Highlights
-
•
Manuscript Highlights.
-
•
HFpEF is associated with reduced ATP production in the myocardium.
-
•
Ubiquinol and d-ribose both contribute to the generation of myocardial ATP.
-
•
Both ubiquinol and d-ribose are being studied as supplemental treatments for patients with HFpEF.
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) is a complex syndrome in which the patient has a normal or near normal left ventricular ejection fraction. This type of heart disease has a high rate of morbidity and mortality [1,2], and patients with HFpEF are often misdiagnosed because they describe symptoms such as dyspnea on exertion that are non-specific. Thus, health care providers have difficulty distinguishing HFpEF from heart failure with reduced ejection fraction (HFrEF) on a clinical basis alone [3]. The optimal treatment for patients with HFpEF should be related to the underlying biologic mechanisms of the syndrome [4]; however, there is an incomplete understanding of the pathophysiology of HFpEF. Researchers propose that one mechanism for the development of HFpEF is reduced myocardial bioenergetics [5].
Approximately 6.5 million Americans have heart failure, and the prevalence is projected to increase by 8 million by 2030. About 50% of heart failure patients have HFpEF [6]. With this clinical syndrome there is an increased risk of organ dysfunction and other diseases such as hypertension and diabetes mellitus. HFpEF is also more prevalent in women and the elderly [7]. Despite various pharmacological treatments, the incidence of HFpEF is rising. While the patient's symptoms are treated [8], the underlying mechanisms of the syndrome are under investigation. Mitochondrial dysfunction has been identified as the primary factor contributing to the development of HFpEF. In many clinical trials, researchers are now investigating agents that target improving mitochondrial function as a treatment strategy for HFpEF [5,9].
The American College of Cardiology (ACC)/American Heart Association (AHA)/HFSA (Heart Failure Society of American) guidelines have defined HFpEF signs and symptoms. The major symptoms are dyspnea, fatigue, and chest pain that often limit a patient's mobility and activities. With HFpEF, patients have a left ventricular ejection fraction (LVEF) ≥ 50% and evidence of cardiac dysfunction (e.g., increased atrial and ventricular stiffness and elevated filling pressures). The patients often exhibit elevated jugular venous pressure and pulmonary and lower extremity edema [10]. Patients with HFpEF have common exacerbations that cause elevated LV diastolic pressures [11]. Depending upon the age, gender, and ejection fraction, many of these patients experience atrial fibrillation (AF), which can further limit their LV filling and stroke volume [12]. Many patients also have elevated blood pressures due to arterial stiffening [13] and increased myocardial stiffness [14]. This can impair myocardial relaxation and lead to pulmonary hypertension [15].
There is emerging data regarding the benefits of both ubiquinol and d-ribose in the management of HF [[16], [17], [18], [19]]. These substances may have beneficial properties impacting improvement in mitochondrial function. Due to the challenges in the management of HFpEF and the limited therapeutic options, further investigation of these promising agents is warranted. Our research team is presently undertaking a clinical trial of these supplements in HFpEF subjects (NCT03133793) [9]. Our study is a randomized, double-blinded, placebo controlled, parallel arm protocol utilizing these agents with the outcomes being change in health status defined by the Kansas City Cardiomyopathy Questionnaire and change in mitochondrial ATP production.
Both ubiquinol and d-ribose are over-the-counter supplements that may improve mitochondrial bioenergetics [20]. In our paper, we will review the role of mitochondrial metabolism as it may relate to HFpEF pathophysiology and the potential mechanisms by which ubiquinol and d-ribose may impact mitochondrial function. We will also discuss the present status of the clinical management of HFpEF.
