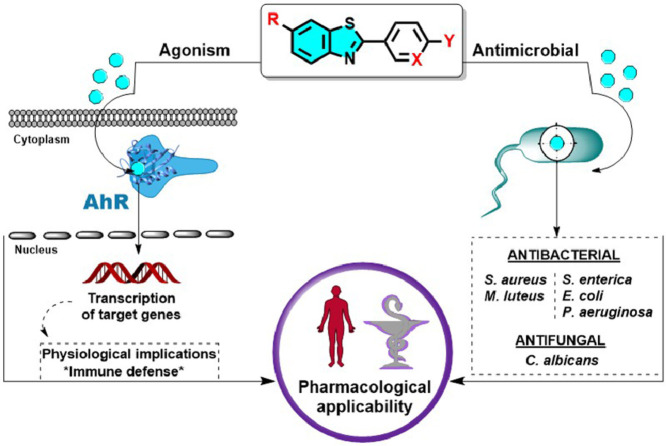
Graphical abstract
Keywords: Benzothiazole, Antibacterial, Antifungal, Antibiofilm, Ah receptor, Agonism
Abstract
Benzothiazole is a privileged scaffold in medicinal chemistry present in diverse bioactive compounds with multiple pharmacological applications such as analgesic, anticonvulsant, antidiabetic, anti-inflammatory, anticancer and radioactive amyloidal imagining agents. We reported in this work the study of sixteen functionalized 2-aryl and 2-pyridinylbenzothiazoles as antimicrobial agents and as aryl hydrocarbon receptor (AhR) modulators. The antimicrobial activity against Gram-positive (S. aureus and M. luteus) and Gram-negative (P. aeruginosa, S. enterica and E. coli) pathogens yielded MIC ranging from 3.13 to 50 μg/mL and against the yeast C. albicans, the benzothiazoles displayed MIC from 12.5 to 100 μg/mL. All compounds showed promising antibiofilm activity against S. aureus and P. aeruginosa. The arylbenzothiazole 12 displayed the greatest biofilm eradication in S. aureus (74%) subsequently verified by fluorescence microscopy. The ability of benzothiazoles to modulate AhR expression was evaluated in a cell-based reporter gene assay. Six benzothiazoles (7, 8-10, 12, 13) induced a significant AhR-mediated transcription and interestingly compound 12 was also the strongest AhR-agonist identified. Structure-activity relationships are suggested herein for the AhR-agonism and antibiofilm activities. Furthermore, in silico predictions revealed a good ADMET profile and druglikeness for the arylbenzothiazole 12 as well as binding similarities to AhR compared with the endogenous agonist FICZ.
1. Introduction
The interaction with microorganisms is fundamental to produce nutrients and to suppress the pathogen colonization in the mucous membranes of healthy hosts. Microbial communities are playing important roles in organ development, metabolism, and immune homeostasis (Sommer and Bäckhed, 2013). However, the diseases triggered by infectious agents have caused most of the biggest health tragedies of mankind. The coronavirus SARS-CoV-2 responsible for the COVID-19 pandemic is a current example (Shereen et al., 2020). The preservation of health against pathogenic colonization is determined by the immune response as well as the adequate biotransformation of toxicants in the host cells (Janeway et al., 2001). The transformation and appearance of new pathogens and the so-called emerging diseases, as well as the adaptive resistance to pharmacological treatments, mean that all efforts are insufficient for the control and/or eradication of infections.
The aryl hydrocarbon receptor (AhR) is a long known chemical sensor highly expressed in the liver and in barrier organs (Esser and Rannug, 2015). Despite the extensive association of AhR activation with dioxin-like toxicity during several decades, nowadays is well known that agonist modulations upon AhR can lead to physiotherapeutical benefits in multiple pathological conditions (Hu et al., 2007; Zhao et al., 2019). AhR activity regulates, among others, the immune system functions, through direct or indirect paths, and the differentiation of diverse immune cell types (Esser et al., 2009). Important roles of AhR are suggested in infective disease tolerance and in defense pathways. As an example, the influence of AhR in primary LPS responsiveness and in fact the induction of endotoxin tolerance has been demonstrated using a Streptococcus-induced multifocal septic arthritis model. The endotoxin-tolerant state LPS triggered was found to protect mice against immunopathology in Gram-positive infections, steering the contribution of AhR to host fitness (Bessede et al., 2014) . Moreover, AhR plays a key role in innate defense against bacteria. Bacterial pigmented virulence factors such as the phenazines from P. aeruginosa and the naphthoquinone phthiocol from M. tuberculosis, have been characterized, as ligands of AhR. During ligand binding, AhR activation leads to degradation of virulence factor and regulation of cytokine and chemokine production. Therefore, AhR recognition of these bacterial pigments is reported as a prominent alternative path to control the antibacterial response. It has also been shown that AhR activation regulates inflammatory leukocyte recruitment to the infected lung and control of bacterial replication demonstrating that AhR plays a central role in defense against both acute and chronic bacterial infection (Moura-Alves et al., 2014). Besides its protective effect against intestinal pathogenic bacteria, AhR is also involved in resistance to fungi. It has been described that some indole-type AhR ligands dramatically inhibit Candida albicans biofilm formation, as well as attachment to intestinal epithelial cells suggesting that AhR activation may be a potential therapeutic target to combat bacterial and fungal intestinal infection (Oh et al., 2012).
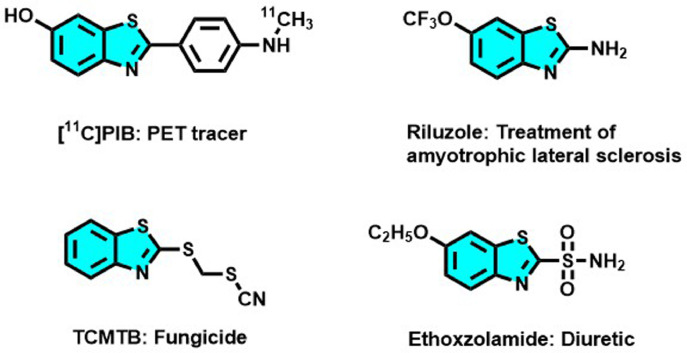
Benzothiazole (BT) is a heterocyclic structure bearing a benzene ring fused with a five-membered ring containing nitrogen and sulfur atoms. The BT nucleus is considered a privileged structure particularly important in medicinal chemistry due to the wide range of pharmacological applications ascribed to its derivatives (Ali and Siddiqui, 2013; Bondock et al., 2010; Choudhary et al., 2017; Gill et al., 2015). Anticancer, antimicrobial, and anti-inflammatory are among the most extensively reported clinical uses of this versatile fused heterocyclic scaffold (Kamal et al., 2015). Furthermore, BT skeleton is present in imaging agents and as radiotracers of positron emission tomography (PET) used to diagnose neurological diseases and in biocidal agents employed as industrial chemicals (Mathis et al., 2003; Muthusubramanian et al., 2001). Some examples of widely used benzothiazoles are represented in Figure 1 .
Figure 1.
Examples of marketed benzothiazoles and their main applications (Hergesheimer et al., 2015; Mathis et al., 2003; Moyer and Ford, 1958; Muthusubramanian et al., 2001)
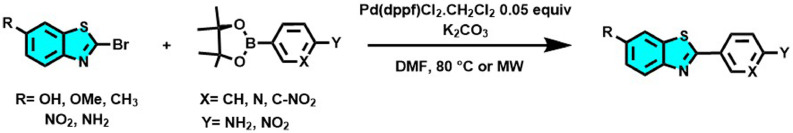
In 2013, encouraged by the high interest in [11C] PIB for diagnosing Alzheimer's disease, we developed a new series of 2-arylbenzothiazoles and 2-pyridinylbenzothiazole derivatives by Szuki-Miyaura coupling reaction. The one-step protocol was performed from commercially available reactants to obtain the desired radiopharmaceutical precursors in high yields under thermal conditions or microwave activation, Scheme 1 (Bort et al., 2013).
Scheme 1.
