To the Editor,
Sir, Madam,
There is currently no specific treatment with demonstrated efficacy against the respiratory infection outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) that affected more than 4000,000 persons and killed 300,000 around the world during the last 6 months (1,2). Like Peiris and al. suggested with the SARS-CoV1, we believe that an effective antiviral agent is needed to decrease the viral load and direct cytolytic damage during the first phase of infection, and in turn reduce the immunologic storm during the second phase with the risk of progression to acute respiratory distress syndrome (3). Among existing antiviral therapeutics tested, protease inhibitors seemed promising, and ritonavir-boosted lopinavir (LPV/r) has been shown to inhibit the replication of SARS-CoV-2 in vitro and in hospitalized patients (4, 5, 6).
Here we report the viral dynamics in multiple clinical samples in regards to pharmacological LPV/r levels during and after treatment in a SARS-CoV-2-infected patient. This first SARS-CoV-2 infection in a French resident was diagnosed in our department on January 29th 2020, six days after his exposure to a laboratory-confirmed case from Asia (7).
We performed monitoring of SARS-CoV-2 infection from day 2 (D2) after onset of symptoms in different sequential clinical samples by real-time RT-PCR targeting E gene (8). Viral loads were estimated with the cycle threshold (Ct) values: Ct > 50 was considered as negative. Detection of specific antibodies was performed on plasma specimens with the Abbott SARS-CoV-2 IgG assay. When chest CT-scan confirmed small areas of ground-glass opacities in both lower lungs on D9, the patient started ritonavir-boosted lopinavir (LPV/r) 400/100 mg BID until hospital discharge on D18. LPV plasma concentration (Cmin) was measured by liquid chromatography tandem mass spectrometry method (LC-MS/MS); the limit of quantification (LOQ) was 15 ng/mL.
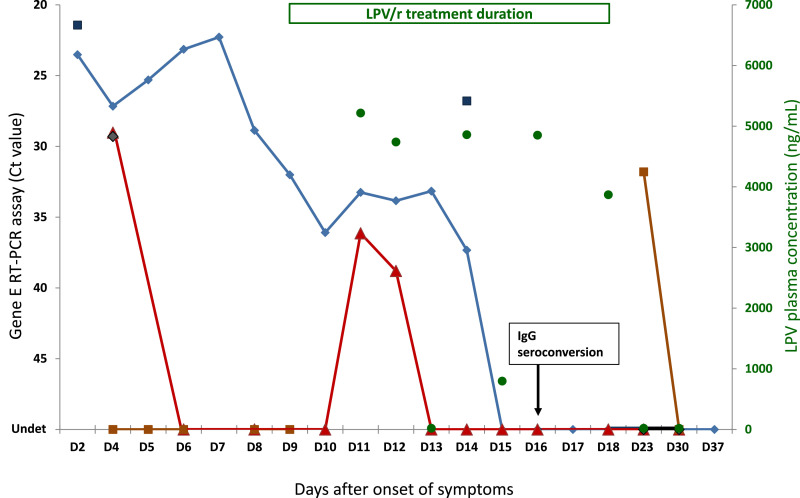
The outcome of the patient was good. He experienced the typical pattern of COVID-19 symptoms, such as sore throat, muscle pain, headaches and anosmia, then lung infection signs but did not develop severe pneumonia and never required supportive treatments with oxygen or immunomodulators. During the whole period of viral monitoring, SARS-CoV-2 RNA was detected not only in nasopharyngeal swab (NPS), but also in induced sputum, saliva, plasma, and stool (Fig. 1 ). However, SARS-CoV-2 RNA was never detected in urine. The whole genome sequence obtained from positive NPS sample is available in Global Initiative on Sharing All Influenza Data (GISAID) with the sequence number EPI_ISL_408,431. Between D2 and D4, high viral loads (Ct<30) were detected in NPS, induced sputum, saliva, and plasma. Viral load decreased gradually in NPS to become undetectable on D15, after 6 days of treatment. In plasma, after a rapid initial drop, a low-level rebound (Ct>35) occurred on D11 and D12, corresponding to a transient plateau in NPS. This phenomenon was observed between 2 and 3 days after the start of LPV/r treatment and despite expected LPV Cmin. On D14, SARS-CoV-2 RNA was still detected at high level (Ct<30) in sputum, but at low level (Ct>35) in NPS, illustrating differential compartmentalization of SARS-CoV-2 in upper and lower respiratory tracts. SARS-CoV-2 RNA was detected once in stool sample on D23, after LPV/r removal. Further additional samples (i.e., NPS, saliva, plasma and stool) collected on D30 and D90 were negative for SARS-CoV-2. In terms of immunity, IgG seroconversion was evidenced on D16 (Fig. 1).