2. Mitochondria function
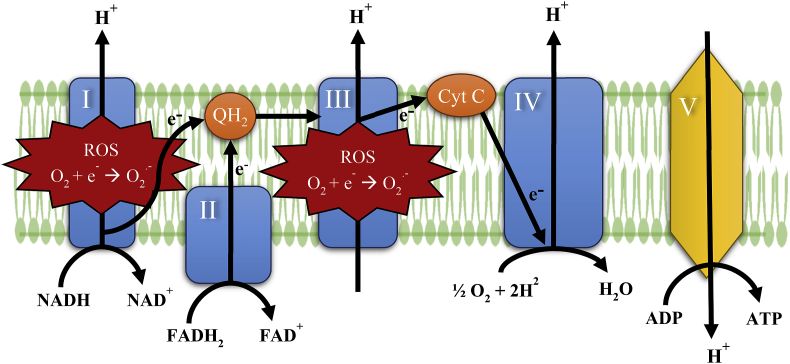
Mitochondria have several functions, the main one being production of ATP by a sequence of biochemical reactions that form the basis of aerobic cellular respiration [21]. Mitochondria are intracellular organelles enclosed by two membranes, and they contain their own DNA (mtDNA) in order to synthesize proteins to carry out their role in bioenergetics [22]. Some of the proteins produced contribute to the formation of the mitochondria's electron transport chain (ETC) complexes that are located within the cristae of the inner membrane [23]. The close proximity and the increased surface area of the cristae allows the protein complexes of the ETC to function and maximize energy production [24]. Reducing agents NADH and FADH2 that are generated from glycolysis and the tricarboxylic acid cycle transport electrons to complexes I (NADH, ubiquinone oxidoreductase) and II (succinate dehydrogenase) [25,26]. Ubiquinol, the reduced form of CoQ10, transfers electrons from complexes I and II to complex III (ubiquinol-cytochrome c oxidoreductase). Complex III transfers electrons to complex IV (cytochrome c oxidase) where oxygen accepts the electrons and reacts with hydrogen to form water [23]. The movement of electrons through complexes I, II, and IV enables hydrogen ions to be pumped out of the matrix to the inner membrane space. The pumping of hydrogen generates a temporary electrochemical gradient to power complex V (ATP synthase) in the oxidative phosphorylation of ADP to ATP (Fig. 1) [23,27].
Fig. 1.
Mitochondrial ETC – electrons are transferred through complexes I–IV with the final electron acceptor being oxygen. Complexes I and III generate the majority of ROS.
The ETC is known for producing reactive oxygen species (ROS) and is hypothesized to be a major cause of diseases associated with mitochondrial dysfunction and aging [22]. Oxidative stress can lead to low levels of CoQ10 and high levels of ROS. Deficiencies in CoQ10 and elevated ROS production have been observed in patients with heart failure [21,28]. Complexes I and III generate the majority of the ROS (Fig. 1) that include superoxide and hydrogen peroxide formed by electron and hydrogen leaking [22,29]. The ability of CoQ10 to be oxidized and reduced allows it to function as an antioxidant to stabilize ROS [21]. The myocardium contains an abundance of mitochondria in order to produce the energy needed to function. Damage to the myocardiocytes may led to low CoQ10 levels and high ROS levels that contribute to a decrease in ATP production [30]. Supplementation of ubiquinol could improve the disease progression of heart failure by increasing depleted levels of CoQ10, thus decreasing ROS levels and increasing ATP [30,31]. Further research trials are needed to study ubiquinol as a potential treatment for heart failure.
The pathophysiology of HFpEF is complex and heterogeneous with “phenotypes” identified based on the predominant symptoms and comorbidities [32]. A signature feature of HFpEF is the presence of a metabolic syndrome manifested by impaired mitochondrial respiration, oxidative stress with associated inflammation, decreased mitochondrial calcium, and depressed oxygen supply. This diminished oxygen availability directly reduces ATP synthesis, with consequent energy impairment [5,33]. In patients with HFpEF, several factors contribute to mitochondrial dysfunction: (1) increased sympathetic tone with associated hypertension [5]; (2) oxidative stress with associated inflammation [34]; (3) pro-inflammatory cytokines with microvascular dysfunction [35]; and (5) a buildup of lipids in the myocardium due to diabetes [36]. Present strategies for altering mitochondrial function include stimulation of mitochondrial biogenesis, reduction of oxidative stress, and several mitochondrial-targeted therapies including nitrites, resveratrol, ubiquinol, and d-ribose [9,37].
