Abstract
Simple synthetic compounds with only S and C donors offer a ligation environment similar to the active site of nitrogenase (FeMoco) and thus demonstrate reasonable mechanisms and geometries for N2 binding and reduction in nature. We recently reported the first example of N2 binding at a mononuclear iron site supported by only S and C donors. In this work, we report experiments that examine the mechanism of N2 binding in this system. The reduction of an iron(II) tris(thiolate) complex with one equivalent of KC8 leads to a new, thermally unstable intermediate and a combination of Mössbauer, EPR and X-ray absorption spectroscopies identify it as a high spin (S = 3/2) iron(I) species that maintains coordination of all three sulfur atoms. DFT calculations suggest that this iron(I) intermediate has a pseudotetrahedral geometry that resembles the S3C iron coordination environment of the belt iron sites in the resting state of the FeMoco. Further reduction to the iron(0) oxidation level under argon causes the dissociation of one of the thiolate donors and gives an η6-arene species which reacts with N2. Thus, in this system the loss of thiolate and binding of N2 require reduction beyond the iron(I) level to the iron(0) level. Further reduction of the iron(0)-N2 complexes gives a reactive, formally iron(-I) species. Treatment of the putative iron(-I) complex with weak acids gives low yields of ammonia and hydrazine, demonstrating that these nitrogenase products can be generated from N2 at a synthetic Fe-S-C site. Catalytic N2 reduction is not observed, which is attributed to protonation of the supporting ligand and degradation of the complex via ligand dissociation. Identification of the challenges in this system give insight into the design features needed for functional biomimetic complexes.
Graphical Abstract

INTRODUCTION
Biological fixation of dinitrogen to ammonia occurs at the iron- and sulfur-containing cofactors of nitrogenase enzymes.1-2 The most thoroughly studied nitrogenase reduces N2 at the iron-molybdenum cofactor (FeMoco), a cluster that contains seven iron atoms and a molybdenum atom (Figure 1, top).3-5 Studies on the reactions of nitrogenase variants with substrates such as alkynes, propargyl alcohol, carbon monoxide, and selenocyanate strongly implicate the iron sites of the FeMoco as the N2 binding sites.6-13 These iron atoms are coordinated by three sulfides and a carbide in the resting state.
Figure 1.
Top: structure of the resting state of the FeMoco with one of the pseudotetrahedral FeS3C sites emphasized. Bottom: hypothetical structure of an N2-bound species in which a protonated sulfide dissociates from an iron site to enable N2 binding. Though the structure of the N2-bound species is currently unclear, this illustrates the potential role of Fe–S cleavage and carbide coordination during N2 binding.
Starting from the resting state, the FeMoco accumulates four reducing equivalents and four protons before N2 binding occurs, along with reductive elimination of H2.1, 14 This process presumably involves structural changes that create an open coordination site for substrate binding at an iron center. There is growing evidence that Fe–S dissociation within the cofactor is possible (Figure 1, bottom), as supported by the crystallographically observed substitution of the central sulfide bridge in the FeMoco by CO and by selenide.12-13 More recently, Einsle and co-workers reported a structure of vanadium nitrogenase in which one of the bridging sulfides in the iron-vanadium cofactor (FeVco) is displaced from the active site, and a light atom (assigned as N2-derived NH, though it may instead be OH15-16) lies between these two iron centers.17 Computational studies have also suggested that sulfide lability or hemilability may be mechanistically important in nitrogenases.18-23
The unusual structure and reactivity of the FeMoco have encouraged studies of the coordination chemistry of simple synthetic iron complexes with the ability to bind and/or reduce N2.24-33 Notably, catalytic ammonia and hydrazine production from N2 using strong reductants and strong acids has been achieved using phosphine-supported complexes.34-40 Sulfur donors, in the form of thioethers and thiolates,41-45 and carbon donors, in the form of carbanions or N-heterocyclic carbenes,35-36, 46-51 have also been incorporated in ligand systems. However, none of these complexes rely on ligands that contain only the biologically relevant sulfur and carbon donors.
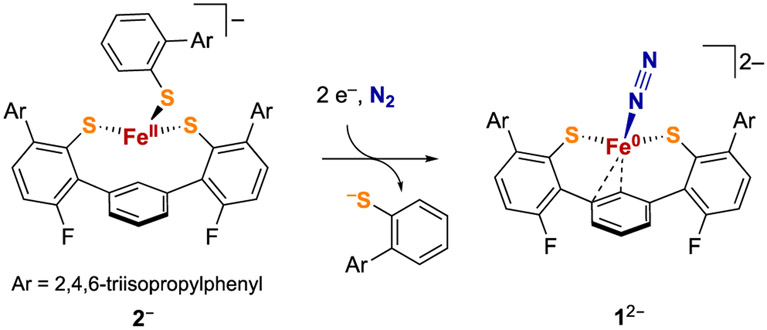
Recently, we reported the first example of a structurally characterized Fe-N2 complex with a mixed sulfur/carbon ligand sphere, which utilizes a bulky bis(thiolate) ligand with a terphenyl core.43 Complex 12− (Scheme 1) features N2 coordinated to a formally iron(0) center in a pseudotetrahedral geometry (considering a C=C bond of the central arene as a single donor). This product could be formed from reduction of the iron(II) tris(thiolate) species 2-K at low temperature under an atmosphere of N2 (Scheme 1). The transformation of 2-K to 1-K2 provides a small-molecule model of Fe-S dissociation that leads to N2 binding. However, the reported study does not distinguish the order of the processes that occur in this transformation (thiolate loss, N2 binding, and two-electron reduction), which could provide insight into the behavior of iron-sulfur-carbon sites under reducing conditions.
Scheme 1.
Reduction of an Iron(II) Tris(thiolate) Complex Involving Thiolate Dissociation and N2 Binding43
Here, we report mechanistic details of the Fe-S dissociation/N2 binding process in this synthetic system through characterization of several thermally sensitive low-valent iron species. Characterization of the iron(I) and iron(0) precursors to N2 binding helps to elucidate the reasons for the weak N2 binding observed in this complex. We also demonstrate the production of ammonia and hydrazine from N2 through further reduction of 1-K2 followed by treatment with weak acids.
RESULTS
Reduction of Iron(II) to Iron(I) Without Thiolate Dissociation.
The product of the one-electron reduction of 2-K was first examined in order to determine whether the Fe–S bond of the monothiolate remains intact at the iron(I) redox level. Treatment of a solution of 2-K in THF with 1.1 equiv of KC8 at −70 °C under N2 results in an immediate color change to dark red. The Mössbauer spectrum of the frozen reaction mixture indicates a single species with δ = 0.80 mm/s and ∣ΔEQ∣ = 2.01 mm/s (Table 1 and Figure 1). In contrast, the previously isolated43 iron(I) bis(thiolate) complex 3-K exhibits a broad, unresolved spectrum in frozen THF under the same conditions (Figures S5-S8). Thus, Mössbauer spectroscopy indicates that one-electron reduction of 2-K produces a species (4-K2) that is distinct from 3-K. Furthermore, the same species is formed in an analogous reduction under an Ar atmosphere (Figure S9), indicating that 4-K2 does not contain N2. Based on these results, we assign 4-K2 as an iron(I) tris(thiolate) complex, in which the iron center is reduced but retains both the monodentate thiolate and bis(thiolate) ligands (Scheme 2).
Table 1.