Synthesis of a new series of arylbenzothiazoles, potential precursors of the radiochemical tracer [11C]PIB, by Suzuki-Miyaura coupling reaction (Bort et al., 2013).
Inspired by the therapeutic potentialities of the benzothiazole scaffold and taking into account the aforementioned pharmacological interest of combining antimicrobial and AhR-modulatory activities we investigate the antimicrobial potential of a series of sixteen arylbenzothiazoles, previously synthesized in our laboratory, against Gram positive and Gram negative pathogens and the opportunist yeast C. albicans. The AhR agonist/antagonist behavior of benzothiazoles was studied using an AhR reporter assay preceded by a viability study. The structure-activity relationships considerations between biocidal and AhR modulatory activities were also discussed herein.
2. Experimental Section
The thirteen 2-arylbenzothiazoles and the three 2-pyridinylbenzothiazoles biologically evaluated in this work were previously prepared by our team from the corresponding 2-bromobenzothiazoles (1 eq.) and phenylboronic acid pinacol ester (1.2 eq.) in anhydrous DMF, and in presence of K2CO3 (6.0 eq.) and Pd(dppf)Cl2.CH2Cl2 (0.05 eq.). The reactions were performed under argon bubbling at 80 °C or under microwave irradiation and monitoring by TLC or by GC-MS. After treatment, the compounds were isolated by filtration as colored powders and the structures were checked by comparison of their NMR, IR, and HRMS data. The detailed synthetic procedures and characterization can be found in the original research article (Bort et al., 2013).
2.1. Biological studies
2.1.1. Antibacterial and antifungal activities
The in vitro antibacterial and antifungal activities of the studied benzothiazoles (1-16) were evaluated by using a microplate broth dilution assay according to the National Committee for Clinical Laboratory Standard (NCCLS. 2002). They were screened against the two Gram-positive pathogens Staphylococcus aureus (ATCC 9144) and Micrococcus luteus (LB14110) and the three Gram-negative Salmonella enterica (NCTC 6017), Pseudomonas aeruginosa (ATCC 9027), and Escherichia coli (ATCC 8739), and the yeast Candida albicans (ATCC 2091) by using microplate broth dilution assay according to the National Committee for Clinical Laboratory Standard (NCCLS. 2002). Serial dilutions of compounds, dissolved in DMSO, were prepared in LB broth medium (1% Bactotryptone, 0.5% Yeast extract, 0.5% NaCl).
A volume of 100 μL of the tested compounds (final concentration ranging from 200-1.15 μg/mL) and 100 μL of bacterial suspension (107 CFU/mL) was dispensed into each well of 96-well plates (Corning) and incubated at 37 C for 20 h. Levofloxacin and fusidic acid were used as positive controls. After the incubation period, the Minimal Inhibitory Concentrations (MICs) were recorded as the lowest concentrations of the compounds at which no visible growth occurred. The 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent was added to each well to evaluate the microbial growth, indicating live cells when a color transformation from yellow to dark blue was observed. The Minimal Bactericidal Concentrations (MBCs) were determined by sampling one loopful from each well and cultured on plate count agar at 30 C for 24 h. The lowest concentration of compounds that resulted in microbial death was reported as their MBCs.
The optical density (OD) was measured at 600 nm using a microplate reader (Varioskan, Thermofisher). The antimicrobial activity was expressed as the inhibition percentage when compared with microbial culture untreated.
2.1.2. Antibiofilm activity
Biofilm formation in S. aureus and P. aeruginosa was determined spectrophotometrically by using the crystal violet test in 96-well plates as previously described (Ricco et al., 2020). The tested compounds (1-16) were assayed at their MIC against S. aureus and P. aeruginosa, respectively, to determine the biofilm anti-adhesion activity. In addition, the biofilm eradication activity of benzothiazoles was evaluated at 200 μg/mL. A volume of 100 μL of each BT/well was delivered into the plates. Then, 100 μL/well of a fresh overnight cultured bacterial suspension diluted was added until obtaining a final OD of 0.1 at 600 nm. After 24 h incubation at 37 ºC, the wells were emptied, gently rinsed twice with PBS, and the plates dried at 60 ºC for 45 min. The biofilm was stained with 150 μL of crystal violet solution (0.2%) for 15 min at room temperature. After staining, plates were rinsed with water and 200 μL/well of glacial acetic acid were added to dissolve the crystals. After 1 h incubation at room temperature, the OD of the adherent biofilm was measured at 570 nm using a microplate reader (Varioskan, Thermofisher). The antibiofilm activity was expressed as the inhibition percentage when compared with the negative control.
The antibiofilm activity against S. aureus of compound 4-(6-methyl-1,3-benzothiazol-2-yl)-2-nitroaniline (12) was also determined by fluorescence microscopy images (OLYMPUS Fluorescent microscope BX50 equipped with a digital camera OLYMPUS DP70). The biofilms grown on glass pieces (Ø 10 mm) and placed in 24-well polystyrene plates were treated with 12 at 50 µg/mL (MIC value against S. aureus) for the anti-adhesion test and at 200 µg/mL for the eradication test. Non-treated wells containing the LB medium served as control. The bacterial inoculation was adjusted to an OD 600 nm of 0.1. Plates were incubated at 37 °C for 24 h. The wells were carefully emptied, the glass slides washed with PBS, and 500 µL/well of acridine orange (0.1% in PBS) were added. A 40× objective using U-MWB2 filter with excitation at 460–490 nm and emission at 520 nm was used for the visualization.
2.1.3. Cell viability evaluation
Cell Line characteristics and maintenance. AhR-HepG2 Lucia™ cell line engineered from the human HepG2 hepatoma cell line was obtained from InvivoGen stably transfected to express the endogenous Ah receptor and thereby screening potential AhR ligands. Lucia luciferase reporter gene is coupled with the human Cyp1a1, while the Lucia luciferase reporter protein is readily measurable in the cell culture supernatant. According to the provider's recommendations, AhR-HepG2 cells were maintained in Minimum Essential Medium containing non-essential amino acids (MEM-NEAA, Gibco ThermoFisher Scientific) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL) in a humidified 5% CO2 atmosphere at 37 °C. Normocin (0.1 mg/mL) and Zeocin (0.2 mg/mL) from Invivogen were added to the culture medium after the third passage.
Cell viability bioassay. The reduction caused by dehydrogenases and other reducing agents present in metabolically active cells was evaluated following the colorimetric MTT assay (Mosmann, 1983), with the aim of avoiding misinterpretations caused by cell damage in AhR activity tests.
Briefly, AhR-HepG2 cells were trypsinized, seeded on 96-well plates at a density of 2.0×105 cells/mL, and cultured overnight in MEM-NEAA. Next, cells were treated during 24 h with 10 μL/well of 5 μM and 10 μM concentrations of the sixteen benzothiazoles, or else with the positive control ligands which were 5,11-dihydroindolo[3,2-b]carbazole-12-carbaldehyde (FICZ) for agonism, and 2-methyl-2H-pyrazole-3-carboxylic acid (CH223191) for antagonism. A volume of 100 µL/well of MTT reagent (0.5 mg/mL) was added to the emptied plates containing the cells attached. Approximately 3 h of incubation at 37 °C allowed the transformation of the yellow MTT to the water-insoluble violet-blue formazan that was dissolved by adding 100 μL/well of DMSO. The OD was measured by reading the absorbance at 490 nm using a microplate reader (VICTORx3, PerkinElmer Inc., USA).
2.1.4. AhR reporter assays
AhR agonist and antagonist bioassay. To measure the effects on AhR transcriptional activity, AhR-HepG2 cells were trypsinized, passed through 18-gauge (18G) needles, and a suspension of 2.0×105 cells/mL was seeded into 96-wells microplates (200 μL/well) in a MEM-NEAA medium without Normocin nor Zeocin. After 24 h of incubation at 37 °C, cells were exposed to the tested compounds during another 24 h. The treatment of cells in agonistic mode was conducted by adding 10 μL/well of 5 μM and 10 μM of the benzothiazoles. In antagonistic mode 10 μL/well of the benzothiazoles (or CH223191) were added followed by 10 μL/well of the EC50 determined for the known AhR agonist FICZ used as positive control.
