Abstract
Background
The COVID-19 pandemic forced surgeons to reconsider concepts of “elective” operations. Perceptions about the time sensitivity and medical necessity of a procedure have taken on greater significance during the pandemic. The evolving ethical and clinical environment requires reappraisal of perioperative factors, such as personal protective equipment conservation; limiting the risk of exposure to COVID-19 for patients, families, and healthcare workers; preservation of hospital beds and ICU resources; and minimizing COVID-19-related perioperative risk to patients.
Study Design
A scaffold for the complex decision-making required for prioritization of medically necessary, time-sensitive (MeNTS) operations was developed for adult patients by colleagues at the University of Chicago. Although adult MeNTS scoring can be applied across adult surgical specialties, some variables were irrelevant in a pediatric population. Pediatric manifestations of chronic diseases and congenital anomalies were not accounted for. To account for the unique challenges children face, we modified the adult MeNTS system for use across pediatric subspecialties.
Results
This pediatric MeNTS scoring system was applied to 101 cases both performed and deferred between March 23 and April 19, 2020 at the University of Chicago Comer Children's Hospital. The pediatric MeNTS scores provide a safe, equitable, transparent, and ethical strategy to prioritize children's surgical procedures.
Conclusions
This process is adaptable to individual institutions and we project it will be useful during the acute phase of the pandemic (maximal limitations), as well as the anticipated recovery phase.
Abbreviations and Acronyms: EBL, estimated blood loss; MeNTS, medically necessary time-sensitive; OR, operating room; OSA, obstructive sleep apnea; pMeNTS, pediatric medically necessary, time-sensitive
Visual Abstract
The COVID-19 pandemic poses unprecedented challenges to healthcare systems around the world. Consistent with advice from national health organizations and surgical societies, most hospitals, including our own, cancelled nonemergency (also termed lower acuity or elective) surgical procedures. Consequently, procedures with a varying degree of urgency were delayed to avoid overwhelming hospital resources during the initial phase of the pandemic. In addressing the surgical needs of patients, hospitals and surgeons had to rely on individualized judgment to determine which procedures should be performed. Hospital and surgical leadership were also required to calibrate the medical necessity of procedures against the following contingencies: risk of lateral COVID-19 transmission to patients and families entering the hospital, relative scarcity of finite and necessary resources (eg personal protective equipment, blood products, hospital beds, and ventilators), and the consequent risk to healthcare providers. Although the initial decrease in nonemergency surgical cases enabled hospitals to immediately preserve hospital capacity, it created a new challenge to thoughtfully manage the surgical needs of the patients in their treatment communities.
The complexity of surgical decision-making during the pandemic prompted a series of questions to which established answers were not available. Given the current constraints, how long can repair of a symptomatic inguinal hernia be safely deferred in an infant? Could we reasonably ask a sickle-cell patient with symptomatic cholelithiasis to postpone laparoscopic cholecystectomy by 4 weeks when demands on hospital resources might or might not be lessened by the passage of time? How long should resection of a stage IV neuroblastoma be deferred once 4 cycles of chemotherapy have been completed? Some of the most difficult decisions during this pandemic have included those around the timing of operations in patients with known or presumed malignancies. Physicians have been forced to shift their entire focus from what is in their patient's best interest to what might be best for public welfare. By so doing, physicians must consider how the impact of changing the timing or sequence of treatments, including operations, affects the outcomes of individual patients.
In light of these considerations, evaluation of the relative effectiveness of nonoperative treatment options has been pushed to the forefront. For example, the growing experience with nonoperative management of uncomplicated appendicitis in both the adult and pediatric populations has shifted treatment patterns in many. As a result, surgeons who might not have previously considered nonoperative management are now offering this approach to patients and families. In the case of pediatric solid tumors, such as Wilms tumor or rhabdomyosarcoma, it makes sense to proceed with diagnostic procedures, and decisions between complete tumor resection and chemotherapy must be made on a case-by-case basis, taking into account local conditions and the child's health status. However, as we shift toward recommending initial nonoperative management of some conditions, we must also consider how long we can safely defer these procedures in the setting of an evolving pandemic with no clear end date.
The timing of a procedure could previously be calibrated according to established standards of care. In the setting of the COVID-19 pandemic, scheduling a procedure requires consideration of all the contingencies that we have outlined, such as medical necessity of the procedure, risk to the patient of disease progression or increased symptoms imposed by delay, risk to the patient and family of lateral transmission in the hospital, perioperative risk to asymptomatic COVID-19 patients if preoperative testing is limited, risk of exposure to healthcare workers, and use of limited resources. Cases once defined simply as “elective” or “urgent” are now assessed by a much more complex set of criteria, and perhaps are more appropriately defined as “medically necessary and time sensitive.” This constitutes a paradigm shift for surgeons caring for children. Our experience suggests that optimal assessment requires some framework to ensure systematic consideration of these unique factors, and that these decisions are made in as ethical and transparent a manner as possible.