Mössbauer parameters for compounds 1 − 7.
| Compound | Solid | Frozen THF Solution |
DFT- Calculated |
|---|---|---|---|
| 1-K2 | δ = 0.74 mm/s a, b ∣ΔEQ∣ = 1.79 mm/s |
δ = 0.70 mm/s ∣ΔEQ∣ = 1.32 mm/s |
δ = 0.77 mm/s ∣ΔEQ∣ = 1.78 mm/s |
| 2-K | δ = 0.69 mm/s a ∣ΔEQ∣ = 1.50 mm/s |
δ = 0.84 mm/s c ∣ΔEQ∣ = 3.00 mm/s |
δ = 0.62 mm/s ∣ΔEQ∣ = 2.76 mm/s |
| 3-K | δ = 0.59 mm/s a ∣ΔEQ∣ = 0.53 mm/s |
Broad, unresolved spectrum | δ = 0.67 mm/s ∣ΔEQ∣ = 0.75 mm/s |
| 4-K2 | Not isolable | δ = 0.80 mm/s ∣ΔEQ∣ = 2.01 mm/s |
δ = 0.78 mm/s d ∣ΔEQ∣ = 1.64 mm/s |
| 5 | δ = 0.89 mm/s a ∣ΔEQ∣ = 3.77 mm/s |
Poor solubility in THF |
δ = 0.78 mm/s e ∣ΔEQ∣ = 3.81 mm/s |
| 6-K2 | δ = 0.97 mm/s b ∣ΔEQ∣ = 2.07 mm/s |
δ = 1.00 mm/s ∣ΔEQ∣ = 2.02 mm/s |
δ = 1.07 mm/s f ∣ΔEQ∣ = 1.86 mm/s |
| 7-K3 | Not isolable | δ = 0.61 mm/s ∣ΔEQ∣ = 2.48 mm/s |
Not calculated |
Mössbauer parameters for solid samples were reported previously.43
As 18-crown-6 adduct.
As previously reported43, 2-K binds THF at low temperature which accounts for the difference between its solid-state and solution Mössbauer parameters (see Figure S4)
Calculated values for S = 3/2 model.
Calculated Mössbauer parameters were previously reported.57
Calculated values for S = 1 model.
Scheme 2.
Iron(I) Thiolate Complexes
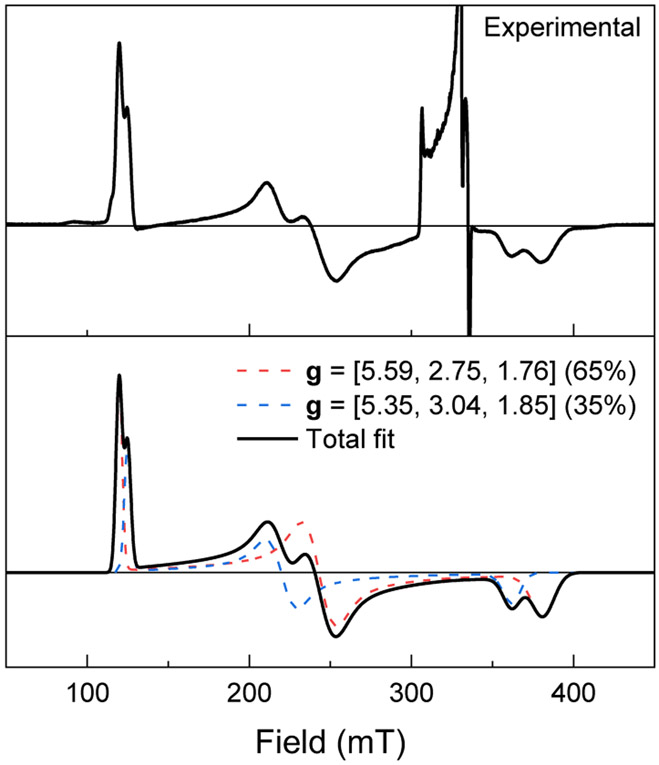
Further insight into the nature of 4-K2 was obtained from EPR spectroscopy. Figure 2 shows the X-band EPR spectrum of the crude reduction product following transfer to a pre-cooled EPR tube in a −70 °C cold well and immediate flash-freezing in liquid nitrogen. Two signals with slightly different rhombicities appear at g = [5.59, 2.75, 1.76] (65%, red) and g = [5.35, 3.04, 1.85] (35%, blue), which are each indicative of an S = 3/2 spin state. The reason for the appearance of two components in the EPR spectrum is not known, but it is possible that the two components represent different conformers of 4-K2 which have similar Mössbauer spectra that cannot be resolved at 80 K at zero field.52-56 In these EPR spectra, the previously characterized43 bis(thiolate) complex 3-K is also present as a 10% impurity (S = 1/2 signal in the g = 2 region), as determined by comparison to a sample of 3-K generated via reduction of the iron(II) bis(thiolate) complex 5 under the same conditions (Figure S20). Warming the reaction mixture to room temperature for 15 minutes results in a decrease in the S = 3/2 signals of 4-K2 (Figure S19) and an increase in the S = 1/2 signal of 3-K (Figure S20), suggesting that the small amount of 3-K present in these samples arises from thermal decomposition of 4-K2 during transfer to the EPR tube. This proposal is also supported by Mössbauer spectroscopy; the spectrum of 4-K2 after stirring at room temperature for 20 minutes contains a broad feature consistent with the presence of 3-K (Figure S10). Thus, all our evidence indicates that 4-K2 is a high-spin iron(I) complex that differs from 3-K, presumably because it retains the monodentate thiolate ligand.
Figure 2.
Mössbauer spectrum of a frozen reaction mixture containing 4-K2 generated by treatment of 2-K with 1.1 equiv of KC8 in THF under N2. The black circles are the experimental data, the red line is a simulation of 4-K2 with the fit parameters shown in Table 1, and the gray line is the residual. The slight asymmetry may be attributed to broadening from paramagnetic relaxation at the measurement temperature of 80 K.
Since attempts to crystallize 4-K2 have not been successful, insight into its structure was obtained from X-ray absorption studies (Figure 3). In order to validate this approach, we began by collecting EXAFS data for 2-K and 3-K, which have previously been characterized crystallographically.43 Fits of the k3-weighted EXAFS of 2-K indicate the presence of 3 Fe-S interactions at 2.33 Å and multiple Fe-C interactions, with the closest at 2.42 Å (Table S3). These are in reasonable agreement with the crystallographically determined bond lengths for this species (Table S4). The 2 Fe-S and 6 Fe-C interactions in 3-K determined by EXAFS (2.25 Å and 2.08 Å, respectively) are also in good agreement with the crystal structure (Tables S5 and S6). The EXAFS fits of 4-K2 (Table S7) require 3 Fe-S interactions at a similar distance to those in 2-K (2.32 Å). Notably, these distances are significantly longer than those of the low-spin iron bis(thiolate) complex 3-K, suggesting that 4-K2 is high-spin (in agreement with the interpretation of the EPR spectra described above). The fit also requires the inclusion of 2 short Fe-C interactions at 2.02 Å, implying η2 binding to the central arene ring.
Figure 3.
X-band EPR spectrum of a frozen reaction mixture of 2-K treated with 1.1 equiv of KC8 in 2-methyltetrahydrofuran recorded at 5 K. The experimental spectrum is shown in the top frame. A simulation for the S = 3/2 species (corresponding to 4-K2) is shown as a solid black line in the bottom frame and the two components of the fit are shown as blue and red dashed lines. The S = 1/2 signal corresponding to 3-K in the g = 2 region is not included in the fit; this signal is power-saturated under these conditions (see Figure S20).