A volume of 20 μL/well of the supernatant of treated cells was transferred to white sterile and flat-bottom 96-wells microplates (Corning). After 50 μL/well of the QUANTI-Luc™ assay reagent (Invivogen) was added and the luminescence immediately measured in a microplate reader (VICTORx3, PerkinElmer Inc., USA). Results were expressed as fold response and as efficiency (Eff) percentage relative to the activity displayed by the positive control FICZ.
2.2. Computational studies
ADMET profile and druglikeness. Physicochemical, biopharmaceutical, and toxicological properties of 12 were predicted using the software ADMET Predictor™ v9.5 (Simulations Plus, Inc., Lancaster, CA, USA) (Ghosh et al., 2016).
Molecular docking simulations. Molecular docking analysis was performed with Autodock Vina (Trott and Olson, 2009) as implemented in YASARA (Krieger and Vriend, 2014). The crystallized protein structure was obtained from the Protein Data Bank (PDB ID 3F1O). Benzothiazole 12 and the known agonist FICZ were the ligands used. Simulations were performed for the entire target structure making stiff the protein and flexible the ligand compounds. The interactions of the best protein/ligand complexes were predicted using the Protein-Ligand Interaction Profiler web server (Salentin et al., 2015). Molecular graphics and analyses were performed with UCSF Chimera (Pettersen et al., 2004).
3. Results and Discussion
3.1. Biological Evaluation
3.1.1. Antimicrobial activity
A set of BTs was screened for antibacterial activity against Gram-positive (M. luteus and S. aureus) and Gram-negative (E. coli, P. aeruginosa, and S. enterica) pathogen strains and the yeast C. albicans. The Minimal Inhibitory Concentration (MIC) and the Minimal Bactericidal Concentration (MBC) for the 16 tested benzothiazoles and for levofloxacin (Lv) and fusidic acid (FA), used as positive controls, are reported in Table 1 . As shown, levofloxacin had a notable potency against all the pathogens with MICs and MBCs lower than 1.52 µg/mL as expected from this broad-spectrum fluoroquinolone (Keam et al., 2005). Meanwhile, the MICs for fusidic acid ranging from 12.5-25 µg/mL and the MBCs were 100 µg/mL in Gram positive bacteria and higher in the other microorganisms studied.
Table 1.
Minimal inhibitory concentration (MIC) and Minimal bactericidal concentration (MBC) values exhibited by the BTs and the positive controls levofloxacin and fusidic acid.
| ID | R | X | Y | [c]a | Gram (+) | Gram (-) | Yeast | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M. luteus | S. aureus | E. coli | P. aeruginosa | S. enterica | C. albicans | |||||
| 1 | OH | CH | NH2 | MIC | [6.25-12.5] | [6.25-12.5] | [12.5-25] | [12.5-25] | [12.5-25] | [12.5-25] |
| MBC | 100 | 100 | >100 | >100 | 100 | >100 | ||||
| 2 | OH | N | NH2 | MIC | [12.5-25] | [12.5-25] | [12.5-25] | [25-50] | [12.5-25] | [12.5-25] |
| MBC | 100 | >100 | >100 | >100 | >100 | >100 | ||||
| 3 | OH | CH | NO2 | MIC | [12.5-25] | [12.5-25] | [12.5-25] | [25-50] | [25-50] | [25-50] |
| MBC | 100 | >100 | >100 | >100 | >100 | >100 | ||||
| 4 | OH | CNO2 | NH2 | MIC | [12.5-25] | [12.5-25] | [25-50] | [50-100] | [25-50] | [50-100] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 5 | OCH3 | CH | NH2 | MIC | [6.25-12.5] | [25-50] | [25-50] | [25-50] | [25-50] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 6 | OCH3 | N | NH2 | MIC | [3.13-6.25] | [25-50] | [25-50] | [25-50] | [25-50] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 7 | CH3 | CH | NO2 | MIC | [6.25-12.5] | [25-50] | [12.5-25] | [12.5-25] | [25-50] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 8 | OCH3 | CNO2 | NH2 | MIC | [6.25-12.5] | [25-50] | [12.5-25] | [12.5-25] | [25-50] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 9 | CH3 | N | NH2 | MIC | [12.5-25] | [25-50] | [12.5-25] | [25-50] | [50-100] | [50-100] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 10 | CH3 | CH | NH2 | MIC | [12.5-25] | [25-50] | [12.5-25] | [25-50] | [50-100] | [50-100] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 11 | OCH3 | CH | NO2 | MIC | [6.25-12.5] | [25-50] | [12.5-25] | [25-50] | [50-100] | [50-100] |
| MBC | >100 | >100 | 100 | >100 | >100 | >100 | ||||
| 12 | CH3 | CNO2 | NH2 | MIC | [6.25-12.5] | [12.5-25] | [12.5-25] | [25-50] | [12.5-25] | [50-100] |
| MBC | >100 | >100 | 100 | >100 | 50 | 100 | ||||
| 13 | NO2 | CH | NH2 | MIC | [6.25-12.5] | [12.5-25] | [25-50] | [12.5-25] | [25-50] | [25-50] |
| MBC | >100 | >100 | 100 | >100 | 50 | 100 | ||||
| 14 | NO2 | CH | NO2 | MIC | [6.25-12.5] | [12.5-25] | [25-50] | [25-50] | [12.5-25] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 15 | NO2 | CNO2 | NH2 | MIC | [6.25-12.5] | [12.5-25] | [25-50] | [25-50] | [25-50] | [25-50] |
| MBC | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 16 | NH2 | CH | NH2 | MIC | [12.5-25] | [12.5-25] | [12.5-25] | [25-50] | [25-50] | [25-50] |
| MBC | 100 | >100 | >100 | 100 | 100 | 100 | ||||
| Levofloxacin | MIC | <1.52 | <1.52 | <1.52 | <1.52 | <1.52 | <1.52 | |||
| MBC | <1.52 | <1.52 | <1.52 | <1.52 | <1.52 | <1.52 | ||||
| Fusidic acid | MIC | [12.5-25] | [12.5-25] | [12.5-25] | [12.5-25] | [12.5-25] | [12.5-25] | |||
| MBC | 100 | 100 | >100 | >100 | >100 | >100 | ||||
[c] Concentration values are expressed in μg/mL.
The functionalized 2-aryl and 2-pyridinylbenzothiazoles assayed showed in general good antimicrobial potency. The MBC/MIC ratio (≥4) indicated predominant bacteriostatic effects. The antibacterial activity did not reveal important differences in the potency of the benzothiazoles against Gram-positive and Gram-negative bacteria, although Gram-positive strains seem to be more sensitive.
The inhibition potency against M. luteus was the most relevant. Hence, against M. luteus the same MICs as FA were estimated for compounds 2-4, 9, 10 and 16, and the majority of benzothiazoles (5, 7, 8, 11-15) showed MICs below that of FA, ranging between 6.25-12.5 μg/mL except for 6 (3.13-6.25 μg/mL). Against S. aureus 1 showed the strongest potency with MIC between 6.25-12.5 μg/mL, while half of the compounds (2-4, 12-16) exhibited the same MIC as FA (12.5-25 μg/mL). Against E. coli, P. aeruginosa and S. enterica, compounds 2, 7, 8, and 12 displayed the higher potency with MICs similar to FA in two bacteria indistinctly, while 1 showed the same MIC as FA for the three Gram-negative strains. Only 12 and 13 displayed MBCs of 50 μg/mL for S. enterica. The antifungal MICs for the 2-arylbenzothiazole 1 and its 2-pyridinyl analogous 2 were comparable with FA (12.5-25 μg/mL). The rest of the benzothiazoles displayed higher inhibitory concentrations against the opportunistic pathogenic yeast C. albicans ranging from 25-100 μg/mL.