3. Ubiquinone and ubiquinol
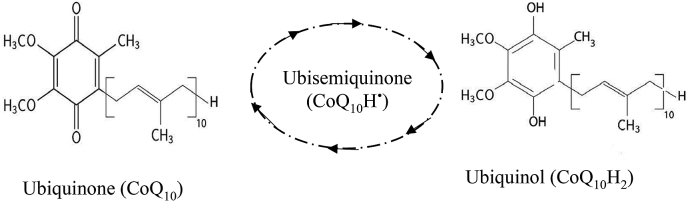
In the US, there is often confusion about these over-the-counter supplements. A bottle labeled CoQ10 is most likely the oxidized form of CoQ10 (ubiquinone) and is produced by different manufacturers that may vary in quality and purity. The reduced form of CoQ10 (ubiquinol) is labeled with different brand names and is mainly manufactured by the Japanese corporation called Kaneka [38]. While both forms are commonly used dietary supplements that have antioxidant properties and the ability to produce ATP, ubiquinol is more readily absorbed in the body [21]. There is no established ideal dosage of CoQ10; in the literature daily dosage widely varies from 50 mg to 1000 mg [39]. Oral CoQ10 is well tolerated and safe with few drug interactions. The most common side effects are gastrointestinal symptoms such as appetite suppression, nausea, vomiting, and abdominal discomfort. CoQ10 is a small lipophilic molecule that is an essential component in the production of cellular energy (ATP) in mitochondria. There are reduction-oxidation states of CoQ10: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol) (Fig. 2). The different forms of this molecule are fundamental to ETC and to the function of free radical scavenging. Ubiquinol has more electrons to donate for free radicals and thus is a much better antioxidant than ubiquinone. With aging, the mitochondrial function is reduced, and the ability to convert ubiquinone to ubiquinol decreases. With this reduction, the antioxidant properties and ATP production declines and the cells are more susceptible to age-related conditions such as heart failure [40].
Fig. 2.
CoQ10 has 3 Redox states: Ubiquinone, the completely oxidized form, a partially reduced intermediate (ubisemiquinone), and ubiquinol, the completely reduced form.
Ubiquinone and ubiquinol used as supplements are available in single capsule doses of 30, 50, 60, 100, 200, and 300 mg. Although there is no established minimum or maximum effective dose for these supplements, approximately 400 mg is needed per day to attain a therapeutic blood level of at least 2.5 mcg/ml [21]. Most studies related to cardiac disease use daily doses from 50 to 600 mg from oral supplementation with varying results. A reduction in CoQ10 production and subsequent mitochondrial dysfunction from the use of statins may result in statin-associated muscle symptoms (SAMS), and CoQ10 supplementation has been found to decrease SAMS in clinical trials [21]. Future clinical trials evaluating the use of CoQ10 may want to consider analyzing subjects on statin therapy separately from the study population due to mitochondrial dysfunction caused by statin use. Meta-analysis of CoQ10 supplementation in heart failure patients by Fotino et al. shows that CoQ10 has been associated with increases in ejection fraction and improved New York Heart Association symptom classification [41]. Mochamad et al. found patients with HFpEF who consumed 100 mg of ubiquinone three times per day versus placebo had improved left ventricular diastolic function after 30 days [42]. These studies suggest a role for CoQ10 in improving symptoms and cardiac function as well as reducing adverse clinical events.
Researchers have shown that ubiquinone and/or ubiquinol supplements reduce the symptoms of mitochondrial dysfunction and of aging because of an improvement in bioenergetics [43]. Over the past 30 years, many researchers have examined the effects of both ubiquinone and ubiquinol at various doses in patients with heart failure. One of the most recent studies called the Q-SYMBIO found a reduction in major adverse cardiovascular events with CoQ10 (ubiquinone) supplementation [18]. From this study came recommendations for powered randomized controlled trials of CoQ10 supplementation in patients with heart failure. Currently, we are funded by NIH and regulated by the FDA to study the effects of ubiquinol (300 mg softgels/twice daily/p.o. with food) and/or d-ribose (15 g/daily/p.o.) in patients with HFpEF [9].
4. d-ribose
d-ribose is monosaccharide that is naturally produced within the body within the pentose pathway. It is a critically important pentose sugar molecule that is part of the DNA and is essential for cellular ATP production. There are other mechanisms for ATP generation such as the pentose phosphate pathway (PPP). Through the PPP, d-ribose is an alternative to ETC to assist with ATP generation. Thus, many investigators are examining d-ribose in different pathologic conditions such as heart failure as well as prolonged physical exercise where ATP concentrations (relative to total nucleotides) are decreased [44].