On March 16, 2020, our institution initially cancelled all nonemergency cases, with the exception of a limited number of medically necessary procedures deemed time sensitive for the patients. As the list of deferred procedures grew, we recognized the need for a system to prioritize the scheduling of select cases. Existing structures for classifying case urgency (eg emergent, urgent, and elective) were not adequate to address the current situation. Our institutional surgical colleagues developed a scoring system for medically necessary, time-sensitive (MeNTS) procedures for adults that has been in use since late March 2020.1 This scoring tool systematically incorporates the novel factors reviewed here (eg resource consumption, COVID-19 transmission risk, and pre-existing conditions affecting both surgical and individual COVID-19 risk). The adult MeNTS system affords a transparent method to rank and triage these deferred cases by assigning a score to each procedure. Scores are then compared among cases, allowing appropriate and transparent allocation of operating room (OR) resources across specialties for the overall benefit of the community of surgical patients. Hospital and surgical leadership can then determine threshold scores for operations to be performed and those whose index of risks and resource use might be excessive based on the hospital resources available in real time. A key advantage of this scoring system is that it provides flexibility for dynamic adjustment of these thresholds based on the current environment. This system can be used not only for the prioritization of cases to be performed during the peak of resource limitations, but also during the anticipated recovery period during which resources gradually return to a more normal status.
We evaluated the adult MeNTS scoring system for our children's hospital operations, and recognized that adjustments would be required to optimize use for pediatric patients. For example, the original adult MeNTS scoring system could not differentiate between the risk of a symptomatic inguinal hernia repair in an 8-week-old ex-31-week preterm infant with chronic lung disease, and the risk of the same operation in an otherwise healthy 14-year-old. Differences in pediatric-specific risk factors, such as congenital diseases, were not accounted for, and several adult comorbidities, such as coronary artery disease or COPD, were essentially irrelevant. However, children (particularly infants) have also been reported to have significant morbidities and death from COVID-19, and it was important to capture these risk factors in the scoring.2 Comorbidities such as congenital heart and lung diseases can increase susceptibility to severe COVID-19 infection.2 , 3 Severe symptoms are also more common among children younger than 5 years of age, particularly those younger than 1 year and those with congenital diseases.4
Given this challenge, we undertook modifying the adult MeNTS scoring system to reflect the needs of a pediatric population, incorporating relevant conditions into the risk stratification system. We hypothesized that modifications to the adult MeNTS scoring system incorporating the unique requirements of pediatric surgical patients would yield a valuable pediatric-specific scoring system for triaging procedures. Based on our early experience with pediatric MeNTS (pMeNTS), we are hopeful that this approach might be applicable across a broad range of hospital settings in which children receive surgical care (academic and community, urban and rural), and that it would provide a mechanism for adapting surgical scheduling to the changing resource environment of the current COVID-19 pandemic. Individual institutions can evaluate their available resources and choose a different pMeNTS cutoff score at different times during the pandemic to reflect their individual situation at the time. This score can be shifted as the situation changes and a backlog of cases builds or is alleviated during different phases of the pandemic. The value of this approach might be amplified by the uncertain and dynamic conditions projected for the next phases of the pandemic. It is not known when or how the pandemic will subside, or at what level it might persist or recur as a “second wave.” Providing a framework for decision-making could reduce the moral stress experienced by healthcare providers making difficult decisions in a period of extended uncertainty.
Methods
We modified the MeNTS scoring system developed by Prachand and colleagues1 to suit the unique needs of pediatric patients. This scoring system was originally developed at our institution to provide guidance during the process of prioritizing adult MeNTS cases during the COVID-19 pandemic. This system assigns a risk score to each procedure, incorporating factors not routinely considered by the operative team, such as resource constraints (eg personal protective equipment and ICU beds), as well as COVID-19-specific risks to the perioperative healthcare team and the patient. This enables cases to be triaged in a way that considers the impact of the procedure, taking into account the needs of the patient and the human and physical resources of the institution.
As proof of concept, the pMeNTS scores of a sampling of procedures performed or deferred during a 4-week period from March 23 through April 19, 2020 were determined. We analyzed the 48 cases that were allowed to proceed as scheduled during this time period, and a random selection of 53 cases that were deferred; a total of 101 procedures were reviewed. The scores were calculated from representatives from across the range of surgical sub-specialties. These pMeNTS scores were compared to evaluate use of the pMeNTS scoring system as a decision aid for pediatric case triage and prioritization in the time of the COVID-19 pandemic or future crises.
To develop a pMeNTS scoring system that could be compared with the adult MeNTS system, we kept the same basic structure for scoring procedures. The same 3 categories of risk (ie procedure, disease, and patient) were modified to maximize use in the pediatric patient population. The same 5-point scale was used, assigning a higher numeric value to worse perioperative outcomes for the patient, an increased risk of COVID-19 transmission to the healthcare team, and greater resource use. Procedures with lower overall numeric scores are prioritized. A thorough description of the rationale behind the adult MeNTS scoring system can be found in the article by Prachand and colleagues.1
By preserving the 21 general categories, we were able to compare our model in a side by side comparison using the same cumulative score range of 21 to 105 points. This facilitated a direct comparison of our pMeNTS scoring system with the scores that would have resulted from application of the adult system to our pediatric patients. As in the adult model, a higher score is associated with a higher risk to the patient and to providers, as well as a greater likelihood of consumption of scarce resources during the COVID-19 pandemic.