These results suggest that the concurrent functionalization of a hydroxyl group and an amino group as substituents in R and Y positions, respectively, has a positive influence on the antibacterial activity against Gram-positive pathogens (compound 1) and on the antifungal activity (compounds 1 and 2). When the hydroxyl function of compound 1 is replaced by a methoxy (compound 5) or a nitro function (compound 13), no difference in activity was observed against M. luteus (MIC values between 6.25-12.5). However, the antimicrobial activity decreases for most pathogens. The introduction of an amino group (compound 16) has no positive influence on the activity. Therefore, a hydroxyl group may be required to exhibit notable antimicrobial effects.
3.1.2. Antibiofilm activity
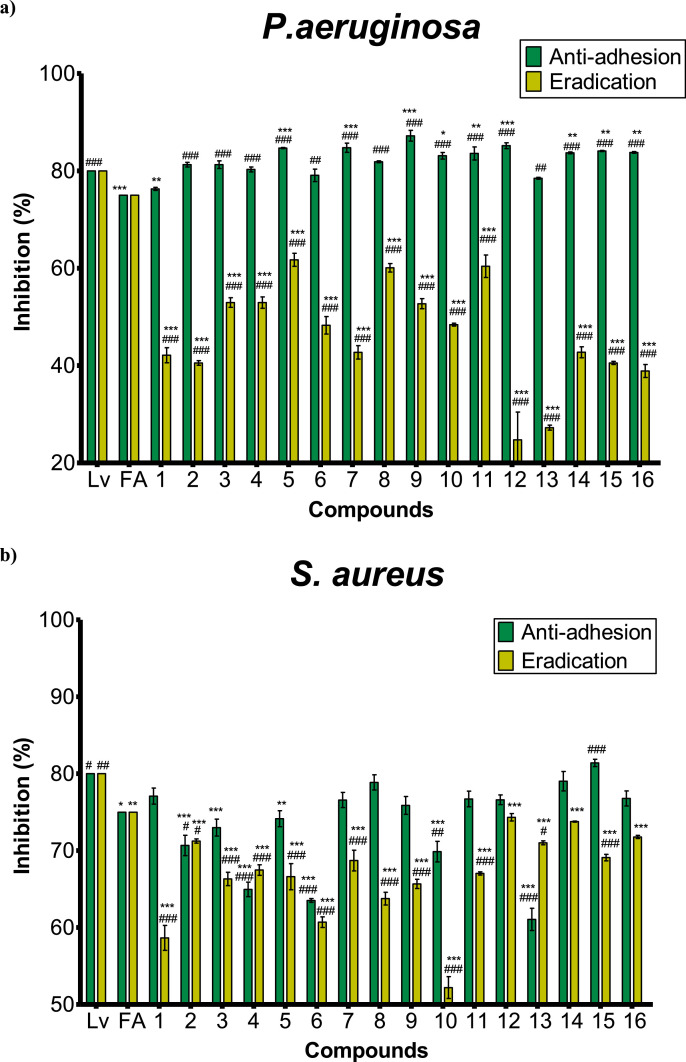
The biocidal effects of the BTs were evaluated through their ability to inhibit the biofilm formation in S. aureus and P. aeruginosa. The early biofilm adherence was studied according to the minimum inhibitory concentration corresponding to each compound. All compounds exhibited antibiofilm effects in both strains. The inhibition percentages were greater than 20% for P. aeruginosa and above 50% for S. aureus, as shown in Figure 2 a) and b), respectively.
Figure 2.
Inhibition percentage of the antibiofilm activity against a) P. aeruginosa b) S. aureus of levofloxacin (Lv), fusidic acid (FA), and benzothiazoles (1-16). Bars represent the effect at MICs (anti-adhesive)-green or at 200 µg/mL (eradication)-yellow of each BT. The percentage is the OD measured at 570 nm of treated bacteria relative to the negative control (untreated). Means ± SD for at least three independent experiments (n = 3) are shown. The levels of significance were determined using one-way ANOVA followed by Dunnett's post-test when compared to Lv: ***p<0.001, **p<0.01 and *p<0.05, or to FA ###p<0.001, ##p<0.01 and #p<0.05 in the anti-adhesion or eradication tests, indistinctly.
The 16 functionalized benzothiazoles exhibited good biocidal effects against Gram-negative P. aeruginosa, showing inhibition percentages at their MIC greater 76%. Compounds 5, 7, and 12 with 85% of anti-adhesive activity and benzothiazole 9 (87%) showed the most prominent anti-biofilm activity on P. aeruginosa, significantly higher (p<0.001) than both positive controls (Lv and FA) used. Unexpectedly, most of the compounds exhibited eradication percentages of P. aeruginosa lower than those obtained for S. aureus, displaying 5 the greater value recorded of 62%.
Meanwhile, against S. aureus, the studied benzothiazoles 1, 7-9, 11, 12, 14, 16 displayed inhibition percentages equivalent to FA at their MIC in the anti-adhesion test as shown in Figure 2 a). In the same, compounds 14 (79%) and 15 (81%) exhibited a biocidal effect similar to levofloxacin (80%) against S. aureus, significantly greater than FA. In the eradication test, the antibiofilm effect of compound 12 (74%) against S. aureus was the most important of the set, being less than Lv but not significantly different from FA (75%).
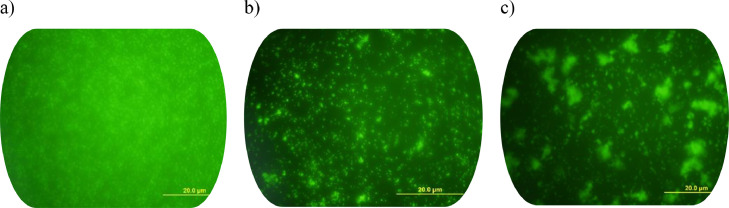
The biocidal activity of 12 over S. aureus was confirmed by fluorescence microscopy (Figure 3 ). The images of the acridine orange staining treated slides with compound 12 confirmed the ability of this benzothiazole to alter the surface characteristics of bacterial cells and at both anti-adhesive (b) and eradication (c) concentrations when compared to untreated bacteria (a).
Figure 3.
Fluorescence microscopy images (9× 40) of S.aureus biofilm formation. Green areas represent the formed biofilm and black areas indicate necrotic zones. a) Non-treated biofilm, b) Biofilm treated with compound 12 at 50 μg/mL (MIC) i.e. anti-adhesion activity, c) Biofilm treated with compound 12 at 200 μg/mL i.e. eradication activity.
3.1.3. Cell viability evaluation
Effects on AhR-HepG2 proliferation caused by the studied 2-aryl and 2-pyridinylbenzothiazoles were determined in vitro by the MTT bioassay. The obtained cell viability expressed as a percentage of non-treated cells is represented in Figure 4 .
Figure 4.
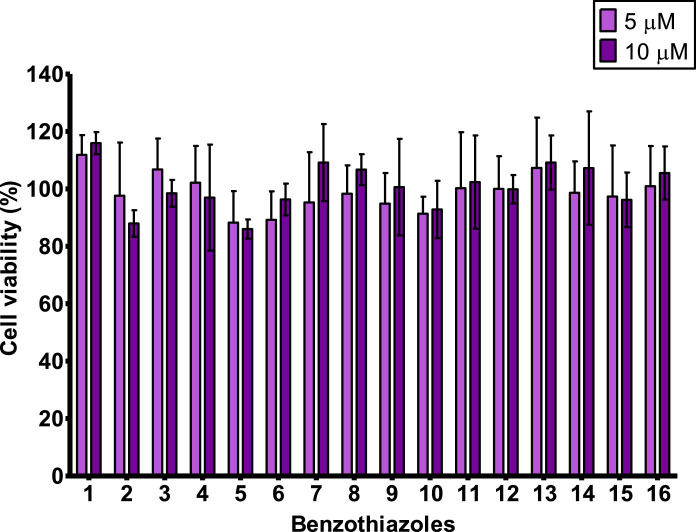
Viability percentages of cells exposed to the BTs by MTT assay. Compounds 1-16 were assayed at 5 μM and 10 μM. Bars represent the mean percentage ± SD from at least four independent experiments (n = 4). Compounds that reduce cell viability below 85% were considered cytotoxic. No significant differences were observed from vehicle control using one-way ANOVA followed by Dunnett's post-test (p<0.05).
