Liver abscess | 2 months |
| 40. 2009 – Chen | 14 M | ALL-B | Normal | Pneumonia, tumor lysis syndrome, MRSA, S. viridans and coagulase-negative Staphylococcus in pleural fluid | 14 days |
| 41. 2009 – Marisavljevic | 63 M | AML-M2 | 46XY, del(6)(q21) | Blood transfusion | 6 months |
| 42. 2010 – Teng | 75 M | AML-M2 | Trisomy 8 | Blood transfusion, pneumonia | 21 weeks |
| 43. 2012 – Xie | 42 M | AML-M5a | Normal | Pneumonia, G-CSF | Blastic plasmacytoid dendritic cell neoplasm 40 months after initial diagnosis |
| 44. 2012 – Müller-Schmah | 61 F | AML-M5a | t(9;11), MLL-AF9 | Fever, S. aureus bacteremia, antibiotics administration | > 10 years |
| 45. 2013 – Zeng | 34 F | Cutaneous myeloid sarcoma | 46 XX, normal | Blood transfusion, fever | 1 month |
| 46. 2013 – Zeng | 31 M | AML-M2 | 46 XY, t(8;21)(q22;q22), del(9)(q22,q34) | Pulmonary infection by Serratia marcescens | 2 months |
| 47. 2014 – Adam | 35 M | AML-M4 | Not disclosed | Blood transfusion, possible infection | 6 weeks |
| 48. 2014 – Kazmierczak | 77 M | AML-M4 | 48 XY, +13, +21 | Blood transfusion, low dose steroids | 7 months |
| 49. 2014 – Purhoit | 46 M | ALL-B | Normal | Acinetobacter spp. bacteremia, infective endocarditis, possible fungal pneumonia | 9 weeks |
| 50. 2015 – Takahashi | 79 F | Aleukemic cutaneous myeloid sarcoma | Trisomy 8 | No associated factor | 2 months |
| 51. 2017 – Hoshino | 49 F | AML-M5a | 46,XX,t(8;16)(p11;p13), MOZ-CBP fusion | None. Received BMT 4 months after SR. | 4 months, at least. |
| 52. 2017 – Kremer | 51 M | AML-M4 | 45 XY, t(3;3)(q21;q26), der(17)t(17;21)(p11.2;q11;2) | Previous lymphoma / discontinuation of lenalidomide | 5 months |
| 53. 2017 – Mozafari | 53 M | AML-M4 | Normal | Pulmonary infection | > 18 months |
| 54. 2018 – Höres | 31 F | ALL | 46XX, del(5)(q13;q22); ACSL6 deletion. | Pregnancy, blood transfusion, GI infection | > 30 months (had SR but also received therapy) |
| 55. 2019 – Grunwald | 72 M | AML- M2 | Normal, Mutated NPM1, RUNX1, NRAS, TET2, U2AF1, PRPF8 | Blood transfusion, leukemia cutis | ~12 months (relapsed) |
| 56. 2019 – Bradley | 58 M | Unclear (had MDS changes) | Normal; Deletion of NF1 gene | GI septicemia | > 24 months (f/u ongoing) |
References
- 1.Rashidi A., Fisher SI. Spontaneous remission of acute myeloid leukemia. Leukemia Lymphoma. 2015;56(6):1727–1734. doi: 10.3109/10428194.2014.970545. [DOI] [PubMed] [Google Scholar]
- 2.Argueles-Grande C., Leon F., Matilla Fuentes J., Dominguez J., Montero J. Steroidal management and serum cytokine profile of a case of alcoholic hepatitis with leukemoid reaction. Scand. J. Gastroenterol. 2002;37:1111–1113. doi: 10.1080/003655202320378347. [DOI] [PubMed] [Google Scholar]
- 3.Leizer T., Cebon J., Layton J.E., Hamilton J.A. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76(10):1989–1996. [PubMed] [Google Scholar]