Modifications were made within each of the 21 categories to adapt each to the unique health concerns of children. For example, the procedure category was slightly modified to reflect the fact that many of the MeNTS procedures involve patients in the neonatal ICU or pediatric ICU who are nearing discharge. The operation would, in fact, expedite the patient's discharge, potentially emancipating an ICU bed and additional resources.
Procedure factors
Procedure-specific factors (Table 1 ) that could be associated with worse patient outcomes, an increase in risk to care providers, or increased hospital resource use were modified for a pediatric population. The total score ranged from 7 to 35. Three variables—OR time, size of surgical team, and intubation probability—were not modified from the original adult MeNTS scoring system. The OR time allows consideration of the length of time clinical resources might be sequestered in a particular procedure. We modified the anticipated length of stay and postoperative ICU need variables to reflect the fact that some neonatal ICU and pediatric ICU patients might be ready for discharge to home or a sub-acute rehabilitation facility within 1 to 4 days after a pMeNTS procedure. Both of these variables quantify the length of time a hospital or ICU bed could be occupied by a patient. Procedures that can be performed on an outpatient basis will inherently receive lower pMeNTS scores. This is also helpful when triaging the constrained resources of hospital and ICU beds during a crisis, such as the COVID-19 pandemic, in particular because performing these procedures can expedite discharge. For example, an inguinal hernia repair in a premature infant who is otherwise ready for discharge, or a surgical gastrostomy tube in a child needed for safe feeding access, would both be predicted to speed discharge and release resources.
Table 1.
Procedure-Specific Factors
| Variable | Category |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| OR time, min | < 30 | 31–60 | 61–120 | 121–180 | ≥ 181 |
| Anticipated LOS | Outpatient or current inpatient, ready for discharge | 23 h | 24–48 h | ≤ 3 d or current inpatient, nearing discharge | >4 d or current inpatient, unknown LOS |
| Postoperative ICU need, % | Very unlikely | < 5 or current NICU/PICU patient, ready for discharge | 5–10 | 11–25 or current NICU/PICU patient, nearing discharge | ≥ 25 or current NICU/PICU patient, unknown LOS |
| Projected EBL∗ (% blood volume) or probability of blood product transfusion | ≤ 2, or transfusion very unlikely | 3–5, or transfusion < 25% likely |
6–10, or transfusion 50% likely | 11–15, or transfusion > 75% likely |
> 15, or multiple transfusions likely |
| Surgical team size, n | 1 | 2 | 3 | 4 | > 4 |
| Intubation needed to perform procedure (probability), % | ≤ 1 | 1–5 | 6–10 | 11–25 | ≥ 25 |
| Surgical site† | None of the following row variables | Abdominopelvic MIS | Abdominopelvic open operation, infra-umbilical | Abdominopelvic open operation, supra-umbilical | Other OHNS, upper GI, thoracic |
Procedure score 7 to 35. Higher scores associated with potentially worse outcomes, increased risk to provider, and/or increased hospital resource use.
GI, gastrointestinal; LOS, length of stay; MIS, minimally invasive surgery; NICU, neonatal ICU; OHNS, otolaryngology-head and neck surgery; OR, operating room; PICU, pediatric ICU.
A weight-based estimated blood loss calculator available at: https://reference.medscape.com/calculator/estimated-blood-volume.
Surgical sites that would fall into category 1 include orthopaedic and neurosurgical cases, as well as head and neck or axillary lymph node biopsies. If the approach also includes one of the other surgical sites, and then the higher scoring site would take precedence in scoring.
The “anticipated blood loss or estimated blood loss” (EBL) variable for the adult MeNTS specified particular blood volumes based on the average 70-kg male patient. We changed this to “projected EBL, or probability of blood product transfusion” to reflect the weight-based approach required for a pediatric population.5 As the preoperative calculation of EBL as a percentage of a patient's weight can be unwieldy, we tried to also align those scoring columns with a surgeon's expectation about the probability that a blood transfusion would be required during the procedure. We hoped that this combination of estimates would yield results that are more consistent. The EBL variable is particularly relevant during this crisis due to concerns that the shelter-in-place requirements are placing constraints on a hospital's ability to maintain blood bank supplies by donation. The “surgical team size” quantifies the increased risk of COVID-19 transmission with larger numbers of people in the ORs.
The only other modification made was to the “surgical site” variable. Proximity to the airway and its secretions containing COVID-19 particles places otolaryngology-head and neck surgery procedures firmly in the highest risk category. Both endotracheal intubation and extubation carry a high risk of viral exposure for healthcare workers due to airway secretion aerosolization.6 , 7 A child with a difficult airway could also place healthcare personnel (eg anesthesia and nursing) at increased risk if exposed to multiple attempts at securing an airway.