The treatment up to 10 μM of exposure concentration with all the compounds allowed the maintenance of cell proliferation above 85%. Therefore, none of the 16 BTs evaluated was considered cytotoxic on the AhR-HepG2 cell line.
3.1.4. AhR reporter assays
The modulation of AhR-mediated expression caused by the set of BTs was evaluated in a cell-based method using AhR-HepG2 cells. In a previous study, we have reported a preliminary in silico/in vitro screening of the AhR agonism of diverse compounds, including some of the studied benzothiazoles herein analyzed (Goya-Jorge et al., 2020). The prominent activity exhibited by these derivatives motivated to elucidate further on the AhR effects caused by BT class of compounds as presented herein. Thus, agonistic and antagonistic bioassays were conducted in vitro for a greater number of benzothiazole derivatives, aiming to analyze the substituents’ influence in AhR-mediated activity.
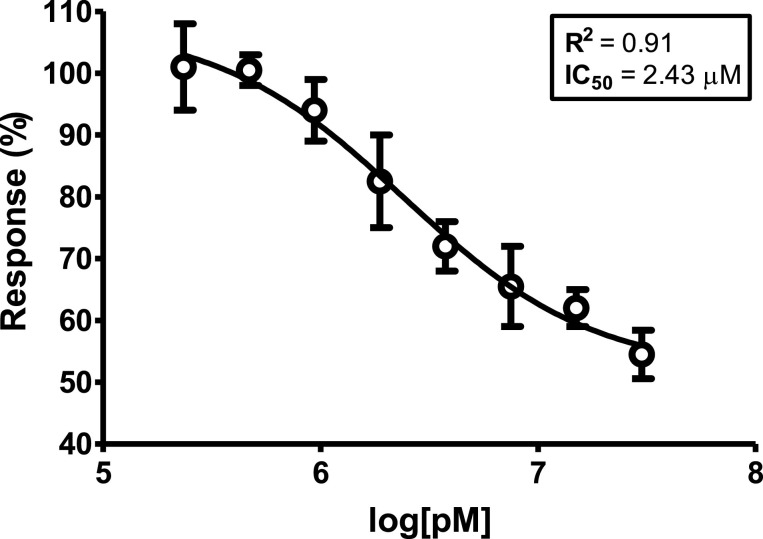
AhR antagonism. The EC50 of the agonist compound FICZ was 9.06 μM in this cell line, as we previously reported (Goya-Jorge et al., 2020). The antagonist bioassays were developed by co-exposure the EC50 of FICZ with each tested BT or else, with the known AhR antagonist CH223191. The dose-response curve obtained for CH223191 is presented in Figure 5 .
Figure 5.
Dose-response curve of compound CH223191 used as positive control in the AhR antagonist assay. The dosage is represented as the logarithm of the concentration expressed in pM (1.0 × 10−12 M), while the effect is expressed as % of FICZ [EC50] ± SEM. The R2 and the IC50 (μM) estimated from the curve are informed.
The dose-response curve obtained for the control CH223191 to validate the competitive antagonist test was consistent with some other results reported in the literature (Mohammadi-Bardbori et al., 2019). However, none of the 16 benzothiazoles assayed exhibited any blockage capacity of AhR transcriptional activity, by the contrary, most of them displayed potential synergism when co-exposure with FICZ.
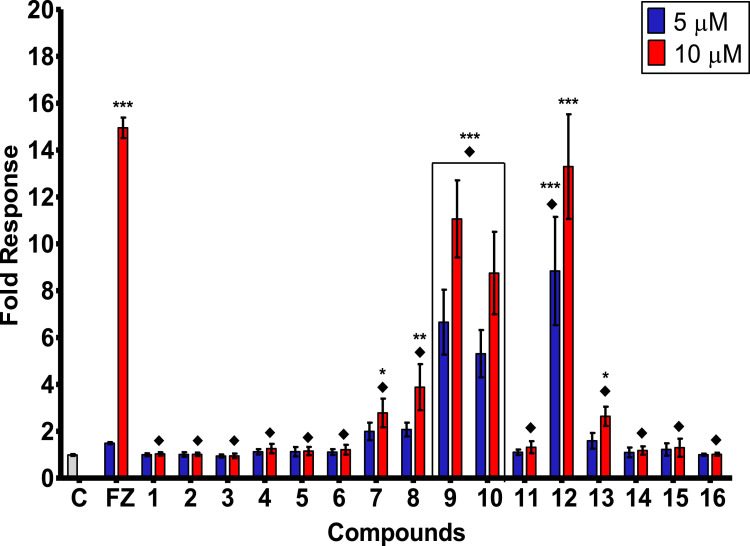
AhR agonism. Results obtained in the AhR agonist bioassay are represented in Figure 6 for the two assayed concentrations of the benzothiazoles, for the positive control FICZ (FZ) and for the vehicle control (C) of non-treated cells.
Figure 6.
AhR agonistic activity (fold response) induced in AhR-HepG2 cells by the set of BTs (1-16). Bars represent means ± SD from at least four individual experiments (n = 4). The levels of significance were determined using one-way ANOVA followed by Dunnett's post-test when compared to vehicle control (C): ***p < 0.001, **p < 0.01 and *p < 0.05, and when compared with FICZ's effect ♦p< 0.001 at the same exposure concentration.
Overall, the set of BT derivatives exhibited a remarkable induction of AhR-mediated expression as displayed in Figure 6. Compounds 9, 10, and 12 at both concentrations tested exhibited a very significant AhR agonist activity (p < 0.001) compared with the vehicle, while the positive control FICZ induced a significant activity only at the highest dosage. Moreover, compounds 7, 8, and 13 showed a notable response when compared with non-treated cells.
The induction of AhR transcriptional activity of benzothiazole 12 at 10 μM was comparable with that displayed by the agonist FICZ, while the rest of the tested compounds exhibited effects significantly lower.
3.2. SAR considerations: antibiofilm activity vs. AhR agonism
The structural differences across the set of BTs and their influence on the antibiofilm activity and the AhR agonism were analyzed and summarized in Table 2 .
Table 2.
Summary of the AhR agonist efficiency and the biocidal activity exhibited by the sixteen benzothiazole derivatives.
 |
As observed in Table 2, the AhR agonism was represented as a percentage of the activity displayed by FICZ (agonist efficiency). All benzothiazoles at 5 μM reached percentages higher than 50% of agonist efficiency. Compounds 7, 8, and 13 exhibited a similar induction (~100%) of AhR transcriptional activity than the positive control at 5 μM, while 9, 10, and 12 displayed AhR agonist efficiencies more than three times stronger than FICZ. At 10 μM, which is slightly above the EC50 determined for FICZ (9.06 μM), only the benzothiazole 12 induced an effect comparable (89%) with the positive control followed by compounds 9 (74%) > 10 (66%) > 8 (22%) > 13 (21%). The rest of BTs induced below 20% of FICZ's response at the highest exposure concentration. In the anti-adhesion tests where the MIC of each compound was assayed against S. aureus and P. aeruginosa, the greatest inhibition percentages in the biofilm formation for P. aeruginosa were identified for 9 (87%) followed by 12 = 7 = 5 (85%). Compounds 12 (74%) and 14 (73%) showed the strongest eradication of S. aureus followed by 16 (72%) > 13 = 2 (71%). Therefore, based on the effects displayed in both antibacterial and AhR agonist effects the following structure-activity considerations stand out:
-
1
AhR agonism does not seem to be determined by belonging to the 2-aryl or to the 2-pyridinylbenzothiazole subsets, noticeable when compared 1 vs. 2 or 5 vs. 6, all of them below 10% of efficiency at 10 μM, and also when compared the strong inducers of AhR agonistic activity 9 vs. 10 whose efficiencies were no significantly different. Moreover, the stronger inducer of AhR-mediated effects was the arylbenzothiazole 12 followed by the pyridinylbenzothiazole 9. Similarly, the biofilm formation of S. aureus and P. aeruginosa was not crucially determined by the aminophenyl or the aminopyridine substituent in position 2 of the benzothiazole, however, the aminopyridinyl derivative 6 exhibited lower inhibitory percentages in both anti-adherence and eradication tests when compared with its phenyl analogous 5.