A more detailed structural model of 4-K2 was obtained from DFT calculations, using a truncated model in which the triisopropylphenyl groups were removed (see Supplementary Information for computational details). This method and level of truncation gave highly accurate models of 1-K2 and 5 in previous studies,43, 57 as well as of 2-K and 3-K (see Tables S10 and S11). Structures of the hypothesized iron(I) tris(thiolate) intermediate in the two possible spin states (S = 1/2 and S = 3/2) were optimized at the BP86/def2-TZVP level. The calculations indicate the S = 3/2 configuration to be more stable with ΔGl.s.-h.s. = +14 kcal/mol, in agreement with the EPR evidence that 4-K2 is high-spin (see above). The calculated Fe-C bond lengths of both models are longer than those determined by EXAFS. However, the S = 3/2 DFT model reproduces the experimental Fe-S distances, whereas the Fe-S bonds are too short in the S = 1/2 DFT model (see Table S8). Furthermore, the calculated Mössbauer parameters for the high spin DFT model (δ = 0.78 mm/s, ∣ΔEQ∣ = 1.64 mm/s) are close to those observed experimentally and agree much better than the parameters for the alternative low spin DFT model (δ = 0.64 mm/s, ∣ΔEQ∣ = 1.04 mm/s).57 The DFT calculations and experimental results therefore both support assigning 42− as an S = 3/2 iron(I) tris(thiolate) complex. In the computationally optimized structure, three thiolate donors and an asymmetrical η2 coordination of the central arene ring (Fe-C distances = 2.02, 2.21 Å) form a pseudotetrahedral ligand environment around the iron(I) site (Figure 4). The immediate coordination sphere of 42− therefore resembles the environment of the individual iron centers in the resting state of the FeMoco, although there are likely to be electronic differences (see Discussion section).
Figure 4.
Non-phase-shift-corrected FT of the Fe K-edge EXAFS spectra (black, solid) and fits (red, dashed) of a) 2-K (3S/5C model), b) 3-K (2S/6C/CC model) and c) 4-K2 (2-3S/6C model). Parameters and details of the fits are provided in the Supplementary Information.
Reduction to Iron(0).
In order to determine whether monothiolate loss is triggered by reduction or occurs only in the presence of N2, the two-electron reductions of the iron(II) complexes 2-K and 5 were examined under argon atmosphere (Scheme 3). Reduction of 2-K with 2.4 equiv of KC8 under an argon atmosphere at −65 °C yields a new species (6-K2) with Mössbauer parameters δ = 1.00 mm/s and ∣ΔEQ∣= 2.02 mm/s) (Figure 6, top). Reduction of the iron(II) bis(thiolate) complex 5 with excess KC8 results in formation of the same species, although samples prepared in this manner are contaminated with the iron(I) bis(thiolate) complex 3-K (Figure S11).
Scheme 3.
Iron(0) thiolate complexes
Figure 6.
Mössbauer spectra of frozen reaction mixtures of 2-K treated with 2.4 equiv of KC8 in THF under argon (top), under argon followed by addition of N2 (middle), and under an N2 atmosphere (bottom). The black circles are the experimental data, and the grey lines are the residuals for the total fits. The solid lines correspond to fits with the parameters given in Table 1.
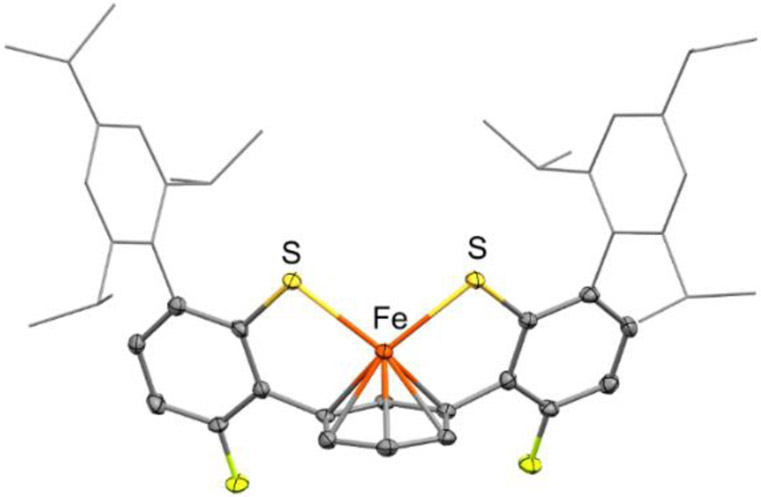
We were unable to crystallize 6-K2 from the reduction of 2-K with excess KC8. However, reduction of 5 with excess KC8 at −65 °C in THF in the presence of 18-crown-6, followed by brief warming, affords 6-[K(18-crown-6)]2, which crystallized after standing overnight at −40 °C. X-ray diffraction analysis shows that the anion in 6 is an iron(0) bis(thiolate) complex with η6 coordination to the central arene ring (Figure 6 and Scheme 3). Complex 6-[K(18-crown-6)]2 is structurally similar to the iron(I) complex 3-K with longer bond lengths to ligated atoms, consistent with a more reduced iron site (Table S14).
Although it was possible to obtain clean Mössbauer spectra of solid 6-[K(18-crown-6)]2 by careful isolation and handling at low temperature (Figure S13), further characterization of this species was hindered by its thermal instability (Figure S14) and low solubility. Insight into the nature of this species was therefore obtained from DFT calculations, using the same methods that were validated above. Geometry optimizations were performed on a truncated model of the anionic portion of 6-[K(18-crown-6)]2 for two possible spin states (S = 0 and S = 1). The calculations predict the two spin states to be close in energy, with the calculated ground state depending on the functional used. However, the Fe-S and Fe-C bond distances for the S = 1 DFT model are closer to those observed crystallographically (see Table S13). Furthermore, the observed Mössbauer parameters of 6-[K(18-crown-6)]2 (δ = 1.00 mm/s and ∣ΔEQ∣= 2.02 mm/s) are closer to those predicted for the S = 1 model (δ = 1.07 mm/s, ∣ΔEQ∣ = 1.86 mm/s) than those predicted for the S = 0 model (δ = 0.76 mm/s, ∣ΔEQ∣ = 2.36 mm/s). Therefore, we favor assigning 62− as an iron(0) complex having a high-spin (S = 1) electronic configuration.
N2 binding to the Iron(0) Complex.
When a reaction mixture containing 6-K2 (generated in situ from 2-K under Ar) is treated with N2 at −65 °C, an immediate color change from dark brown to dark red is observed. Analysis of this reaction mixture using Mössbauer spectroscopy reveals the presence of 1-K2, along with a small amount of the over-reduced product 7-K3 (see below) formed from unreacted KC8 present in the crude reaction mixture. These are the same species observed when the reduction of 2-K is performed under an atmosphere of N2 (Figure 5 middle and bottom). Since the transformation of 6-K2 to 1-K2 occurs rapidly upon addition of N2, the iron(0) bis(thiolate) species 6-K2 is a reasonable and kinetically competent intermediate for the reaction sequence that leads to N2 binding (Scheme 3). It is also significant that reduction of 2-K to 6-K2 was performed at −65 °C, since Mössbauer spectroscopy demonstrates that the spontaneous loss of thiolate from intermediate 4-K2 (forming 3-K) does not occur at low temperature within 40 minutes. Therefore, direct monothiolate loss from 4-K2 is not a kinetically competent step during the formation of 1-K2.
Figure 5.
Computationally optimized structure of a truncated model of the Fe(I) tris(thiolate) complex 42− (S = 3/2 model). Selected bond distances: Fe–C = 2.02, 2.21 Å, Fe–S = 2.32, 2.27, 2.31 Å.