- 4.Sakka V., Tsiodras S., Giamarellos-Bourboulis E.J., Giamarellou H. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur. J. Internal Med. 2006;17:394–398. doi: 10.1016/j.ejim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Grunwald VV, Hentrich M, Schiel X, Dufour A, Schneider S, Neusser M, Subklewe M, Fiegl M, Hiddemann W, Spiekermann K, Rothernberg-Thurley M, Metzeler K (2019) Patients with spontaneous remission of high-risk MDS and AML show persistent preleukemic clonal hematopoiesis. Blood Adv. 3(18):2696-2699. doi: 10.1182/bloodadvances.2019000265. [DOI] [PMC free article] [PubMed]
- 6.Schrader J.W. Colony-stimulating factor. Encyclopedia Immunol. 1998;2:596–599. [Google Scholar]
- 7.Meyerson H.J., Farhi D.C., Rosenthal N.S. Transient increase in blasts mimicking acute leukemia and progressing myelodysplasia in patients receiving growth factor. Am. J. Clin. Pathol. 1998;109(6):675–681. doi: 10.1093/ajcp/109.6.675. [DOI] [PubMed] [Google Scholar]
- 8.Shoushtari, A.N., Wolchock, J., Hellman, M. (2018) Principles of cancer immunotherapy. In Uptodate. Available athttps://www.uptodate.com/contents/principles-of-cancer-immunotherapy. Accessed on Jul 01, 2018.
- 9.Korf, B. (2018) Neurofibromatosis type 1 (NF1): Pathogenesis, clinical features, and diagnosis. UptoDatehttps://www.uptodate.com/contents/neurofibromatosis-type-1-nf1-pathogenesis-clinical-features-and-diagnosis. Accessed on Jul 18, 2018.
- 10.Side L., Taylor B., Cayouette M., Conner E., Thompson P., Luce M., Shannon K. Homozygous inactivation of the NF1 gene in the bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N. Engl. J. Med. 1997;336(24):1713–1720. doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- 11.Eisfeld A.K., Kohlschmidt J., Krzysztof M., Mims A., Waler C., Blachly J., Nicolet D., Orwick S., Maharry S., Carroll A., Powell B., Kolitz J., Wang E., Stone R., de la Chapelle A., Byrd J., Bloomfield C. Springer Nature; 2018. NF1 Mutations are Recurrent in Adult Acute Myeloid Leukemia and Confer Poor Outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkin B., Ouillette P., Wang Y., Liu Y., Wright W., Roulston D., Purkayastha A., Dressel A., Karp J., Bockensted P., Al-Zoubi A., Talpaz M., Kujawaski L., Liu Y., Shedden K., Shakhan S., Li C., Erba H., Malek S.N. NF1 inactivation in adult acute myelogenous leukemia. Clin. Cancer Res. 2010;16(16):4135–4147. doi: 10.1158/1078-0432.CCR-09-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudry-Labis E., Roche-Lestienne C., Nibourel O., Boissel N., Terre C., Perot C., Eclache V., Gachard N., Tiguad I., Plessis G., Cuccuini W., Geoffroy S., Villenet C., Figeac M., Lepretre F., Renneville A., Cheok M., Soulier J., Dombret H., Preudhomme C. Neurofibromatosis-1 gene deletions and mutations in de novo adult acute myeloid leukemia. Am. J. Hematol. 2013;88:306–311. doi: 10.1002/ajh.23403. (2013) [DOI] [PubMed] [Google Scholar]
- 15.Cooms C., Gillis N.K., Tan X., Berg J.S., Ball M.C., Balasis M.E., Montgomery N.D., Bolton K., Parker J.S., Mesa T.E., Yoder S.J., Hayward M.C., Patel N.M., Richards K.L., Walko C.M., Knepper T.C., Soper J.T., Weiss J., Grilley-Olson J.E., Kim W.Y., Earp S., Levine R., Papaemmanuil E., Sehir A., Hayes D.N., Padron E. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin. Cancer Res. 2018 doi: 10.1158/1078-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]