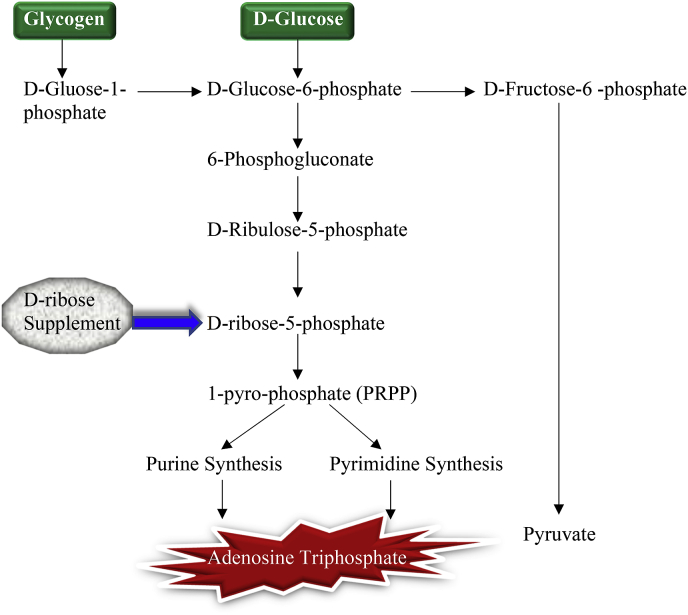
The de novo pathway is one of the first steps in making ATP in which nucleotides are produced using ribose. This is a slow pathway compared to the faster pathway called the salvage pathway in which the mitochondria use ATP metabolites to form new ATP molecules. Ribose is required for these pathways and is produced from glucose through the PPP [45]. The PPP is needed for the creation of an intermediate molecule in the production of ATP called 5-phosphyl-ribose-1-pyrophosphate (PRPP). The PRPP is needed in ATP de novo synthesis that is highly dependent on glucose 6-phosphte dehydrogenase (G6PDH) [46]. With d-ribose supplementation, this rate-limiting enzymatic step in the formation of PRPP is bypassed [47], and there is an increase in ATP production (Fig. 3). Different pathologic myocardial conditions can produce lower levels of ATP, thereby altering cardiac function [19]. Since these pathways are time-consuming and no foods are able to provide sufficient ribose to restore levels quickly [45], d-ribose supplements can replenish the cellular energy deficiency particularly in the myocardium [19,44].
Fig. 3.
Metabolism of carbohydrate with d-ribose supplementation. The long rate-controlling steps of the hexose monophosphate shunt can be bypassed with supplemental d-ribose as an alternate source for PRPP synthesis into purine and pyrimidine for adenosine triphosphate.
Several investigators have examined the effect of d-ribose in different cardiac conditions. Orman et al. studied 15 patients with chronic coronary artery disease and congestive heart failure. These patients received either oral d-ribose (15 g) or placebo over 3 weeks followed by a 1-week wash-out period. They reported that the patients consuming d-ribose had significant enhancement of atrial contribution to left ventricular filling as well as an improvement in their quality of life [48]. MacCarter et al. examined the role of d-ribose (15 g) on ventilation in 16 congestive heart failure patients. After 8 weeks, all of the patients had a significant improvement in the ventilatory parameters [49]. Another study of 11 patients with New York Heart Association class II-IV heart failure received only 5 g of oral d-ribose for 6 weeks. There was improvement in tissue doppler velocity (E′) that was maintained for 9 weeks with some of the patients showing better ratios of early diastolic filling velocity (E) to early annulus relaxation velocity (E’) [50].
In our current clinical trial, we believe supplemental oral d-ribose provides patients with HFpEF the extra substrate to bypass the G6PDH step and potentially increase the myocardial ATP levels. Though we are unable to measure myocardial ATP levels directly, we are obtaining blood ATP measurements in HFpEF patients receiving 15 g of d-ribose or placebo over 12 weeks. Since this current blinded study is still in progress, we are unable to report these data, but we will have a more in-depth metabolic explanation after study completion.
5. Conclusion
HFpEF is a common and often debilitating disease process that may be difficult to diagnose and has limited targeted therapeutic options. Mitochondrial dysfunction appears to play a significant role in the pathophysiologic processes of this clinical syndrome. Improved methods of diagnosis for HFpEF are needed, as are specific effective therapies. Active ongoing research into the roles of ubiquinol and d-ribose in HFpEF may provide clinicians with additional management opportunities.
Provenance and peer review
Not commissioned, externally peer reviewed.