Upper aerodigestive tract and thoracic procedures also carry this increased hazard of viral aerosolization and transmission. We decided that procedures not directly involving oropharyngeal secretions could be deemed lower risk than others located in the head and neck. For that reason, we scored lymph node biopsy or abscess drainage in the thoracic or the head and neck regions as a 1 instead of a 5. In the adult MeNTS system, this is the same approach being used for breast cancer cases, with SLNB receiving a score of 1 in the surgical site coding. Most of the assigned risk categories for the “surgical site” variable were based on potential impact on postoperative respiratory function,8, 9, 10 with higher scores reflecting worse postoperative function. A patient who requires postoperative high-flow nasal cannula or continuous positive airway pressure/bi-level positive airway pressure might also increase risk to healthcare providers by potentially aerosolizing droplets and having a higher risk of postoperative intubation.11
Disease factors
The disease-specific factors (Table 2 ) were chosen to reflect treatment management choices that can affect patient outcomes. The total score range was from 6 to 30 and was not modified from the adult MeNTS scoring system. For each patient, a nonoperative treatment option was evaluated for both its “effectiveness” and the “resource use/exposure risk” to the patient, as well as the care providers involved. For example, a patient with stage IV neuroblastoma who has completed preoperative chemotherapy would be evaluated and scored for efficacy of administering an additional cycle of chemotherapy and delaying operation. This nonoperative approach would also receive a score for the resource use and exposure risk involved for both the patient and healthcare workers. In this case, the patient would require ongoing chemotherapy, which would use a different set of resources and involve a different risk profile than an operation, as the pediatric oncology team would manage this nonoperative treatment strategy. These nonoperative management decisions then receive additional scoring based on an evaluation of the potential time sensitivity of a procedure. The decision for a 2-week or 6-week surgical delay was evaluated for its impact on patient disease outcomes and any anticipated increase in difficulty or risk during an operation due to the delay.
Table 2.
Disease-Specific Factors
| Variable | Category |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Nonoperative treatment option effectiveness∗ | None available | Available, < 40% as effective as operation |
Available, 40% to < 60% as effective as operation | Available, 60% to < 95% as effective as operation | Available, ≥ 95% or equally effective |
| Nonoperative treatment option resource use/exposure risk | Significantly worse/not applicable | Somewhat worse | Equivalent | Somewhat better | Significantly Better |
| Impact of 2-wk delay in disease outcomes | Significantly worse | Worse | Moderately worse | Slightly worse | No worse |
| Impact of 2-wk delay in surgical difficulty/risk | Significantly worse | Worse | Moderately worse | Slightly worse | No worse |
| Impact of 6-wk delay in disease outcomes | Significantly worse | Worse | Moderately worse | Slightly worse | No worse |
| Impact of 6-wk delay in surgical difficulty/risk | Significantly worse | Worse | Moderately worse | Slightly worse | No worse |
Disease score 6 to 30. Higher score equates with less harm to patient if nonoperative treatment is pursued and/or operation delayed. Limited resources might be better deployed for diseases where nonoperative treatment is less effective or not available, or delayed surgical treatment leads to worse disease outcomes and/or increases surgical risk. Consideration of disease factors at 2 different time points integrates natural history of disease, significance of patient symptoms, and time sensitivity of operation into the decision-making process.
Patients scoring 1 point under “nonoperative treatment option effectiveness” will also score 1 point under “nonoperative treatment option resource use/exposure risk” because the question would be “not applicable.”
These questions integrate a measurement of the time sensitivity of a procedure, accounting for the natural history of the patient's disease as well as any perceived changes to the difficulty or risk of the procedure after surgical delay. We considered shortening the time interval for the second question pair to a 4-week delay, but ultimately kept the timeline aligned with the adult version of the MeNTS scoring for consistency. In actual practice, this incorporation of potential disease progression, and its associated impact on surgical safety and technical feasibility, will lead to a higher score when a nonoperative or deferred operative approach is anticipated to be of minimal risk to the patient. Those patients with equally efficacious nonoperative management options with minimal increased risk to the healthcare team or to the patient should have their operation delayed. This can guide deployment of constrained OR resources during the pandemic.
Patient factors
The patient-specific factors (Table 3 ) are those that are associated with worse perioperative outcomes in adult or pediatric patients. The total score range was 8 to 40. The impact of COVID-19 infection on a surgical patient is better understood in the adult population; however, there are increasing reports on the impact of COVID-19 infection on children.4 In some instances we extrapolated adult risk factors, such as pulmonary and cardiac disease, as pediatric equivalents appear to be relevant. For example, adult COVID-19 patients who required care in the ICU or ventilator support had more severe illnesses and significantly worse outcomes, including increased mortality risk. We modified the categories to fit a population of children that includes infants and newborns. We thought this was particularly important, as the true increase in perioperative risk for asymptomatic COVID-positive patients, which might be the majority of COVID-infected pediatric patients, is unknown, and we must use emerging data as they present.4 For this group of variables, only 2 were not modified for our pMeNTS score: “influenza-like illness symptoms, COVID-19 symptoms” and “exposure to known COVID-19-positive patient (14 days).”
Table 3.