In 6-[K(18-crown-6)]2, there is η6-binding of the arene which shifts to η2-binding in 1-[K(18-crown-6)]2. Changes in hapticity to a central arene have also been observed by Agapie and co-workers in structurally similar terphenyl diphosphine systems.58-59 This observation suggests that the binding of N2 competes with additional strong bonding to the arene of the ligand. Correspondingly, the N2 ligand in 1-K2, despite engaging in strong π-backbonding based on its low N-N stretching frequency (1880 cm−1), is very labile; the N-N stretching band in the infrared spectrum of 1-[K(18-crown-6)]2 begins to disappear upon warming the compound above −40 °C.43 Additionally, warming of solid 1-[K(18-crown-6)]2 to room temperature under vacuum results in partial formation of 6-[K(18-crown-6)]2, along with other decomposition products (Figure S15). The presence of π-backbonding interactions with both N2 and the central arene ring is also reflected by the requirement for reduction to the iron(0) level in order to observe N2 binding, in contrast to other systems where N2 can bind at the iron(II) or iron(I) redox level.26-27, 33
Further understanding of the bonding in 12− was obtained from DFT calculations. As reported previously, the DFT model reproduces the metrical parameters from the crystallographic structure of 1-[K(18-crown-6)]2 well.43 The DFT-predicted Mössbauer parameters are also in good agreement with experimental values (Table 1). In order to determine whether the N2 and/or the central arene act as redox non-innocent ligands, we examined the unrestricted corresponding orbitals (UCOs)60 of 12− at the B3LYP/def2-TZVP level of theory (Figure 7). The spatial overlaps between the α and β spin orbitals (Sαβ) are close to unity, indicating that neither the N2 nor the arene act as redox-active ligands, although there is some spin polarization. The DFT calculation also indicates π-backbonding interactions between the iron and both the N2 and the central arene, supporting the idea that binding of N2 competes with binding to the central arene of the supporting ligand.
Figure 7.
X-ray crystallographic structure of the anionic portion of Fe(0) bis(thiolate) 6-[K(18-crown-6)]2 with thermal ellipsoids shown at the 50% probability level. Hydrogen atoms are omitted and the triisopropylphenyl groups are shown in wireframe representation for clarity. Selected bond distances and angles: Fe–C = 2.083(2) − 2.194(2) Å; Fe–S = 2.4036(9), 2.4050(7) Å; S−Fe−S = 104.10(3)°.
Further Reduction and Ammonia Production.
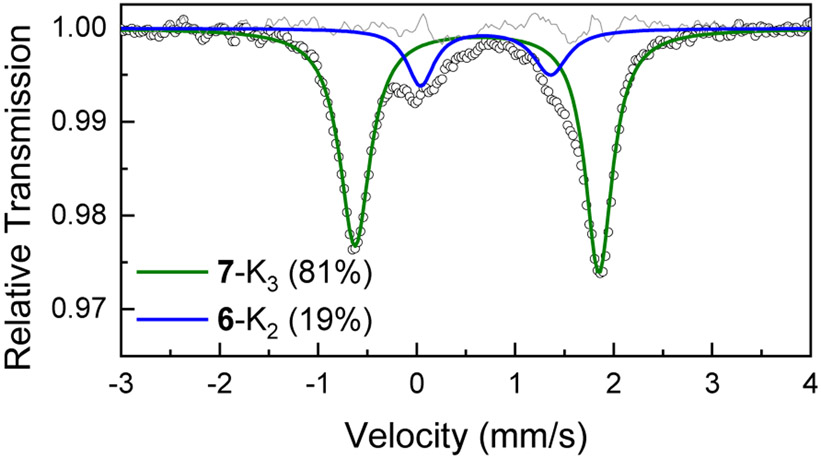
Mössbauer spectra of samples obtained after the treatment of 2-K with 3.6 equiv of KC8 at −65 °C for 40 min under an atmosphere of Ar are identical to those using 2.4 equiv of KC8 (Figure S16), indicating that no reduction beyond the Fe(0) level occurs without N2. However, under an atmosphere of N2, reduction of 2-K with 3.6 equiv of KC8 gives a new species (7-K3) as observed by Mossbauer spectroscopy (Figure 8). This indicates that the iron(0)-N2 complex 1-K2 can be reduced by an additional electron to give a formally iron(-I) species. We were unsuccessful in isolating 7-K3 due to its instability (see Figures S18 and S21), but the requirement for N2 during its generation indicates that it is also an Fe-N2 complex. Further reduction of 7-K3 was not feasible, even with excess KC8 (Figure S17).
Figure 8.
Unrestricted corresponding orbitals (UCOs) for 12−, as calculated at the B3LYP/def2-TZVP level. Sαβ indicates the spatial overlap between the α and β spin orbitals.
Treatment of the reaction mixtures containing 1-K2 with 20 equiv of a variety of proton sources gives 1% or less of the N2 reduction products ammonia (NH3) and hydrazine (N2H4). However, treatment of 7-K3 with the weak acids 2,6-di-tert-butyl-4-methylphenol (BHT) and H2O produces substoichiometric amounts of ammonia and hydrazine, respectively (Table 2 and Scheme 4). Treatment with concentrated H2SO4 also results in formation of a small amount of N2H4. These products were not detected when 7-K3 was warmed to room temperature (resulting in its decomposition) prior to acid addition, supporting the idea that 7-K3 itself, rather than some impurity in the system, mediates N2 reduction.
Table 2.
Yields of NH3 and N2H4 (per equivalent of iron complex) after treatment of crude reaction mixtures containing 1-K2 or 7-K3 with 20 equiv of acid. The yields given in bold are an average of multiple runs and the errors correspond to the range of observed values (see Table S2); all other entries are for a single run.
| 1-K2 | 7-K3 | |
|---|---|---|
| [H(OEt2)2][BArF4] | no NH3 no N2H4 |
no NH3 no N2H4 |
| 2,6-di-tert-butyl-4-methylphenol | 0.02 NH3 no N2H4 |
0.17 ± 0.06 NH3 no N2H4 |
| H2O | 0.01 NH3 no N2H4 |
0.02 NH3 0.26 ± 0.04 N2H4 |
| Monothiola | no NH3 no N2H4 |
no NH3 no N2H4 |
| H2SO4 | no NH3 0.01 N2H4 |
no NH3 0.06 N2H4 |
Monothiol = 2’,4’,6’-triisopropyl-[1,1’-biphenyl]-2-thiol
Scheme 4.
Protonation of N2 Complexes
Addition of the strong acid [H(OEt2)2][BArF4] (ArF = 3,5-bis(trifluoromethyl)phenyl) to crude reaction mixtures containing 1-K2 or 7-K3 results in bleaching of the deep red color characteristic of these species (Figure S2), which suggests decomposition of the iron complexes via protonation of the supporting ligand. Correspondingly, the 1H NMR spectrum of a hexanes extract of the crude reaction mixture indicates the presence of the bis(thiol) (Figure S3). No N2 reduction products were detected from the reactions of 1-K2 or 7-K3 with [H(OEt2)2][BArF4].
Attempts to perform catalytic N2 reduction by treating 2-K with 50 equiv of KC8 and 50 equiv of acid, under conditions similar to those utilized by Peters and others,34-40 were unsuccessful. Using 2-K as the prospective catalyst, NH3 is the only product detected, in a substoichiometric yield (0.13, 0.41, and 0.22 equiv of NH3 per Fe using [H(OEt2)2][BArF4], BHT, and H2O, respectively, as proton sources).
DISCUSSION
Mechanism of Fe-Based N2 Binding and Reduction.