-
2
The p,m-disubstitutions with amino and nitro groups of 2-phenyl benzothiazoles, positively contributed to the AhR agonism and to the biocidal activity when compared 12 vs. 10. In 8 the disubstitution significantly contributed to the AhR agonism compared to 5, but non important differences were observed regarding the antiadhesive or eradication effects. Lastly, the p,m-disubstitution positively contributed to the antibiofilm activity of 15 when compared with 13, contrary to the AhR agonism, which was much higher in the monosubstituted 13 than in 15.
-
3
The occurrence of AhR-mediated transcription was significantly higher for methyl substituents in the benzothiazole skeleton compared with all the corresponding analogous hydroxy, methoxy, nitro and amino for both 2-phenyl and 2-pyridinyl subseries (10 vs. 1, 5, 13, 16; 12 vs. 4, 8, 15; 9 vs. 2, 6; 7 vs. 3, 11, 14).
3.3. Computational studies
3.3.1. ADMET and druglikeness profile
Benzothiazole 12 was selected by its attractive antimicrobial and AhR agonist activities to predict in silico its ADMET and druglikeness profile. Physicochemical and pharmacokinetic properties, as well as potential metabolic reactions predicted for 12, are presented in Table 3 .
Table 3.
ADME-related physicochemical parameters, pharmacokinetic properties, and metabolic reactions predicted for benzothiazole 12.
| PHYSICOCHEMICAL | ||||||||||
| logPa | pKab | Swc | DiffCd | MolVole | MDCKf | Peffg | Skinh | Corneai | BBBj | logBBk |
| 4.23 | 3.73 | 4.79 | 8.29 | 266 | 5.45 | 4.90 | 1.25 | 1.48 | High | 0.15 |
| PHARMACOKINETIC | ||||||||||
| Hum_fupl | RBPm | Vdn | ECCSo | CL_Metbp | CL_Renalq | CL_Uptaker | ||||
| 1.06% | 0.12 | 0.31 | Class 2 | Yes (99%) | No (95%) | No (99%) | ||||
| METABOLISM | ||||||||||
| Phase 1. Oxidations | CYP1A2 | CYP2A6 | CYP2B6 | CYP2C8 | CYP2C9 | CYP2C19 | CYP2D6 | CYP2E1 | CYP3A4 | |
| Substratet | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | |
| Confidenceu | 88% | 60% | 55% | 79% | 81% | 98% | 82% | 97% | 83% | |
| Kmv | 23.02 | - | - | - | - | - | 63.02 | - | 55.73 | |
| Vmaxw | 7.10 | - | - | - | - | - | 3.48 | - | 0.41 | |
| CLintx | 16.04 | - | - | - | - | - | 0.44 | - | 0.82 | |
| Phase 2. Glucuronidationy | UGT1A1 | UGT1A3 | UGT1A4 | UGT1A6 | UGT1A8 | UGT1A9 | UGT1A10 | UGT2B7 | UGT2B15 | |
| Substratet | Yes | No | No | No | Yes | Yes | No | No | No | |
| Confidenceu | 90% | 51% | 72% | 75% | 87% | 88% | 86% | 56% | 98% |
logP: octanol-water partition coefficient (lipophilicity)
pKa: dissociation constant using submodels to predict protic ionization microconstants for all identified ionizable groups in a molecule.
Sw (mg/mL×10−4): native water solubility.
DiffC (cm2/s×106): molecular diffusion coefficient in water
MolVol (cm3/mol): molal volume at the normal boiling point
MDCK (cm2/s×105): apparent MDCK COS permeability
Peff (cm2/s×104): human effective jejunal permeability
Skin (cm2/s×105): permeability through human skin
Cornea (cm2/s×105): permeability through rabbit cornea
BBB: qualitative likelihood (High/Low) of crossing the blood-brain barrier (98% confidence)
logBB: logarithm of the brain/blood partition coefficient.
Hum_fup: percent unbound to blood plasma proteins in human.
RBP: blood-to-plasma concentration ratio in human.
Vd (L/kg): volume of distribution in humans at steady state.
ECCS Class: Extended Clearance Classification System (ECCS) assignment
CL_Metab: predicts whether or not metabolism will be critical to clearance
CL_Renal: predicts whether or not renal elimination will be critical to clearance.
CL_Uptake: predicts whether or not hepatic uptake will be critical to clearance.
Most common chemical reaction of Phase 1 of metabolism (oxidation) by Cytochromes P450 (CYP) enzymes
Substrate: substrate classification models (yes/no) for human CYPxxx or UGTxxx
Confidence of predictions
Km (μM): estimated Michaelis-Menten Km constant for predicted sites of metabolism by human CYPxxx
Vmax: [nmol/min/nmol enzyme]: estimated Michaelis-Menten Vmax constant for predicted sites of metabolism by CYPxxx
CLint (μL/min/mg human liver microsomes (HLM) protein): estimated intrinsic clearance for predicted sites of metabolism by CYPxxx
Most common chemical reaction of Phase 2 of metabolism (glucuronidation) by uridine 5′-diphospho-glucuronosyltransferase (UGT)
As reported in Table 3, 12 showed acceptable physicochemical parameters with not excessive lipophilicity (log P = 4.23), low solubility (Sw), dissociation (pKa) and diffusion in water (DiffC) standards as well as a not overly large structure as demonstrated by the molal volume (MolVol) of 266 cm3/mol. High passive permeability (predicted Madin-Darby Canine Kidney (MDCK) model) and jejunal permeability (Peff) were predicted for 12. The skin and cornea permeabilities were normal while the blood-brain barrier (BBB) penetration was identified as high.
In addition, Lipinski's rule of 5 revealed that 12 complied with molecular weight < 500, partition coefficient (logP) < 5, hydrogen bond donor ≤ 5, and hydrogen bond acceptors ≤ 10, suggesting favorable oral bioavailability for this arylbenzothiazole (data no shown).
Concerning the pharmacokinetic properties, only 1.06% was predicted as unlikely to be bond to blood plasma proteins in human (hum_fup%) with a blood to plasma ratio (RBP) of 0.12. The Volume of distribution (Vd) at steady state in humans was estimated as adequate and the Extended Clearance Classification System (ECCS) assessed a Class 2 to 12. Such classification places metabolism as a primary clearance mechanism which is very common for drug-like compounds (Varma et al., 2015). The clearance parameters (CL_Metb, CL_Renal, CL_Uptake) corroborated the critical role of metabolism and the non-relevant contributions of the renal elimination nor the hepatic uptake for the studied molecule.
Compound 12 was predicted to be a substrate for several Phase I metabolizing enzymes of cytochrome P450 (CYP). The metabolic rate constants Km and Vmax were above 23 μM and below 8 nmol/min/nmol enzyme, respectively. For CYP1A2, the lowest Km and the highest Vmax and intrinsic clearance (CLint) were predicted. Comparable Km values were estimated for CYP2D6 and CYP3A4 (63.02 and 55.73 μM) and, while a higher Vmax was predicted for CYP2D6 (3.48 vs. 0.41 nmol/min/nmol enzyme), a greater clearance was predicted for CYP3A4 (0.82 vs. 0.44 μL/min/mg human liver microsomes protein).
In Phase II of metabolism, 12 was predicted to be a substrate of three out of nine uridine 5′-diphospho-glucuronosyltransferase (UGT) isoforms studied. Thus, with predictive confidence above 87%, the arylbenzothiazole is probably metabolized in glucuronidation reactions mediated by UGT1A1, UGT1A8, and UGT1A9.
Finally, the global parameter ADMET_Risk was calculated in ADME Predictor software, which concisely summarizes the information from other properties and descriptors predicted to identify important liabilities in drug candidates. The risk assessment is conducted by comparing with a reference set of 2270 commercial drugs from the World Drug Index (WDI) of which 90% have an ADMET_Risk score lower than 7. For 12, a score of 3.75 was predicted as ADMET_Risk.