Patient-Specific Factors
| Variable | Category |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Child's age | 12 y to 20 y | 5 y to younger than 12 y | 6 mo to younger than 5 y | Full term, or preterm infant ≥ 50 wk post conception age and younger than 6 mo |
Preterm infant < 50 wk post conception |
| Pediatric chronic lung disease, or cystic fibrosis, or pHTN | None | Mild BPD or asthma (no supplemental O2 required after 36 wk), or history of pHTN (resolved) | Intermittent bronchodilators, inhaled steroids, or mild to moderate pHTN (no current medications but needed for procedures) | Daily diuretics, bronchodilators, inhaled steroids, or moderate pHTN (single medication) | On supplemental O2 or history of respiratory hospitalizations or single lung or severe pHTN (multiple current medications) |
| OSA/difficult airway, AHI | No OSA or earlier intubation without airway concern | Mild OSA (AHI 1–5) | BMI < 15 kg/m2 Moderate OSA (AHI 6–10) |
BMI ≥ 25 kg/m2 Severe OSA (AHI 11–20) |
On CPAP or greater support, or ASA ≥ 3, or known difficult airway (AHI > 20) |
| Pediatric congenital heart disease | None | Minor (eg PFO or PDA) or repaired Moderate defect | Moderate (eg ASD, VSD) or repaired major defect | Major (eg transposition great vessels, pulmonic stenosis) or repaired severe defect | Severe (eg hypoplastic left heart, single ventricle) |
| Diabetes | None | — | Mild (no medications) | Moderate (po meds only) | More than moderate (insulin) |
| Immunocompromised∗ | No | — | Mild (pregnancy, single immunosuppressive medication) | Moderate (multiple immunosuppressive meds, chronic steroids) | Severe (malignancy, transplantation, recent chemotherapy) |
| ILI or COVID-19 symptoms (fever, cough, sore throat, body aches, diarrhea, etc) | None (asymptomatic) | — | — | — | Yes |
| Exposure to known COVID-positive patient (past 14 d) | No | Probably not | Possibly | Probably | Yes |
Patient score 8 to 40. Total combined score = procedure + disease + patient. Higher score is associated with potentially worse outcomes, increased risk to providers, and/or increased hospital resource use.
AHI, apnea hypopnea index; ASA, American Society of Anesthesiologists; ASD, atrial septal defect; BPD, bronchopulmonary dysplasia; ILI, influenza-like illness; OSA, obstructive sleep apnea; PDA, patent ductus arteriosus; PFO, patent foramen ovale; pHTN, pulmonary hypertension; VSD, ventricular septal defect.
Hematologic malignancy, stem cell transplantation, solid organ transplantation, active cytotoxic chemotherapy, anti-tumor necrosis factor-α, immunosuppressants, steroid use, congenital immunodeficiency, hypogammaglobulinemia on IV immunoglobulin, HIV with CD4 < 200 cells/mm3.
The “child's age” was adjusted using common cut points for the impact of age on a procedure, such as pediatric inguinal hernia repair, with younger children scored higher, consistent with increased surgical risk. The “pediatric chronic lung disease, or pulmonary hypertension” variable incorporates evidence from a study of the determinants of chronic lung disease severity and estimates of the impact of pulmonary hypertension.12 Obstructive sleep apnea (OSA) is relatively common in adults and can put patients at increased risk of postoperative respiratory impairment,13 , 14 which increases the risk of exposure to healthcare workers due to the aerosolization risk associated with some positive airway pressure devices.11 In our pediatric model, we modified OSA classifications to include mild, moderate, and severe OSA based on the apnea hypopnea index.15 Patients with severe OSA have an increased risk of hospital admission as well as postoperative complications, thereby increasing the overall risk of the procedure. We also added other factors associated with potentially difficult airways, as these tend to increase intubation time, and potentially prolong the postoperative intubation course.16 The adult MeNTS variable “cardiovascular disease” was changed to reflect the greater impact of congenital heart disease in vulnerable children. “Pediatric congenital heart disease” was categorized by severity and baseline risk to the patient. Although there are limited data about the effect of congenital heart disease on COVID-19 infection in children, there does seem to be an increased risk for adults with a history of congenital heart disease.2 Finally, the variable “immunocompromised” was slightly modified to provide pediatric-specific examples.
Results
We captured scheduled or deferred procedures for this 4-week period occurring from the time of pMeNTS implementation in late March onward. A total of 101 cases were captured during the study period. These consisted of 48 cases that were completed as scheduled, and a random sample of 53 deferred cases. The 48 completed cases include all emergency procedures, as well as the urgent cases deemed safe and appropriate during the COVID-19 shutdown. All decisions were made on a case by case basis as they presented. The pMeNTS score was calculated and each procedure was classified by specialty and categorized as either “completed” or “deferred” (Fig. 1 ). The cases represent a broad range of pediatric surgical specialties, including general surgery, surgical oncology, ophthalmology, orthopaedic surgery, otorhinolaryngology, neurosurgery, urology, and plastic surgery. Cases were reviewed and scored by representatives of each specialty or the medical director of the pediatric ORs.