We have identified very reactive iron(I) and iron(0) intermediates that lie on the route to N2 binding at a sulfur- and carbon-ligated iron(0) iron center. The iron(I) tris(thiolate) intermediate has a high-spin configuration, and DFT calculations suggest a pseudotetrahedral iron-sulfur-carbon environment reminiscent of the coordination environment in the resting state of the FeMoco. Further reduction to iron(0) results in Fe-S bond cleavage and N2 coordination. Combining the observations that (a) reduction of iron(II) tris(thiolate) 2-K to iron(I) tris(thiolate) 4-K2 takes place without loss of thiolate or coordination of N2 at −70 °C, (b) reduction of iron(I) tris(thiolate) 4-K2 under argon leads to spontaneous thiolate ejection at −65 °C to give the iron(0) complex 6-K2, and (c) 6-K2 binds N2 to give the iron(0)-N2 complex 1-K2, we propose the mechanism in Scheme 5 as the most reasonable pathway for the conversion of 2-K to 1-K2. Our observations do not rule out an alternative associative mechanism in which N2 binds prior to ejection of thiolate (bypassing 6-K2), but we favor the mechanism shown in Scheme 5 because all of the individual steps have been observed.
Scheme 5.
Proposed Mechanism for Formation of 12− Through Electronation and N2 Binding
Further reduction of 1-K2 results in formation of the highly unstable formally iron(-I) species 7-K3. Although rare, there have been several reports of iron-dinitrogen complexes at this redox level. Ung and Peters have described a cyclic alkyl amino carbene (CAAC) supported system in which an iron(0) can be reduced to iron(-I) under N2.36 In this case, the iron(-I)–N2 species was isolable and thus was characterized in more detail. Their analysis indicated substantial spin delocalization onto the CAAC ligand. More recently, Schild and Peters reported a phosphine-supported system where reduction gave formally iron(-I) or iron(-II) species that were more reactive at the N2 ligands.61 The structure of 7-K3 is not clear based on the limited spectroscopic characterization we were able to obtain. It is possible that the central arene (rather than the Fe-N2 unit) is reduced; it is also possible that one of the thiolate donors dissociates, as observed for highly reduced molybdenum complexes supported by a structurally similar terphenyl bis(phosphine) ligand.58-59, 62
Addition of proton sources to 7-K3 results in release of substoichiometric NH3 or N2H4. The distribution of N2-derived products depends on the acid used, with NH3 as the major product from treatment with BHT and N2H4 from treatment with H2O. However, the reasons for this reactivity difference are unclear because the yields of NH3 and N2H4 in these reactions are low. We note that BHT can act as a hydrogen atom donor as well as an acid, and a related phenol can donate electrons and protons to an N2-derived NH group.63 There have been several recent reports of using hydrogen-atom donors to functionalize transition-metal coordinated N2 ligands . These include classical H atom donors such as TEMPO-H64, as well as H atom donors generated in situ from samarium diiodide and alcohols65-66 or metallocenes and weak acids.40, 67-68 In reactions with 1-K2 and 7-K3, stronger acids such as [H(OEt2)2][BArF4] give rapid decomposition via ligand protonation, even at low temperature. It is likely that achieving catalytic N2 reduction with biomimetic sulfur-carbon supporting ligands will require the design of ligand systems that are less susceptible to acid-induced degradation.
Relevance to Nitrogenase.
As described in the Introduction, recent biochemical, spectroscopic, and crystallographic studies have implicated a S3CFe site as the N2 binding site on the FeMoco. The FeMoco is the only known case of a carbide being incorporated into an iron-sulfur cluster. In a very recent synthetic achievement, Rauchfuss and coworkers have isolated an iron-carbide-carbonyl cluster that incorporates a single sulfide ligand.69 However, no clusters are yet known that have the characteristic S3CFe or S2CFe coordination of the resting state and putative N2-binding state of the FeMoco. It is also important to note that replicating the exact primary coordination sphere of the FeMoco may not replicate its reactivity. For example, when extracted from nitrogenase the FeMoco can catalyze the reduction of alternative nitrogenase substrates (e.g. acetylene, CO2, CO, and CN−) but not N2.70-71
The only examples of well-defined iron complexes containing carbon donors that catalyze N2 reduction contain CAAC36 or tris(phosphino)alkyl35 ligands but do not also incorporate sulfur donors. Similarly, there are few examples of N2 reduction by any molecular iron complexes with ligands containing only S donors. An early report from Tanaka and co-workers indicated that controlled potential electrolysis of [Fe4S4(SPh)4]2− or [Mo2Fe6S8(SPh)9]3− under N2 atmosphere results in the formation of NH3 with low Faradaic yield.72 More recently, photochemical reduction of N2 to NH3 by chalcogels containing MoFeS clusters was reported.73-74 Ohki and co-workers have also demonstrated catalytic silylation of N2 by [Mo2Fe2] hydride clusters with monothiolate ligands.75 However, the exact identities of the active species in all of these systems are unknown, and the key Fe-N2 complexes were not isolable.
To the best of our knowledge, the results described here represent the first example of NH3 and/or N2H4 production from a well-defined synthetic Fe-N2 complex with a supporting ligand containing only sulfur and carbon donors. Though the yields are low, control experiments show that N2 is the source of these reduced products.
The complexes described here have an S3C ligand environment, but the sulfur donors are thiolates rather than sulfides, and the carbon donor is an arene rather than a carbide. Ligand K-edge X-ray absorption studies indicate that iron-sulfide bonds are generally more covalent than iron-thiolate bonds.76-77 The iron-carbide bond in the FeMoco is also thought to be highly covalent.78-80 Since neither the electronic structure of the FeMoco nor the role of the carbide in catalysis are well-understood,81-83 it is difficult to evaluate the similarity of our model to the FeMoco. There are also other important differences: for example, the model complexes presented here have a single iron atom, while the FeMoco is a multimetallic cluster. In addition, the terphenyl supporting ligand has π-backbonding capacity that is not present in the FeMoco. Furthermore, secondary sphere effects, which are not present in the model system, could play a role in the FeMoco for N2 binding and activation or stabilize important intermediates through hydrogen bonding. Nevertheless, our results represent the first step towards the development of synthetic iron complexes containing only S and C donors capable of N2 binding and functionalization.
Another relevant comparison is to the oxidation states in the FeMoco. Hoffman has argued that only two oxidation levels are accessed in the FeMoco, with additional reducing equivalents stored on hydrides.1 Thus, the intermediates (like the resting state) likely have formally iron(II) and iron(III) ions,81-83 whereas the complexes described here bind N2 only upon reduction to iron(0). If the iron(0) oxidation level is feasible in the FeMoco, it would only be transiently available (perhaps from reductive elimination of H2 from an iron site, which is formally a two-electron reduction of the metal). N2 binding is relatively weak in the iron(0) complex 1-K2, as shown by its loss of N2 at room temperature. Both the lability of N2 and the requirement for reduction to the iron(0) redox level in the synthetic system may be due to the competition between backbonding to N2 and backbonding to the internal arene of the supporting ligand (supported by DFT calculations above), rather than any deficiency in the electron-richness of the iron complex in this ligand field. Scaffolds that present a biomimetic C donor without π-backbonding capability, for example using alkyl donors,35, 46 may be more effective for N2 binding and reduction.
CONCLUSIONS
We have examined the mechanism of N2 binding to the iron(II) tris(thiolate) complex 2-K. Reduction to the iron(0) level results in monothiolate dissociation, which produces a complex that is competent for N2 binding. These results demonstrate the feasibility of dissociation of RS− leading to a low-coordinate sulfur-carbon iron site that binds N2, which has been proposed for the FeMoco. Further reduction of the N2 complex to the formally iron(-I) redox level gives a species that reacts with acids to give NH3 or N2H4 in low yield, showing that it is possible to bind and reduce N2 at iron without incorporating abiological donor atoms. The challenges associated with this system include the presence of a π-accepting arene in the ligand backbone, which results in weak N2 binding, and the high acid lability of the ligand, which results in decomposition under catalytic conditions for N2 reduction. We are currently designing new multidentate sulfur/carbon ligands in an effort to create more suitable functional models for the FeMoco.