Toxicological indexes for humans and for ecosystems were also predicted for 12 (Table 4 ). Hence, the arylbenzothiazole was not predicted as a potential trigger of phospholipidosis nor as a reproductive or mutagenic agent. Regarding the hepatotoxicity, elevated levels of the two transaminases (AST and ALT) and normal levels of the rest of the enzymes were predicted. Therefore, 12 was not predicted to cause relevant harm to humans. Meanwhile, the ecotoxicological parameters in silico estimated for 12, standing out the prediction of a poor biodegradability and an unlike probability of causing endocrine disruption via the estrogen receptor.
Table 4.
Human and environmental toxicological parameters predicted for benzothiazole 12
| HUMAN TOXICITY | ||||||||
| MaxRTDa | Plipidosisb | Developmental and genetic toxicity | Human liver adverse effects | |||||
| Repro_Toxc | Mutagenesisd | AlkPhose | GGTf | LDHg | ASTh | ALTi | ||
| 3.16 | Nontoxic | Nontoxic | Negative | Normal | Normal | Normal | Elevated | Elevated |
| 96% | 99% | 69% | 99% | 65% | 97% | 74% | 85% | 86% |
| ECOTOXICITY | ||||||||
| Bioconcnj | Biodegradnk | Daphnia_LC50l | Minnow_LC50m | Estrogenicm | ||||
| 68.75 | No (95%) | 0.11 | 7.53 | Nontoxic (98%) | ||||
n Fathead minnow (Pimephales promelas) toxicity
MaxRTD (mg/kg/day): qualitative assessment of the Maximum Recommended Therapeutic Dose administered as an oral dose.
Plipidosis: qualitative estimation of potential for causing phospholipidosis.
Repro_Tox: qualitative estimation of reproductive / developmental toxicity, (confidence %).
Mutagenesis: classification model for the mutagenicity of pure compounds in S. typhimurium strain TA102 or E. coli strain WP2 uvrA, (confidence%).
AlkPhos: human liver adverse effect as the likelihood of causing elevation in the levels of Alkaline Phosphatase enzyme, (confidence %).
GGT: human liver adverse effect as the likelihood of causing elevation in the levels of gamma-glutamyl transferase (GGT) enzyme, (confidence %).
LDH: human liver adverse effect as the likelihood of causing elevation in the levels of lactate dehydrogenase (LDH) enzyme, (confidence %).
AST: human liver adverse effect as the likelihood of causing elevation in the levels of serum glutamic oxaloacetic transaminase (SGOT) enzyme, (confidence %).
ALT: human liver adverse effect as the likelihood of causing elevation in the levels of serum glutamic pyruvic transaminase (SGTP) enzyme, (confidence %).
Bioconcn (Cfish/Cwater): bioconcentration factor - partition coefficient between fish tissues and environmental water at steady state.
Biodegradn: likelihood of biodegradation in the environment expressed as relative biological oxygen demand.
Daphnia_LC50 (mg/L): LC50 for Daphnia magna (water flea) lethal toxicity after 48 hours of exposure.
Estrogenic: classification model for predicting antiestrogen activity in rats (confidence %).
3.3.2. Molecular docking analysis
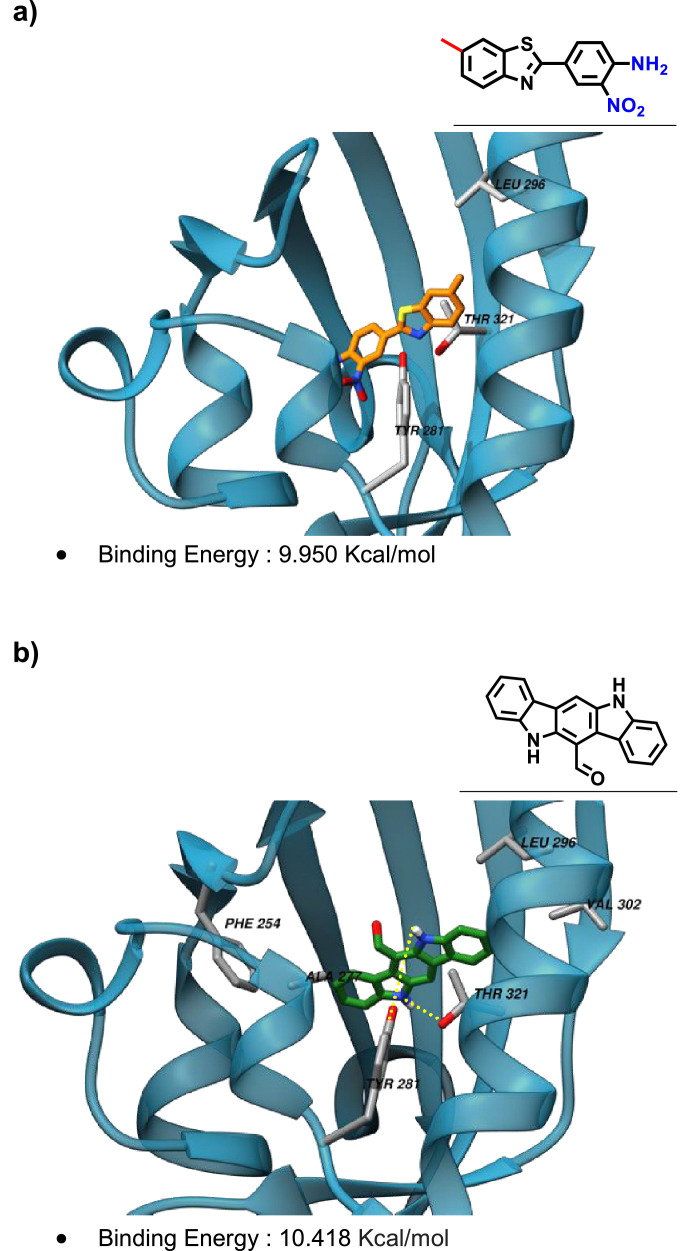
The binding interactions with the ligand binding domain of AhR were explored through molecular docking for 12 and for the known agonist FICZ and the outcome is presented in Figure 7 a) and b), respectively.
Figure 7.
Molecular docking analyses for a) Benzothiazole 12 and b) FICZ with AhR-Ligand Binding Domain (PBD ID: 3F1O). Left side: the structure of the protein is represented as transparent blue ribbons and the best pose obtained for 12 and FICZ are displayed as sticks. The residues involved in hydrophobic interactions are labeled. Hydrogen bond interactions are represented in b) as yellow dot lines. Right side: the binding pocket residues are listed, in bold are highlight the matching between the two ligands.
The binding energy with AhR, as well as the pocket residues identified in the molecular docking simulations, were similar for the arylbenzothiazole and for the ligand/agonist FICZ as represented in Figure 7. Furthermore, the THR 321 residue was predicted to interact hydrophobically with 12 and FICZ, while TYR 281 and LEU 296 seemed to establish hydrophobic interactions with 12 and hydrogen bonds with FICZ.
4. Conclusions
Ah receptor is an important chemical sensor that integrates dietary, environmental, metabolic, and microbial signals to regulate transcriptional programs in a context, cell type, and ligand-specific manner. The association of AhR with the immune system has been widely studied, particularly important in gut microbiota and barrier tissues, where crucial immune responses are related to AhR expression. The dual association of antimicrobial and AhR modulatory effects could be potentially beneficial in drug discovery. Hence, in this work, both effects were evaluated for a set of functionalized benzothiazoles yielding a promising activity for one derivative 12 as biocidal against S. aureus and as AhR agonist. Structure-activity analyses revealed general headlines on the substituent's contributions. Finally, computational studies with benzothiazole 12 predicted an adequate ADMET profile and a potentially similar binding to AhR when compared with the known agonist FICZ.