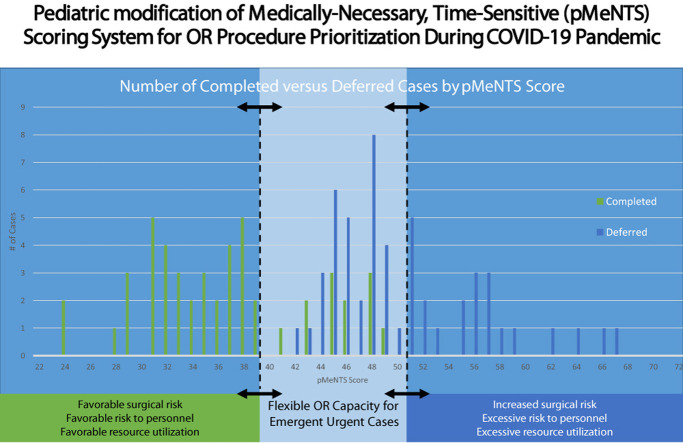
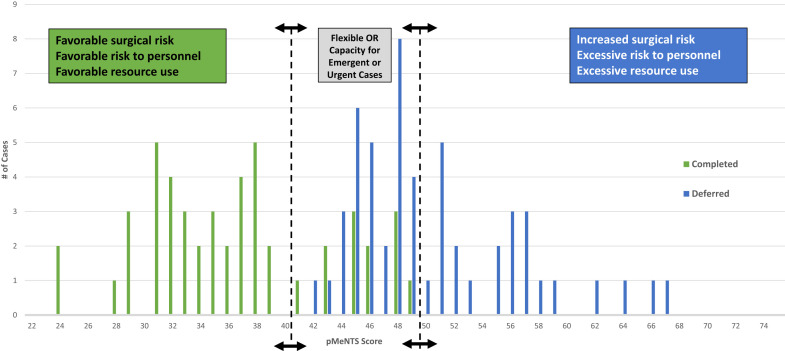
Figure 1.
Number of completed vs deferred cases by pediatric medically necessary, time-sensitive (pMeNTS) score. The pMeNTS scoring system was applied to all 101 completed and deferred procedures during the initial restrictions on elective operations at Comer Children's Hospital, The University of Chicago Medicine from March 23 through April 19, 2020. The y-axis represents the number of cases with a specific pMeNTS score. The arrows with dotted lines represent the upper and lower threshold pMeNTS scores that were allowed to proceed or that were deferred during this period. The threshold can be dynamically adjusted to respond to changes in operating room capacity, resource availability, and risk tolerance of the individual institution. This facilitates preservation of operating room capacity for trauma, emergency, and highly urgent cases.
The range of possible pMeNTS scores was unchanged from the adult model (21 to 105), and application of the scoring system to our pediatric patients yielded a lowest recorded pMeNTS score of 24 and a highest of 67 (mean 42.2, interquartile range 11.5) (Fig. 1). For those cases completed as scheduled, the pMeNTS scores ranged from 24 to 49 (mean 36.3, interquartile range 8.0), and the deferred procedures scored from 42 to 67 (mean 49.8, interquartile range 4.5).
A sample of 21 cases also underwent scoring in both the adult MeNTS and the pMeNTS models to evaluate differences in the scoring systems. When we compared these scores, the adult MeNTS scores were higher for the majority but not all cases. The mean difference was a decrease of 4 points from the adult to pediatric scoring, but it ranged from –10 to +3 for the patients reviewed. This seems consistent with the modification of the adult criteria, as many pediatric patients are healthy with no comorbidities. However, we did observe the greatest difference in scores for those pediatric patients that are younger, premature, or have significant comorbidities.
Discussion
Our adaptation of the adult MeNTS system into a pediatric-specific scoring system better reflects the needs of the pediatric patient population, and still accounts for the resource limitations and transmission risks during the COVID-19 pandemic. We found that our pMeNTS risk-stratification system was a valuable decision tool that helped us balance the complex array of factors affecting patients, healthcare providers, and hospital resources within the context of an overburdened healthcare system.
Upon review of the scores, we noted that all of the overlap between completed and deferred cases occurred between the pMeNTS scores of 42 and 49. As we used the pMeNTS scoring system, scores between 40 and 50 received closer scrutiny to ensure they were correctly scored and that the decision to allow the procedure to proceed as scheduled seemed reasonable within the current overall context of the pandemic and its local impact. At the outset, OR and resource capacity could only accommodate patients with scores in the low 40s and we deferred procedures with higher scores. As we were able to secure more resources, such as personal protective equipment and ventilators, and ICU capacity expanded, we were able to accommodate pMeNTS scores in the high 40s. A similar and parallel effect was noted in the adult hospital where they started with scores in the mid 40s and progressed to scheduling procedures with scores in the mid 50s. This adaptability of the pMeNTS system is one of its key strengths. The pMeNTS scoring system has helped us to facilitate difficult decisions about case prioritization by incorporating procedure, disease, and patient factors into the decision-making process, while accounting for the novel and evolving risk factors that have emerged during the COVID-19 pandemic. We have also found that implementation of pMeNTS scoring has relieved the children's hospital surgical leadership from some of the moral distress associated with triaging care resources in the current context.