Funding
Sponsoring Information: Supported by the Department of Health and Human Services, National Institutes of Health, National Institute on Aging (Grant Number: 1R01AG054486-01A1). Trial registration: ClinicalTrials.gov Identifier: NCT03133793.
Declaration of competing interest
There is no conflict of interest for any of the authors to declare.
References
- 1.Fini M L.M., Karoor V., Sullivan T., Stenmark K.R. International Conference, American Journal of Respiratory and Critical Care Medicine; Dallas, TX: 2019. Looking outside of the Lung to Treat Pulmonary Hypertension Due to Left Heart Disease; Lessons from a Novel Humanized Model of HFpEF-PH, American Thoracic Society 2019. [Google Scholar]
- 2.Kriegel A.J., Gartz M., Afzal M.Z., de Lange W.J., Ralphe J.C., Strande J.L. Molecular approaches in HFpEF: MicroRNAs and iPSC-derived cardiomyocytes. J. Cardiovasc. Transl. Res. 2017;10(3):295–304. doi: 10.1007/s12265-016-9723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tromp J., MacDonald M.R., Tay W.T., Teng T.K., Hung C.L., Narasimhan C., Shimizu W., Ling L.H., Ng T.P., Yap J., McMurray J.J.V., Zile M.R., Richards A.M., Anand I.S., Lam C.S.P. Heart failure with preserved ejection fraction in the young. Circulation. 2018;138(24):2763–2773. doi: 10.1161/CIRCULATIONAHA.118.034720. [DOI] [PubMed] [Google Scholar]
- 4.Rech M., Barandiaran Aizpurua A., van Empel V., van Bilsen M., Schroen B. Pathophysiological understanding of HFpEF: microRNAs as part of the puzzle. Cardiovasc. Res. 2018;114(6):782–793. doi: 10.1093/cvr/cvy049. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A.A., Kelly D.P., Chirinos J.A. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139(11):1435–1450. doi: 10.1161/CIRCULATIONAHA.118.036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., Isasi C.R., Jimenez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Mackey R.H., Matsushita K., Mozaffarian D., Mussolino M.E., Nasir K., Neumar R.W., Palaniappan L., Pandey D.K., Thiagarajan R.R., Reeves M.J., Ritchey M., Rodriguez C.J., Roth G.A., Rosamond W.D., Sasson C., Towfighi A., Tsao C.W., Turner M.B., Virani S.S., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.H., Alger H.M., Wong S.S., Muntner P., American Heart Association Statistics C., Stroke Statistics S. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ. Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., E.S.C.S.D. Group ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Pierce J.D., Mahoney D.E., Hiebert J.B., Thimmesch A.R., Diaz F.J., Smith C., Shen Q., Mudaranthakam D.P., Clancy R.L. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose. BMC Cardiovasc. Disord. 2018;18(1):57. doi: 10.1186/s12872-018-0796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Colvin M.M., Drazner M.H., Filippatos G.S., Fonarow G.C., Givertz M.M., Hollenberg S.M., Lindenfeld J., Masoudi F.A., McBride P.E., Peterson P.N., Stevenson L.W., Westlake C. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J. Am. Coll. Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. (2017) [DOI] [PubMed] [Google Scholar]
- 11.Gori M., Iacovoni A., Senni M. Haemodynamics of heart failure with preserved ejection fraction: a clinical perspective. Card. Fail. Rev. 2016;2(2):102–105. doi: 10.15420/cfr.2016:17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartipy U., Dahlstrom U., Fu M., Lund L.H. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5(8):565–574. doi: 10.1016/j.jchf.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Beale A.L., Nanayakkara S., Kaye D.M. Impact of sex on ventricular-vascular stiffness and long-term outcomes in heart failure with preserved ejection fraction: TOPCAT trial substudy. J. Am. Heart Assoc. 2019;8(13) doi: 10.1161/JAHA.119.012190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zile M.R., Baicu C.F., Ikonomidis J.S., Stroud R.E., Nietert P.J., Bradshaw A.D., Slater R., Palmer B.M., Van Buren P., Meyer M., Redfield M.M., Bull D.A., Granzier H.L., LeWinter M.M. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131(14):1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzman D.W., Herrington D.M., Brubaker P.H., Moore J.B., Eggebeen J., Haykowsky M.J. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61(1):112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen S.A., Rosenfeldt F., Kumar A., Dolliner P., Filipiak K.J., Pella D., Alehagen U., Steurer G., Littarru G.P., Investigators Q.S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Omran H., Illien S., MacCarter D., St Cyr J., Luderitz B. D-Ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur. J. Heart Fail. 2003;5(5):615–619. doi: 10.1016/s1388-9842(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A., Fonarow G.C., Butler J., Ezekowitz J.A., Felker G.M. Coenzyme Q10 and heart failure: a state-of-the-art review. Circ. Heart Fail. 2016;9(4) doi: 10.1161/CIRCHEARTFAILURE.115.002639. [DOI] [PubMed] [Google Scholar]
- 19.Shecterle L.M., Terry K.R., St Cyr J.A. Potential clinical benefits of D-ribose in ischemic cardiovascular disease. Cureus. 2018;10(3) doi: 10.7759/cureus.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiebert J.B., Shen Q., Thimmesch A., Pierce J. Impaired myocardial bioenergetics in HFpEF and the role of antioxidants. Open Cardiovasc. Med. J. 2016;10:158–162. doi: 10.2174/1874192401610010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raizner A.E. Coenzyme Q10. Methodist Debakey Cardiovasc. J. 2019;15(3):185–191. doi: 10.14797/mdcj-15-3-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyuna L.A., Albuquerque R.P.E., Chen C.H., Mochly-Rosen D., Ferreira J.C.B. Targeting mitochondrial dysfunction and oxidative stress in heart failure: challenges and opportunities. Free Radic. Biol. Med. 2018;129:155–168. doi: 10.1016/j.freeradbiomed.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros M.H., McStay G.P. Modular biogenesis of mitochondrial respiratory complexes. Mitochondrion. 2019;50:94–114. doi: 10.1016/j.mito.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Baker N., Patel J., Khacho M. Linking mitochondrial dynamics, cristae remodeling and supercomplex formation: how mitochondrial structure can regulate bioenergetics. Mitochondrion. 2019;49:259–268. doi: 10.1016/j.mito.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Bezawork-Geleta A., Rohlena J., Dong L., Pacak K., Neuzil J. Mitochondrial complex II: at the crossroads. Trends Biochem. Sci. 2017;42(4):312–325. doi: 10.1016/j.tibs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Hekimi S. The complexity of making ubiquinone. Trends Endocrinol. Metabol. 2019;30(12):929–943. doi: 10.1016/j.tem.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Haran M., Gross A. Balancing glycolysis and mitochondrial OXPHOS: lessons from the hematopoietic system and exercising muscles. Mitochondrion. 2014:3–7. doi: 10.1016/j.mito.2014.09.007. 19 Pt A. [DOI] [PubMed] [Google Scholar]
- 28.Sheeran F.L., Pepe S. Mitochondrial bioenergetics and dysfunction in failing heart. Adv. Exp. Med. Biol. 2017;982:65–80. doi: 10.1007/978-3-319-55330-6_4. [DOI] [PubMed] [Google Scholar]
- 29.Thummasorn S., Shinlapawittayatorn K., Khamseekaew J., Jaiwongkam T., Chattipakorn S.C., Chattipakorn N. Humanin directly protects cardiac mitochondria against dysfunction initiated by oxidative stress by decreasing complex I activity. Mitochondrion. 2018;38:31–40. doi: 10.1016/j.mito.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Kloer H.U., Belardinelli R., Ruchong O., Rosenfeldt F. Heart Lung Circ; 2019. Combining Ubiquinol with a Statin May Benefit Hypercholesterolaemic Patients with Chronic Heart Failure. [DOI] [PubMed] [Google Scholar]
- 31.Fragaki K., Chaussenot A., Benoist J.F., Ait-El-Mkadem S., Bannwarth S., Rouzier C., Cochaud C., Paquis-Flucklinger V. Coenzyme Q10 defects may be associated with a deficiency of Q10-independent mitochondrial respiratory chain complexes. Biol. Res. 2016;49:4. doi: 10.1186/s40659-015-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman D.N., Shah S.J. Treatment of heart failure with preserved ejection fraction (HFpEF): the phenotype-guided approach. Curr. Treat. Options Cardiovasc. Med. 2019;21(4):20. doi: 10.1007/s11936-019-0709-4. [DOI] [PubMed] [Google Scholar]