Fig. 1.
Viral dynamics in multiple and sequential clinical samples and kinetics of lopinavir plasma concentrations in a patient with confirmed SARS-CoV-2 infection and treated with oral ritonavir boosted lopinavir. Real-time RT-PCR targeting viral E gene, presented by reverse Ct values on left vertical axis, was performed in serial different types of clinical samples collected from the patient: nasopharyngeal swab (◆), induced sputum (■), saliva (◆), plasma (▴), and stool (■). Lopinavir concentration (●), expressed in ng/mL on right vertical axis, was measured in sequential plasma samples by liquid chromatography tandem mass spectrometry method. Range of lopinavir minimal plasma concentrations: 4.660 ± 2.250 ng/mL Duration of ritonavir-boosted lopinavir (400/100 mg) treatment (D9 to D18) is indicated on the top of the graph. SARS-CoV-2 antibody response (IgG seroconversion) in indicated on the graph (D16). Undet: undetectable (Ct>50).
In a retrospective cohort study, 96 patients infected with SARS-CoV-2, the median duration of virus detection in NPS samples varied from 14 to 21 days according to disease severity (9). A recent study showed that SARS-CoV-2 RNA could not be detected in NPS from half of non-severe patients after 14 days of LPV/r treatment (10. However, in a randomized trial involving 199 patients, LPV/r treatment did not significantly improve clinical symptoms or survival, nor diminish throat viral RNA detectability in late-presenters patients with severe pneumonia (11). Interestingly, in a post-hoc analysis of the subgroup of patients treated less than 12 days after the onset of symptoms, clinical cure was obtained after 16 days in the LPV/r arm versus 17 days and the mortality rate was 19.0% versus 27.1%, without statistical significance, possibly due to the weak study power. In our study, the viral clearance in NPS of this single patient was obtained within 2 weeks, coinciding with the antibody response (D16). The rebound of viral load observed in NPS during treatment could be explained by the transient subtherapeutic levels of LPV/r between D13 and D15. The SARS-CoV-2 RT-PCR became positive in stool only after the end of treatment, addressing the question of longer treatment need for oro-fecal transmission prevention.
Our findings suggest that, if LPV/r treatment does not seem to constitute the treatment of choice for salvage therapy in patients with severe COVID-19, it could be effective in early presenting non-severe patients to decrease the SARS-CoV-2 load and to prevent the secondary immune-related severe evolution.
Ritonavir-boosted lopinavir (LPV/r) use in SARS-CoV-2′s infected patients should be better evaluated in a prospective controlled study including multisite drug dosages and pharmacokinetic/pharmacodynamic (PK/PD) study, and treatment should be given for at least 14 days to reduce long-term viral carriage and related transmission risks.
Ethics
The patient signed an informed consent accepting the extra tests performed for this study. The Infectious Diseases Department obtained the clearance from the Hospital's Ethic Committee to proceed with COVID-19 cohort follow-up.
Transparency declarations
All authors contributed towards the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
No public or private funds were used for the current study.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
On behalf of the « COVID SMIT PSL STUDY GROUP »
References
- 1.WHO. Coronavirus disease (COVID-19): situation Report – 114 Data as received by WHO from national authorities by 10:00 CEST, 13 May 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200513-covid-19-sitrep-114.pdf?sfvrsn=17ebbbe_4
- 2.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - Navigating the Uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv. 2020 doi: 10.1101/2020.03.20.999730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prajapat M., Sarma P., Shekhar N., Avti P. Drug targets for corona virus: a systematic review. Indian J Pharmacol. 2020;52(1):56–65. doi: 10.4103/ijp.IJP_115_20. https://www.ncbi.nlm.nih.gov/pubmed/32201449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan K.S., Lai S.T., Chu C.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. https://www.ncbi.nlm.nih.gov/pubmed/14660806 [PubMed] [Google Scholar]
- 6.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., IHung F.N., Poon L.L.M. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klement E., Godefroy N., Burrel S., Kornblum D., Monsel G., Bleibtreu A. The first locally acquired novel case of 2019-nCoV infection in a healthcare worker in the Paris area. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.By Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan D, Liu X-y, Zhu Y-n, et al. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in patients with SARS-CoV-2 infection. 2020 10.1101/2020.03.22.20040832. [DOI] [PMC free article] [PubMed]
- 11.Cao Bin, Wang Yeming, Wen Danning, Liu Wen, Wang Jingli, Fan Guohui. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020:0028–4793. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]