Abreviation
- ADMET
absorption, distribution, metabolism, and excretion - toxicity
- AhR
aryl hydrocarbon receptor
- AhR-HepG2, Lucia™
human hepatoma cell line stably transfected to express AhR
- BT
benzothiazole
- CH223191
2-methyl-2H-pyrazole-3-carboxylic acid
- CYP
cytochrome P450
- Eff
efficiency
- FA
fusidic acid
- FICZ
5,11-dihydroindolo[3,2-b]carbazole-12-carbaldehyde
- Lv
levofloxacin
- MBC
Minimal Bactericidal Concentration
- MIC
Minimal Inhibitory Concentration
- MTT
3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- OD
optical density
Author Contributions
All authors contributed to the drafting and revision of the article and approved the final version.
Credit Author Statement
E.G.J accomplished biological experiments to evaluated AhR expression ability, data curation, and structure-activity relationship analyses. F. A performed antibacterial, antifungal, and biocidal evaluation. L.E.C accomplished molecular docking studies. R. M. G designed and supervised biological studies. M.S-IV was responsible for chemicals supply, structure-activity and biological analysis and supervised the project. All authors contributed to the drafting and revision of the article and approved the final version presented.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledged the “PHC Utique” programme of the French Ministry of Foreign Affairs and Ministry of higher education, research and innovation and the Tunisian Ministry of higher education and scientific research in the CMCU project number 17G1215 for financial support of this work. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 722634. FA thanks to Mouna Jlidi for assisting her during the antibiofilm activity evaluation.
References
- Ali R., Siddiqui N. Biological aspects of emerging benzothiazoles: A short review. J. Chem. 2013 doi: 10.1155/2013/345198. 2013. [DOI] [Google Scholar]
- Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M.C., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M.L., Orabona C., Bianchi R., Lanz T.V., Platten M., Della Fazia M.A., Piobbico D., Zelante T., Funakoshi H., Nakamura T., Gilot D., Denison M.S., Guillemin G.J., Duhadaway J.B., Prendergast G.C., Metz R., Geffard M., Boon, L., Pirro M., Iorio A., Veyret B., Romani L., Grohmann U., Fallarino F., Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondock S., Fadaly W., Metwally M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010;45:3692–3701. doi: 10.1016/j.ejmech.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Bort G., Sylla-Iyarreta Veitía M., Ferroud C. Straightforward synthesis of PET tracer precursors used for the early diagnosis of Alzheimers disease through Suzuki-Miyaura cross-coupling reactions. Tetrahedron. 2013;69:7345–7353. doi: 10.1016/j.tet.2013.06.085. [DOI] [Google Scholar]
- Choudhary S., Jeyabalan G., Kalra N. A Review: Therapeutic and Biological Activity of Benzothiazole Derivatives. Int. J. Recent Adv. Sci. Technol. 2017;4:8–20. doi: 10.30750/ijrast.432. [DOI] [Google Scholar]
- Esser C., Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- Esser C., Rannug A., Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ghosh J., Lawless M.S., Waldman M., Gombar V., Fraczkiewicz R. In: Silico Methods for Predicting Drug Toxicity. Benfenati E., editor. Springer; New York, New York, NY: 2016. Modeling ADMET; pp. 63–83. [DOI] [Google Scholar]
- Gill R.K., Rawal R.K., Bariwal J. Recent advances in the chemistry and biology of benzothiazoles. Arch. Pharm. (Weinheim). 2015;348:155–178. doi: 10.1002/ardp.201400340. [DOI] [PubMed] [Google Scholar]
- Goya-Jorge E., Giner R.M., Veitía M.S.-I., Gozalbes R., Barigye S.J. Predictive modeling of aryl hydrocarbon receptor (AhR) agonism. Chemosphere. 2020;256 doi: 10.1016/j.chemosphere.2020.127068. [DOI] [PubMed] [Google Scholar]
- Goya-Jorge E., Rampal C., Loones N., Barigye S.J., Carpio L.E., Gozalbes R., Ferroud C., Veitía M.S.-I., Giner R.M. Targeting the Aryl Hydrocarbon Receptor with a novel set of Triarylmethanes. Eur. J. Med. Chem. 2020 doi: 10.1016/j.ejmech.2020.112777. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- Hergesheimer R., Lanznaster D., Vourc'h P., Andres C., Bakkouche S., Beltran S., Blasco H., Corcia P., Couratier P. Advances in pharmacotherapy for the treatment of gout. Expert Opin. Pharmacother. 2015;6566:1–8. doi: 10.1517/14656566.2015.997213. [DOI] [PubMed] [Google Scholar]
- Hu W., Sorrentino C., Denison M.S., Kolaja K., Fielden M.R. Induction of Cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007;71:1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- Janeway C., Travers P., Walport M. Garland Science; New York: 2001. Manipulating the immune response to fight infection, in: Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- Kamal A., Syed M.A.H., Mohammed S.M. Therapeutic potential of benzothiazoles: A patent review (2010-2014) Expert Opin. Ther. Pat. 2015;25:335–349. doi: 10.1517/13543776.2014.999764. [DOI] [PubMed] [Google Scholar]
- Keam S.J., Croom K.F., Keating G.M. Levofloxacin. A review of its use in the treatment of bacterial infections in the United States. Drugs. 2005;63:2769–2802. doi: 10.2165/00003495-200565050-00007. [DOI] [PubMed] [Google Scholar]
- Krieger E., Vriend G. YASARA View - molecular graphics for all devices - from smartphones to workstations. Bioinformatics. 2014;30:2981–2982. doi: 10.1093/bioinformatics/btu426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C.A., Wang Y., Holt D.P., Huang G.F., Debnath M.L., Klunk W.E. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Bardbori A., Omidi M., Arabnezhad M.R. Impact of CH223191-Induced Mitochondrial Dysfunction on Its Aryl Hydrocarbon Receptor Agonistic and Antagonistic Activities. Chem. Res. Toxicol. 2019;32:691–697. doi: 10.1021/acs.chemrestox.8b00371. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moura-Alves P., Faé K., Houthuys E., Dorhoi A., Kreuchwig A., Furkert J., Barison N., Diehl A., Munder A., Constant P., Skrahina T., Guhlich-Bornhof U., Klemm M., Koehler A.B., Bandermann S., Goosmann C., Mollenkopf H.J., Hurwitz R., Brinkmann V., Fillatreau S., Daffe M., Tümmler B., Kolbe M., Oschkinat H., Krause G., Kaufmann S.H.E. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- Moyer J.H., Ford R.V. Laboratory and clinical observations on ethoxzolamide (Cardrase) as a diuretic agent. Am. J. Cardiol. 1958;1:497–504. doi: 10.1016/0002-9149(58)90121-8. [DOI] [PubMed] [Google Scholar]
- Muthusubramanian L., Rao V.S.S., Mitra R.B. Efficient synthesis of 2-(thiocyanomethylthio)benzothiazole. J. Clean. Prod. 2001;9:65–67. doi: 10.1016/S0959-6526(00)00031-7. [DOI] [Google Scholar]
- Oh S., Go G.W., Mylonakis E., Kim Y. The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans. J. Appl. Microbiol. 2012;113:622–628. doi: 10.1111/j.1365-2672.2012.05372.x. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Ricco C., Abdmouleh F., Riccobono C., Guenineche L., Martin F., Goya-Jorge E., Lagarde N., Liagre B., Ali M.Ben, Ferroud C., Arbi M.El, Veitía M.S.-I. Pegylated triarylmethanes: Synthesis, antimicrobial activity, anti-proliferative behavior and in silico studies. Bioorg. Chem. 2020;96 doi: 10.1016/j.bioorg.2020.103591. [DOI] [PubMed] [Google Scholar]
- Salentin S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015;43:W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F., Bäckhed F. The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Trott O., Olson A.. Software News and Update. AutoDock Vina Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M.V., Steyn S.J., Allerton C., El-Kattan A.F. Predicting Clearance Mechanism in Drug Discovery: Extended Clearance Classification System (ECCS) Pharm. Res. 2015;32:3785–3802. doi: 10.1007/s11095-015-1749-4. [DOI] [PubMed] [Google Scholar]
- Zhao H., Chen L., Yang T., Feng Y.L., Vaziri N.D., Liu B.L., Liu Q.Q., Guo Y., Zhao Y.Y. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J. Transl. Med. 2019;17:1–14. doi: 10.1186/s12967-019-2054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]