We sought to develop a pediatric-specific scoring system because many of the variables initially identified for the adult system did not apply to children (eg COPD and advanced age). When we scored some of the pediatric cases within the adult MeNTS system, this observation was confirmed. The fact that there were not significant differences between the pMeNTS and adult MeNTS scores might represent successful calibration of our pediatric scoring system, indicating that it is relatively proportionate to the adult system.
Although these scoring systems attempt to provide as much objective criteria as possible to create reproducible and accurate scores, there must be an element of subjectivity. Surgeons must exercise clinical expertise in each case, and assess the timeliness of the operation and its projected effect on the individual patient. However, this inevitable element of subjectivity raises the possibility of “gaming” the system. As anyone involved in OR management knows from experience, active management to offset “gaming” behavior is necessary to maintain equitable provision of care to appropriate patients and conservation of resources.
The pMeNTS scoring system allows us to prioritize patient access to time-sensitive procedures, while balancing the additional risks of exposing the healthcare team to potential viral transmission and consumption of scarce medical resources. Managing the risk to healthcare providers has been one of the most challenging aspects of the COVID-19 pandemic. Although durable medical equipment and consumable supplies can be manufactured with urgency and output increased in the short-term, albeit within regional and supply chain constraints, the rapid reconstitution of a highly trained workforce, including specialized surgeons, anesthesiologists, OR nurses, and other personnel, is essentially impossible. This workforce is undoubtedly foundational to ongoing medical care. The potential impact of COVID-19 on the healthcare workforce includes the short-term effects of exposure and the need to quarantine infectious individuals, but also the longer-term ramifications of extended recovery required by severe illness and actual attrition as a consequence of severe morbidities. This need to preserve a functioning workforce must be included in the calculus governing procedural scheduling and the pMeNTS system enables us to consider it.
An additional consideration is the potential impact of operation and anesthesia on an asymptomatic or presymptomatic COVID-19-positive patient. The patient is entrusting their health to us during the operation, and we must be forthcoming about what is known about COVID-19, and perhaps more importantly, what remains unknown. This possibility has informed the discussions we conduct with patients and families, so that the possible risks of proceeding with the operation, given the uncertainties about COVID-19 in children, are included and limitations of COVID-19 testing are recognized (eg shifting availability of testing supplies and experience with test sensitivity). We have modified our informed consent documentation to reflect these important conversations.17 In patients for whom higher-risk operations are needed, we have also modified our consent discussions with families to address the possibility of rapid deterioration and increased mortality in COVID-19-positive patients undergoing procedures.17 , 18 This is particularly applicable to patients with malignancies, in whom unanticipated and catastrophic perioperative outcomes have been reported in cancer patients.19
We recognize that there are limitations inherent in a scoring system such as this. Whenever possible, we relied on published data to decide how to weight each of the 21 factors in the pMeNTS score. Outcomes data for COVID-19-positive children undergoing procedures is, for the most part, limited to case reports and case series. We acknowledge that weighting of these factors might need to be revised over time, and we encourage institutions and subspecialties to do so to serve the needs of each practice or institution. As more research emerges, it might become apparent that additional factors need to be considered. This will not be the final word about surgical procedure prioritization in the setting of this pandemic, but it is a useful system to help triage procedures by medical necessity and time sensitivity in a thoughtful and potentially more objective way.
Our surgeons assign a pMeNTS score to their intended procedure and they submit the score to their section chief. If deemed appropriate to schedule, the pMeNTS worksheet is reviewed by the medical director of the OR before scheduling. We recognize that there is a risk that these scores appear to be objective and free of individual bias or gamesmanship. It is for this reason that the assigned numerical values are also reviewed by the section chiefs and medical director of the OR who have final approval on the appropriateness of proceeding in the current clinical context of the pandemic. Nonetheless, our retrospective review of cases performed supports the utility of this system, and we have successfully used it across all of our pediatric surgical specialties to date. Future research would be important to evaluate the pMeNTS system in a prospective study to ensure that this is true in other institutions.
This system is adaptable to changes in the healthcare environment, and we encourage additional modification of the pMeNTS system. The impact of COVID-19 on pediatric patients does not play out in isolated institutions and what happens within children's hospitals can affect nearby adult hospitals. Because of the projected needs of the adult population, even stand-alone children's hospitals deferred nonemergency surgical procedures to conserve hospital capacity and resources across communities. The need to assess surgical risk for pediatric patients applies not only to children's services that are embedded within adult systems, but also to dedicated children's facilities.
The system is flexible and can be adapted to the individual institution's needs based on the changing resource environment of the COVID-19 pandemic. During the first phase of the pandemic, when the number of COVID-positive patients was rising quickly, we set a very low score as the threshold for scheduling operations. A procedure had to have a pMeNTS score in the low 40s to be scheduled. As we reached the second phase of the pandemic, and the number of COVID-positive patients plateaued, our hospital resources appeared stabilized. We thought that we could accommodate a slight increase in the number of scheduled procedures each day and we added procedures for patients with higher pMeNTS scores in the upper 40s. Individual institutions can use this approach to evaluate their available resources and choose a different pMeNTS cutoff score at different times during the pandemic to reflect their individual situation at the time. This score can be shifted higher or lower as the situation changes and a backlog of cases ebbs and flows during different phases of the pandemic. The value of this approach might be amplified by the uncertain and dynamic conditions projected for the future phases of the pandemic (Fig. 1).