Supplementary Material
Figure 9.
Mössbauer spectrum of a frozen reaction mixture of 2-K treated with 3.6 equiv of KC8 in THF under N2. The black circles are the experimental data, and the grey line is the residual for the total fit. The green and blue lines are fits corresponding to 7-K3 and 6-K2, respectively, with the parameters shown in Table 1.
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health (R01-GM065313 to P.L.H. and F32-GM123658 to A.L.S.). We thank David Vinyard (Yale University, Brudvig group), Avery Vilbert (Cornell University, Lancaster group), and Edward Badding (Massachusetts Institute of Technology, Suess Group) for assistance measuring EPR spectra. C.V.S. and S.D. would like thank Stefan Hugenbruch for assistance in the collection of XAS data, and the Max-Planck Society for funding. S.D. acknowledges funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC Grant Agreement No. 615414 and the DFG SPP 1927 “Iron Sulfur for Life” project (Project No. DE 1877/1–1). C.V.S. additionally thanks the IMPRS-RECHARGE for funding.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website. The crystallographic information (CIF format) has been deposited at the CCDC with deposition number 1916106.
The authors declare no competing financial interests.
REFERENCES
- 1.Hoffman BM; Lukoyanov D; Yang Z-Y; Dean DR; Seefeldt LC Mechanism of Nitrogen Fixation by Nitrogenase:The Next Stage. Chem. Rev 2014, 114, 4041–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eady RR Structure–Function Relationships of Alternative Nitrogenases. Chem. Rev 1996, 96, 3013–3030. [DOI] [PubMed] [Google Scholar]
- 3.Einsle O; Tezcan FA; Andrade SLA; Schmid B; Yoshida M; Howard JB; Rees DC Nitrogenase MoFe-Protein at 1.16 Å Resolution: A Central Ligand in the FeMo-Cofactor. Science 2002, 297, 1696–1700. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster KM; Roemelt M; Ettenhuber P; Hu Y; Ribbe MW; Neese F; Bergmann U; DeBeer S X-ray Emission Spectroscopy Evidences a Central Carbon in the Nitrogenase Iron-Molybdenum Cofactor. Science 2011, 334, 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spatzal T; Aksoyoglu M; Zhang L; Andrade SLA; Schleicher E; Weber S; Rees DC; Einsle O Evidence for Interstitial Carbon in Nitrogenase FeMo Cofactor. Science 2011, 334, 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos PC; Igarashi RY; Lee H-I; Hoffman BM; Seefeldt LC; Dean DR Substrate Interactions with the Nitrogenase Active Site. Acc. Chem. Res 2005, 38, 208–214. [DOI] [PubMed] [Google Scholar]
- 7.George SJ; Barney BM; Mitra D; Igarashi RY; Guo Y; Dean DR; Cramer SP; Seefeldt LC EXAFS and NRVS Reveal a Conformational Distortion of the FeMo-cofactor in the MoFe Nitrogenase Propargyl Alcohol Complex. J. Inorg. Biochem 2012, 112, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton PMC; Laryukhin M; Mayer SM; Hoffman BM; Dean DR; Seefeldt LC Localization of a Substrate Binding Site on the FeMo-Cofactor in Nitrogenase: Trapping Propargyl Alcohol with an α-70-Substituted MoFe Protein. Biochemistry 2003, 42, 9102–9109. [DOI] [PubMed] [Google Scholar]
- 9.Lee H-I; Igarashi RY; Laryukhin M; Doan PE; Dos Santos PC; Dean DR; Seefeldt LC; Hoffman BM An Organometallic Intermediate during Alkyne Reduction by Nitrogenase. J. Am. Chem. Soc 2004, 126, 9563–9569. [DOI] [PubMed] [Google Scholar]
- 10.Barney BM; Igarashi RY; Dos Santos PC; Dean DR; Seefeldt LC Substrate Interaction at an Iron-Sulfur Face of the FeMo-cofactor during Nitrogenase Catalysis. J. Biol. Chem 2004, 279, 53621–53624. [DOI] [PubMed] [Google Scholar]
- 11.Lee H-I; Hales BJ; Hoffman BM Metal-Ion Valencies of the FeMo Cofactor in CO-Inhibited and Resting State Nitrogenase by 57Fe Q-Band ENDOR. J. Am. Chem. Soc 1997, 119, 11395–11400. [Google Scholar]
- 12.Spatzal T; Perez KA; Einsle O; Howard JB; Rees DC Ligand binding to the FeMo-cofactor: Structures of CO-bound and reactivated nitrogenase. Science 2014, 345, 1620–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spatzal T; Perez KA; Howard JB; Rees DC Catalysis-dependent selenium incorporation and migration in the nitrogenase active site iron-molybdenum cofactor. eLife 2015, 4, e11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seefeldt LC; Hoffman BM; Dean DR Mechanism of Mo-Dependent Nitrogenase. Annu. Rev. Biochem 2009, 78, 701–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benediktsson B; Thorhallsson AT; Bjornsson R QM/MM calculations reveal a bridging hydroxo group in a vanadium nitrogenase crystal structure. Chem. Commun 2018, 54, 7310–7313. [DOI] [PubMed] [Google Scholar]
- 16.Skubi KL; Holland PL So Close, yet Sulfur Away: Opening the Nitrogenase Cofactor Structure Creates a Binding Site. Biochemistry 2018, 57, 3540–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sippel D; Rohde M; Netzer J; Trncik C; Gies J; Grunau K; Djurdjevic I; Decamps L; Andrade SLA; Einsle O A bound reaction intermediate sheds light on the mechanism of nitrogenase. Science 2018, 359, 1484–1489. [DOI] [PubMed] [Google Scholar]
- 18.Hallmen PP; Kästner J N2 Binding to the FeMo-Cofactor of Nitrogenase. Z. Anorg. Allg. Chem 2015, 641, 118–122. [Google Scholar]
- 19.Schimpl J; Petrilli HM; Blöchl PE Nitrogen Binding to the FeMo-Cofactor of Nitrogenase. J. Am. Chem. Soc 2003, 125, 15772–15778. [DOI] [PubMed] [Google Scholar]
- 20.Raugei S; Seefeldt LC; Hoffman BM Critical computational analysis illuminates the reductive-elimination mechanism that activates nitrogenase for N2 reduction. Proc. Natl. Acad. Sci. USA 2018, 115, E10521–E10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dance I How feasible is the reversible S-dissociation mechanism for the activation of FeMo-co, the catalytic site of nitrogenase? Dalton Trans. 2019, 48, 1251–1262. [DOI] [PubMed] [Google Scholar]
- 22.Varley JB; Wang Y; Chan K; Studt F; Norskov JK Mechanistic insights into nitrogen fixation by nitrogenase enzymes. Phys. Chem. Chem. Phys 2015, 17, 29541–29547. [DOI] [PubMed] [Google Scholar]
- 23.Kästner J; Blöchl PE Ammonia Production at the FeMo Cofactor of Nitrogenase: Results from Density Functional Theory. J. Am. Chem. Soc 2007, 129, 2998–3006. [DOI] [PubMed] [Google Scholar]