- 33.Hohendanner F., Bode D. Mitochondrial Calcium in heart failure with preserved ejection fraction-friend or foe? Acta Physiol. 2019 doi: 10.1111/apha.13415. [DOI] [PubMed] [Google Scholar]
- 34.Aimo A., Castiglione V., Borrelli C., Saccaro L.F., Franzini M., Masi S., Emdin M., Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2019 doi: 10.1177/2047487319870344. 2047487319870344. [DOI] [PubMed] [Google Scholar]
- 35.Mocan M., Mocan Hognogi L.D., Anton F.P., Chiorescu R.M., Goidescu C.M., Stoia M.A., Farcas A.D. Biomarkers of inflammation in left ventricular diastolic dysfunction. Dis. Markers. 2019;2019:7583690. doi: 10.1155/2019/7583690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura M., Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2019:1–17. doi: 10.1113/JP276747. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V., Kumar T.R.S., Kartha C.C. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail. Rev. 2019;24(2):255–267. doi: 10.1007/s10741-018-9756-2. [DOI] [PubMed] [Google Scholar]
- 38.Kettawan A., Kunthida C., Takahashi T., Kishi T., Chikazawa J., Sakata Y., Yano E., Watabe K., Yamamoto Y., Okamoto T. The quality control assessment of commercially available coenzyme q(10)-containing dietary and health supplements in Japan. J. Clin. Biochem. Nutr. 2007;41(2):124–131. doi: 10.3164/jcbn.2007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung C.K., Billings F.T.t., Claessens A.J., Roshanravan B., Linke L., Sundell M.B., Ahmad S., Shao B., Shen D.D., Ikizler T.A., Himmelfarb J. Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrol. 2015;16:183. doi: 10.1186/s12882-015-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mortensen A.L., Rosenfeldt F., Filipiak K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: a sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019;26(2):147–156. doi: 10.5603/CJ.a2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fotino A.D., Thompson-Paul A.M., Bazzano L.A. Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am. J. Clin. Nutr. 2013;97(2):268–275. doi: 10.3945/ajcn.112.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobirin M.A., Herry Y., Sofia S.N., Uddin I., Rifqi S., Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov. Ther. 2019;13(1):38–46. doi: 10.5582/ddt.2019.01004. [DOI] [PubMed] [Google Scholar]
- 43.Barcelos I.P., Haas R.H. CoQ10. Aging, Biol. (Basel) 2019;8(2) doi: 10.3390/biology8020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifert J.G., Brumet A., St Cyr J.A. The influence of D-ribose ingestion and fitness level on performance and recovery. J. Int. Soc. Sports Nutr. 2017;14:47. doi: 10.1186/s12970-017-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez M.J., Seyfried T., Nicolson G.L., Barclay B.J., Matta J., Vasquez A., D'Agostino D., Olalde J., Duconge J., Hunninghake R., Berdiel M.J., Cintrón A. Mitochondrial correction: a new therapeutic paradigm for cancer and degenerative diseases. J. Orthomol. Med. 2018;33(4) [Google Scholar]
- 46.Dodd S.L., Johnson C.A., Fernholz K., St Cyr J.A. The role of ribose in human skeletal muscle metabolism. Med. Hypotheses. 2004;62(5):819–824. doi: 10.1016/j.mehy.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Shecterle L., St Cyr J. Myocardial ischemia: alterations in myocardial cellular energy and diastolic function, a potential role for D-ribose. In: Lakshmanadoss U., editor. Novel Strategies in Ischemic Heart Disease. IntechOpen; 2012. [Google Scholar]
- 48.Omran H., Illien S., MacCarter D., St Cyr J., Luderitz B. D-Ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur. J. Heart Fail. 2003;5(5):615–619. doi: 10.1016/s1388-9842(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 49.MacCarter D., Vijay N., Washam M., Shecterle L., Sierminski H., St Cyr J.A. D-ribose aids advanced ischemic heart failure patients. Int. J. Cardiol. 2009;137(1):79–80. doi: 10.1016/j.ijcard.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Bayram M., St Cyr J.A., Abraham W.T. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther. Adv. Cardiovasc. Dis. 2015;9(3):56–65. doi: 10.1177/1753944715572752. [DOI] [PMC free article] [PubMed] [Google Scholar]