One certainty is that the current situation will change. If resource constraints diminish, or our ability to prevent disease transmission changes, the upper and lower pMeNTS score thresholds can be adjusted to calibrate procedure volumes. This approach offers dynamic flexibility while simultaneously preserving OR capacity for the most urgent cases. One approach to implementation of the pMeNTS system might be to look for natural thresholds in the preoperative case scores. For example, our case series has natural clusters of scores below the low 40s and again at scores above the low 50s (Fig. 1). As the curve of the COVID-19 pandemic bends away from peak resource use, we anticipate moving from deferring cases with scores above the low 40s to deferring those with scores above the 50s.
Conclusions
Although we have learned much about COVID-19 in the past few months, the range of manifestations of this virus in children remains incompletely understood. We sought to report our experience in adapting the adult MeNTS scoring system for use in the children's hospital ORs. The pMeNTS scoring tool is intended to be adaptable across a range of surgical specialties and practice environments. The upper and lower pMeNTS score thresholds can be shifted each day based on changes in personnel and resource availability, as well as the changes at the state level. This dynamic flexibility allows MeNTS procedures to be prioritized in a manner that preserves OR capacity for emergency cases. We believe that pMeNTS provides a safe, equitable, efficient, transparent, and ethical approach to difficult decisions in the current environment of the COVID-19 pandemic. Finally, the inherently adaptive design of pMeNTS leads us to believe that this conceptual framework is adaptable across different healthcare settings and might also be applicable to future health system challenges.
Author Contributions
Study conception and design: Slidell, Kandel, Prachand, Baroody, Gundeti, Reid, Angelos, Matthews, Mak
Acquisition of data: Slidell, Prachand, Baroody, Gundeti, Reid, Mak
Analysis and interpretation of data: Slidell, Kandel, Prachand, Baroody, Gundeti, Reid, Angelos, Matthews, Mak
Drafting of manuscript: Slidell, Mak
Critical revision: Slidell, Kandel, Prachand, Baroody, Gundeti, Reid, Angelos, Matthews, Mak
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Prachand receives consulting fees fromMedtronicand WL Gore and receives payment for lectures from WL Gore.
References
- 1.Prachand V.N., Milner R., Angelos P. Medically-necessary, time-sensitive procedures: a scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020 Apr 9 doi: 10.1016/j.jamcollsurg.2020.04.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. 2020 Mar 31 doi: 10.1002/jmv.25807. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson J.F. Systematic review of COVID-19 in children show milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estimated blood volume Medscape. https://reference.medscape.com/calculator/estimated-blood-volume Available at:
- 6.Tran K., Cimon K., Severn M. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raboud J., Shigayeva A., McGeer A. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig D.B. Postoperative recovery of pulmonary function. Anesth Analg. 1981;60:46–52. [PubMed] [Google Scholar]
- 9.Bablekos G.D., Michaelides S.A., Analitis A. Comparative changes in tissue oxygenation between laparoscopic and open cholecystectomy. J Clin Med Res. 2015;7:232–241. doi: 10.14740/jocmr2086w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellström M., Olsén M.F., Olsson J.H. Pain and pulmonary function following laparoscopic and abdominal hysterectomy: a randomized study. Acta Obstet Gynecol Scand. 1998;77:923–928. [PubMed] [Google Scholar]
- 11.Simonds A.K., Hanak A., Chatwin M. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14(46):131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 12.Gage S., Kan P., Oehlert J. Determinants of chronic lung disease severity in the first year of life; a population based study. Pediatr Pulmonol. 2015;50:878–888. doi: 10.1002/ppul.23148. [DOI] [PubMed] [Google Scholar]
- 13.Chan M.T.V., Wang C.Y., Seet E. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321:1788–1798. doi: 10.1001/jama.2019.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Bustamante A., Bartels K., Clavijo C. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth Analg. 2017;125:593–602. doi: 10.1213/ANE.0000000000002241. [DOI] [PubMed] [Google Scholar]
- 15.Iber C., Ancoli-Israel S., Chesson A.L. American Academy of Sleep Medicine; Westchester, IL: 2007. The American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events. [Google Scholar]
- 16.Oofuvong M., Ratprasert S., Chanchayanon T. Risk prediction tool for use and predictors of duration of postoperative oxygen therapy in children undergoing non-cardiac surgery: a case- control study. BMC Anesthesiol. 2018;18:137. doi: 10.1186/s12871-018-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan A.F., Milner R., Roggin K.K. Unknown unknowns: surgical consent during the covid-19 pandemic. Ann Surg. 2020 Apr 29 doi: 10.1097/SLA.0000000000003995. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aminian A., Safari S., Razeghian-Jahromi A. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020 Mar 26 doi: 10.1097/SLA.0000000000003925. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]