- 24.Holland PL Low-coordinate iron complexes as synthetic models of nitrogenase. Can. J. Chem 2005, 83, 296–301. [Google Scholar]
- 25.MacKay BA; Fryzuk MD Dinitrogen Coordination Chemistry: On the Biomimetic Borderlands. Chem. Rev 2004, 104, 385–402. [DOI] [PubMed] [Google Scholar]
- 26.Crossland JL; Tyler DR Iron–dinitrogen coordination chemistry: Dinitrogen activation and reactivity. Coord. Chem. Rev 2010, 254, 1883–1894. [Google Scholar]
- 27.Hazari N Homogeneous iron complexes for the conversion of dinitrogen into ammonia and hydrazine. Chem. Soc. Rev 2010, 39, 4044–4056. [DOI] [PubMed] [Google Scholar]
- 28.MacLeod KC; Holland PL Recent developments in the homogeneous reduction of dinitrogen by molybdenum and iron. Nature Chem. 2013, 5, 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoenkhoen N; de Bruin B; Reek JNH; Dzik WI Reactivity of Dinitrogen Bound to Mid- and Late-Transition-Metal Centers. Eur. J. Inorg. Chem 2015, 2015, 567–598. [Google Scholar]
- 30.Köthe C; Limberg C Late Metal Scaffolds that Activate Both, Dinitrogen and Reduced Dinitrogen Species NxHy. Z. Anorg. Allg. Chem 2015, 641, 18–30. [Google Scholar]
- 31.Nishibayashi Y Recent Progress in Transition-Metal-Catalyzed Reduction of Molecular Dinitrogen under Ambient Reaction Conditions. Inorg. Chem 2015, 54, 9234–9247. [DOI] [PubMed] [Google Scholar]
- 32.Čorić I; Holland PL Insight into the Iron–Molybdenum Cofactor of Nitrogenase from Synthetic Iron Complexes with Sulfur, Carbon, and Hydride Ligands. J. Am. Chem. Soc 2016, 138, 7200–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piascik AD; Ashley AE, Group 8 Transition Metal–Dinitrogen Complexes In Transition Metal–Dinitrogen Complexes: Preparation and Reactivity, Nishibayashi Y, Ed. Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2019; pp 285–335. [Google Scholar]
- 34.Anderson JS; Rittle J; Peters JC Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 2013, 501, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Creutz SE; Peters JC Catalytic Reduction of N2 to NH3 by an Fe–N2 Complex Featuring a C-Atom Anchor. J. Am. Chem. Soc 2014, 136, 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ung G; Peters JC Low-Temperature N2 Binding to Two-Coordinate L2Fe0 Enables Reductive Trapping of L2FeN2− and NH3 Generation. Angew. Chem. Int. Ed 2015, 54, 532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Castillo TJ; Thompson NB; Peters JC A Synthetic Single-Site Fe Nitrogenase: High Turnover, Freeze-Quench 57Fe Mössbauer Data, and a Hydride Resting State. J. Am. Chem. Soc 2016, 138, 5341–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuriyama S; Arashiba K; Nakajima K; Matsuo Y; Tanaka H; Ishii K; Yoshizawa K; Nishibayashi Y Catalytic transformation of dinitrogen into ammonia and hydrazine by iron-dinitrogen complexes bearing pincer ligand. Nat. Commun 2016, 7, 12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill PJ; Doyle LR; Crawford AD; Myers WK; Ashley AE Selective Catalytic Reduction of N2 to N2H4 by a Simple Fe Complex. J. Am. Chem. Soc 2016, 138, 13521–13524. [DOI] [PubMed] [Google Scholar]
- 40.Chalkley MJ; Del Castillo TJ; Matson BD; Roddy JP; Peters JC Catalytic N2-to-NH3 Conversion by Fe at Lower Driving Force: A Proposed Role for Metallocene-Mediated PCET. ACS Cent. Sci 2017, 3, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bart SC; Lobkovsky E; Bill E; Wieghardt K; Chirik PJ Neutral-Ligand Complexes of Bis(imino)pyridine Iron: Synthesis, Structure, and Spectroscopy. Inorg. Chem 2007, 46, 7055–7063. [DOI] [PubMed] [Google Scholar]
- 42.Takaoka A; Mankad NP; Peters JC Dinitrogen Complexes of Sulfur-Ligated Iron. J. Am. Chem. Soc 2011, 133, 8440–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Čorić I; Mercado BQ; Bill E; Vinyard DJ; Holland PL Binding of dinitrogen to an iron-sulfur-carbon site. Nature 2015, 526, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Creutz SE; Peters JC Diiron Bridged-Thiolate Complexes That Bind N2 at the FeIIFeII, FeIIFeI, and FeIFeI Redox States. J. Am. Chem. Soc 2015, 137, 7310–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu NX; Oyala PH; Peters JC An S = 1/2 Iron Complex Featuring N2, Thiolate, and Hydride Ligands: Reductive Elimination of H2 and Relevant Thermochemical Fe–H Parameters. J. Am. Chem. Soc 2018, 140, 6374–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rittle J; Peters JC Fe–N2/CO complexes that model a possible role for the interstitial C atom of FeMo-cofactor (FeMoco). Proc. Natl. Acad. Sci. USA 2013, 110, 15898–15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang Z; Cheng J; Li L; Bao X; Deng L High-Spin Iron(I) and Iron(0) Dinitrogen Complexes Supported by N-Heterocyclic Carbene Ligands. Chem. Eur. J 2016, 22, 14162–14165. [DOI] [PubMed] [Google Scholar]
- 48.Danopoulos AA; Wright JA; Motherwell WB Molecular N2 complexes of iron stabilised by N-heterocyclic ‘pincer’ dicarbene ligands. Chem. Commun 2005, 784–786. [DOI] [PubMed] [Google Scholar]
- 49.Reiners M; Baabe D; Zaretzke M-K; Freytag M; Walter MD Reversible dinitrogen binding to [Cp′Fe(NHC)] associated with an N2-induced spin state change. Chem. Commun 2017, 53, 7274–7277. [DOI] [PubMed] [Google Scholar]
- 50.Lindley BM; Jacobs BP; MacMillan SN; Wolczanski PT Neutral Fe(IV) alkylidenes, including some that bind dinitrogen. Chem. Commun 2016, 52, 3891–3894. [DOI] [PubMed] [Google Scholar]
- 51.Hickey AK; Muñoz SB; Lutz SA; Pink M; Chen C-H; Smith JM Arrested α-hydride migration activates a phosphido ligand for C–H insertion. Chem. Commun 2017, 53, 412–415. [DOI] [PubMed] [Google Scholar]
- 52.We note that EPR spectroscopy is much more sensitive to small perturbations in zero-field splitting than Mössbauer spectroscopy. For example, Yousif et. al. recently reported an iron tetrazene complex which is a single species by Mössbauer spectroscopy but has a distributed E/D based on high-field EPR.54 We earlier reported an iron β-diketiminate system in which the spin Hamiltonian parameters are highly sensitive to minute structural changes.55 This phenomenon has also been observed in cobalt tris(phosphine) complexes56 and chromium tetra(siloxide) complexes.57.
- 53.Yousif M; Wannipurage D; Huizenga CD; Washnock-Schmid E; Peraino NJ; Ozarowski A; Stoian SA; Lord RL; Groysman S Catalytic Nitrene Homocoupling by an Iron(II) Bis(alkoxide) Complex: Bulking Up the Alkoxide Enables a Wider Range of Substrates and Provides Insight into the Reaction Mechanism. Inorg. Chem 2018, 57, 9425–9438. [DOI] [PubMed] [Google Scholar]
- 54.Stoian SA; Smith JM; Holland PL; Münck E; Bominaar EL Mössbauer, Electron Paramagnetic Resonance, and Theoretical Study of a High-Spin, Four-Coordinate Fe(II) Diketiminate Complex. Inorg. Chem 2008, 47, 8687–8695. [DOI] [PubMed] [Google Scholar]
- 55.Krzystek J; Ozarowski A; Zvyagin SA; Telser J High Spin Co(I): High-Frequency and -Field EPR Spectroscopy of CoX(PPh3)3 (X = Cl, Br). Inorg. Chem 2012, 51, 4954–4964. [DOI] [PubMed] [Google Scholar]
- 56.Bucinsky L; Breza M; Malček M; Powers DC; Hwang SJ; Krzystek J; Nocera DG; Telser J High-Frequency and -Field EPR (HFEPR) Investigation of a Pseudotetrahedral CrIV Siloxide Complex and Computational Studies of Related CrIVL4 Systems. Inorg. Chem 2019, 58, 4907–4920. [DOI] [PubMed] [Google Scholar]
- 57.McWilliams SF; Brennan-Wydra E; MacLeod KC; Holland PL Density Functional Calculations for Prediction of 57Fe Mössbauer Isomer Shifts and Quadrupole Splittings in β-Diketiminate Complexes. ACS Omega 2017, 2, 2594–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buss JA; Agapie T Four-electron deoxygenative reductive coupling of carbon monoxide at a single metal site. Nature 2015, 529, 72–75. [DOI] [PubMed] [Google Scholar]
- 59.Buss JA; Oyala PH; Agapie T Terminal Molybdenum Phosphides with d Electrons: Radical Character Promotes Coupling Chemistry. Angew. Chem. Int. Ed 2017, 56, 14502–14506. [DOI] [PubMed] [Google Scholar]
- 60.Neese F Definition of corresponding orbitals and the diradical character in broken symmetry DFT calculations on spin coupled systems. J. Phys. Chem. Solids 2004, 65, 781–785. [Google Scholar]
- 61.Schild DJ; Peters JC Light Enhanced Fe-Mediated Nitrogen Fixation: Mechanistic Insights Regarding H2 Elimination, HER, and NH3 Generation. ACS Catal. 2019, 9, 4286–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horak KT; Velian A; Day MW; Agapie T Arene non-innocence in dinuclear complexes of Fe, Co, and Ni supported by a para-terphenyl diphosphine. Chem. Commun 2014, 50, 4427–4429. [DOI] [PubMed] [Google Scholar]
- 63.MacLeod KC; McWilliams SF; Mercado BQ; Holland PL Stepwise N–H bond formation from N2-derived iron nitride, imide and amide intermediates to ammonia. Chem. Sci 2016, 7, 5736–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kendall AJ; Johnson SI; Bullock RM; Mock MT Catalytic Silylation of N2 and Synthesis of NH3 and N2H4 by Net Hydrogen Atom Transfer Reactions Using a Chromium P4 Macrocycle. J. Am. Chem. Soc 2018, 140, 2528–2536. [DOI] [PubMed] [Google Scholar]
- 65.Ashida Y; Arashiba K; Nakajima K; Nishibayashi Y Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 2019, 568, 536–540. [DOI] [PubMed] [Google Scholar]
- 66.Ashida Y; Arashiba K; Tanaka H; Egi A; Nakajima K; Yoshizawa K; Nishibayashi Y Molybdenum-Catalyzed Ammonia Formation Using Simple Monodentate and Bidentate Phosphines as Auxiliary Ligands. Inorg. Chem 2019, 58, 8927–8932. [DOI] [PubMed] [Google Scholar]
- 67.Chalkley MJ; Del Castillo TJ; Matson BD; Peters JC Fe-Mediated Nitrogen Fixation with a Metallocene Mediator: Exploring pKa Effects and Demonstrating Electrocatalysis. J. Am. Chem. Soc 2018, 140, 6122–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chalkley MJ; Oyala PH; Peters JC Cp* Noninnocence Leads to a Remarkably Weak C–H Bond via Metallocene Protonation. J. Am. Chem. Soc 2019, 141, 4721–4729. [DOI] [PubMed] [Google Scholar]
- 69.Liu L; Rauchfuss TB; Woods TJ Iron Carbide–Sulfide Carbonyl Clusters. Inorg. Chem 2019, 58, 8271–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson RA Mechanistic Studies on Synthetic Fe−S-Based Clusters and Their Relevance to the Action of Nitrogenases. Chem. Rev 2005, 105, 2365–2438. [DOI] [PubMed] [Google Scholar]
- 71.Lee CC; Hu Y; Ribbe MW Catalytic Reduction of CN−, CO, and CO2 by Nitrogenase Cofactors in Lanthanide-Driven Reactions. Angew. Chem. Int. Ed 2015, 54, 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka K; Hozumi Y; Tanaka T Dinitrogen Fixation Catalyzed by the Reduced Species of [Fe4S4(SPh)4]2− and [Mo2Fe6S8(SPh)9]3−. Chem. Lett 1982, 11, 1203–1206. [Google Scholar]
- 73.Banerjee A; Yuhas BD; Margulies EA; Zhang Y; Shim Y; Wasielewski MR; Kanatzidis MG Photochemical Nitrogen Conversion to Ammonia in Ambient Conditions with FeMoS-Chalcogels. J. Am. Chem. Soc 2015, 137, 2030–2034. [DOI] [PubMed] [Google Scholar]
- 74.Liu J; Kelley MS; Wu W; Banerjee A; Douvalis AP; Wu J; Zhang Y; Schatz GC; Kanatzidis MG Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc. Natl. Acad. Sci. USA 2016, 113, 5530–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohki Y; Araki Y; Tada M; Sakai Y Synthesis and Characterization of Bioinspired [Mo2Fe2]–Hydride Cluster Complexes and Their Application in the Catalytic Silylation of N2. Chem. Eur. J 2017, 23, 13240–13248. [DOI] [PubMed] [Google Scholar]
- 76.Rose K; Shadle SE; Glaser T; de Vries S; Cherepanov A; Canters GW; Hedman B; Hodgson KO; Solomon EI Investigation of the Electronic Structure of 2Fe−2S Model Complexes and the Rieske Protein Using Ligand K-Edge X-ray Absorption Spectroscopy. J. Am. Chem. Soc 1999, 121, 2353–2363. [Google Scholar]
- 77.Glaser T; Hedman B; Hodgson KO; Solomon EI Ligand K-Edge X-ray Absorption Spectroscopy: A Direct Probe of Ligand−Metal Covalency. Acc. Chem. Res 2000, 33, 859–868. [DOI] [PubMed] [Google Scholar]
- 78.Pollock CJ; Tan LL; Zhang W; Lancaster KM; Lee SC; DeBeer S Light-Atom Influences on the Electronic Structures of Iron–Sulfur Clusters. Inorg. Chem 2014, 53, 2591–2597. [DOI] [PubMed] [Google Scholar]
- 79.Rees JA; Bjornsson R; Kowalska JK; Lima FA; Schlesier J; Sippel D; Weyhermüller T; Einsle O; Kovacs JA; DeBeer S Comparative electronic structures of nitrogenase FeMoco and FeVco. Dalton Trans. 2017, 46, 2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grunenberg J The Interstitial Carbon of the Nitrogenase FeMo Cofactor is Far Better Stabilized than Previously Assumed. Angew. Chem. Int. Ed 2017, 56, 7288–7291. [DOI] [PubMed] [Google Scholar]
- 81.Bjornsson R; Lima FA; Spatzal T; Weyhermüller T; Glatzel P; Bill E; Einsle O; Neese F; DeBeer S Identification of a spin-coupled Mo(iii) in the nitrogenase iron–molybdenum cofactor. Chem. Sci 2014, 5, 3096–3103. [Google Scholar]
- 82.Spatzal T; Schlesier J; Burger E-M; Sippel D; Zhang L; Andrade SLA; Rees DC; Einsle O Nitrogenase FeMoco investigated by spatially resolved anomalous dispersion refinement. Nat. Commun 2016, 7, 10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bjornsson R; Neese F; DeBeer S Revisiting the Mössbauer Isomer Shifts of the FeMoco Cluster of Nitrogenase and the Cofactor Charge. Inorg. Chem 2017, 56, 1470–1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.